Arginine-Induced Self-Assembly of Protoporphyrin to Obtain Effective Photocatalysts in Aqueous Media Under Visible Light
Abstract
:1. Introduction
2. Results
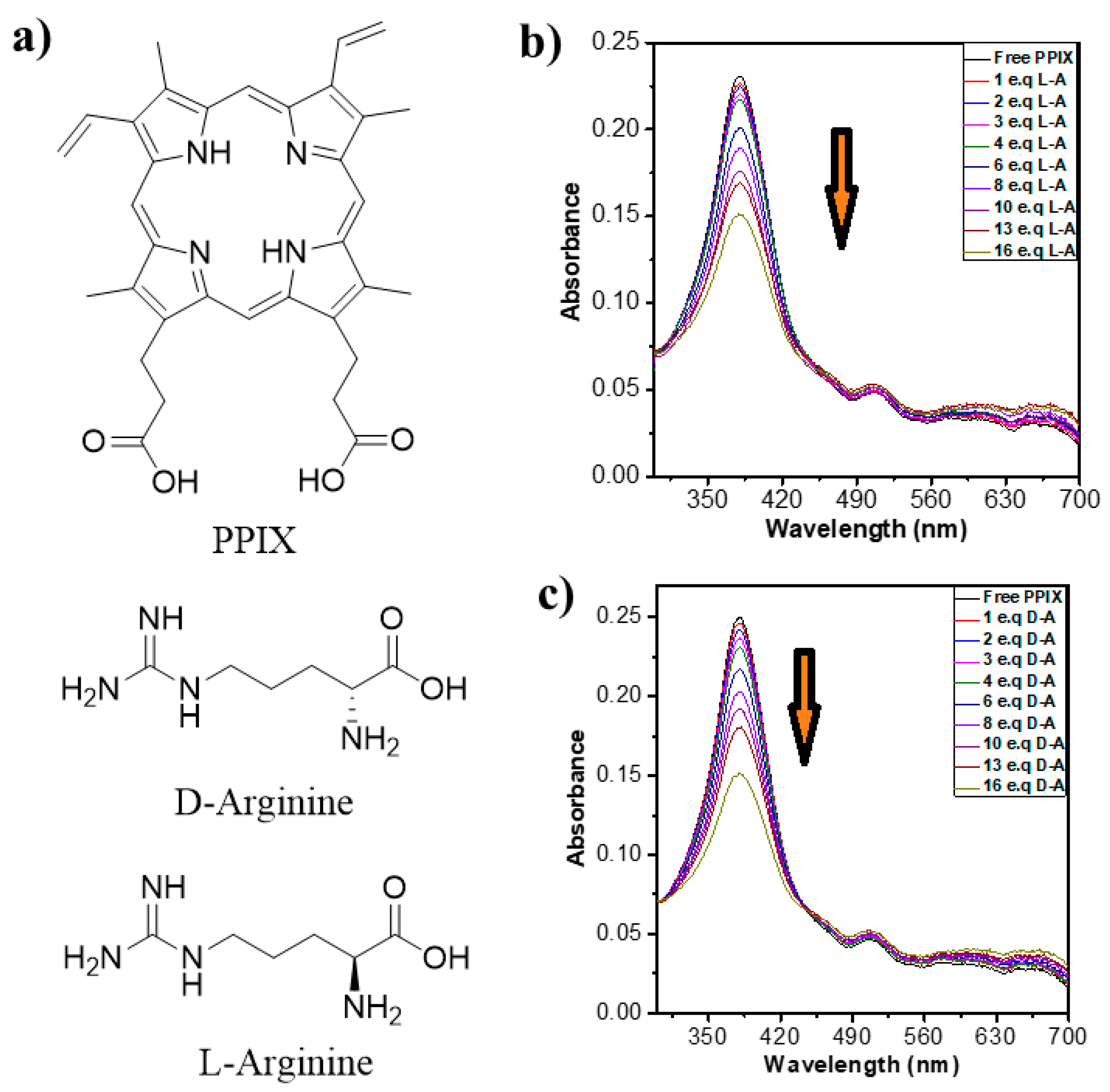
2.1. Photophysical Properties
2.1.1. UV-Vis Absorption Properties
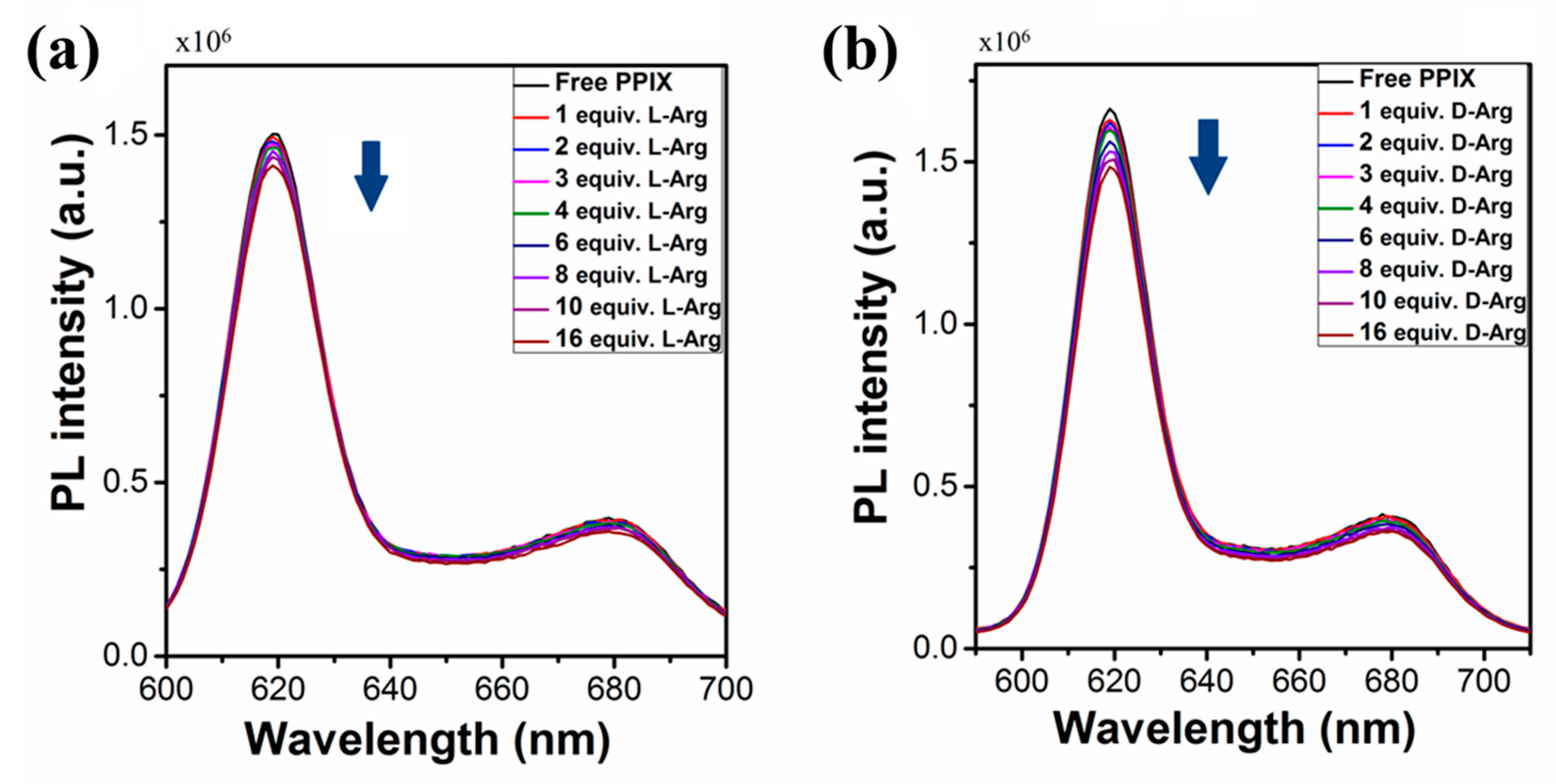
2.1.2. Fluorescent Emission Properties
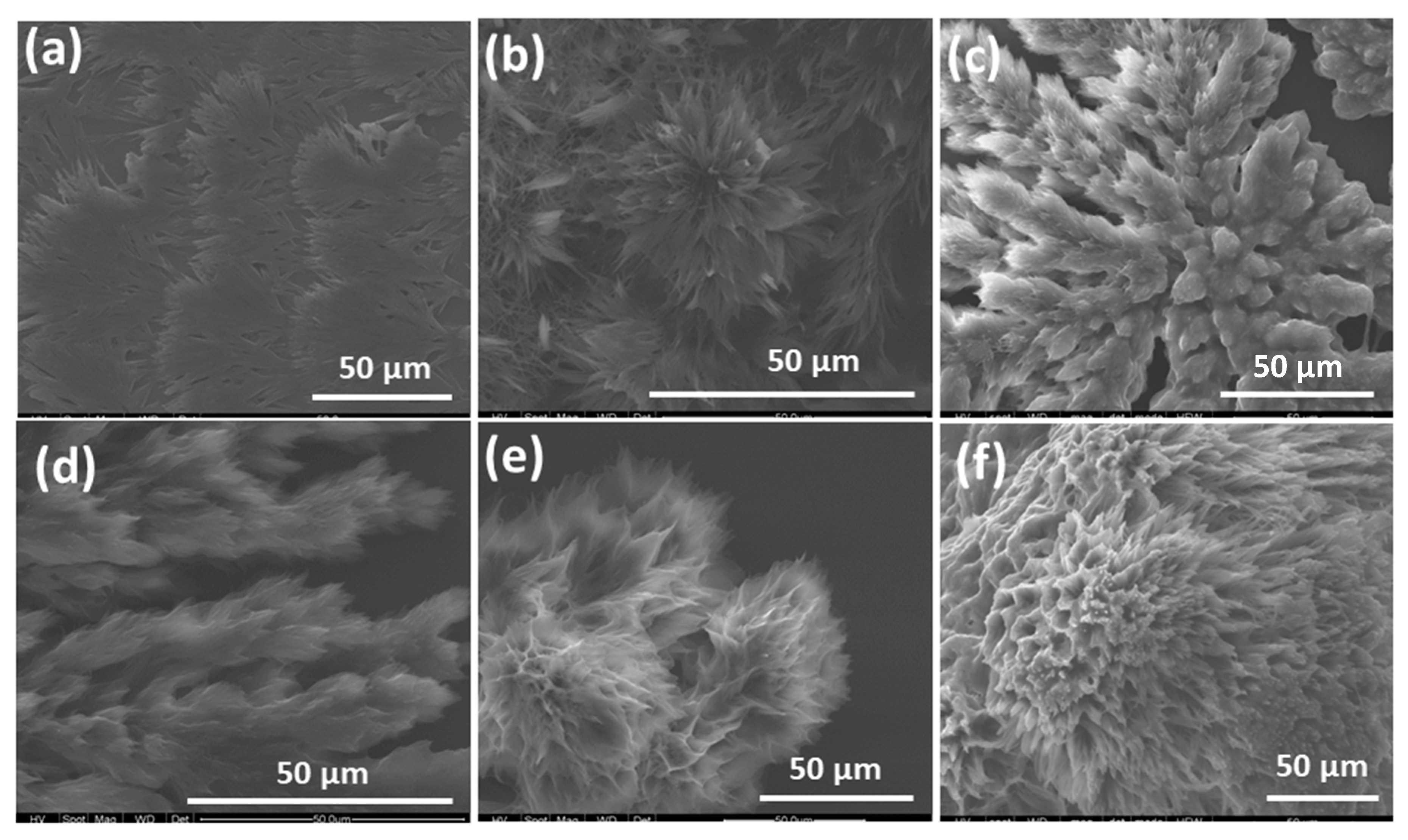
2.2. Scanning Electron Microscopy
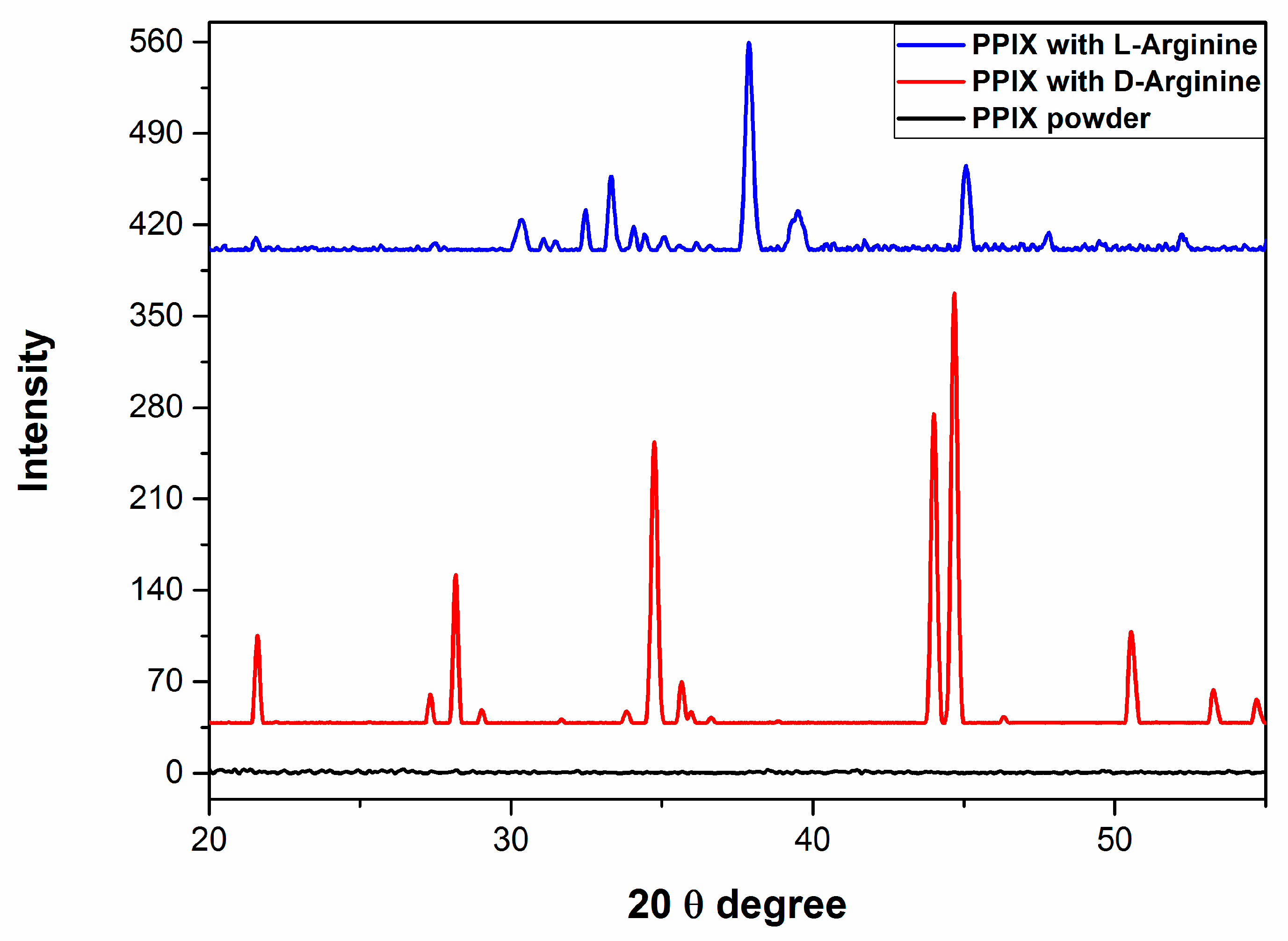
2.3. X-ray Diffraction Properties
2.4. Dynamic Light Scattering Measurements
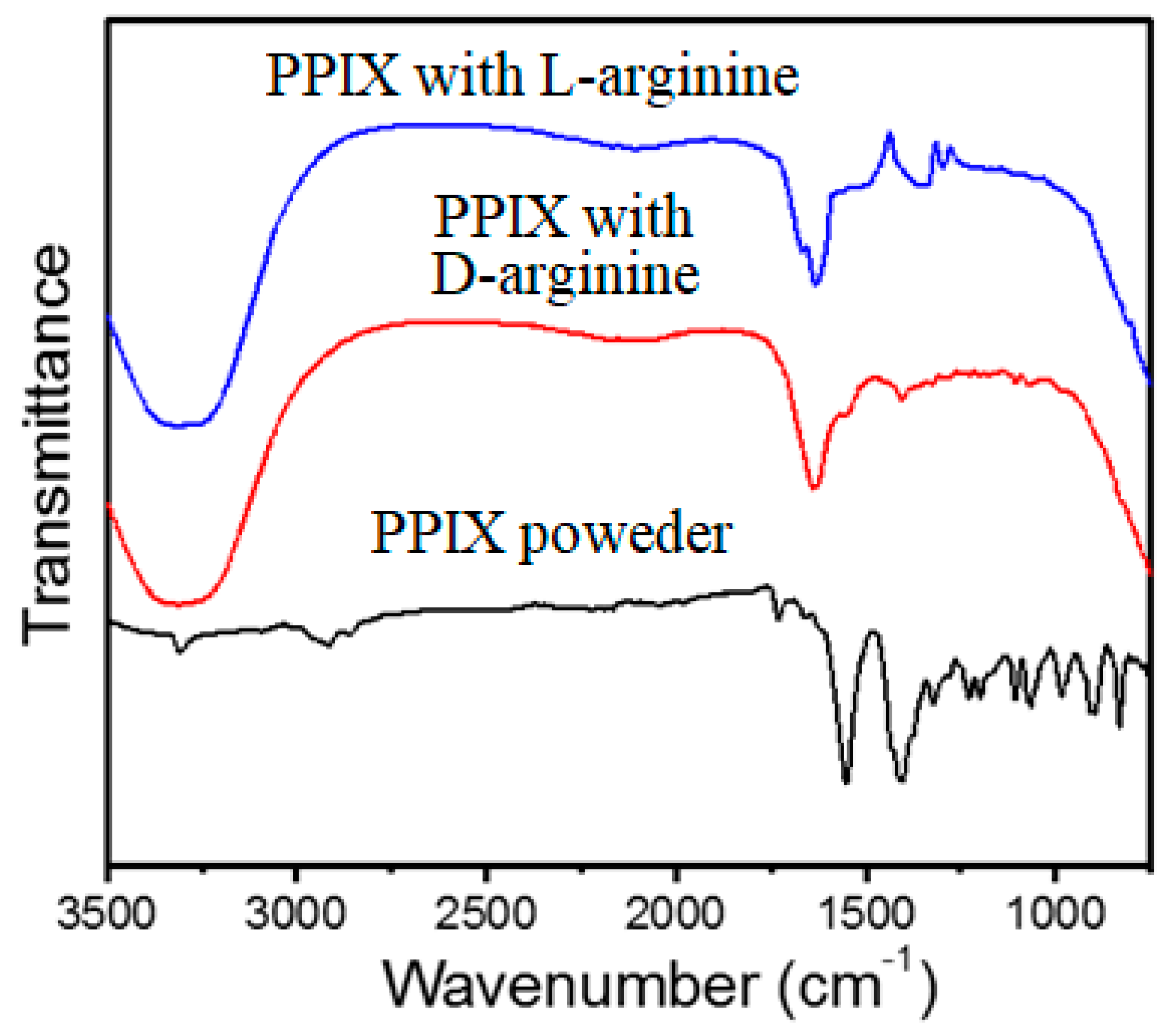
2.5. Fourier Transform Infrared Spectroscopy (FT-IR)
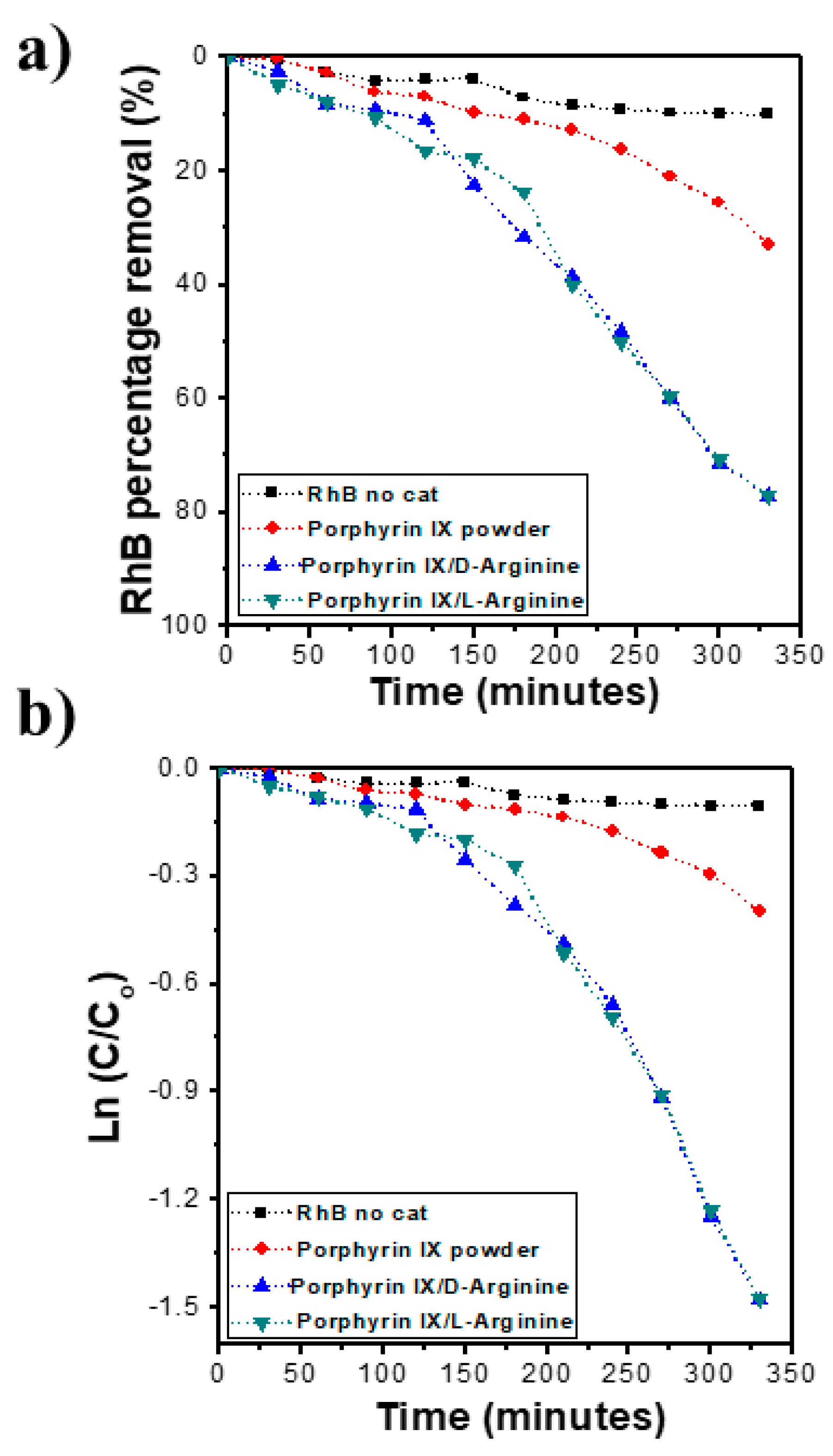
2.6. Photocatalytic Performance
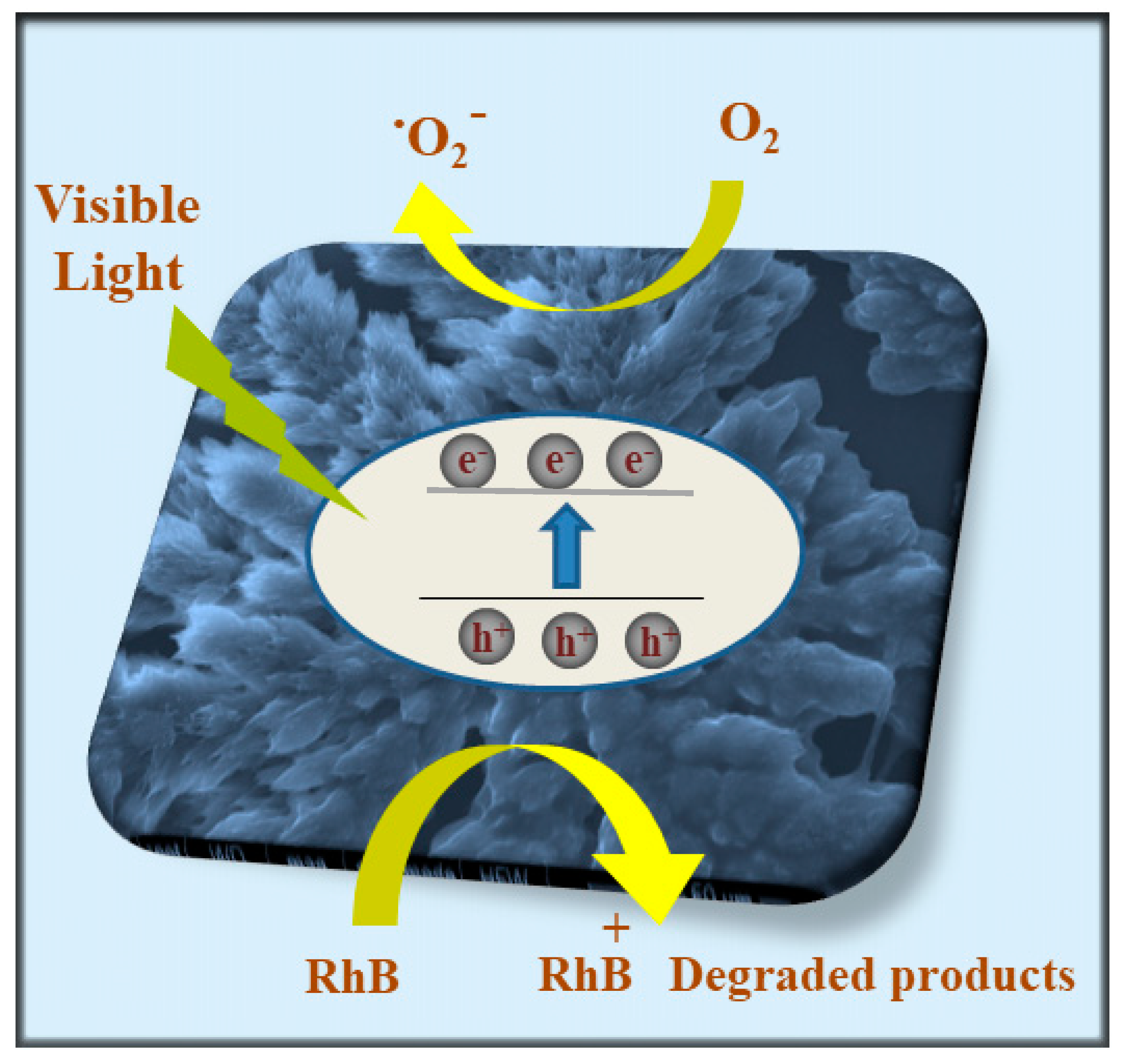
2.7. Photocatalytic Activity Mechanism
3. Experimental Section
3.1. Materials and Instruments
3.2. UV-Vis Absorbance and Fluorescence Spectrometry
3.3. Scanning Electron Microscopy
3.4. XRD Measurement
3.5. Photocatalytic Investigation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bhatia, D.; Sharma, N.R.; Singh, J.; Kanwar, R.S. Biological methods for textile dye removal from wastewater: A review. Crit. Rev. Environ. Sci. Technol. 2017, 47, 1836–1876. [Google Scholar] [CrossRef]
- Nidheesh, P.V.; Rajan, R. Removal of rhodamine B from a water medium using hydroxyl and sulphate radicals generated by iron loaded activated carbon. RSC Adv. 2016, 6, 5330–5340. [Google Scholar] [CrossRef]
- Puvaneswari, N.; Muthukrishnan, J.; Gunasekaran, P. Toxicity assessment and microbial degradation of azo dyes. Indian J. Exp. Biol. 2006, 44, 618–626. [Google Scholar] [PubMed]
