Copper bis-Dipyridoquinoxaline Is a Potent DNA Intercalator that Induces Superoxide-Mediated Cleavage via the Minor Groove
Abstract
1. Introduction
2. Experimental
2.1. Materials and Methods
2.2. Synthesis of [Cu(DPQ)2NO3](NO3)
2.3. X-Ray Crystallography
2.4. DNA Binding Studies
2.5. DNA Damage Studies
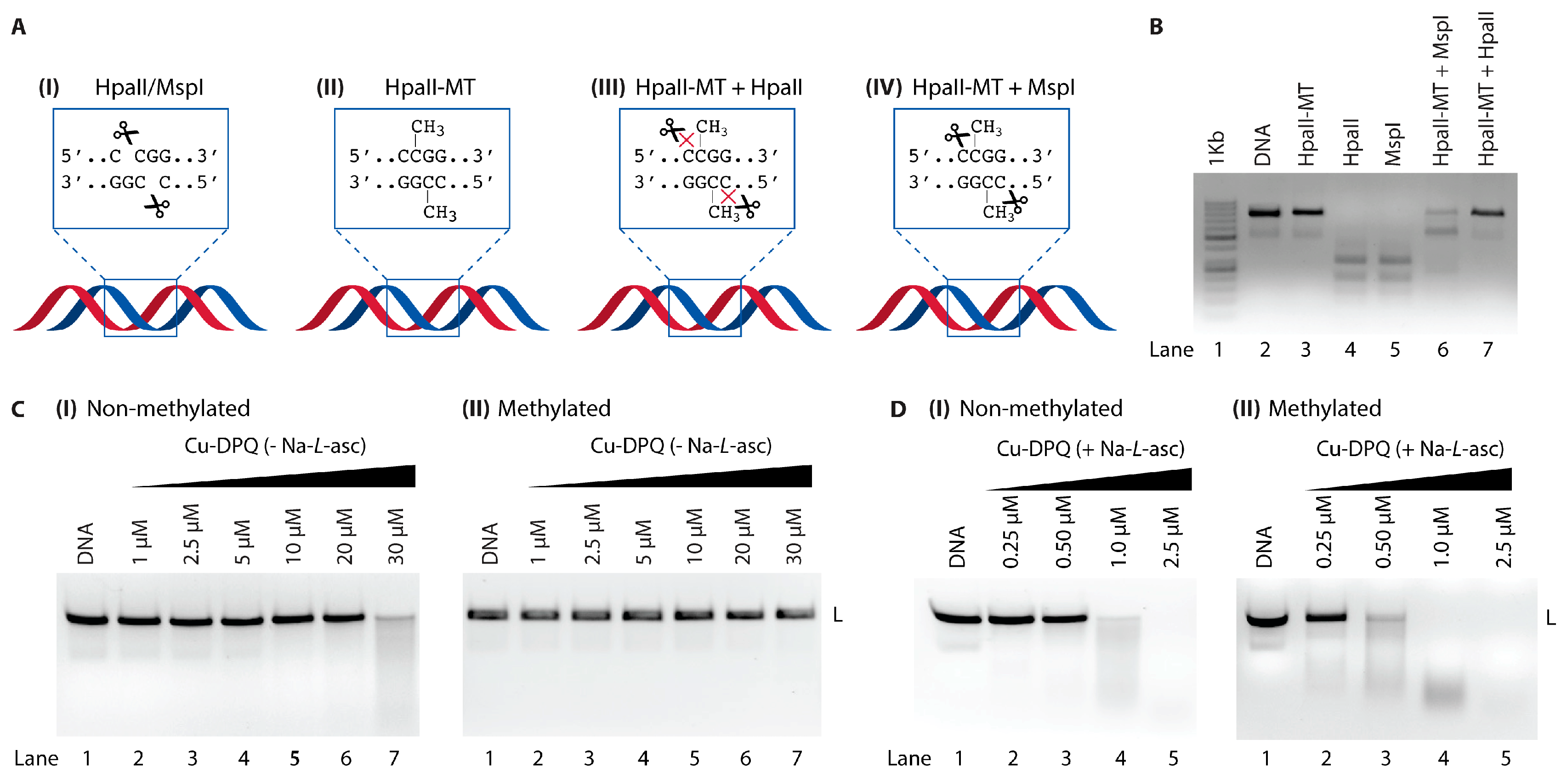
3. CpG Sequence Studies
3.1. PCR Primer Design
3.2. Restriction Enzyme Studies
3.3. DNA Damage
4. Results and Discussion
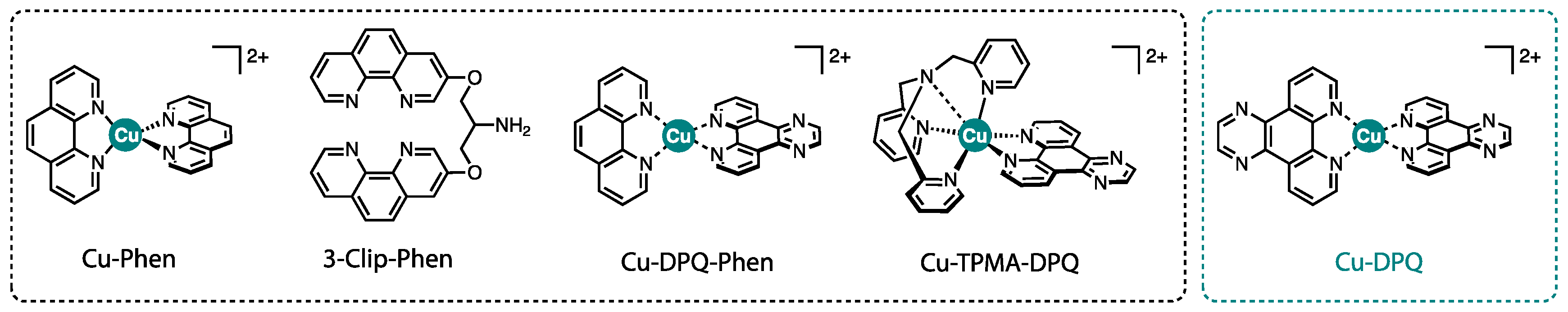
4.1. Preparation and Characterisation of Cu-DPQ
4.2. DNA Binding Experiments
4.3. DNA Damage Studies on SC pUC19
5. Studies with CpG and Methylated CpG Islands
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sigman, D.S.; Graham, D.R.; D’Aurora, V.; Stern, A.M. Oxygen-dependent cleavage of DNA by the 1,10-phenathroline cuporous complex. Inhibition of Escherichia coli DNA polymerase I. J. Biol. Chem. 1979, 254, 12269–12272. [Google Scholar] [PubMed]
- Thederahn, T.B.; Kuwabara, M.D.; Larsen, T.A.; Sigman, D.S. Nuclease activity of 1,10-phenanthroline-copper: Kinetic mechanism. J. Am. Chem. Soc. 1989, 111, 4941–4946. [Google Scholar] [CrossRef]
- Sigman, D.S. Nuclease Activity of 1,10-Phenanthroline-Copperion. Acc. Chem. Res. 1986, 19, 180–186. [Google Scholar] [CrossRef]
- Goyne, T.E.; Sigman, D.S. Nuclease activity of 1,10-phenanthroline-copper ion. Chemistry of deoxyribose oxidation. J. Am. Chem. Soc. 1987, 109, 2846–2848. [Google Scholar] [CrossRef]
- Sigman, D.S. Chemical Nucleases. Biochemistry 1990, 29, 9097–9105. [Google Scholar] [CrossRef]
- Sigman, D.S.; Mazumder, A.; Perrin, D.M. Chemical Nucleases. Chem. Rev. 1993, 93, 2295–2316. [Google Scholar] [CrossRef]
- Pratviel, G.; Bernadou, J.; Meunier, B. Carbon—Hydrogen Bonds of DNA Sugar Units as Targets for Chemical Nucleases and Drugs. Angew. Chem. Int. Ed. 1995, 34, 746–769. [Google Scholar] [CrossRef]
- Chen, T.; Greenberg, M.M. Model Studies Indicate That Copper Phenanthroline Induces Direct Strand Breaks via β-Elimination of the 2′-Deoxyribonolactone Intermediate Observed in Enediyne Mediated DNA Damage. J. Am. Chem. Soc. 1998, 120, 3815–3816. [Google Scholar] [CrossRef]
- Pitié, M.; Boldron, C.; Pratviel, G. DNA Oxidation by Copper and Manganese Complexes. Adv. Inorg. Chem. 2006, 58, 77–130. [Google Scholar]
- Pitié, M.; Pratviel, G. Activation of DNA Carbon−Hydrogen Bonds by Metal Complexes. Chem. Rev. 2010, 110, 1018–1059. [Google Scholar] [CrossRef]
- Kellett, A.; Molphy, Z.; Slator, C.; McKee, V. Recent advances in anticancer copper compounds. In Metal-Based Anticancer Agents; Casini, A., Vessières, A., Meier-Menches, S.M., Eds.; RSC Metallobiology; Royal Society of Chemistry: Cambridge UK, 2019; pp. 91–119. [Google Scholar]
