Isonicotinamide-Based Compounds: From Cocrystal to Polymer
Abstract
1. Introduction
2. Results and Discussion
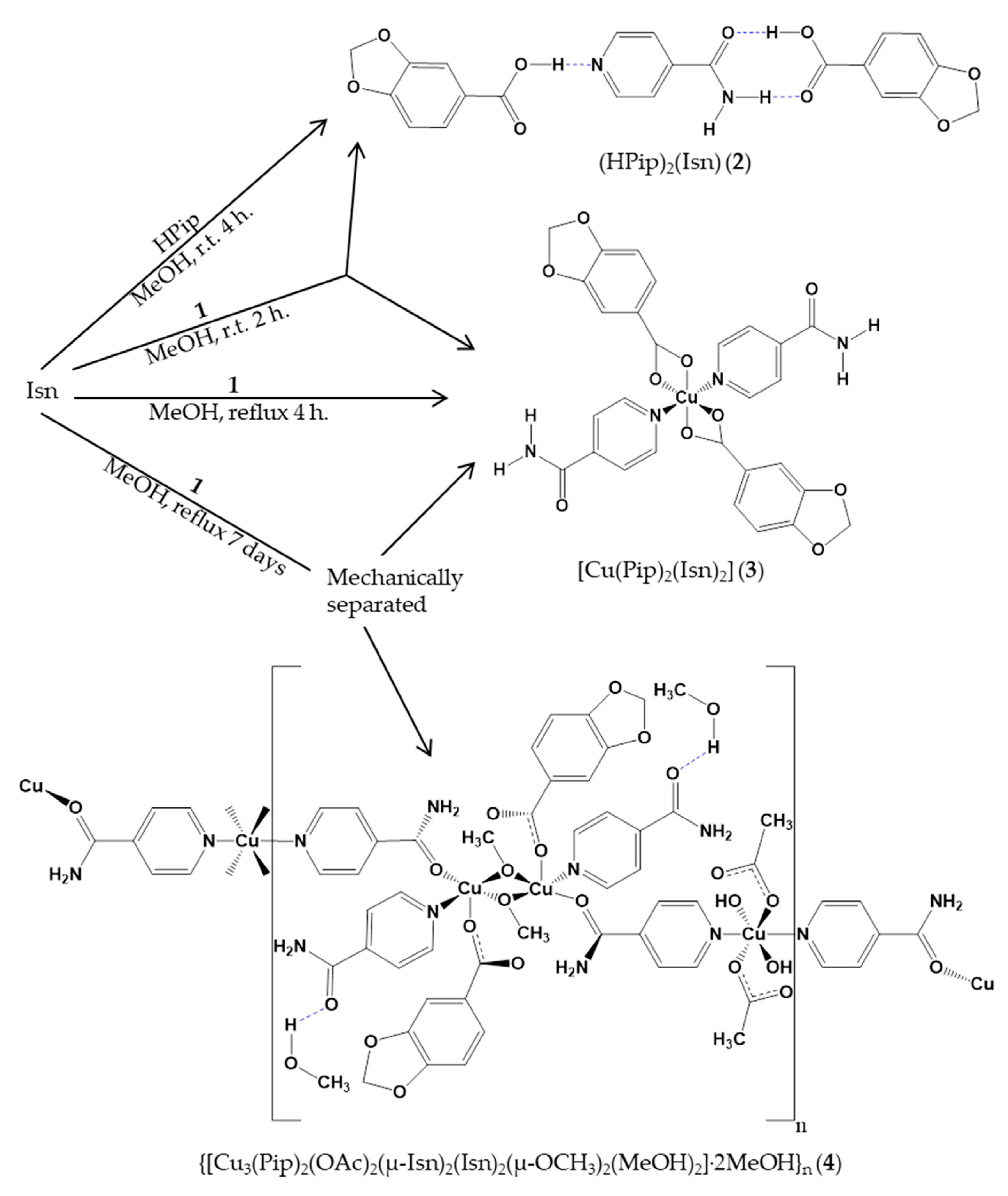
2.1. Synthesis and Characterization of Cocrystal 2
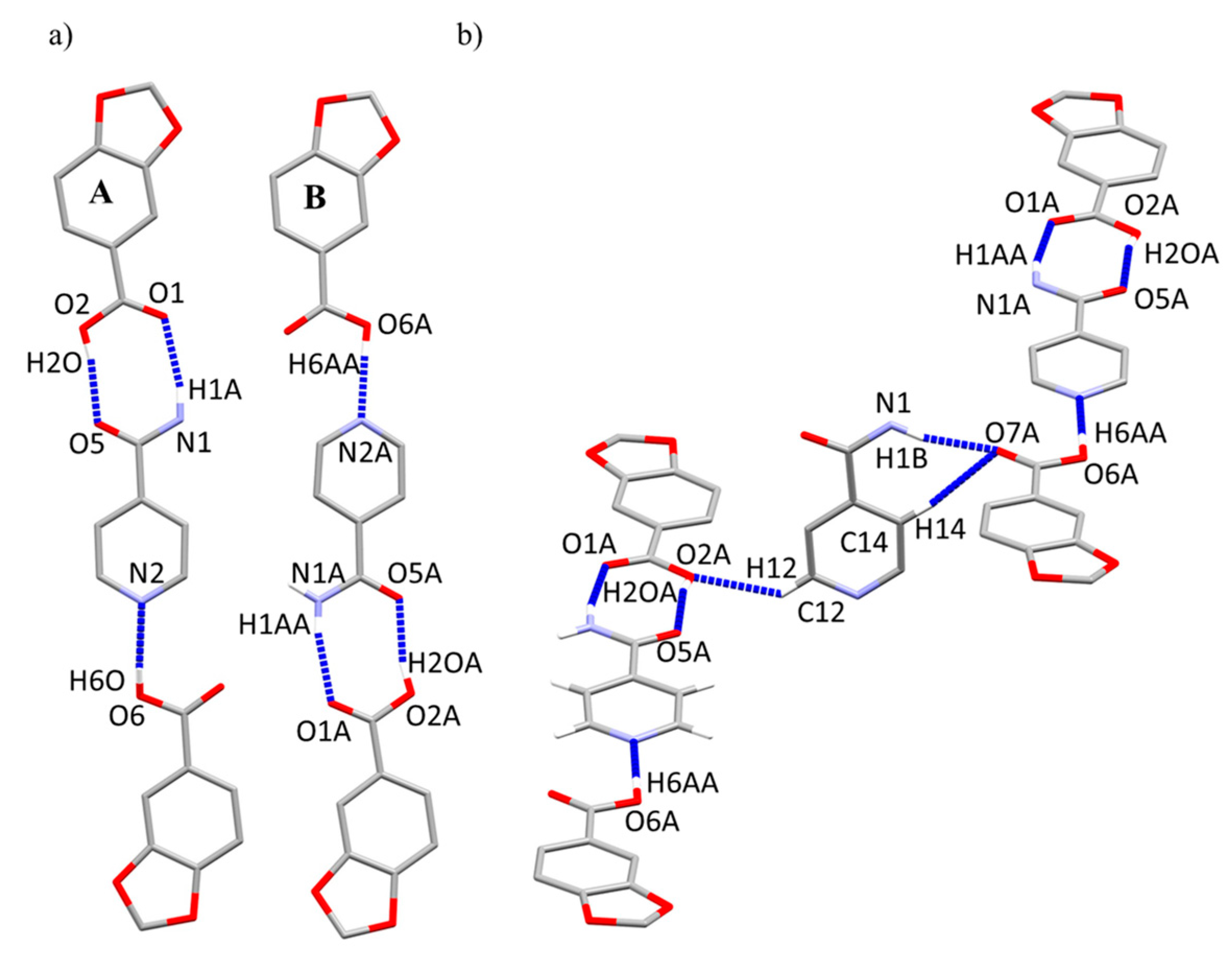
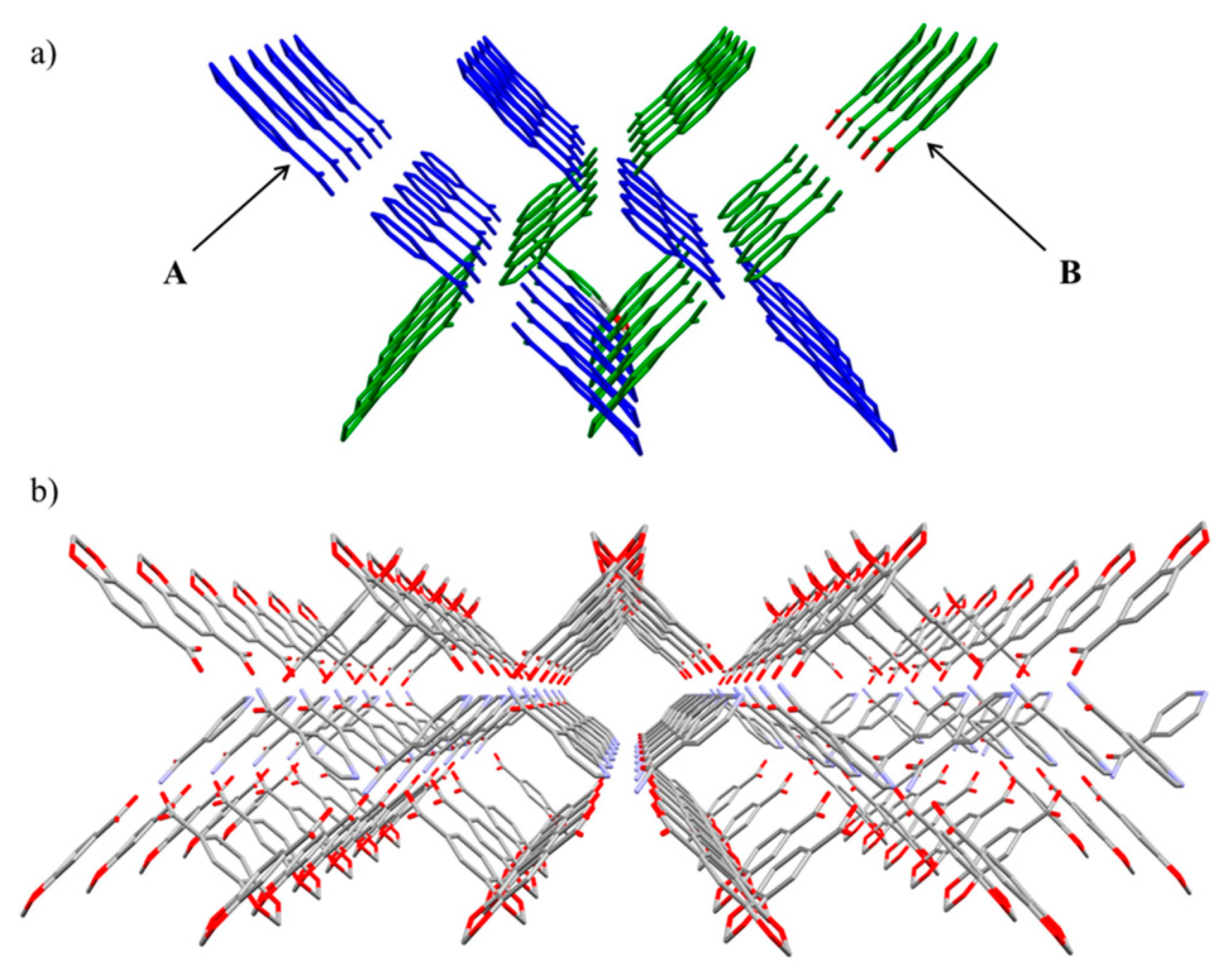
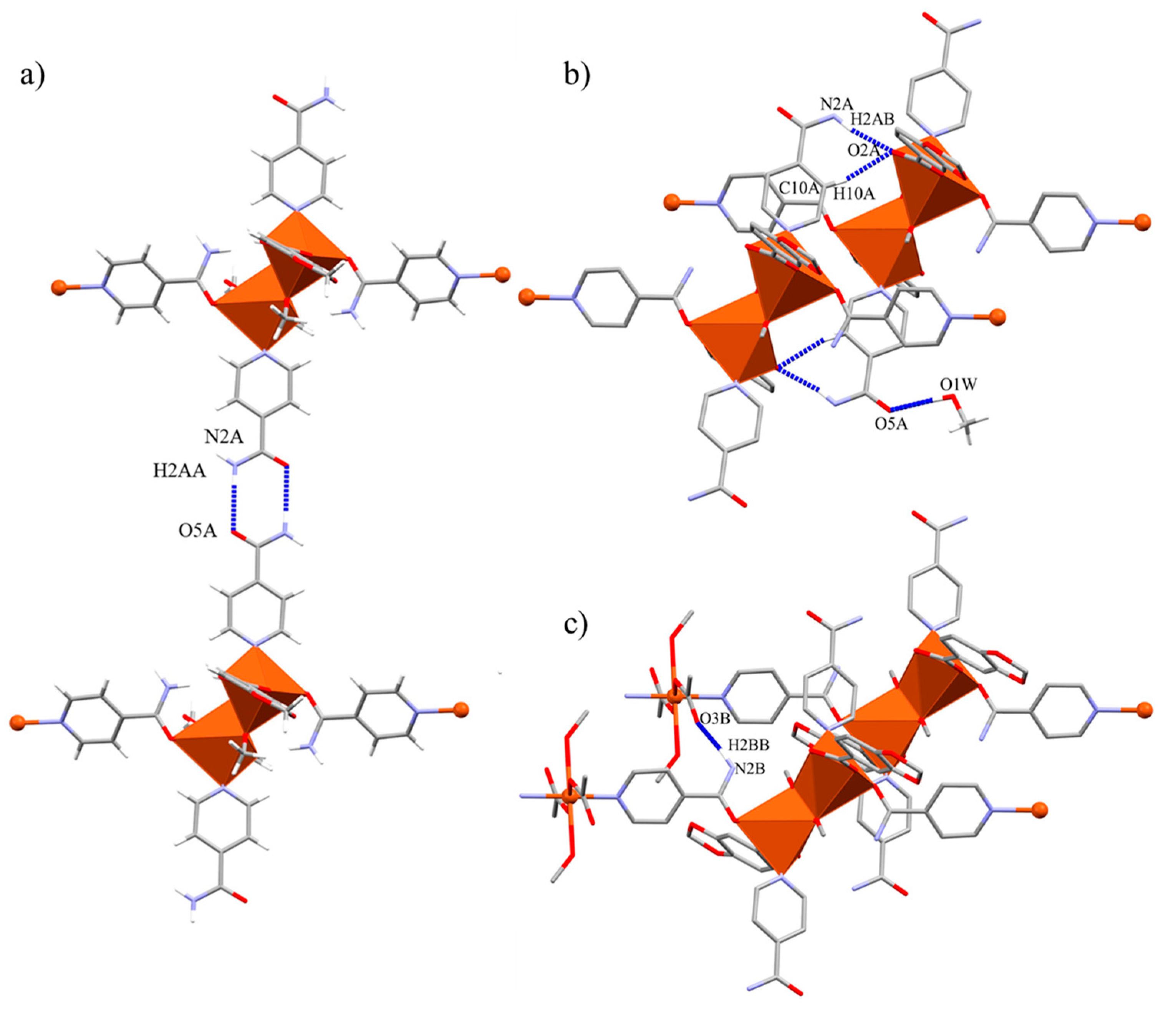
2.2. Crystal Structure of Compound 2
2.3. Synthesis and Characterization of the Coordination Complexes 3 and 4
2.4. Crystal and Extended Structure of Compound 3a
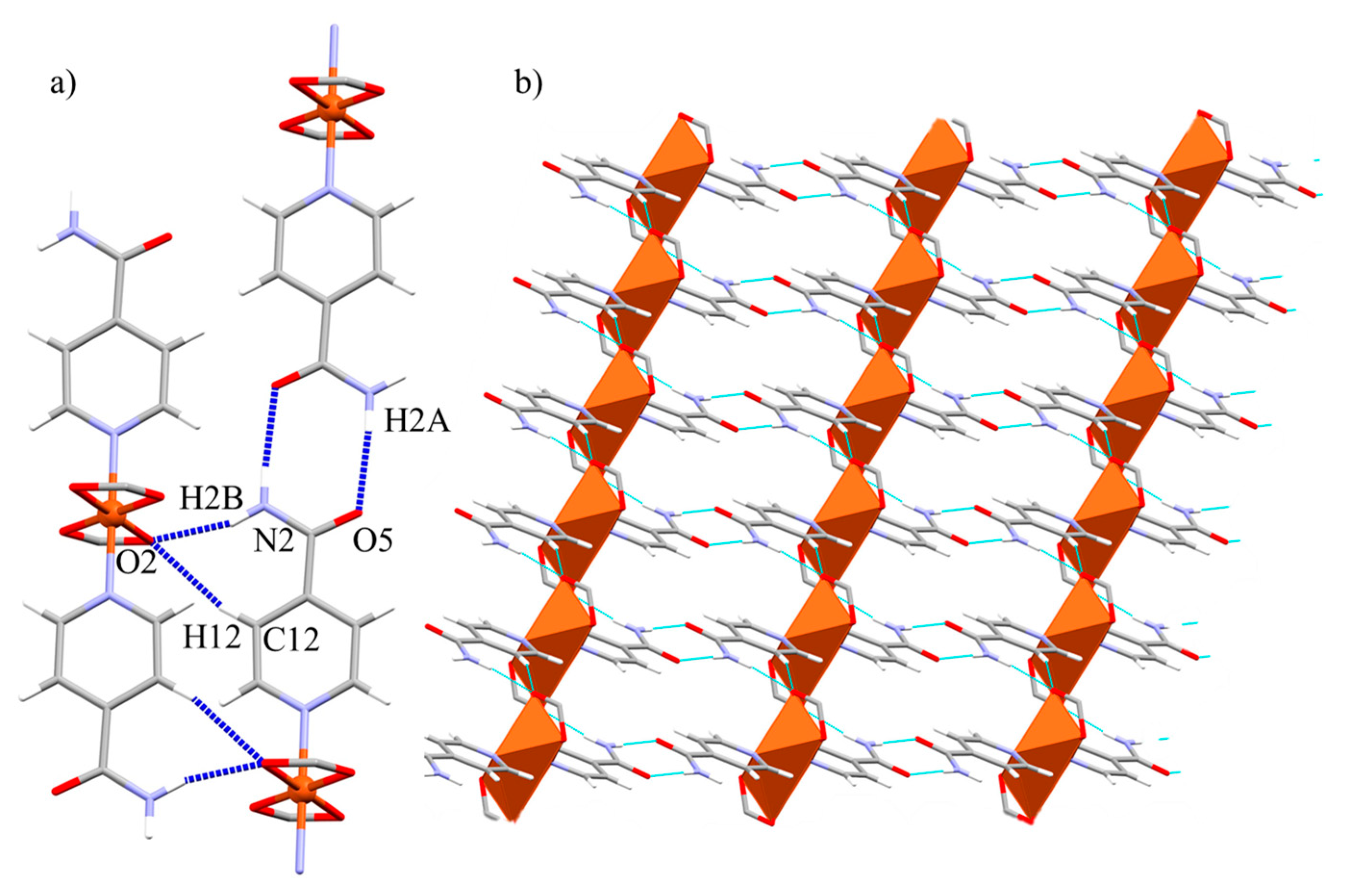
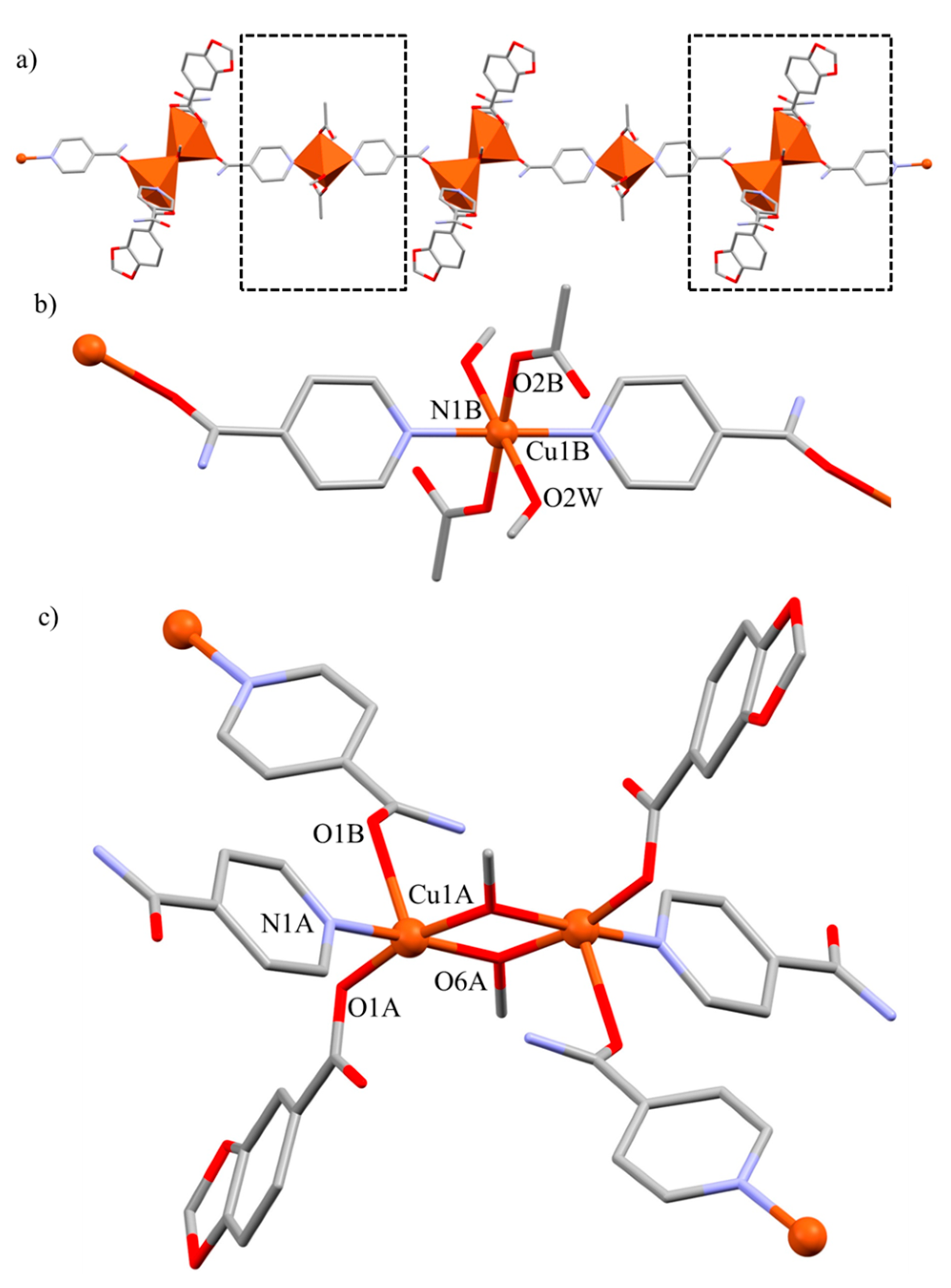
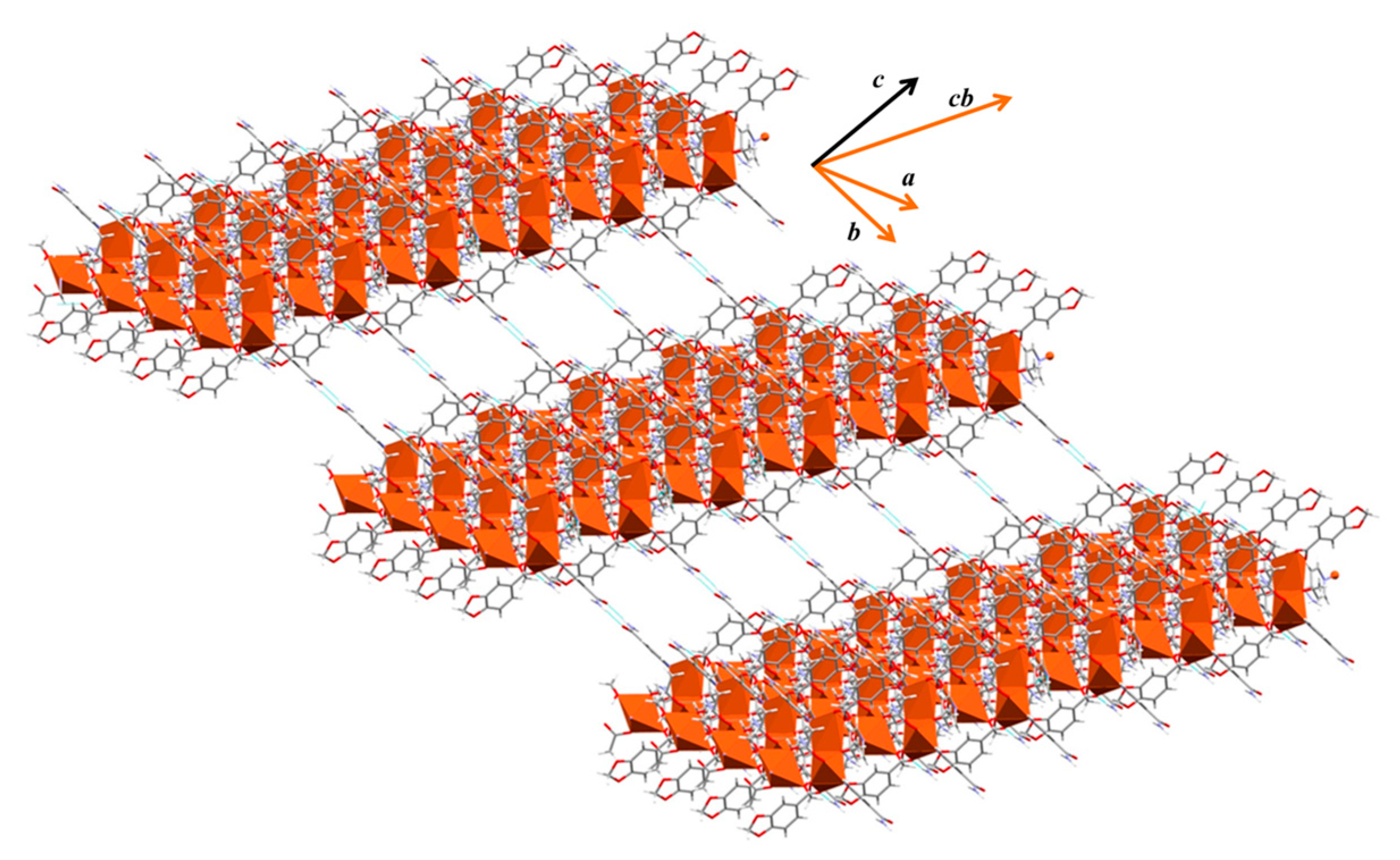
2.5. Crystal and Extended Structure of Compound 4
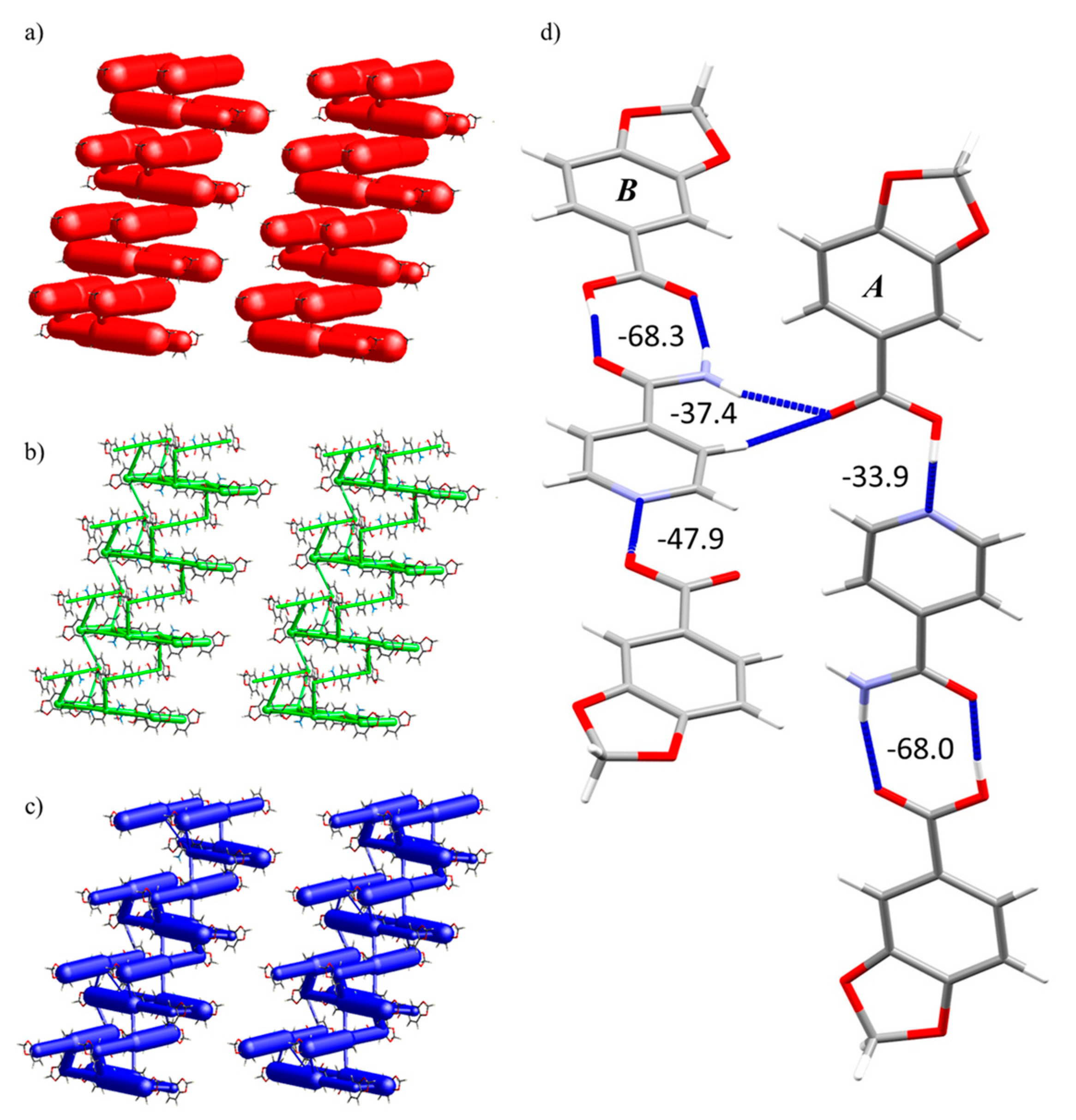
2.6. Hirshfeld Surfaces Analysis and Energy Frameworks Calculations
3. Conclusions
4. Experimental Section
5. Crystallographic Data
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Swiegers, G.F.; Malefetse, T.J. New Self-Assembled Structural Motifs in Coordination Chemistry. Chem. Rev. 2000, 100, 3483–3538. [Google Scholar] [CrossRef]
- Desiraju, G.R. Hydrogen bonds and other intermolecular interactions in organometallic crystals. J. Chem. Soc. Dalton Trans. 2000, 21, 3745–3751. [Google Scholar] [CrossRef]
- Cambridge Structural Database (CSD) (Version 5.37). 2016. Available online: https://libguides.caltech.edu/crystallography/CSD#Applications (accessed on 14 November 2019).
- Ye, B.-H.; Tong, M.-L.; Chen, X.-M. Metal-organic molecular architectures with 2,2′-bipyridyl-like and carboxylate ligands. Coord. Chem. Rev. 2005, 249, 545–565. [Google Scholar] [CrossRef]
- Etter, M.C. Encoding and decoding hydrogen-bond patterns of organic compounds. Acc. Chem. Res. 1990, 23, 120–126. [Google Scholar] [CrossRef]
- Desiraju, G.R. Supramolecular Synthons in Crystal Engineering—A New Organic Synthesis. Angew. Chem., Int. Ed. 1995, 34, 2311–2327. [Google Scholar] [CrossRef]
- Nangia, A.; Desiraju, G.R. Supramolecular Synthons and Pattern Recognition. In Design of Organic Solids; Topics in Current Chemistry, 198; Weber, E., Aoyama, Y., Caira, M.R., Desiraju, G.R., Glusker, J.P., Hamilton, A.D., Meléndez, R.E., Nangia, A., Eds.; Springer: Berlin/Heidelberg, Germany, 1998; pp. 57–95. [Google Scholar]