- Brown, M.A.; De Vito, S.C. Predicting azo dye toxicity. Crit. Rev. Environ. Sci. Technol. 2009, 23, 249–324. [Google Scholar] [CrossRef]
- Tee, H.-C.; Lim, P.E.; Seng, C.E.; Nawi, M.A.; Adnan, R. Enhancement of azo dye Acid Orange 7 removal in newly developed horizontal subsurface-flow constructed wetland. J. Environ. Manage. 2015, 147, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Guimaraes, J.R.; Maniero, M.G.; Araujo, R.N.D. A comparative study on the degradation of RB-19 dye in an aqueous medium by advanced oxidation processes. J. Environ. Manage. 2012, 110, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Ravikumar, K.; Krishnan, S.; Ramalingam, S.; Balu, K. Optimization of process variables by the application of response surface methodology for dye removal using a novel adsorbent. Dyes Pigment. 2007, 72, 66–74. [Google Scholar] [CrossRef]
- Wang, L.; Li, J. Adsorption of C.I. Reactive Red 228 dye from aqueous solution by modified cellulose from flax shive: Kinetics, equilibrium, and thermodynamics. Ind. Crop. Prod. 2013, 42, 153–157. [Google Scholar] [CrossRef]
- Moghaddam, S.S.; Moghaddam, M.R.A.; Arami, M. Coagulation/flocculation process for dye removal using sludge from water treatment plant: Optimization through response surface methodology. J. Hazard. Master. 2010, 175, 651–657. [Google Scholar] [CrossRef]