- McGivern, T.J.P.; Slator, C.; Kellett, A.; Marmion, C.J. Innovative DNA-Targeted Metallo-prodrug Strategy Combining Histone Deacetylase Inhibition with Oxidative Stress. Mol. Pharmaceutics 2018, 15, 5058–5071. [Google Scholar] [CrossRef] [PubMed]
- Prisecaru, A.; McKee, V.; Howe, O.; Rochford, G.; McCann, M.; Colleran, J.; Pour, M.; Barron, N.; Gathergood, N.; Kellett, A. Regulating Bioactivity of Cu2+ Bis-1,10-phenanthroline Artificial Metallonucleases with Sterically Functionalized Pendant Carboxylates. J. Med. Chem. 2013, 56, 8599–8615. [Google Scholar] [CrossRef] [PubMed]
- McCann, M.; Kellett, A.; Kavanagh, K.; Devereux, M.; Santos, A.L.S. Deciphering the Antimicrobial Activity of Phenanthroline Chelators. Curr. Med. Chem. 2012, 19, 2703–2714. [Google Scholar] [CrossRef] [PubMed]
- McCann, M.; Santos, A.L.S.; da Silva, B.A.; Romanos, M.T.V.; Pyrrho, A.S.; Devereux, M.; Kavanagh, K.; Fichtner, I.; Kellett, A. In vitro and in vivo studies into the biological activities of 1,10-phenanthroline, 1,10-phenanthroline-5,6-dione and its copper(II) and silver(I) complexes. Toxicol. Res. 2012, 1, 47–54. [Google Scholar] [CrossRef]
- Larragy, R.; Fitzgerald, J.; Prisecaru, A.; McKee, V.; Leonard, P.; Kellett, A. Protein engineering with artificial chemical nucleases. Chem. Commun. 2015, 51, 12908–12911. [Google Scholar] [CrossRef]
- Pitié, M.; Donnadieu, B.; Meunier, B. Preparation of the New Bis(phenanthroline) Ligand “Clip-Phen” and Evaluation of the Nuclease Activity of the Corresponding Copper Complex. Inorg. Chem. 1998, 37, 3486–3489. [Google Scholar]
- Pitié, M.; Sudres, B.; Meunier, B. Dramatic increase of the DNA cleavage activity of Cu(Clip-phen) by fixing the bridging linker on the C3 position of the phenanthroline units. Chem. Commun. 1998, 0, 2597–2598. [Google Scholar] [CrossRef]
- Bailly, C.; Henichart, J.P. DNA recognition by intercalator-minor-groove binder hybrid molecules. Bioconjugate Chem. 1991, 2, 379–393. [Google Scholar] [CrossRef]
- Bailly, C.; Chaires, J.B. Sequence-Specific DNA Minor Groove Binders. Design and Synthesis of Netropsin and Distamycin Analogues. Bioconjugate Chem. 1998, 9, 513–538. [Google Scholar] [CrossRef]
- Pitié, M.; Burrows, C.J.; Meunier, B. Mechanisms of DNA cleavage by copper complexes of 3-Clip-Phen and of its conjugate with a distamycin analogue. Nucleic Acids Res. 2000, 28, 4856–4864. [Google Scholar] [CrossRef]