- Wang, Y.-Y.; Shi, Q.; Shi, Q.-Z.; Gao, Y.-C.; Zhou, Z.-Y. Syntheses, characterization and crystal structure of copper(II) α,β-unsaturated carboxylate complexes with imidazole. Polyhedron 1999, 18, 2009–2015. [Google Scholar] [CrossRef]
- Choudhury, A.R.; Desiraju, G.R.; Dikundwar, A.G.; Dubey, R.; Duggirala, N.; Ghogale, P.P.; Ghosh, S.; Goswami, P.K.; Goud, N.R.; Jetti, R.R.K.R.; et al. Polymorphs, Salts, and Cocrystals: What’s in a Name? Cryst. Growth Des. 2012, 12, 2147–2152. [Google Scholar]
- Germán-Acacio, J.M.; Hernández-Ortega, S.; Aakeröy, C.B.; Valdés-Martínez, J. Using Lewis acidity differences in chelating ligands to control molecular structure and supramolecular assembly of Cu(II) complexes. Inorg. Chim. Acta 2009, 362, 4087–4090. [Google Scholar] [CrossRef]
- Ɖakovic, M.; Jagličić, Z.; Kozlevčar, B.; Popović, Z. Association of copper(II) isonicotinamide moieties via different anionic bridging ligands: Two paths of ferromagnetic interaction in the azide coordination compound. Polyhedron 2010, 29, 1910–1917. [Google Scholar] [CrossRef]
- Ataç, A.; Yurdakul, Ş.; Ide, S. Synthesis and vibrational spectroscopic studies of isonicotinamide metal(II) halide complexes. J. Mol. Struct. 2006, 783, 79–87. [Google Scholar] [CrossRef]
- Mukherjee, A. Building upon Supramolecular Synthons: Some Aspects of Crystal Engineering. Cryst. Growth Des. 2015, 15, 3076–3085. [Google Scholar] [CrossRef]
- Yenikaya, C.; Poyraz, M.; Sari, M.; Demirci, F.; İlkimen, H.; Büyükgüngör, O. Synthesis, characterization and biological evaluation of a novel Cu(II) complex with the mixed ligands 2,6-pyridinedicarboxylic acid and 2-aminopyridine. Polyhedron 2009, 28, 3526–3532. [Google Scholar] [CrossRef]
- Cruz-Cabeza, A.J. Acid–base crystalline complexes and the pKa rule. CrystEngComm. 2012, 14, 6362–6365. [Google Scholar] [CrossRef]
- Bhogala, B.R.; Basavoju, S.; Nangia, A. Tape and layer structures in cocrystals of some di- and tricarboxylic acids with 4,4′-bipyridines and isonicotinamide. From binary to ternary cocrystals. CrystEngComm. 2005, 7, 551–562. [Google Scholar] [CrossRef]
- Liu, C.-S.; Wang, J.-J.; Yan, L.-F.; Chang, Z.; Bu, X.-H.; Señudo, E.C.; Ribas, J. Copper(II), Cobalt(II), and Nickel(II) Complexes with a Bulky Anthracene-Based Carboxylic Ligand: Syntheses, Crystal Structures, and Magnetic Properties. Inorg. Chem. 2007, 46, 6299–6310. [Google Scholar] [CrossRef]