- Li, S.X.; Cai, S.J.; Zheng, F.-Y. Self-assembled TiO2 with 5-sulfosalicylic acid for improvement its surface properties and photodegradation activity of dye. Dyes Pigment. 2012, 95, 188–193. [Google Scholar] [CrossRef]
- La, D.D.; Ramanathan, R.; Rananaware, A.; Bansal, V.; Bhosale, S.V. Nanostructured charge transfer complex of CuTCNQF4 for efficient photo-removal of hexavalent chromium. RSC Adv. 2016, 6, 33931–33936. [Google Scholar] [CrossRef]
- Naik, A.P.; Salkar, A.V.; Majik, M.S.; Morajkar, P.P. Enhanced photocatalytic degradation of Amaranth dye on mesoporous anatase TiO2: Evidence of C-N, N=N bond cleavage and identification of new intermediates. Photochem. Photobiol. Sci. 2017, 16, 1126–1138. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.N.; Haider, W. Heterogeneous photocatalysis and its potential applications in water and wastewater treatment: A review. Nanotechnology 2018, 29, 342001–342031. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Shi, Y.; Ma, Q.; Gao, D.; Zhang, J.; Li, J.; Wang, F.; He, Y.; Wang, R. A flower-like TiO2 with photocatalytic hydrogen evolution activity modified by Zn(II) porphyrin photocatalysts. J. Mater. SCI-Mater. El. 2017, 28, 2123–2127. [Google Scholar] [CrossRef]
- La, D.D.; Bhosale, S.V.; Jones, L.A.; Bhosale, S.V. Arginine-induced porphyrin-based self-assembled nanostructures for photocatalytic applications under simulated sunlight irradiation. Photochem. Photobio. Sci. 2017, 16, 151–154. [Google Scholar] [CrossRef]