- Pitié, M.; Van Horn, J.D.; Brion, D.; Burrows, C.J.; Meunier, B. Targeting the DNA Cleavage Activity of Copper Phenanthroline and Clip-Phen to A·T Tracts via Linkage to a Poly-N-methylpyrrole. Bioconjugate Chem. 2000, 11, 892–900. [Google Scholar] [CrossRef] [PubMed]
- Bales, B.C.; Kodama, T.; Weledji, Y.N.; Pitié, M.; Meunier, B.; Greenberg, M.M. Mechanistic studies on DNA damage by minor groove binding copper–phenanthroline conjugates. Nucleic Acids Res. 2005, 33, 5371–5379. [Google Scholar] [CrossRef] [PubMed]
- Boldron, C.; Ross, S.A.; Pitié, M.; Meunier, B. Acridine Conjugates of 3-Clip-Phen: Influence of the Linker on the Synthesis and the DNA Cleavage Activity of Their Copper Complexes. Bioconjugate Chem. 2002, 13, 1013–1020. [Google Scholar] [CrossRef] [PubMed]
- Laurent, A.; Wright, M.; Laayoun, A.; Meunier, B.; Tissot, L.; Pitie, M. Clip-Phen Conjugates for the Specific Cleavage of Nucleic Acids. Nucleosides Nucleotides Nucleic Acids 2007, 26, 927–930. [Google Scholar] [CrossRef]
- Kellett, A.; O’Connor, M.; McCann, M.; McNamara, M.; Lynch, P.; Rosair, G.; McKee, V.; Creaven, B.; Walsh, M.; McClean, S.; et al. Bis-phenanthroline copper(II) phthalate complexes are potent in vitro antitumour agents with ‘self-activating’ metallo-nuclease and DNA binding properties. Dalton Trans. 2011, 40, 1024–1027. [Google Scholar] [CrossRef]
- Kellett, A.; O’Connor, M.; McCann, M.; Howe, O.; Casey, A.; McCarron, P.; Kavanagh, K.; McNamara, M.; Kennedy, S.; May, D.D.; et al. Water-soluble bis(1,10-phenanthroline) octanedioate Cu2+ and Mn2+ complexes with unprecedented nano and picomolar in vitro cytotoxicity: Promising leads for chemotherapeutic drug development. MedChemComm 2011, 2, 579–584. [Google Scholar] [CrossRef]
- O’Connor, M.; Kellett, A.; McCann, M.; Rosair, G.; McNamara, M.; Howe, O.; Creaven, B.S.; McClean, S.; Kia, A.F.-A.; O’Shea, D.; et al. Copper(II) complexes of salicylic acid combining superoxide dismutase mimetic properties with DNA binding and cleaving capabilities display promising chemotherapeutic potential with fast acting in vitro cytotoxicity against cisplatin sensitive and resista. J. Med. Chem. 2012, 55, 1957–1968. [Google Scholar] [CrossRef]
- Kellett, A.; Howe, O.; O’Connor, M.; McCann, M.; Creaven, B.S.; McClean, S.; Foltyn-Arfa Kia, A.; Casey, A.; Devereux, M. Radical-induced DNA damage by cytotoxic square-planar copper(II) complexes incorporating o-phthalate and 1,10-phenanthroline or 2,2′-dipyridyl. Free Radic. Biol. Med. 2012, 53, 564–576. [Google Scholar] [CrossRef]
- Prisecaru, A.; Devereux, M.; Barron, N.; McCann, M.; Colleran, J.; Casey, A.; McKee, V.; Kellett, A. Potent oxidative DNA cleavage by the di-copper cytotoxin: [Cu2(μ-terephthalate)(1,10-phen)4]2+. Chem. Commun. 2012, 48, 6906–6908. [Google Scholar] [CrossRef]
