Two-Step Azidoalkenylation of Terminal Alkenes Using Iodomethyl Sulfones
Abstract
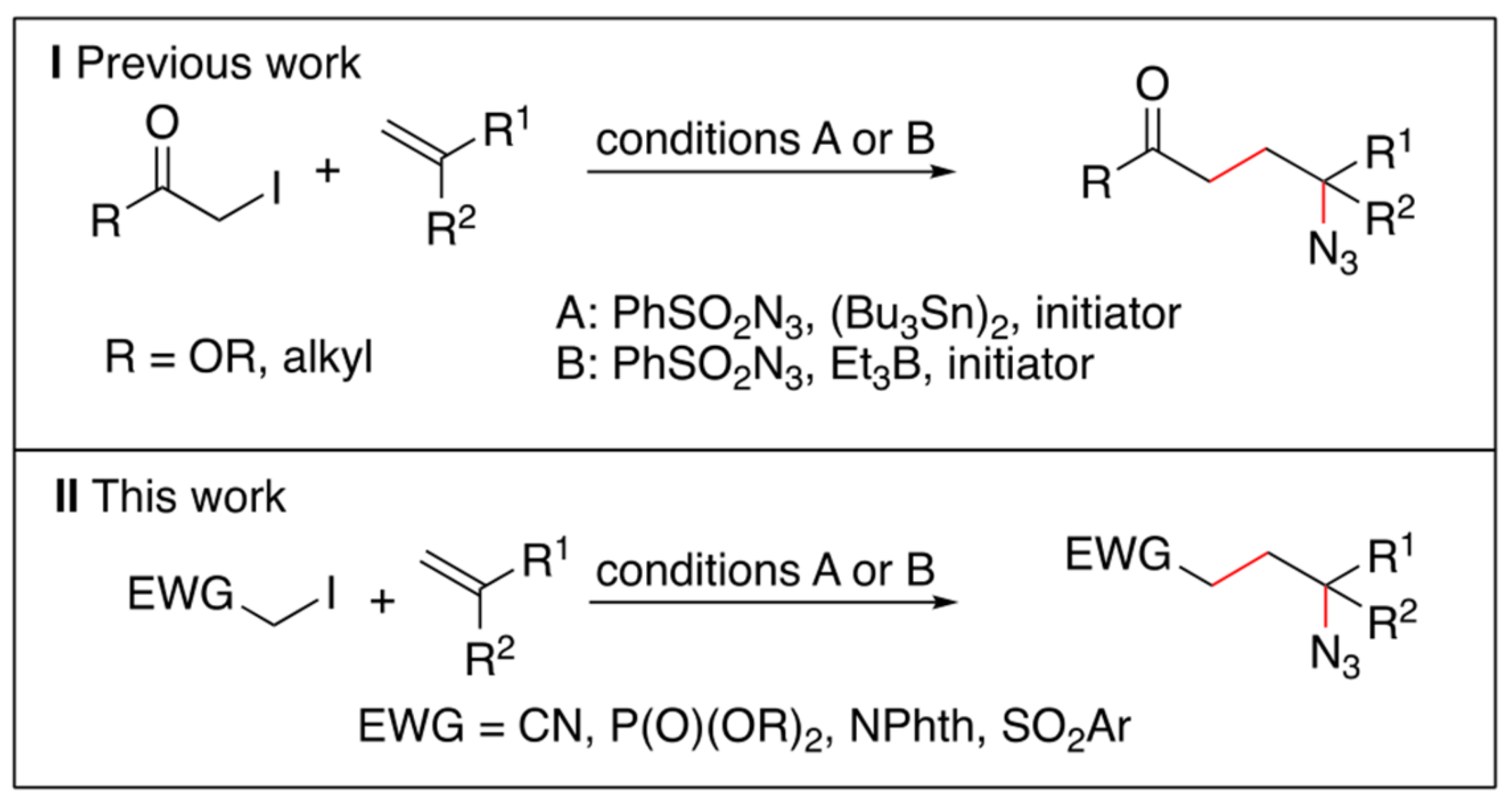
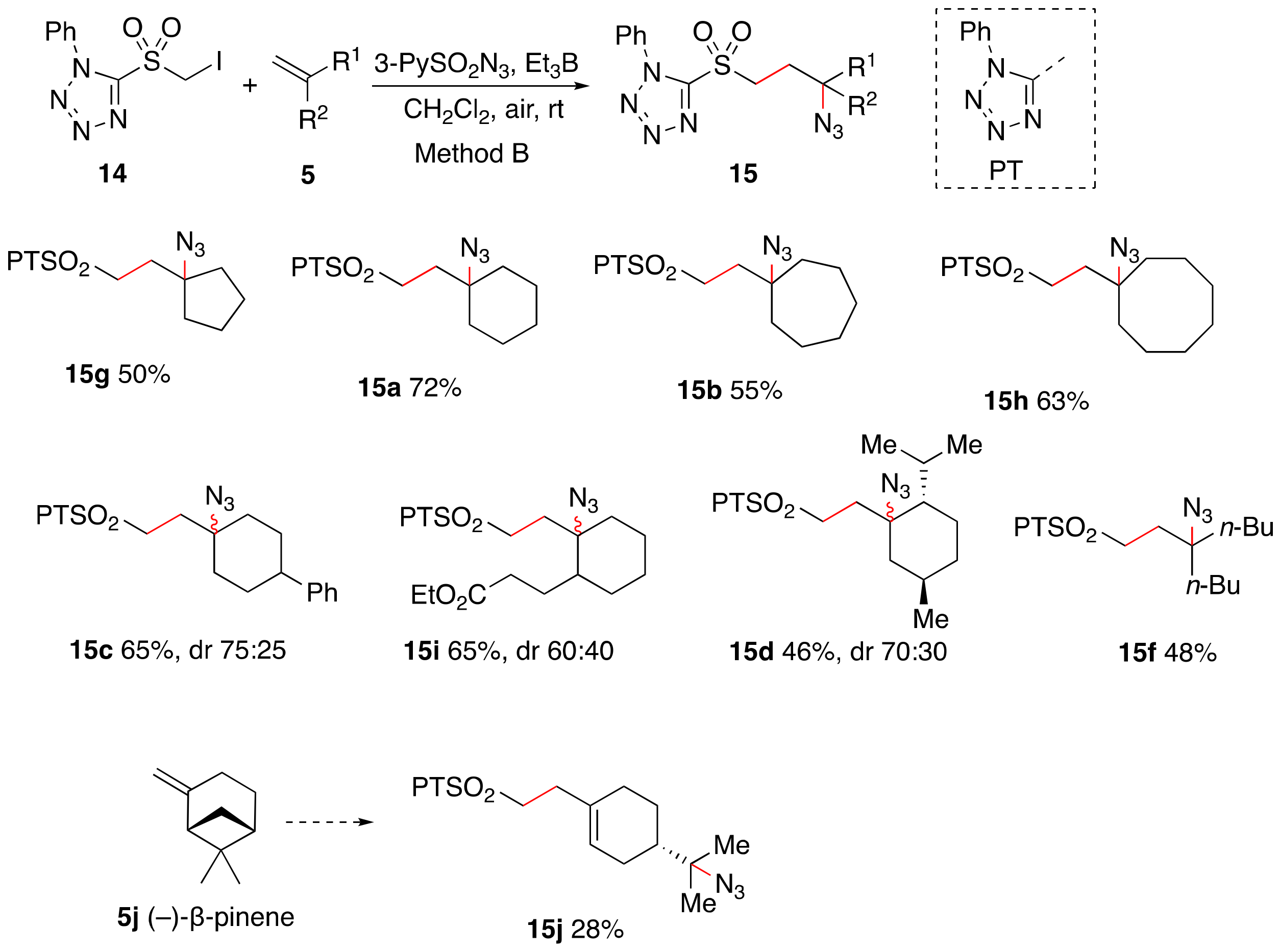
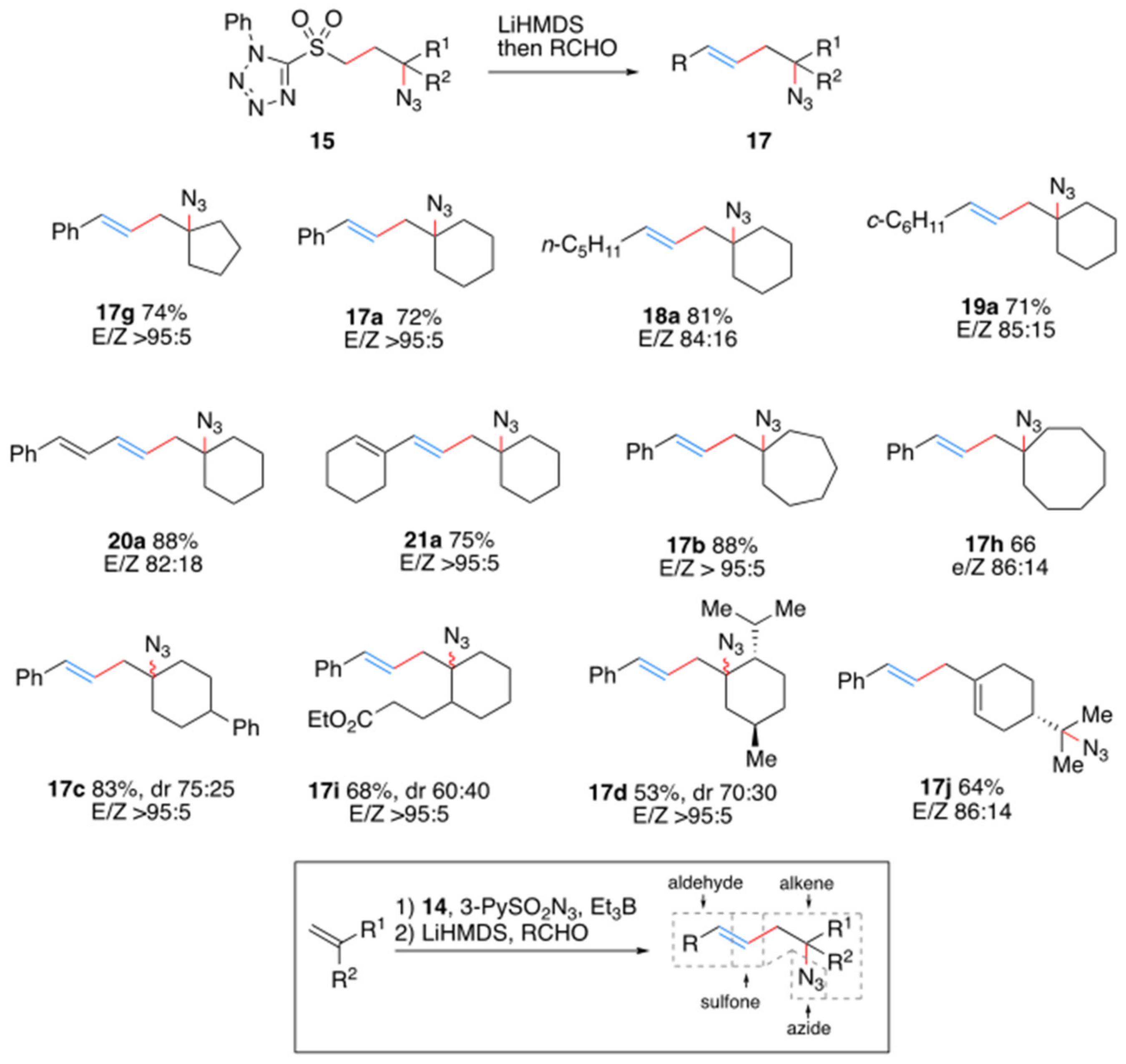
1. Introduction
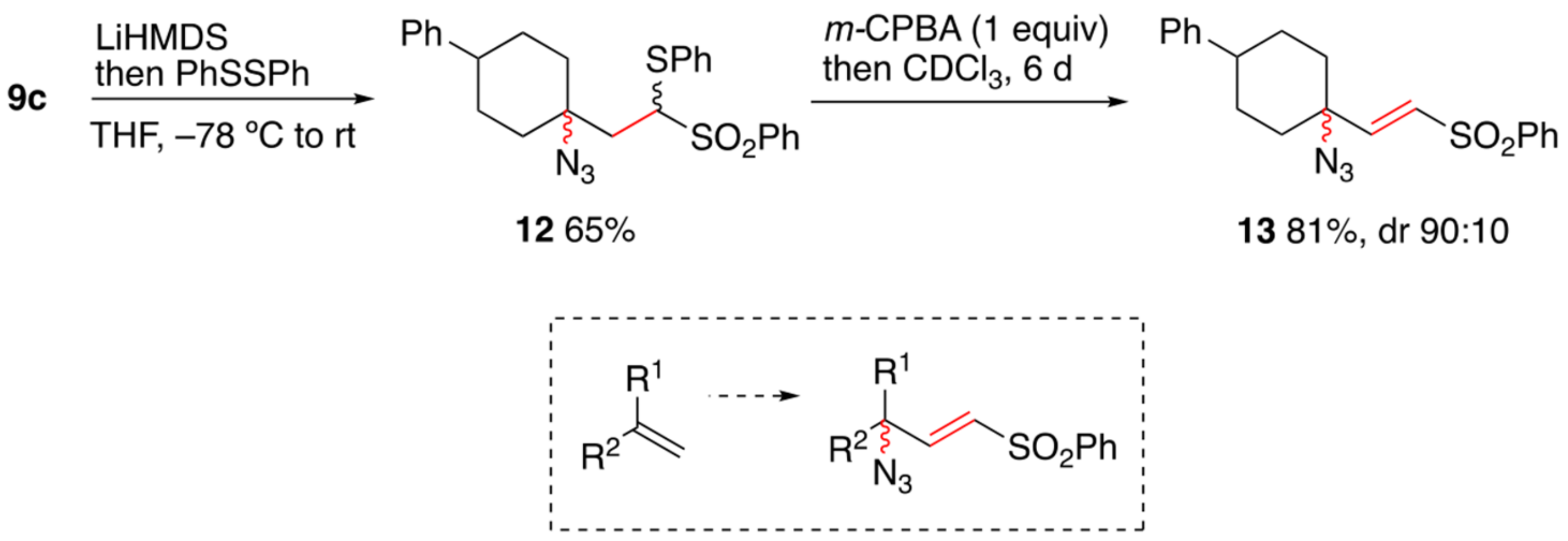
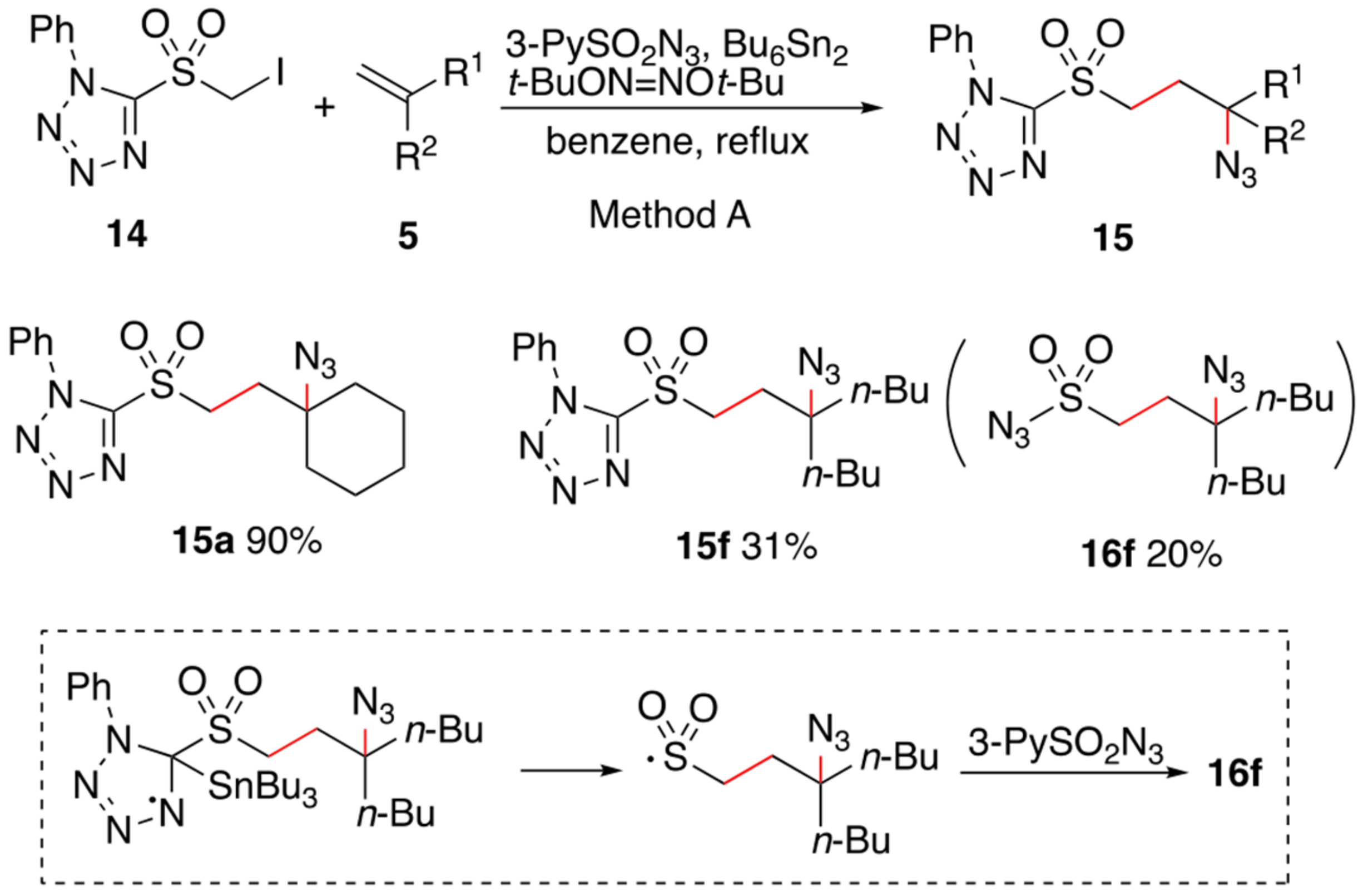
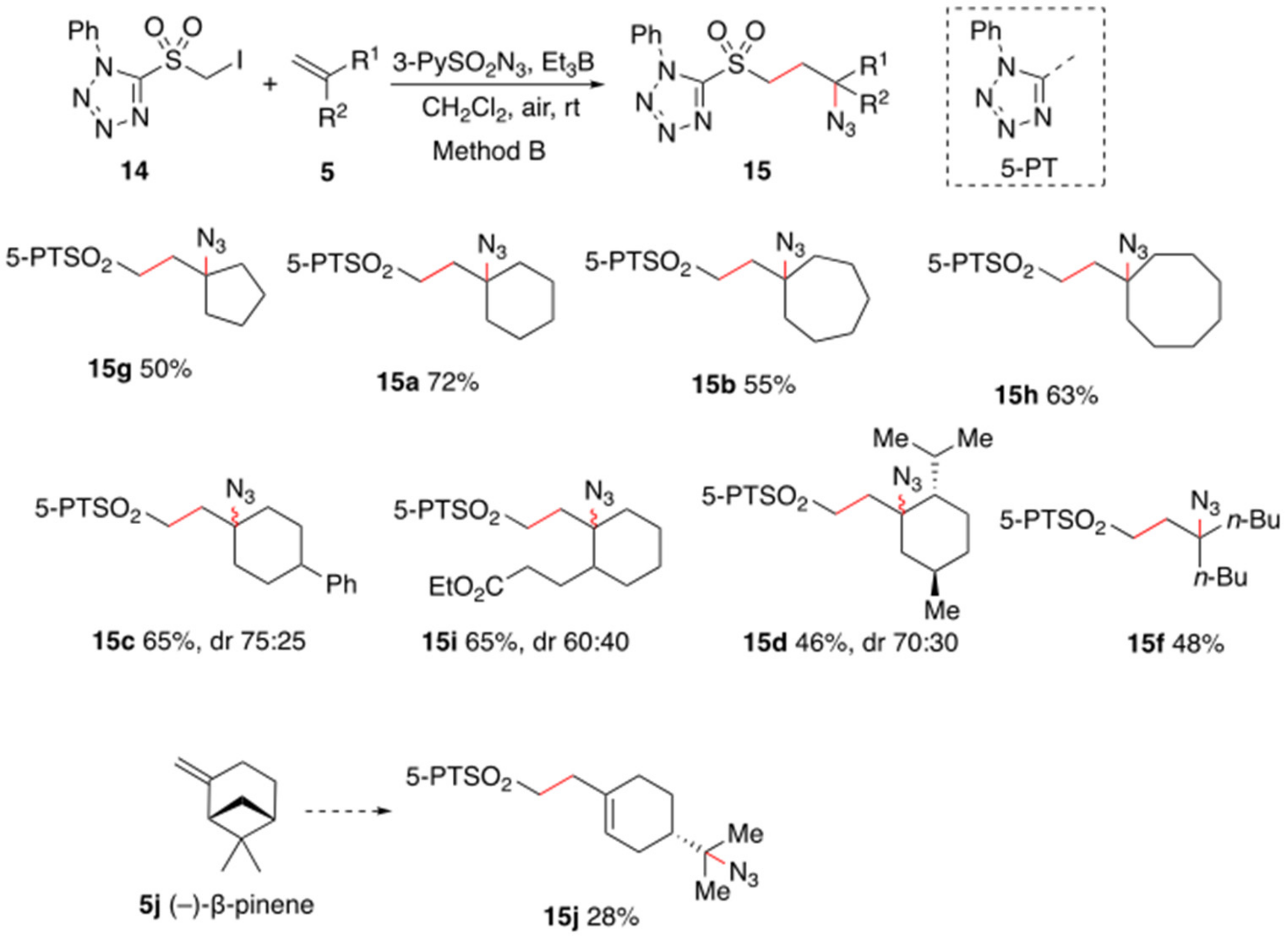
2. Results
3. Experimental Procedures
3.1. General Methods
3.2. General Procedures
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bräse, S.; Banert, K. Organic Azides: Syntheses and Applications; John Wiley & Sons: Chichester, UK, 2010; ISBN 978-0-470-51998-1. [Google Scholar]
- Tanimoto, H.; Kakiuchi, K. Recent applications and developments of organic azides in total synthesis of natural products. Nat. Prod. Commun. 2013, 8, 1021–1034. [Google Scholar] [CrossRef]
- Chiba, S. Application of organic azides for the synthesis of nitrogen-containing molecules. Synlett 2012, 23, 21–44. [Google Scholar] [CrossRef]
- Bräse, S.; Gil, C.; Knepper, K.; Zimmermann, V. Organic azides. An exploding diversity of a unique class of compounds. Angew. Chem. Int. Ed. 2005, 44, 5188–5240. [Google Scholar] [CrossRef]
- Gritsan, N.; Platz, M. Photochemistry of azides: The azide/nitrene interface. In Organic Azides—Syntheses and Applications; Bräse, S., Banert, K., Eds.; John Wiley & Sons: Chichester, UK, 2010; pp. 311–372. ISBN 978-0-470-68251-7. [Google Scholar]
- Kim, S. Radical cyclizations involving the evolution of nitrogen. Pure Appl. Chem. 1996, 68, 623–626. [Google Scholar] [CrossRef]