- Aljabri, M.D.; La, D.D.; Jadhav, R.W.; Jones, L.A.; Nguyen, D.D.; Chang, S.W.; Tran, L.D.; Bhosale, S.V. Supramolecular nanomaterials with photocatalytic activity obtained via self-assembly of fluorinated porphyrin derivative. Fuel 2019, 254, 115639–115645. [Google Scholar] [CrossRef]
- La, D.D.; Rananaware, A.; Salimimarand, M.; Bhosale, S.V. Well–dispersed assembled porphyrin nanorods on graphene for the enhanced photocatalytic performance. ChemistrySelect. 2015, 1, 4430–4434. [Google Scholar] [CrossRef]
- La, D.D.; Bhosale, S.V.; Jones, L.A.; Ravaprasadu, N.; Bhosale, S.V. Fabrication of a Graphene@TiO2@Porphyrin Hybrid Material and Its Photocatalytic Properties under Simulated Sunlight Irradiation. ChemistrySelect 2017, 2, 3329–3333. [Google Scholar] [CrossRef]
- La, D.D.; Hangarge, R.V.; Bhosale, S.V.; Jones, L.A.; Ninh, H.D.; Bhosale, S.V. Arginine-Mediated Self-Assembly of Porphyrin on Graphene: A Photocatalyst for Degradation of Dyes. Appl. Sci. 2017, 7, 643. [Google Scholar] [CrossRef]
- Guo, P.; Chen, P.; Liu, M. One-dimensional porphyrin nanoassemblies assisted via graphene oxide: Sheetlike functional surfactant and enhanced photocatalytic behaviors. ACS Appl. Mater. Inter. 2013, 5, 5336–5345. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, Z.H.; Yue, M.; Kang, F. Integrating porphyrin nanoparticles into a 2D graphene matrix for free-standing nanohybrid films with enhanced visible-light photocatalytic activity. Nanoscale. 2014, 6, 978–985. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Nayak, S.K.; Mallampalli, S.; Patra, A. Surfactant-assisted porphyrin based hierarchical nano/micro assemblies and their efficient photocatalytic behavior. ACS Appl. Mater. Inter. 