- Slator, C.; Barron, N.; Howe, O.; Kellett, A. [Cu(o-phthalate)(phenanthroline)] Exhibits Unique Superoxide-Mediated NCI-60 Chemotherapeutic Action through Genomic DNA Damage and Mitochondrial Dysfunction. ACS Chem. Biol. 2016, 11, 159–171. [Google Scholar] [CrossRef]
- Molphy, Z.; Prisecaru, A.; Slator, C.; Barron, N.; McCann, M.; Colleran, J.; Chandran, D.; Gathergood, N.; Kellett, A. Copper Phenanthrene Oxidative Chemical Nucleases. Inorg. Chem. 2014, 53, 5392–5404. [Google Scholar] [CrossRef] [PubMed]
- Molphy, Z.; Slator, C.; Chatgilialoglu, C.; Kellett, A. DNA oxidation profiles of copper phenanthrene chemical nucleases. Front. Chem. 2015, 3, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zuin Fantoni, N.; Molphy, Z.; Slator, C.; Menounou, G.; Toniolo, G.; Mitrikas, G.; McKee, V.; Chatgilialoglu, C.; Kellett, A. Polypyridyl-Based Copper Phenanthrene Complexes: A New Type of Stabilized Artificial Chemical Nuclease. Chem. Eur. J. 2019, 25, 221–237. [Google Scholar] [CrossRef] [PubMed]
- Toniolo, G.; Louka, M.; Menounou, G.; Fantoni, N.Z.; Mitrikas, G.; Efthimiadou, E.K.; Masi, A.; Bortolotti, M.; Polito, L.; Bolognesi, A.; et al. [Cu(TPMA)(Phen)](ClO4)2: Metallodrug Nanocontainer Delivery and Membrane Lipidomics of a Neuroblastoma Cell Line Coupled with a Liposome Biomimetic Model Focusing on Fatty Acid Reactivity. ACS Omega 2018, 3, 15952–15965. [Google Scholar] [CrossRef] [PubMed]
- Slator, C.; Molphy, Z.; McKee, V.; Long, C.; Brown, T.; Kellett, A. Di-copper metallodrugs promote NCI-60 chemotherapy via singlet oxygen and superoxide production with tandem TA/TA and AT/AT oligonucleotide discrimination. Nucleic Acids Res. 2018, 46, 2733–2750. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. SHELXT. Acta Crystallogr. 2015, A71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXL. Acta Crystallogr. 2015, C71, 3–8. [Google Scholar] [CrossRef]
- McCann, M.; McGinley, J.; Ni, K.; O’Connor, M.; Kavanagh, K.; McKee, V.; Colleran, J.; Devereux, M.; Gathergood, N.; Barron, N.; et al. A new phenanthroline-oxazine ligand: Synthesis, coordination chemistry and atypical DNA binding interaction. Chem. Commun. 2013, 49, 2341–2343. [Google Scholar] [CrossRef]
- Slator, C.; Molphy, Z.; McKee, V.; Kellett, A. Triggering Autophagic Cell Death with a di-Manganese(II) Developmental Therapeutic. Redox Biol. 2017, 12, 150–161. [Google Scholar] [CrossRef]