- Kim, S.; Joe, G.H.; Do, J.Y. Novel radical cyclizations of alkyl azides. A new route to N-Heterocycles. J. Am. Chem. Soc. 1994, 116, 5521–5522. [Google Scholar] [CrossRef]
- Montevecchi, P.C.; Navacchia, M.L.; Spagnolo, P. A study of vinyl radical cyclization onto the azido group by addition of sulfanyl, stannyl, and silyl radicals to alkynyl azides. Eur. J. Org. Chem. 1998, 1219–1226. [Google Scholar] [CrossRef]
- Wyler, B.; Brucelle, F.; Renaud, P. Preparation of the core structure of aspidosperma and strychnos alkaloids from aryl azides by a cascade radical cyclization. Org. Lett. 2016, 18, 1370–1373. [Google Scholar] [CrossRef] [PubMed]
- Brucelle, F.; Renaud, P. Synthesis of a leucomitosane via a diastereoselective radical cascade. J. Org. Chem. 2013, 78, 6245–6252. [Google Scholar] [CrossRef] [PubMed]
- Minozzi, M.; Nanni, D.; Spagnolo, P. From azides to nitrogen-centered radicals: Applications of azide radical chemistry to organic synthesis. Chem. Eur. J. 2009, 15, 7830–7840. [Google Scholar] [CrossRef] [PubMed]
- Wrobleski, A.; Coombs, T.C.; Huh, C.W.; Li, S.-W.; Aubé, J. The Schmidt reaction. Org. React. 2012, 78, 1–320. [Google Scholar]
- Aubé, J.; Fehl, C.; Liu, R.; McLeod, M.C.; Motiwala, H.F. Hofmann, Curtius, Schmidt, Lossen, and related reactions. Compr. Org. Synth. 2014, 6, 598–635. [Google Scholar]
