Marine Collagen Peptides Promote Cell Proliferation of NIH-3T3 Fibroblasts via NF-κB Signaling Pathway
Abstract
1. Introduction
2. Results and Discussions
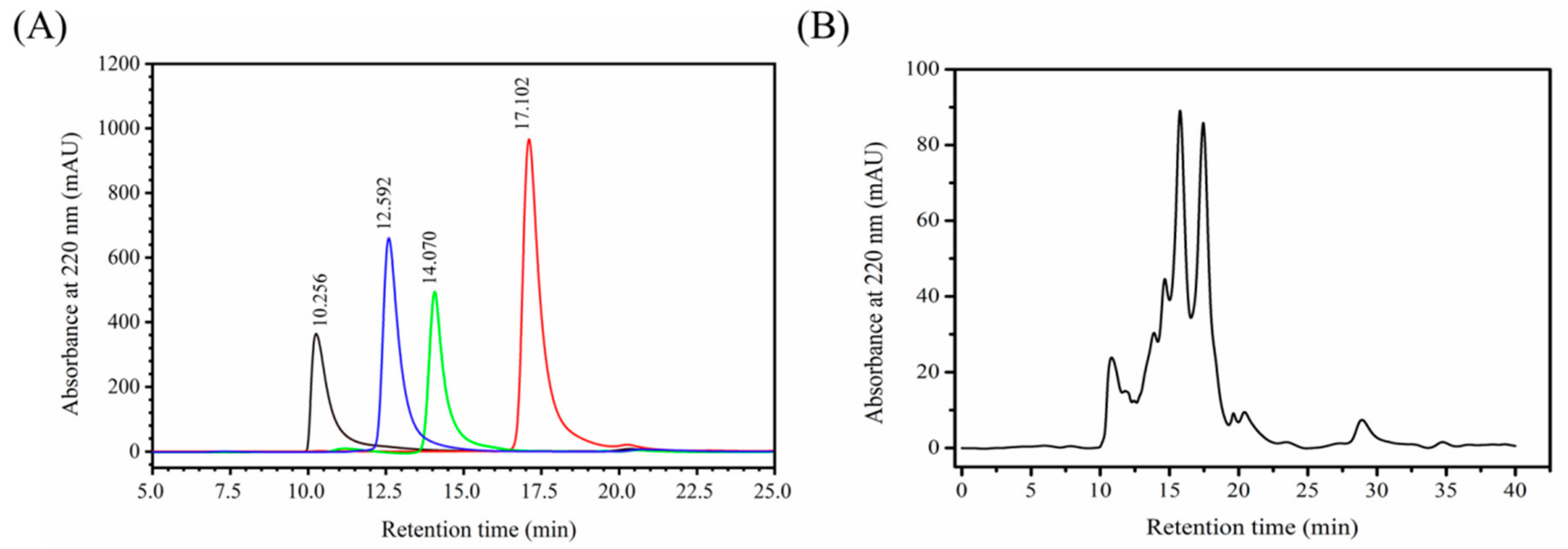
2.1. Determination of Molecular Weight Distribution of MCPs
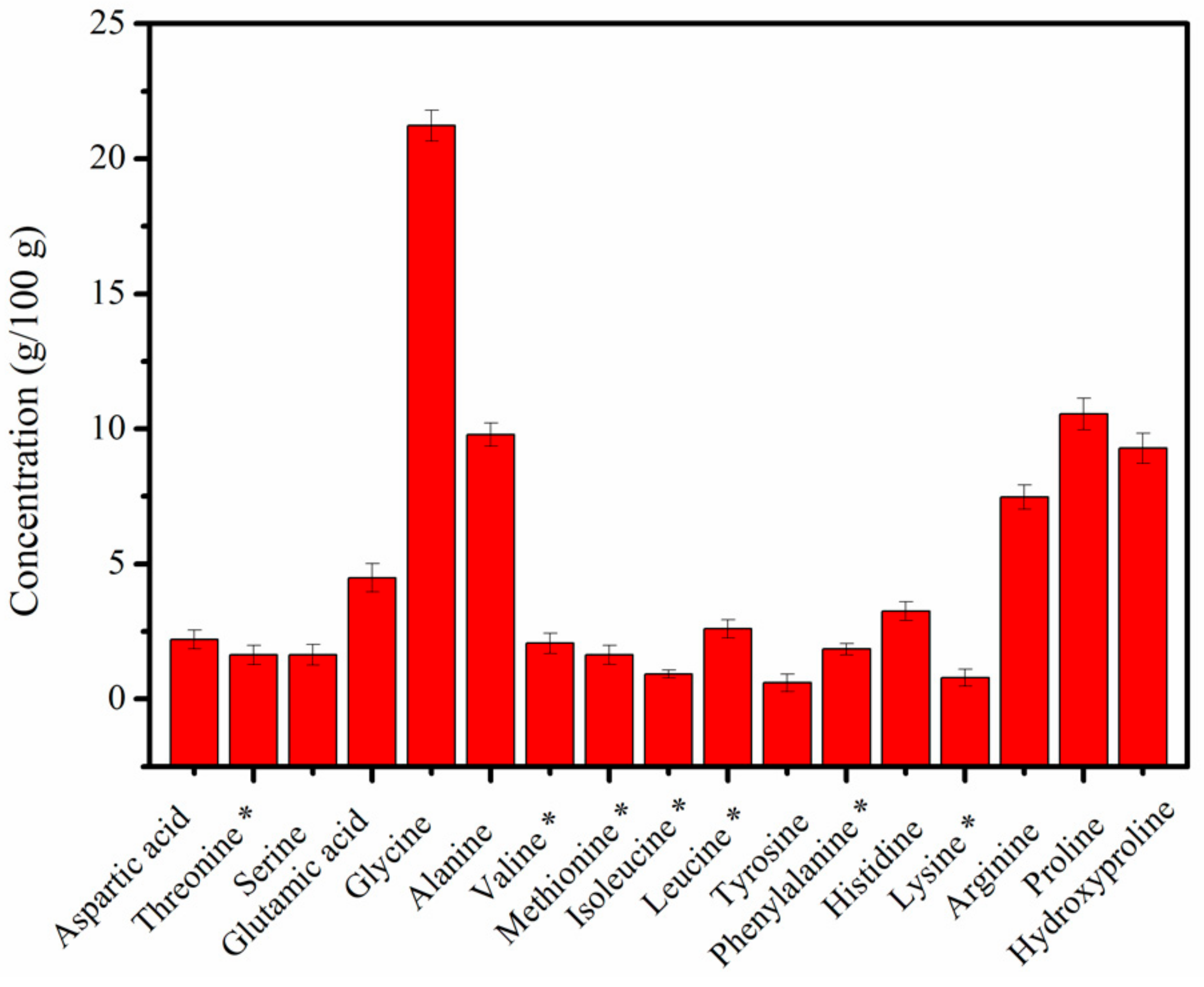
2.2. Amino Acid Content of MCPs
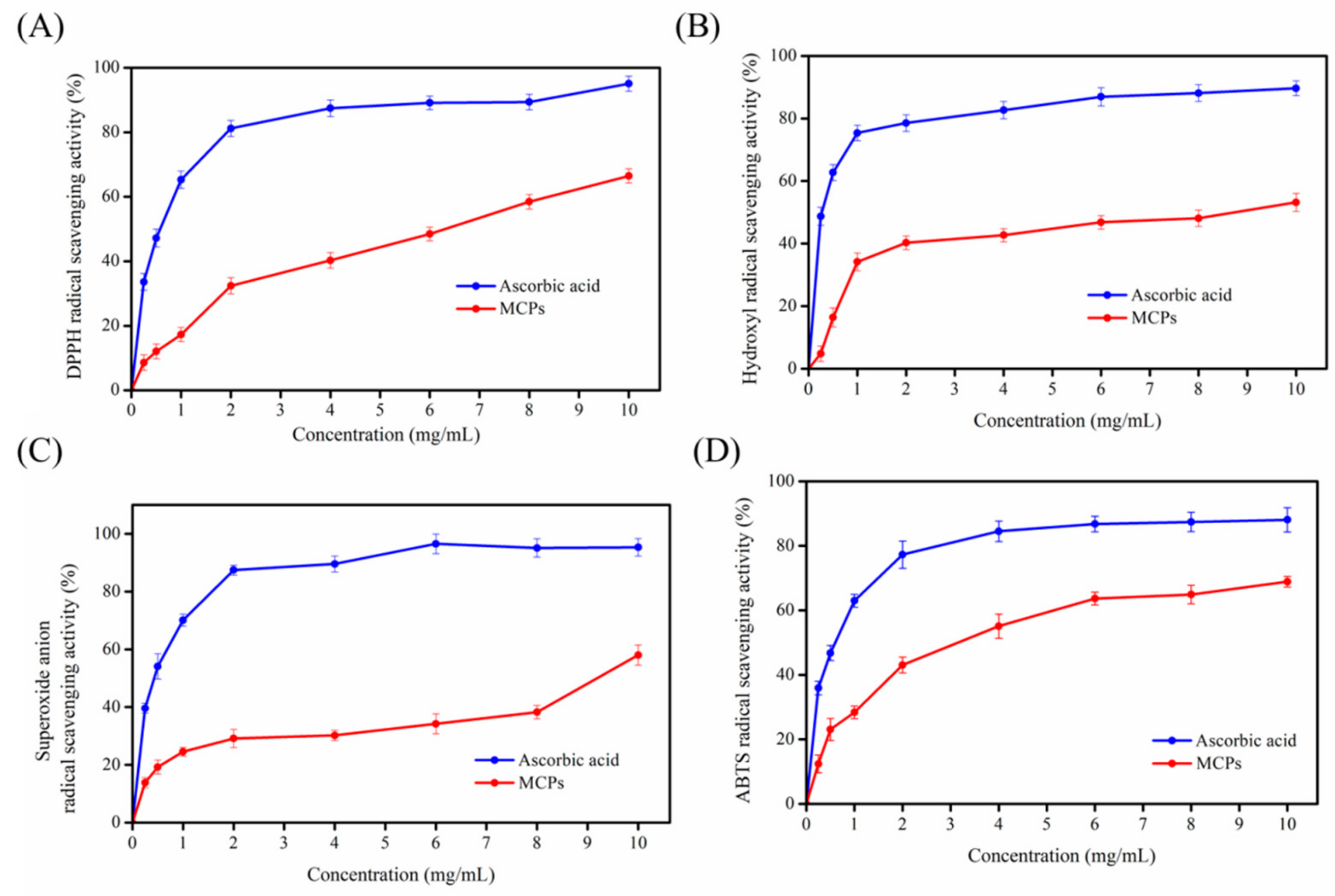
2.3. Antioxidant Activity of MCPs
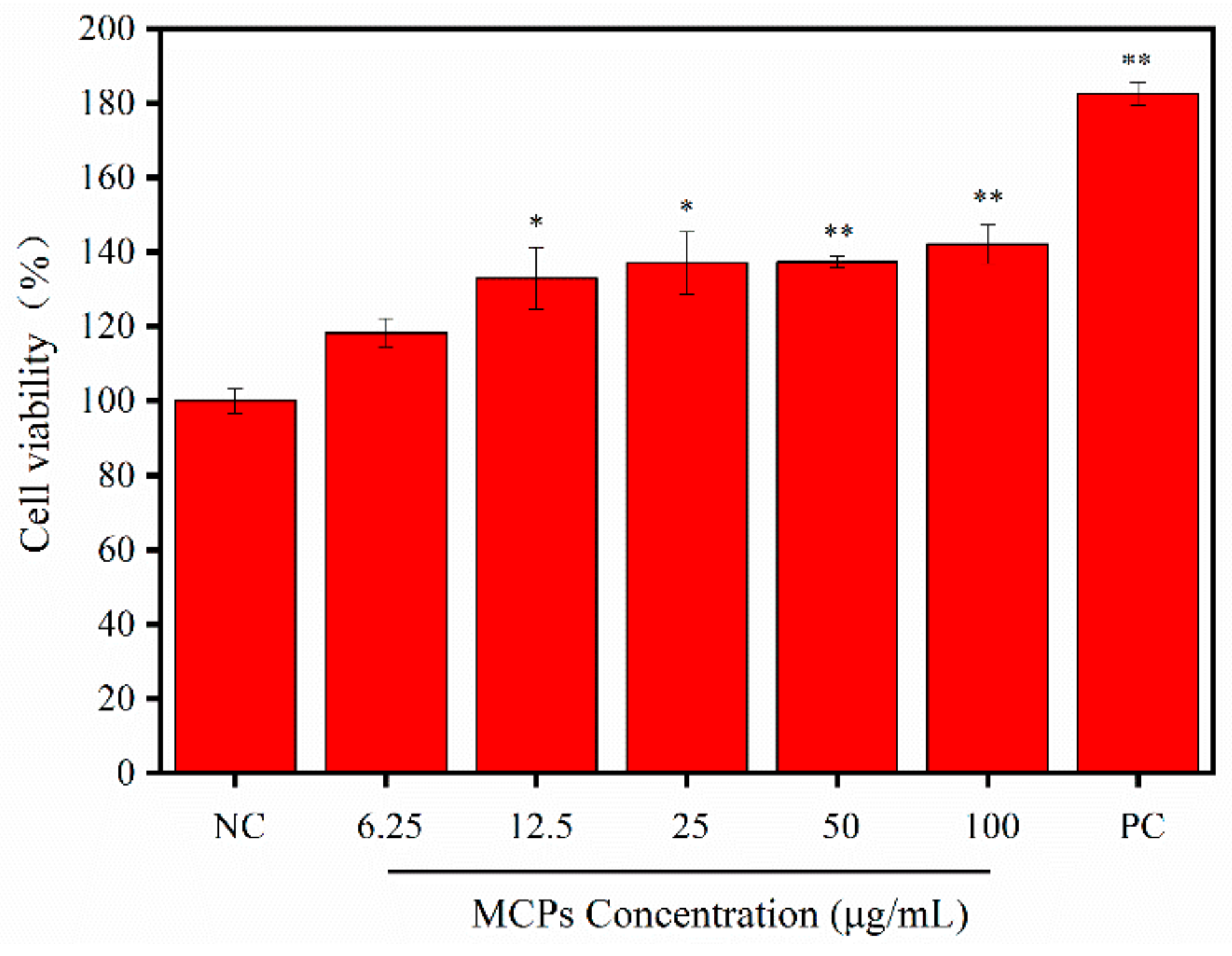
2.4. Cell Proliferation of NIH-3T3
2.5. Effect of MCPs on the Scratch Wound Closure In Vitro
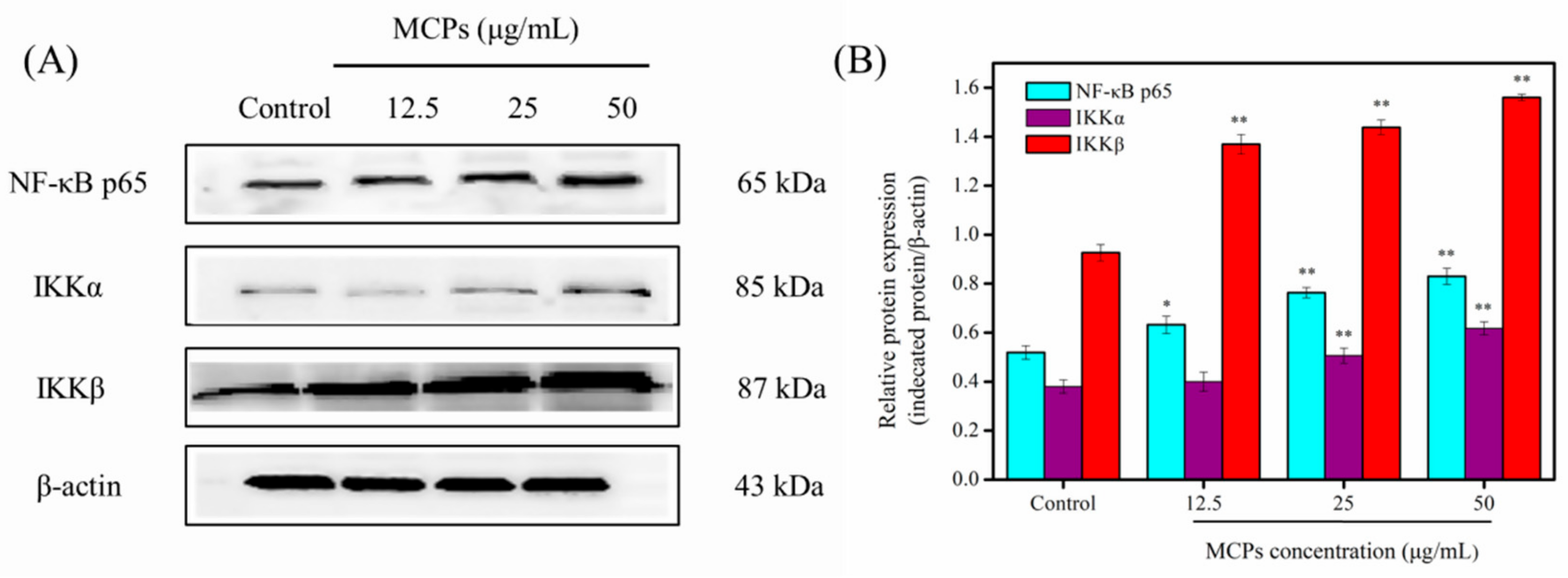
2.6. MCPs Activated the NF-κB Signaling Pathway in NIH-3T3 Fibroblasts
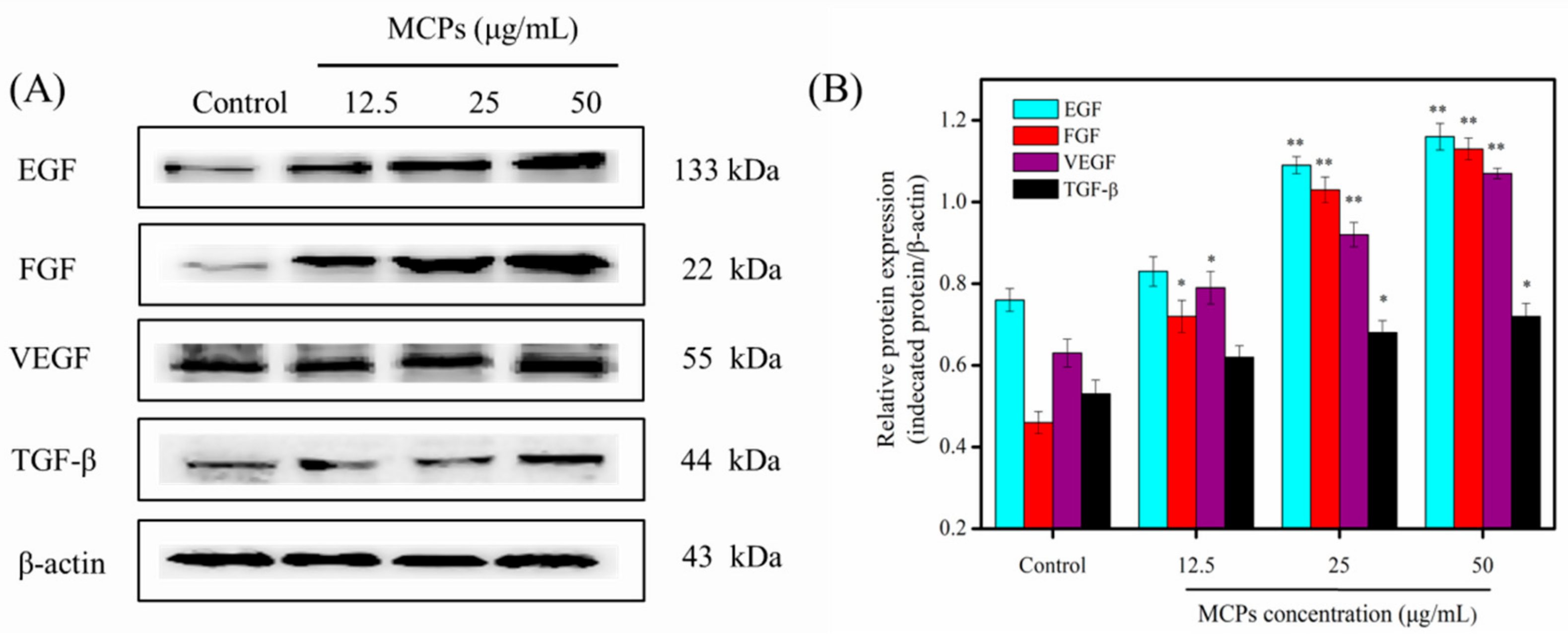
2.7. Western Blot Analysis of Growth Factors
3. Materials and Methods
3.1. Materials
3.2. Preparation of MCPs from N. japonica skin
3.3. Determination of the Molecular Weight Distribution of MCPs
3.4. Amino Acid Content
3.5. Antioxidant Activity of MCPs
3.6. Proliferation of NIH-3T3 Fibroblasts in Presence of MCPs
3.7. In Vitro Scratch Wound Assay
3.8. Western Blot Analysis
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lapena, D.; Vuoristo, K.S.; Kosa, G.; Horn, S.J.; Eijsink, V.G.H. Comparative Assessment of Enzymatic Hydrolysis for Valorization of Different Protein-Rich Industrial Byproducts. J. Agric. Food Chem. 2018, 66, 9738–9749. [Google Scholar] [CrossRef]
- Shavandi, A.; Hou, Y.; Carne, A.; McConnell, M.; Bekhit, A.E.-D.A. Marine Waste Utilization as a Source of Functional and Health Compounds. Adv. Food Nutr. Res. 2019, 87, 187–254. [Google Scholar]