2013, 6, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, C.; Zhang, X.; Ou, X.; Zhang, X. One-step growth of organic single-crystal p-n nano- heterojunctions with enhanced visible-light photocatalytic activity. Chem. Commun. 2013, 49, 9200–9202. [Google Scholar] [CrossRef] [PubMed]
- Inamura, I.; Uchida, K. Association Behaviour of Protoporphyrin IX in Water and Aqueous poly (N-vinylpyrrolidone) Solution. Interaction between Protoporphyrin IX and poly(N-vinylpyrrolidone). Bull. Chem. Soc. Jpn. 1991, 64, 2005–2007. [Google Scholar] [CrossRef]
- Scolaro, L.M.; Castriciano, M.; Romeo, A.; Patane, S.; Cafail, E.; Allegrini, M. Aggregation Behavior of Protoporphyrin IX in Aqueous Solutions: Clear Evidence of Vesicle Formation. J. Phys. Chem. B. 2002, 106, 2453–2459. [Google Scholar] [CrossRef]
- Seo, J.; Jang, J.; Wanrke, S.; Gewinner, S.; Schollkopf, W.; Halden, G.V. Stacking Geometries of Early Protoporphyrin IX Aggregates Revealed by Gas-Phase Infrared Spectroscopy. J. Am. Chem. Soc. 2016, 50, 16315–16321. [Google Scholar] [CrossRef]
- Bhosale, S.V.; Kalyankar, M.B.; Nalage, S.V.; Bhosale, S.V.; Lalander, C.H.; Langford, S.J. Supramolecular self-assembled nanowires by the aggregation of a protoporphyrin derivative in low- polarity solvents. Supramol. L. Chem. 2011, 23, 563–569. [Google Scholar] [CrossRef]
- Bhosale, S.V.; Kalyankar, M.B.; Bhosale, S.V.; Patil, S.G.; Lalander, C.H.; Langford, S.J. Supramolecular self-assembly of protoporphyrin IX amphiphiles into worm-like and particular aggregates. Supramol. L. Chem. 2011, 23, 263–268. [Google Scholar] [CrossRef]