- Nyfeler, E.; Renaud, P. Intramolecular Schmidt reaction: Applications in natural product synthesis. CHIMIA Int. J. Chem. 2006, 60, 276–284. [Google Scholar] [CrossRef]
- Palacios, F.; Alonso, C.; Aparicio, D.; Rubiales, G.; de los Santos, J.M. Aza-Wittig reaction in natural product syntheses. In Organic Azides; Wiley: Chichester, UK, 2010; pp. 437–467. ISBN 978-0-470-68251-7. [Google Scholar]
- Binder, W.H.; Kluger, C. Azide/alkyne-”click” reactions: Applications in material science and organic synthesis. Curr. Org. Chem. 2006, 10, 1791–1815. [Google Scholar] [CrossRef]
- Schilling, C.; Jung, N.; Bräse, S. Cycloaddition Reactions with azides: An overview. In Organic Azides—Syntheses and Applications; Bräse, S., Banert, K., Eds.; John Wiley & Sons: Chichester, UK, 2010; pp. 269–284. ISBN 978-0-470-68251-7. [Google Scholar]
- Pinho e Melo, T.M.V.D. Synthesis of azides. In Organic Azides—Syntheses and Applications; Bräse, S., Banert, K., Eds.; John Wiley & Sons: Chichester, UK, 2010; pp. 53–94. ISBN 978-0-470-68251-7. [Google Scholar]
- Jimeno, C.; Renaud, P. Radical chemistry with azides. In Organic Azides—Syntheses and Applications; Bräse, S., Banert, K., Eds.; John Wiley & Sons: Chichester, UK, 2010; pp. 239–267. ISBN 978-0-470-68251-7. [Google Scholar]
- Lapointe, G.; Kapat, A.; Weidner, K.; Renaud, P. Radical azidation reactions and their application in the synthesis of alkaloids. Pure Appl. Chem. 2012, 84, 1633–1641. [Google Scholar] [CrossRef]