- Wang, B.; Wang, Y.M.; Chi, C.F.; Luo, H.Y.; Deng, S.G.; Ma, J.Y. Isolation and characterization of collagen and antioxidant collagen peptides from scales of croceine croaker (Pseudosciaena crocea). Mar. Drugs 2013, 11, 4641–4661. [Google Scholar] [CrossRef]
- Li, X.J.; Tang, Y.P.; Yu, F.M.; Sun, Y.; Huang, F.F.; Chen, Y.; Yang, Z.S.; Ding, G.F. Inhibition of Prostate Cancer DU-145 Cells Proliferation by Anthopleura anjunae Oligopeptide (YVPGP) via PI3K/AKT/mTOR Signaling Pathway. Mar. Drugs 2018, 16, 16. [Google Scholar] [CrossRef]
- Zhou, Q.J.; Wang, J.; Mao, Y.; Liu, M.; Su, Y.Q.; Ke, Q.Z.; Chen, J.; Zheng, W.Q. Molecular structure, expression and antibacterial characterization of a novel antimicrobial peptide NK-lysin from the large yellow croaker Larimichthys crocea. Aquaculture 2019, 500, 315–321. [Google Scholar] [CrossRef]
- Yu, F.M.; Zhang, Z.W.; Luo, L.W.; Zhu, J.X.; Huang, F.F.; Yang, Z.S.; Tang, Y.P.; Ding, G.F. Identification and Molecular Docking Study of a Novel Angiotensin-I Converting Enzyme Inhibitory Peptide Derived from Enzymatic Hydrolysates of Cyclina sinensis. Mar. Drugs 2018, 16, 411. [Google Scholar] [CrossRef]
- Li, W.; Ye, S.; Zhang, Z.; Tang, J.; Jin, H.; Huang, F.; Yang, Z.; Tang, Y.; Chen, Y.; Ding, G.; et al. Purification and Characterization of a Novel Pentadecapeptide from Protein Hydrolysates of Cyclina sinensis and Its Immunomodulatory Effects on RAW264.7 Cells. Mar. Drugs 2019, 17, 30. [Google Scholar] [CrossRef]