- Moulton, B.; Zaworotko, M.J. From Molecules to Crystal Engineering: Supramolecular Isomerism and Polymorphism in Network Solids. Chem. Rev. 2001, 101, 1629–1658. [Google Scholar] [CrossRef]
- Arici, M.; Yeşilel, O.Z.; Acar, E.; Dege, N. Synthesis, characterization and properties of nicotinamide and isonicotinamide complexes with diverse dicarboxylic acids. Polyhedron 2017, 127, 293–301. [Google Scholar] [CrossRef]
- Moncol, J.; Mudra, M.; Lönnecke, P.; Hewitt, M.; Valko, M.; Morris, H.; Svorec, J.; Melnik, M.; Mazur, M.; Koman, M. Crystal structures and spectroscopic behavior of monomeric, dimeric and polymeric copper(II) chloroacetate adducts with isonicotinamide, N-methylnicotinamide and N,N-diethylnicotinamide. Inorg. Chim. Acta 2007, 360, 3213–3225. [Google Scholar] [CrossRef]
- Bhogala, B.R.; Thallapally, P.K.; Nangia, A. 1:2 and 1:1 Ag(I)-Isonicotinamide coordination compounds: Five-fold interpenetrated CdSO4 network and the first example of (pyridine)N-Ag-O(amide) bonds. Crystal Growth & Design, 4, 215-218. Cryst. Growth Des. 2004, 4, 215–218. [Google Scholar]
- Lynch, M.B.; Lawrence, S.E.; Nolan, M. Predicting Nucleation of Isonicotinamide from the Solvent–Solute Interactions of Isonicotinamide in Common Organic Solvents. J. Phys. Chem. A 2018, 122, 3301–3312. [Google Scholar] [CrossRef]
- Vishweshwar, P.; Nangia, A.; Lynch, V.M. Molecular complexes of homologous alkanedicarboxylic acids with isonicotinamide: X-ray crystal structures, hydrogen bond synthons, and melting point alternation. Cryst. Growth Des. 2003, 3, 783–790. [Google Scholar] [CrossRef]
- Mohamed, S.; Tocher, D.A.; Vickers, M.; Karamertzanis, P.G.; Price, S.L. Salt or Cocrystal? A New Series of Crystal Structures Formed from Simple Pyridines and Carboxylic Acids. Cryst. Growth Des. 2009, 9, 2881–2889. [Google Scholar] [CrossRef]
- Sánchez-Férez, F.; Bayés, L.; Font-Bardia, M.; Pons, J. Solvent dependent formation of Cu(II) complexes based on isonicotinamide ligand. Inorg. Chim. Acta 2019, 494, 112–122. [Google Scholar] [CrossRef]