- Panchaud, P.; Chabaud, L.; Landais, Y.; Ollivier, C.; Renaud, P.; Zigmantas, S. Radical amination with sulfonyl azides: A powerful method for the formation of CN bonds. Chem. Eur. J. 2004, 10, 3606–3614. [Google Scholar] [CrossRef]
- Renaud, P.; Ollivier, C.; Panchaud, P. Radical carboazidation of alkenes: An efficient tool for the preparation of pyrrolidinone derivatives. Angew. Chem. Int. Edit. 2002, 41, 3460–3462. [Google Scholar] [CrossRef]
- Panchaud, P.; Ollivier, C.; Renaud, P.; Zigmantas, S. Radical carboazidation: Expedient assembly of the core structure of various alkaloid families. J. Org. Chem. 2004, 69, 2755–2759. [Google Scholar] [CrossRef]
- Chabaud, L.; Landais, Y.; Renaud, P. Total synthesis of hyacinthacine A(1) and 3-epi-hyacinthacine A(1). Org. Lett. 2005, 7, 2587–2590. [Google Scholar] [CrossRef]
- Schär, P.; Renaud, P. Total synthesis of the marine alkaloid (+/−)-lepadiformine via a radical carboazidation. Org. Lett. 2006, 8, 1569–1571. [Google Scholar] [CrossRef]
- Weidner, K.; Giroult, A.; Panchaud, P.; Renaud, P. Efficient carboazidation of alkenes using a radical desulfonylative azide transfer process. J. Am. Chem. Soc. 2010, 132, 17511–17515. [Google Scholar] [CrossRef]
- Lapointe, G.; Schenk, K.; Renaud, P. Concise synthesis of pyrrolidine and indolizidine alkaloids by a highly convergent three-component reaction. Chem. Eur. J. 2011, 17, 3207–3212. [Google Scholar] [CrossRef]
- Lapointe, G.; Schenk, K.; Renaud, P. Total synthesis of (±)-cylindricine C. Org. Lett. 2011, 13, 4774–4777. [Google Scholar] [CrossRef]