- Nouvong, A.; Ambrus, A.M.; Zhang, E.R.; Hultman, L.; Coller, H.A. Reactive oxygen species and bacterial biofilms in diabetic wound healing. Physiol. Genomics 2016, 48, 889–896. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, R.; Wen, Z.Y. Bioactive compounds and biological functions of sea cucumbers as potential functional foods. J. Funct. Foods 2018, 49, 73–84. [Google Scholar] [CrossRef]
- Umnyakova, E.S.; Gorbunov, N.P.; Zhakhov, A.V.; Krenev, I.A.; Ovchinnikova, T.V.; Kokryakov, V.N.; Berlov, M.N. Modulation of Human Complement System by Antimicrobial Peptide Arenicin-1 from Arenicola marina. Mar. Drugs 2018, 16, 480. [Google Scholar] [CrossRef]
- Tang, Y.; Jin, S.; Li, X.; Li, X.; Hu, X.; Chen, Y.; Huang, F.; Yang, Z.; Yu, F.; Ding, G. Physicochemical Properties and Biocompatibility Evaluation of Collagen from the Skin of Giant Croaker (Nibea japonica). Mar. Drugs 2018, 16, 222. [Google Scholar] [CrossRef]
- Khong, N.M.H.; Yusoff, F.M.; Jamilah, B.; Basri, M.; Maznah, I.; Chan, K.W.; Armania, N.; Nishikawa, J. Improved collagen extraction from jellyfish (Acromitus hardenbergi) with increased physical-induced solubilization processes. Food Chem. 2018, 251, 41–50. [Google Scholar] [CrossRef]
- Hu, Z.; Yang, P.; Zhou, C.; Li, S.; Hong, P. Marine Collagen Peptides from the Skin of Nile Tilapia (Oreochromis niloticus): Characterization and Wound Healing Evaluation. Mar. Drugs 2017, 15, 102. [Google Scholar] [CrossRef]