- Addison, A.W.; Rao, T.N.; Reedijk, J.; van Rijn, J.; Verschoor, G.C. Synthesis, structure, and spectroscopic properties of copper(II) compounds containing nitrogen–sulphur donor ligands; the crystal and molecular structure of aqua[1,7-bis(N-methylbenzimidazol-2′-yl)-2,6-dithiaheptane]copper(II) perchlorate. J. Chem. Soc. Dalton Trans. 1984, 1349–1356. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Cryst. B. 2016, 72, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Roy, M.; Dhar, S.; Maity, B.; Chakravarty, A.R. Dicopper(II) complexes showing DNA hydrolase activity and monomeric adduct formation with bis(4-nitrophenyl)phosphate. Inorg. Chim. Acta 2011, 375, 173–180. [Google Scholar] [CrossRef]
- Ghosh, M.; Biswas, P.; Flörke, U. Structural, spectroscopic and redox properties of transition metal complexes of dipyrido[3,2-f:2′,3′-h]-quinoxaline (dpq). Polyhedron 2007, 26, 3750–3762. [Google Scholar] [CrossRef]
- Reddy, P.A.N.; Santra, B.K.; Nethaji, M.; Chakravarty, A.R. Synthesis, crystal structure and nuclease activity of bis(dipyridoquinoxaline)copper(I) perchlorate. Indian J. Chem. A. 2003, 42A, 2185–2190. [Google Scholar]
- Biswas, P.; Dutta, S.; Ghosh, M. Influence of counter anions on structural, spectroscopic and electrochemical behaviours of copper(II) complexes of dipyrido[3,2-f: 2′,3′-h]-quinoxaline (dpq). Polyhedron 2008, 27, 2105–2112. [Google Scholar] [CrossRef]
- Kellett, A.; Molphy, Z.; Slator, C.; McKee, V.; Farrell, N.P. Molecular methods for assessment of non-covalent metallodrug–DNA interactions. Chem. Soc. Rev. 2019, 48, 971–988. [Google Scholar] [CrossRef]
Sample Availability: Please contact the corresponding author as required. |



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molphy, Z.; McKee, V.; Kellett, A. Copper bis-Dipyridoquinoxaline Is a Potent DNA Intercalator that Induces Superoxide-Mediated Cleavage via the Minor Groove. Molecules 2019, 24, 4301. https://doi.org/10.3390/molecules24234301
Molphy Z, McKee V, Kellett A. Copper bis-Dipyridoquinoxaline Is a Potent DNA Intercalator that Induces Superoxide-Mediated Cleavage via the Minor Groove. Molecules. 2019; 24(23):4301. https://doi.org/10.3390/molecules24234301
Chicago/Turabian StyleMolphy, Zara, Vickie McKee, and Andrew Kellett. 2019. "Copper bis-Dipyridoquinoxaline Is a Potent DNA Intercalator that Induces Superoxide-Mediated Cleavage via the Minor Groove" Molecules 24, no. 23: 4301. https://doi.org/10.3390/molecules24234301
APA StyleMolphy, Z., McKee, V., & Kellett, A. (2019). Copper bis-Dipyridoquinoxaline Is a Potent DNA Intercalator that Induces Superoxide-Mediated Cleavage via the Minor Groove. Molecules, 24(23), 4301. https://doi.org/10.3390/molecules24234301