- Bhosale, S.V.; Nalage, S.V.; Booth, J.M.; Gupta, A.; Bhargave, S.K.; Bhosale, S.V. Solvent induced ordered-supramolecular assembly of highly branched protoporphyrin IX derivative. Supramol. L. Chem. 2012, 24, 779–786. [Google Scholar] [CrossRef]
- Knoben, W.; Crego-Calama, M.; Brongersma, S.H. Comparison of nitric oxide binding to different pure and mixed protoporphyrin IX monolayers. Sens. Actuators B Chem. 2012, 166, 349–356. [Google Scholar] [CrossRef]
- Krieg, M.; Whitten, D.G. Self-sensitized Photooxidation of ProtoporphyrinIX and Related Free-Base Porphyrins Porphyrins in Natural and Model Membrane Systems. Evidence for Novel Photooxidation Pathways Involving Amino Acids. J. Am. Chem. Soc. 1984, 106, 2477–2479. [Google Scholar]
- Xu, Y.; Wang, L.; Zhu, X.; Wang, C.-Q. Hierarchical self-assembly of protoporphyrin IX-bridged Janus particles into photoresponsive vesicles. RSC Adv. 2016, 6, 31053–31058. [Google Scholar] [CrossRef]
- Kathiravan, A.; Raghavendra, V.; Kumar, R.A.; Ramamurthy, P. Protoporphyrin IX on TiO2 electrode: A spectroscopic and photovoltaic investigation. Dyes Pigment. 2013, 96, 196–203. [Google Scholar] [CrossRef]
- Umemura, T.; Hotta, H.; Abe, T.; Takahashi, Y.; Takiguchi, H.; Uehara, M.; Odake, T.; Tsunoda, K. Slab Optical Waveguide High-Acidity Sensor Based on an Absorbance Change of Protoporphyrin IX. Anal. Chem. 2006, 78, 7511–7516. [Google Scholar] [CrossRef]