- Soldevila-Sanmartín, J.; Ayllón, J.A.; Calvet, T.; Font-Bardía, M.; Domingo, C.; Pons, J. Synthesis, crystal structure and magnetic properties of a Cu(II) paddle-wheel complex with mixed bridges. Inorg. Chem. Commun. 2016, 71, 90–93. [Google Scholar] [CrossRef]
- Sánchez-Férez, F.; Guerrero, M.; Ayllón, J.A.; Calvet, T.; Font-Bardía, M.; Giner Planas, J.; Pons, J. Reactivity of homoleptic and heteroleptic core paddle wheel Cu(II) compounds. Inorg. Chim. Acta 2019, 487, 295–306. [Google Scholar]
- Williams, D.H.; Fleming, I. Spectroscopic Methods in Organic Chemistry; McGrawHill: London, UK, 1995. [Google Scholar]
- Dey, A.; Kirchner, M.T.; Vangala, V.R.; Desiraju, G.R.; Mondal, R.; Howard, J.A.K. Crystal Structure Prediction of Aminols: Advantages of a Supramolecular Synthon Approach with Experimental Structures. J. Am. Chem. Soc. 2005, 127, 10545–10559. [Google Scholar] [CrossRef]
- Bavishi, D.D.; Borkhataria, C.H. Spring and parachute: How cocrystals enhance solubility. Prog. Cryst. Growth Charact. Mater. 2016, 62, 1–8. [Google Scholar] [CrossRef]
- Shishkin, O.V.; Zubatyuk, R.I.; Shishkina, S.V.; Dyakonenko, V.V.; Medviediev, V.V. Role of supramolecular synthons in the formation of the supramolecular architecture of molecular crystals revisited from an energetic viewpoint. Phys. Chem. Chem. Phys. 2014, 16, 6773–6786. [Google Scholar] [CrossRef]
- Seo, M.; Park, J.; Kim, S.Y. Self-assembly driven by an aromatic primary amide motif. Org. Biomol. Chem. 2012, 10, 5332–5342. [Google Scholar] [CrossRef]
- Tothadi, S.; Desiraju, G.R. Unusual co-crystal of isonicotinamide: The structural landscape in crystal engineering. Philos. Trans. R. Soc. A 2012, 370, 2900–2915. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds. Applications in Coordination, Organometallic, and Bioinorganic Chemistry, 6th ed.; Wiley Interscience: New York, NY, USA, 2009. [Google Scholar]
- Sutton, D. Electronic Spectra of Transition Metal Complexes, 1st ed.; McGraw-Hill: New York, NY, USA, 1968. [Google Scholar]
- Morse, P.M.; Girolami, G.S. Are d0 ML6 Complexes Always Octahedral? The X-ray Structure of Trigonal-Prismatic [Li(tmed)]2[ZrMe6]. J. Am. Chem. Soc. 1989, 111, 4114–4116. [Google Scholar] [CrossRef]