- You, L.; Zhao, M.; Regenstein, J.M.; Ren, J. Purification and identification of antioxidative peptides from loach (Misgurnus anguillicaudatus) protein hydrolysate by consecutive chromatography and electrospray ionization-mass spectrometry. Food Res. Int. 2010, 43, 1167–1173. [Google Scholar] [CrossRef]
- Peng, Z.; Hou, H.; Zhang, K.; Li, B.F. Effect of calcium-binding peptide from Pacific cod (Gadus macrocephalus) bone on calcium bioavailability in rats. Food Chem. 2017, 221, 373–378. [Google Scholar] [CrossRef]
- Byun, H.G.; Kim, S.K. Purification and characterization of angiotensin I converting enzyme (ACE) inhibitory peptides from Alaska pollack (Theragra chalcogramma) skin. Process. Biochem. 2001, 36, 1155–1162. [Google Scholar] [CrossRef]
- Felician, F.F.; Xia, C.L.; Qi, W.Y.; Xu, H.M. Collagen from Marine Biological Sources and Medical Applications. Chem. Biodivers. 2018, 15, 1700557. [Google Scholar] [CrossRef]
- Ouyang, Q.Q.; Hu, Z.; Lin, Z.P.; Quan, W.Y.; Deng, Y.F.; Li, S.D.; Li, P.W.; Chen, Y. Chitosan hydrogel in combination with marine peptides from tilapia for burns healing. Int. J. Biol. Macromol. 2018, 112, 1191–1198. [Google Scholar] [CrossRef]
- Tingting, Y.; Kai, Z.; Bafang, L.; Hu, H. Effects of oral administration of peptides with low molecular weight from Alaska Pollock (Theragra chalcogramma) on cutaneous wound healing. J. Funct. Foods 2018, 48, 682–691. [Google Scholar]