- Gonçalves-Martin, M.G.; Zigmantas, S.; Renaud, P. Formal synthesis of (−)-cephalotaxine. Helv. Chim. Acta 2012, 95, 2502–2514. [Google Scholar] [CrossRef]
- Huang, W.-Y.; Lü, L. The reaction of perfluoroalkanesulfinates VII. Fenton reagent-initiated addition of sodium perfluoroalkanesulfinates to alkenes. Chin. J. Chem. 1992, 10, 365–372. [Google Scholar] [CrossRef]
- Wei, X.-H.; Li, Y.-M.; Zhou, A.-X.; Yang, T.-T.; Yang, S.-D. Silver-catalyzed carboazidation of arylacrylamides. Org. Lett. 2013, 15, 4158–4161. [Google Scholar] [CrossRef]
- Bunescu, A.; Ha, T.M.; Wang, Q.; Zhu, J. Copper-catalyzed three-component carboazidation of clkenes with acetonitrile and sodium azide. Angew. Chem. Int. Ed. 2017, 56, 10555–10558. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-Y.; Wu, C.-S.; Wang, Z.; Luo, Y. Fe-Catalyzed three-component carboazidation of alkenes with alkanes and trimethylsilyl azide. Chem. Commun. 2018, 54, 11013–11016. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.; Ramkumar, N.; Chiou, M.-F.; Jian, W.; Li, Y.; Su, J.-H.; Zhang, X.; Bao, H. Iron-catalyzed carboazidation of alkenes and alkynes. Nat. Commun. 2019, 10, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ollivier, C.; Renaud, P. Formation of carbon-nitrogen bonds via a novel radical azidation process. J. Am. Chem. Soc. 2000, 122, 6496–6497. [Google Scholar] [CrossRef]