- Friese, J.C.; Krol, A.; Puke, C.; Kirschbaum, K.; Giolando, D.M. Trigonal Prismatic vs Octahedral Coordination Geometry: Syntheses and Structural Characterization of Hexakis(arylthiolato) Zirconate Complexes. Inorg. Chem. 2000, 39, 1496–1500. [Google Scholar] [CrossRef] [PubMed]
- Falvello, L.R. Jahn–Teller effects in solid-state co-ordination chemistry. J. Chem. Soc. Dalton Trans. 1997, 1, 4463–4475. [Google Scholar] [CrossRef]
- Nimmermark, A.; Öhrström, L.; Reedijk, J. Metal-ligand bond lengths and strengths: Are they correlated? A detailed CSD analysis. Z. Kristallogr. 2013, 228, 311–317. [Google Scholar] [CrossRef]
- Adison, A.W.; Rao, T.N. Synthesis, structure, and spectroscopic properties of copper(II) compounds containing nitrogen–sulphur donor ligands; the crystal and molecular structure of aqua[1,7-bis(N-methylbenzimidazol-2′-yl)-2,6-dithiaheptane]copper(II) perchlorate. J. Chem. Soc. Dalton. Trans. 1984, 1, 1349–1356. [Google Scholar] [CrossRef]
- Gör, K.; Kürkçüoğlu, G.S.; Yeşilel, O.Z.; Büyükgüngör, O. One-dimensional bimetallic cyano complexes with nicotinamide and isonicotinamide ligands. J. Mol. Struct. 2014, 1060, 166–175. [Google Scholar] [CrossRef]
- Gudavarthy, R.V.; Burla, N.; Kulp, E.A.; Limmer, S.J.; Sinn, E.; Switzer, J.A. Epitaxial electrodeposition of chiral CuO films from copper(II) complexes of malic acid on Cu(111) and Cu(110) single crystals. J. Mater. Chem. 2011, 21, 6209–6216. [Google Scholar] [CrossRef]
- Wolff, S.K.; Grimwood, D.J.; McKinnon, J.J.; Jayatilaka, D. and Spackman, M.A. CrystalExplorer17.5; University of Western, Australia: Crawley, Australia, 2017. [Google Scholar]
- Évora, A.O.L.; Bernades, C.E.S.; Piedade, M.F.M.; Conceição, A.C.L.; Minas da Piedade, M.E. Energetics of Glycine Cocrystal or Salt Formation with Two Regioisomers: Fumaric Acid and Maleic Acid. Cryst. Growth Des. 2019, 19, 5054–5064. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Macrae, C.F.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Shields, G.; Taylor, R.; Towler, M.; Van de Streek, J.J. Mercury: Visualization and analysis of crystal structures. Appl. Crystallogr. 2006, 39, 453–457. [Google Scholar] [CrossRef]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, I.; Taylor, R.; Towler, M.; van de Streek, J.; et al. Mercury CSD 2.0—New features for the visualization and investigation of crystal structures. Appl. Crystallogr. 2008, 41, 466–470. [Google Scholar] [CrossRef]