- Sun, B.; Zhang, K.; Chen, L.; Guo, L.; Ai, S. A novel photoelectrochemical sensor based on PPIX-functionalized WO3–rGO nanohybrid-decorated ITO electrode for detecting cysteine. Biosens. Bioelectron. 2013, 44, 48–51. [Google Scholar] [CrossRef]
- Kathiravan, A. Excited state electron transfer reactions of ProtoporphyrinIX with fullerene. Synthetic Metal. 2014, 194, 77–81. [Google Scholar] [CrossRef]
- Deng, S.; Lie, J.; Huang, Y.; Cheng, Y.; Ju, H. Electrochemiluminescent Quenching of Quantum Dots for Ultrasensitive Immunoassay through Oxygen Reduction Catalyzed by Nitrogen-Doped Graphene-Supported Hemin. Anal. Chem. 2013, 85, 5390–5396. [Google Scholar] [CrossRef]
- Li, T.; Wang, E.; Dong, S. Parallel G-Quadruplex-Specific Fluorescent Probe for Monitoring DNA Structural Changes and Label-Free Detection of Potassium Ion. Anal. Chem. 2010, 82, 7576–7580. [Google Scholar] [CrossRef]
- Steed, J.W.; Atwood, J.L. Supramolecular Chemistry; John Wiley & Sons Ltd.: Chichester, West Sussex, UK, 2000. [Google Scholar]
- Doziuk, H. Introduction to Supramolecular Chemistry; Kluwer Academic Publisher: Dordrecht, The Netherlands, 2002. [Google Scholar]
- Koren, A.; Curtis, M.; Francis, A.; Kampf, J. Intermolecular Interactions in π-Stacked Conjugated Molecules. Synthesis, Structure, and Spectral Characterization of Alkyl Bithiazole Oligomers. J. Am. Chem. Soc. 2003, 125, 5040–5050. [Google Scholar] [CrossRef]
- Zhao, Y.; Shang, Q.; Yu, J.; Zhang, Y.; Liu, S. Nanostructured 2D diporphyrin honeycomb film: Photoelectrochemistry, photodegradation, and antibacterial activity. ACS Appl. Mater. Inter. 2015, 7, 11783–11791. [Google Scholar] [CrossRef]