- Park, Y.R.; Sultan, M.T.; Park, H.J.; Lee, J.M.; Ju, H.W.; Lee, O.J.; Lee, D.J.; Kaplan, D.L.; Park, C.H. NF-kappa B signaling is key in the wound healing processes of silk fibroin. Acta Biomater. 2018, 67, 183–195. [Google Scholar] [CrossRef]
- Wang, L.; Wu, X.; Shi, T.; Lu, L. Epidermal growth factor (EGF)-induced corneal epithelial wound healing through nuclear factor kappaB subtype-regulated CCCTC binding factor (CTCF) activation. J. Biol. Chem. 2013, 288, 24363–24371. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Ye, L.; Yang, X.; Wang, B.; Yang, C.; Gu, J.; Yu, H. Reconstruction of the Catalytic Pocket and Enzyme-Substrate Interactions To Enhance the Catalytic Efficiency of a Short-Chain Dehydrogenase/Reductase. Chemcatchem 2016, 8, 3229–3233. [Google Scholar] [CrossRef]
- Yu, F.; Zong, C.; Jin, S.; Zheng, J.; Chen, N.; Huang, J.; Chen, Y.; Huang, F.; Yang, Z.; Tang, Y.; et al. Optimization of Extraction Conditions and Characterization of Pepsin-Solubilised Collagen from Skin of Giant Croaker (Nibea japonica). Mar. Drugs 2018, 16, 29. [Google Scholar] [CrossRef] [PubMed]
- Noman, A.; Xu, Y.S.; Al-Bukhaiti, W.Q.; Abed, S.M.; Ali, A.H.; Ramadhan, A.H.; Xia, W.S. Influence of enzymatic hydrolysis conditions on the degree of hydrolysis and functional properties of protein hydrolysate obtained from Chinese sturgeon (Acipenser sinensis) by using papain enzyme. Process. Biochem. 2018, 67, 19–28. [Google Scholar] [CrossRef]
- Gbogouri, G.A.; Linder, M.; Fanni, J.; Parmentier, M. Influence of hydrolysis degree on the functional properties of salmon byproducts hydrolysates. J. Food Sci. 2004, 69, 615–622. [Google Scholar] [CrossRef]