- Persistence of Vision Pty. Ltd. Persistence of Vision (TM) Raytracer; Persistence of Vision Pty. Ltd.: Williamstown, Australia, 2004; Available online: http://www.povray.org/ (accessed on 14 November 2019).

| 2 | 3a | 4 | ||||
|---|---|---|---|---|---|---|
| BSM A | BSM B | |||||
| Bond | Length (Å) | Distance (Å) | Bond | Length (Å) | Bond | Distance (Å) |
| C=OIsn | 1.253(4) | 1.241(5) | C=OIsn | 1.236(2) | C=OIsn coord | 1.234(4) |
| C=OHPip···Npy | 1.233(4) | 1.226(4) | C=OPip | 1.281(3) | C=OIsn | 1.240(5) |
| C=OHPip···H-Namide | 1.217(5) | 1.228(5) | C=OPip | 1.250(3) | C=OPip | 1.330(5) |
| C-N | 1.321(4) | 1.326(4) | C-N | 1.328(3) | C=OPip | 1.317(6) |
| C-OHHPip···Npy | 1.300(5) | 1.314(4) | C-NIsn coord | 1.276(6) | ||
| C-OHHPip···H-Namide | 1.324(4) | 1.318(4) | C-N | 1.257(5) | ||
| Intra-BSM A | ||||
|---|---|---|---|---|
| H···A (Å) | D···A (Å) | D-H (Å) | >D-H···A (°) | |
| O6-H6O···N2 | 1.916 | 2.611 | 0.840 | 139.37 |
| N1-H1A···O1 | 1.979 | 2.840 | 0.881 | 165.44 |
| O2-H2O···O5 | 1.835 | 2.645 | 0.840 | 161.52 |
| Intra-BSM B | ||||
| O6A-H6AA···N2A | 1.778 | 2.615 | 0.841 | 173.73 |
| N1A-H1AA···O1A | 1.969 | 2.831 | 0.881 | 166.02 |
| O2A-H2OA···O5A | 1.819 | 2.634 | 0.840 | 163.11 |
| Inter-BSM A | ||||
| N1-H1B···O7A | 2.033 | 2.880 | 0.880 | 161.48 |
| C14-H14···O7A | 2.342 | 3.279 | 0.950 | 168.81 |
| C12-H12···O2A | 2.374 | 3.099 | 0.950 | 132.81 |
| Inter-BSM B | ||||
| N1A-H1AB···O7 | 2.031 | 2.879 | 0.880 | 161.40 |
| C13A-H13A···O2 | 2.376 | 3.097 | 0.950 | 132.40 |
| C11A-H11A···O7 | 2.344 | 3.281 | 0.950 | 168.84 |
| Bond Length (Å) | |||
|---|---|---|---|
| Cu(1)-O(1)#1 | 1.9673(14) | Cu(1)-N(1)#1 | 1.9988(16) |
| Cu(1)-O(2) | 2.5069(16) | Cu···Cu | 8.023 |
| Bond Angles (°) | |||
| O(1)#1-Cu(1)-O(1) | 180 | O(2)-Cu(1)-O(1) | 57.69(6) |
| O(1)#1-Cu(1)-N(1) | 89.88(6) | O(2)-Cu(1)-O(1)#2 | 122.31(6) |
| O(1)-Cu(1)-N(1) | 90.12(6) | N(1)-Cu(1)-O(2) | 91.26(6) |
| N(1)-Cu(1)-N(1)#1 | 180 | N(1)-Cu(1)-O(2)#1 | 88.74(6) |
| O(2)-Cu(1)-O(2)#1 | 180 | ||
| 3b | H···A (Å) | D···A (Å) | D-H (Å) | >D-H···A (°) |
|---|---|---|---|---|