- Ollivier, C.; Renaud, P. A novel approach for the formation of carbon - nitrogen bonds: Azidation of alkyl radicals with sulfonyl azides. J. Am. Chem. Soc. 2001, 123, 4717–4727. [Google Scholar] [CrossRef]
- Panchaud, P.; Renaud, P. A convenient tin-free procedure for radical carboazidation and azidation. J. Org. Chem. 2004, 69, 3205–3207. [Google Scholar] [CrossRef] [PubMed]
- Panchaud, P.; Renaud, P. 3-Pyridinesulfonyl azide. In Encyclopedia of Reagents for Organic Synthesis; John Wiley Sons, Ltd.: Chichester, UK, 2006; ISBN 978-0-470-84289-8. [Google Scholar]
- Aminophosphonic and Aminophosphinic Acids: Chemistry and Biological Activity; Kukhar, V.P., Hudson, H.R., Eds.; John Wiley Sons, Ltd.: Chichester, UK, 2000; ISBN 978-0-471-89149-9. [Google Scholar]
- Cren, S.; Schär, P.; Renaud, P.; Schenk, K. Diastereoselectivity control of the radical carboazidation of substituted methylenecyclohexanes. J. Org. Chem. 2009, 74, 2942–2946. [Google Scholar] [CrossRef] [PubMed]
- Barton, D.H.R.; Chern, C.Y.; Jaszberenyi, J.C. The Invention of radical reactions. XXXIII. Homologation reactions of carboxylic acids by radical chain chemistry. Aust. J. Chem. 1995, 48, 407–425. [Google Scholar] [CrossRef]
- Vita, M.V.; Caramenti, P.; Waser, J. Enantioselective synthesis of homoallylic azides and nitriles via palladium-catalyzed decarboxylative allylation. Org. Lett. 2015, 17, 5832–5835. [Google Scholar] [CrossRef] [PubMed]
- Blakemore, P.R.; Cole, W.J.; Kocieński, P.J.; Morley, A. A stereoselective synthesis of trans-1,2-sisubstituted alkenes based on the condensation of aldehydes with metallated 1-phenyl-1H-tetrazol-5-yl sulfones. Synlett 1998, 1998, 26–28. [Google Scholar] [CrossRef]