- Son, D.; Yang, D.; Sun, J.; Kim, S.; Kang, N.; Kang, J.; Choi, Y.-H.; Lee, J.; Moh, S.; Shin, D.; et al. A Novel Peptide, Nicotinyl-Isoleucine-Valine-Histidine (NA-IVH), Promotes Antioxidant Gene Expression and Wound Healing in HaCaT Cells. Marine Drugs 2018, 16, 262. [Google Scholar] [CrossRef]
- Schafer, M.; Werner, S. Oxidative stress in normal and impaired wound repair. Pharmacol. Res. 2008, 58, 165–171. [Google Scholar] [CrossRef]
- Pozzolini, M.; Millo, E.; Oliveri, C.; Mirata, S.; Salis, A.; Damonte, G.; Arkel, M.; Scarfì, S. Elicited ROS scavenging activity, photoprotective, and wound-healing properties of collagen-derived peptides from the marine sponge Chondrosia reniformis. Mar. Drugs 2018, 16, 465. [Google Scholar] [CrossRef]
- Eming, S.A.; Martin, P.; Tomic-Canic, M. Wound repair and regeneration: Mechanisms, signaling, and translation. Sci. Transl. Med. 2014, 6, 16. [Google Scholar] [CrossRef]
- Liu, H.; Mu, L.X.; Tang, J.; Shen, C.B.; Gao, C.; Rong, M.Q.; Zhang, Z.Y.; Liu, J.; Wu, X.Y.; Yu, H.N.; et al. A potential wound healing-promoting peptide from frog skin. Int. J. Biochem. Cell Biol. 2014, 49, 32–41. [Google Scholar] [CrossRef]
- Chen, J.; Chen, Y.; Chen, Y.; Yang, Z.; You, B.; Ruan, Y.C.; Peng, Y. Epidermal CFTR Suppresses MAPK/NF-kappaB to Promote Cutaneous Wound Healing. Cell Physiol. Biochem. 2016, 39, 2262–2274. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Chen, X.; Shen, X.; He, Y.; Chen, W.; Luo, Q.; Ge, W.; Yuan, W.; Tang, X.; Hou, D.; et al. Preparation of chitosan-collagen-alginate composite dressing and its promoting effects on wound healing. Int. J. Biol. Macromol. 2018, 107, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Kuroyanagi, M.; Yamamoto, A.; Shimizu, N.; Ishihara, E.; Ohno, H.; Takeda, A.; Kuroyanagi, Y. Development of cultured dermal substitute composed of hyaluronic acid and collagen spongy sheet containing fibroblasts and epidermal growth factor. J. Biomater. Sci. Polym. Ed. 2014, 25, 1133–1143. [Google Scholar] [CrossRef] [PubMed]
- Yoo, Y.; Hyun, H.; Yoon, S.J.; Kim, S.Y.; Lee, D.W.; Um, S.; Hong, S.O.; Yang, D.H. Visible light-cured glycol chitosan hydrogel dressing containing endothelial growth factor and basic fibroblast growth factor accelerates wound healing in vivo. J. Ind. Eng. Chem. 2018, 67, 365–372. [Google Scholar] [CrossRef]
- Ning, J.L.; Zhao, H.L.; Chen, B.; Mi, E.Z.L.; Yang, Z.; Qing, W.H.; Lam, K.W.J.; Yi, B.; Chen, Q.; Gu, J.T.; et al. Argon Mitigates Impaired Wound Healing Process and Enhances Wound Healing In Vitro and In Vivo. Theranostics 2019, 9, 477–490. [Google Scholar] [CrossRef]
- Tsoupras, A.B.; Iatrou, C.; Frangia, C.; Demopoulos, C.A. The implication of platelet activating factor in cancer growth and metastasis: Potent beneficial role of PAF-inhibitors and antioxidants. Infect. Disord. Drug Targets 2009, 9, 390–399. [Google Scholar] [CrossRef]