| N2-H2A···O5 | 2.013 | 2.887 | 0.880 | 172.27 |
| N2-H2B···O2 | 1.986 | 2.842 | 0.880 | 164.42 |
| C12-H12···O2 | 2.330 | 3.251 | 0.950 | 163.28 |
| Bond Length (Å) | |||
|---|---|---|---|
| Cu(1A)-O(6A) | 1.936(3) | Cu(1B)-N(1B)#1 | 2.006(3) |
| Cu(1A)-O(1A) | 1.957(3) | Cu(1B)-O(2W) | 2.546(3) |
| Cu(1A)-O(6A)#2 | 1.957(2) | Cu(1A)···Cu(1A) | 3.052(9) |
| Cu(1A)-O(1B) | 2.395(3) | Cu(1A)···Cu(1B) | 10.820(9) |
| Cu(1A)-N(1A) | 2.011(3) | Cu(1A)···Cu(1B) | 9.139(9) |
| Cu(1B)-O(2B)#1 | 1.964(3) | ||
| Bond Angles (°) | |||
| O(6A)-Cu(1A)-O(1A) | 167.18(11) | O(1A)-Cu(1A)-O(1B) | 84.80(10) |
| O(6A)-Cu(1A)-O(6A)#2 | 76.74(11) | O(6A)#2-Cu(1A)-O(1B) | 97.61(10) |
| O(1A)-Cu(1A)-O(6A)#2 | 93.70(11) | N(1A)-Cu(1A)-O(1B) | 90.83(11) |
| O(6A)-Cu(1A)-N(1A) | 94.18(12) | O(2B)#1-Cu(1B)-O(2B) | 180.00(15) |
| O(1A)-Cu(1A)-N(1A) | 94.24(12) | O(2B)#1-Cu(1B)-N(1B) | 91.01(12) |
| O(6A)#2-Cu(1A)-N(1A) | 168.90(12) | O(2B)#1-Cu(1B)-N(1B)#1 | 88.99(12) |
| O(6A)-Cu(1A)-O(1B) | 104.73(10) | N(1B)-Cu(1B)-N(1B)#1 | 180.0 |
| O(1B)-Cu(1A)-O(6A) | 104.71(10) | O(1B)-Cu(1A)-N(1A) | 90.82(11) |
| H···A (Å) | D···A (Å) | D-H (Å) | >D-H···A (°) | |
|---|---|---|---|---|
| N(2B)-H(2BA)···O(6A) | 2.14 | 2.883(4) | 0.88 | 142 |
| O(1W)-H(1W)···O(5A) | 1.90 | 2.739(6) | 0.84 | 173 |
| N(2B)-H(2BB)···O(3B) | 2.02 | 2.791(4) | 0.88 | 145 |
| O(2W)-H(2W)···O(3B) | 1.86(7) | 2.654(5) | 0.81(7) | 167(7) |
| N(2A)-H(2AA)···O(5A) | 2.04 | 2.916(5) | 0.88 | 178 |
| N(2A)-H(2AB)···O(2A) | 1.92 | 2.781(5) | 0.88 | 164 |
| 2 | 3a | 4 | |
|---|---|---|---|
| Empirical formula | C22H18N2O9 | C33H34CuN4O11 | C50H62Cu3N8O22 |
| Formula weigh | 454.38 | 726.18 | 1317.69 |
| T (K) | 100(2) K | 100(2) | 100(2) |
| Wavelength (Å) | 0.71073 Å | 0.71073 | 0.71073 |
| System, space group | Triclinic, P | Triclinic, P | Triclinic, P |
| Unit cell dimensions | |||
| a (Å) | 9.6976(3) | 8.0226(8) | 7.8402(3) |
| b (Å) | 12.6877(6) | 11.4099(12) | 13.7091(6) |
| c (Å) | 16.1988(7) | 11.9187(13) | 14.3107(6) |
| α (°) | 82.329(2) | 1.114(4) | 109.2060(10) |
| β (°) | 81.3340(10) | 96.344(4) | 97.8040(10) |
| γ (°) | 89.9900(10) | 107.632(3) | 99.7130(10) |
| V (Å3) | 1952.31(14) | 988.10(18) | 1401.15(10) |
| Z | 4 | 1 | 1 |
| Dcalc (g × cm3) | 1.546 | 1.220 | 1.562 |