- Marko, I.; Pospisil, J. Julia, Julia–Kocienski, and related sulfur-based alkenations. In Category 6, Compounds with All-Carbon Functions, Science of Synthesis; Neier, R., Bellus, D., Eds.; G. Thieme Verlag: Stuttgart, Germany, 2010; Volume 47a, ISBN 978-3-13-119011-6. [Google Scholar]
- Lebrun, M.-E.; Le Marquand, P.; Berthelette, C. Stereoselective synthesis of Z alkenyl halides via Julia olefination. J. Org. Chem. 2006, 71, 2009–2013. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Bowman, E.J.; Bowman, B.J.; Porco, J.A. Total synthesis of the salicylate enamide macrolide oximidine III: Application of relay ring-closing metathesis. Angew. Chem. Int. Ed. 2004, 43, 3601–3605. [Google Scholar] [CrossRef]
- Kamijo, S.; Kamijo, K.; Murafuji, T. Aryl ketone mediated photoinduced radical coupling for the alkylation of benzazoles employing saturated heterocyclic compounds. Synthesis 2019, 51, 3859–3864. [Google Scholar]
- David Mendenhall, G. The Lewis acid catalyzed reaction of trans-hyponitrite ion with alkyl halides. Tetrahedron Lett. 1983, 24, 451–452. [Google Scholar] [CrossRef]
- Panchaud, P.; Renaud, P. 3-Pyridinesulfonyl azide: A useful reagent for radical azidation. Adv. Synth. Catal. 2004, 346, 925–928. [Google Scholar] [CrossRef]