- Zhao, W.H.; Chi, C.F.; Zhao, Y.Q.; Wang, B. Preparation, Physicochemical and Antioxidant Properties of Acid- and Pepsin-Soluble Collagens from the Swim Bladders of Miiuy Croaker (Miichthys miiuy). Mar. Drugs 2018, 16, 161. [Google Scholar] [CrossRef]
- Jiang, S.; Jia, Y.; Tang, Y.; Zheng, D.; Han, X.; Yu, F.; Chen, Y.; Huang, F.; Yang, Z.; Ding, G. Anti-Proliferation Activity of a Decapeptide from Perinereies aibuhitensis toward Human Lung Cancer H1299 Cells. Mar. Drugs 2019, 17, 122. [Google Scholar] [CrossRef]
Sample Availability: Samples are available from the first or corresponding author. |

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, F.; Jin, S.; Tang, Y. Marine Collagen Peptides Promote Cell Proliferation of NIH-3T3 Fibroblasts via NF-κB Signaling Pathway. Molecules 2019, 24, 4201. https://doi.org/10.3390/molecules24224201
Yang F, Jin S, Tang Y. Marine Collagen Peptides Promote Cell Proliferation of NIH-3T3 Fibroblasts via NF-κB Signaling Pathway. Molecules. 2019; 24(22):4201. https://doi.org/10.3390/molecules24224201
Chicago/Turabian StyleYang, Fei, Shujie Jin, and Yunping Tang. 2019. "Marine Collagen Peptides Promote Cell Proliferation of NIH-3T3 Fibroblasts via NF-κB Signaling Pathway" Molecules 24, no. 22: 4201. https://doi.org/10.3390/molecules24224201
APA StyleYang, F., Jin, S., & Tang, Y. (2019). Marine Collagen Peptides Promote Cell Proliferation of NIH-3T3 Fibroblasts via NF-κB Signaling Pathway. Molecules, 24(22), 4201. https://doi.org/10.3390/molecules24224201