| µ (mm−1) | 0.122 | 0.609 | 1.215 |
| F(000) | 944 | 377 | 681 |
| Crystal size (mm3) | 0.242 × 0.082 × 0.081 | 0.164 × 0.093 × 0.072 | 0.251 × 0.071 × 0.046 |
| hkl ranges | −11 ≤ h ≤ 12 −16 ≤ k ≤ 16 −21 ≤ l ≤ 21 | −11 ≤ h ≤ 11 −16 ≤ k ≤ 16 −17 ≤ l ≤ 17 | −9 ≤ h ≤ 8 −15 ≤ k ≤ 15 −16 ≤ l ≤ 16 |
| 2θ range (°) | 2.198 to 28.299 | 1.959 to 30.583 | 2.603 to 24.403 |
| Reflections collected/ unique/[Rint] | 69395/9659 [R(int) = 0.0953] | 51118/6046 [R(int) = 0.0428] | 32892/4577 [R(int) = 0.0309] |
| Completeness to θ = 25.242° | 99.9 | 99.6 | 99.6 |
| Absorption Correction | Semi-empirical | Semi-empirical | Semi-empirical |
| Max. and min. transmis. | 0.7457 and 0.6910 | 0.7461 and 0.6872 | 0.7451 and 0.6975 |
| Refinement method | Full-matrix least-squares on |F|2 | Full matrix least-squares on |F|2 | Full matrix least-squares on |F|2 |
| Data/restrains/parameters | 9659/1/597 | 6046/0/250 | 4577/3/382 |
| Goodness of fit on |F|2 | 1.039 | 1.112 | 1.095 |
| Final R indices [I > 2σ(I)] | R1 = 0.0888, wR2 = 0.2189 | R1 = 0.0541, wR2 = 0.1690 | R1 = 0.0444, wR2 = 0.1110 |
| R indices (all data) | R1 = 0.1360, wR2 = 0.2559 | R1 = 0.0591, wR2 = 0.1746 | R1 = 0.0519, wR2 = 0.1172 |
| Extinction coefficient | n/a | n/a | n/a |
| Largest. Diff. peak and hole (e Å−3) | 1.329 and −0.722 | 2.077 and −0.717 | 1.472 and −0.700 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Férez, F.; Ejarque, D.; Calvet, T.; Font-Bardia, M.; Pons, J. Isonicotinamide-Based Compounds: From Cocrystal to Polymer. Molecules 2019, 24, 4169. https://doi.org/10.3390/molecules24224169
Sánchez-Férez F, Ejarque D, Calvet T, Font-Bardia M, Pons J. Isonicotinamide-Based Compounds: From Cocrystal to Polymer. Molecules. 2019; 24(22):4169. https://doi.org/10.3390/molecules24224169
Chicago/Turabian StyleSánchez-Férez, Francisco, Daniel Ejarque, Teresa Calvet, Mercè Font-Bardia, and Josefina Pons. 2019. "Isonicotinamide-Based Compounds: From Cocrystal to Polymer" Molecules 24, no. 22: 4169. https://doi.org/10.3390/molecules24224169
APA StyleSánchez-Férez, F., Ejarque, D., Calvet, T., Font-Bardia, M., & Pons, J. (2019). Isonicotinamide-Based Compounds: From Cocrystal to Polymer. Molecules, 24(22), 4169. https://doi.org/10.3390/molecules24224169