- Harrowven, D.C.; Guy, I.L. KF–Silica as a stationary phase for the chromatographic removal of tin residues from organic compounds. Chem. Commun. 2004, 1968–1969. [Google Scholar] [CrossRef] [PubMed]
- Merchant, R.R.; Edwards, J.T.; Qin, T.; Kruszyk, M.M.; Bi, C.; Che, G.; Bao, D.-H.; Qiao, W.; Sun, L.; Collins, M.R.; et al. Modular radical cross-coupling with sulfones enables access to sp3-rich (fluoro)alkylated scaffolds. Science 2018, 360, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.M.E.; Fier, P.S. Desulfonylative arylation of redox-active alkyl sulfones with aryl bromides. Org. Lett. 2019, 21, 5650–5654. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples are not available from the authors. |

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Millius, N.; Lapointe, G.; Renaud, P. Two-Step Azidoalkenylation of Terminal Alkenes Using Iodomethyl Sulfones. Molecules 2019, 24, 4184. https://doi.org/10.3390/molecules24224184
Millius N, Lapointe G, Renaud P. Two-Step Azidoalkenylation of Terminal Alkenes Using Iodomethyl Sulfones. Molecules. 2019; 24(22):4184. https://doi.org/10.3390/molecules24224184
Chicago/Turabian StyleMillius, Nicolas, Guillaume Lapointe, and Philippe Renaud. 2019. "Two-Step Azidoalkenylation of Terminal Alkenes Using Iodomethyl Sulfones" Molecules 24, no. 22: 4184. https://doi.org/10.3390/molecules24224184
APA StyleMillius, N., Lapointe, G., & Renaud, P. (2019). Two-Step Azidoalkenylation of Terminal Alkenes Using Iodomethyl Sulfones. Molecules, 24(22), 4184. https://doi.org/10.3390/molecules24224184