- Guo, P.; Chen, P.; Ma, W.; Liu, M. Morphology-dependent supramolecular photocatalytic performance of porphyrin nanoassemblies: From molecule to artificial supramolecular nanoantenna. J. Mater. Chem. 2012, 22, 20243–20249. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aljabri, M.D.; Gosavi, N.M.; Jones, L.A.; Morajkar, P.P.; La, D.D.; Bhosale, S.V. Arginine-Induced Self-Assembly of Protoporphyrin to Obtain Effective Photocatalysts in Aqueous Media Under Visible Light. Molecules 2019, 24, 4172. https://doi.org/10.3390/molecules24224172
Aljabri MD, Gosavi NM, Jones LA, Morajkar PP, La DD, Bhosale SV. Arginine-Induced Self-Assembly of Protoporphyrin to Obtain Effective Photocatalysts in Aqueous Media Under Visible Light. Molecules. 2019; 24(22):4172. https://doi.org/10.3390/molecules24224172
Chicago/Turabian StyleAljabri, Mahmood D., Nilesh M. Gosavi, Lathe A. Jones, Pranay P. Morajkar, Duong D. La, and Sheshanath V. Bhosale. 2019. "Arginine-Induced Self-Assembly of Protoporphyrin to Obtain Effective Photocatalysts in Aqueous Media Under Visible Light" Molecules 24, no. 22: 4172. https://doi.org/10.3390/molecules24224172
APA StyleAljabri, M. D., Gosavi, N. M., Jones, L. A., Morajkar, P. P., La, D. D., & Bhosale, S. V. (2019). Arginine-Induced Self-Assembly of Protoporphyrin to Obtain Effective Photocatalysts in Aqueous Media Under Visible Light. Molecules, 24(22), 4172. https://doi.org/10.3390/molecules24224172







