In Silico Molecular Studies of Antiophidic Properties of the Amazonian Tree Cordia nodosa Lam.
Abstract
1. Introduction
2. Results
2.1. Ethnobotanical Survey
2.2. Chemical and Activity Prospection: Results of the Bibliographic Review
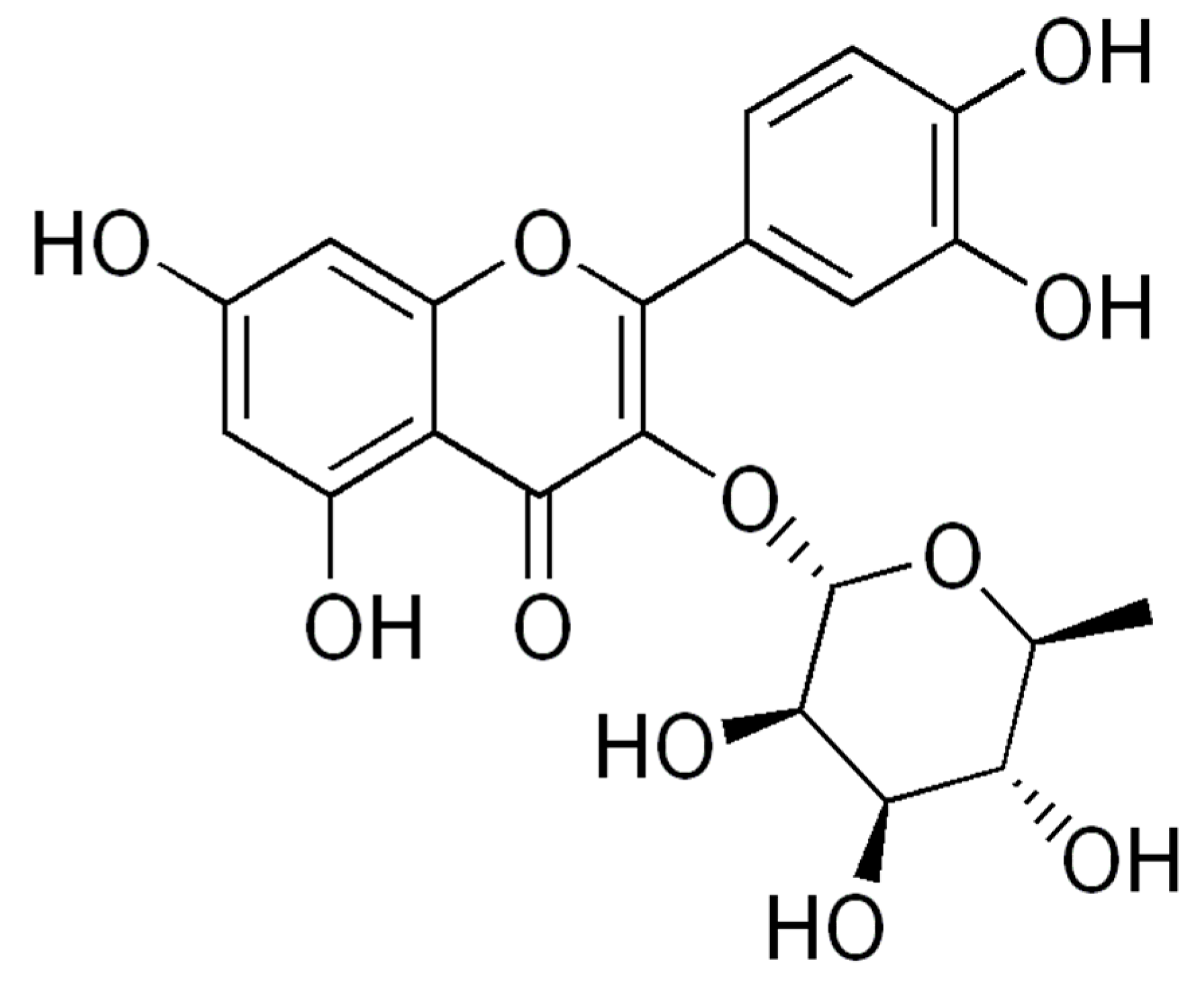
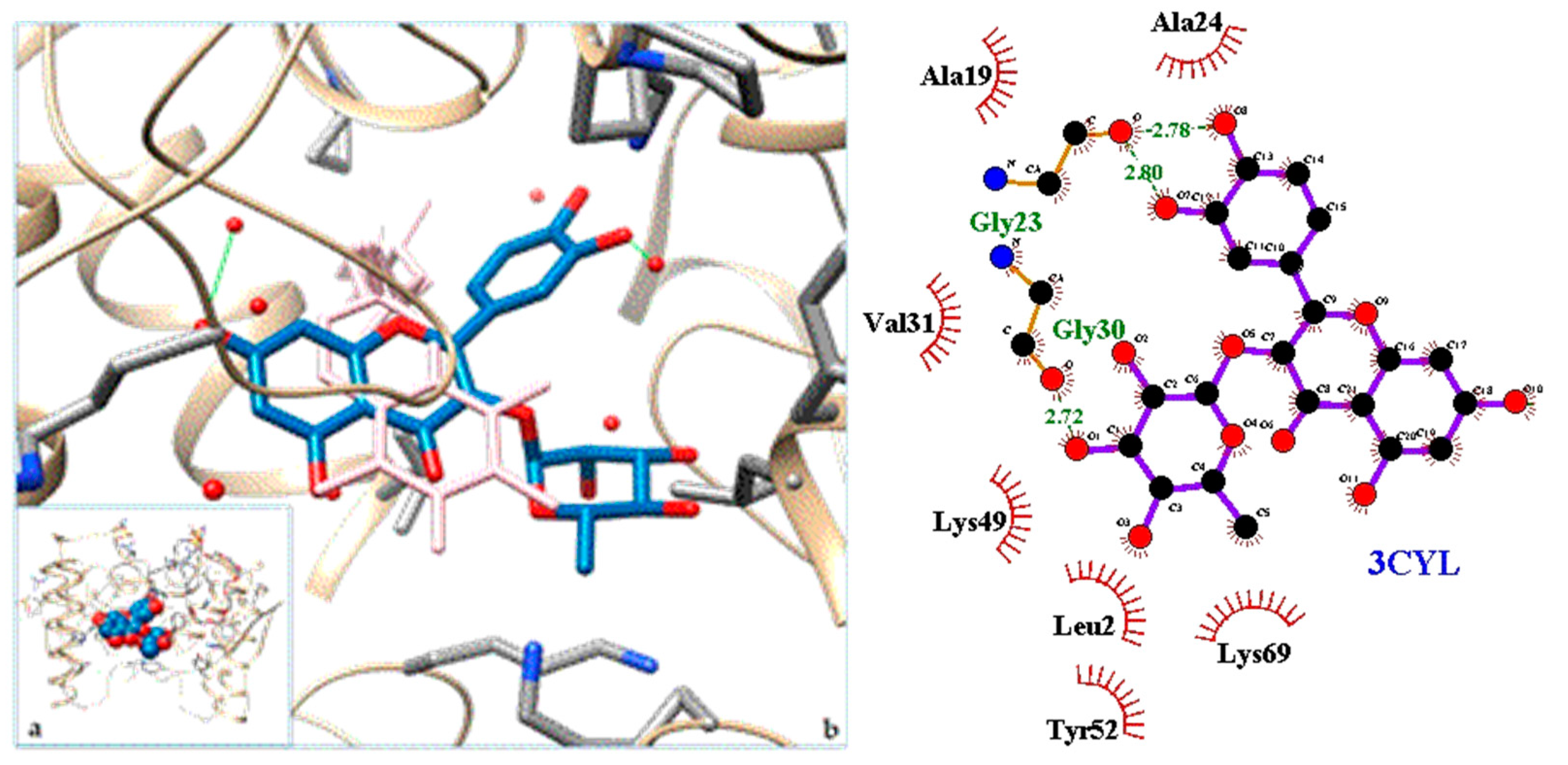
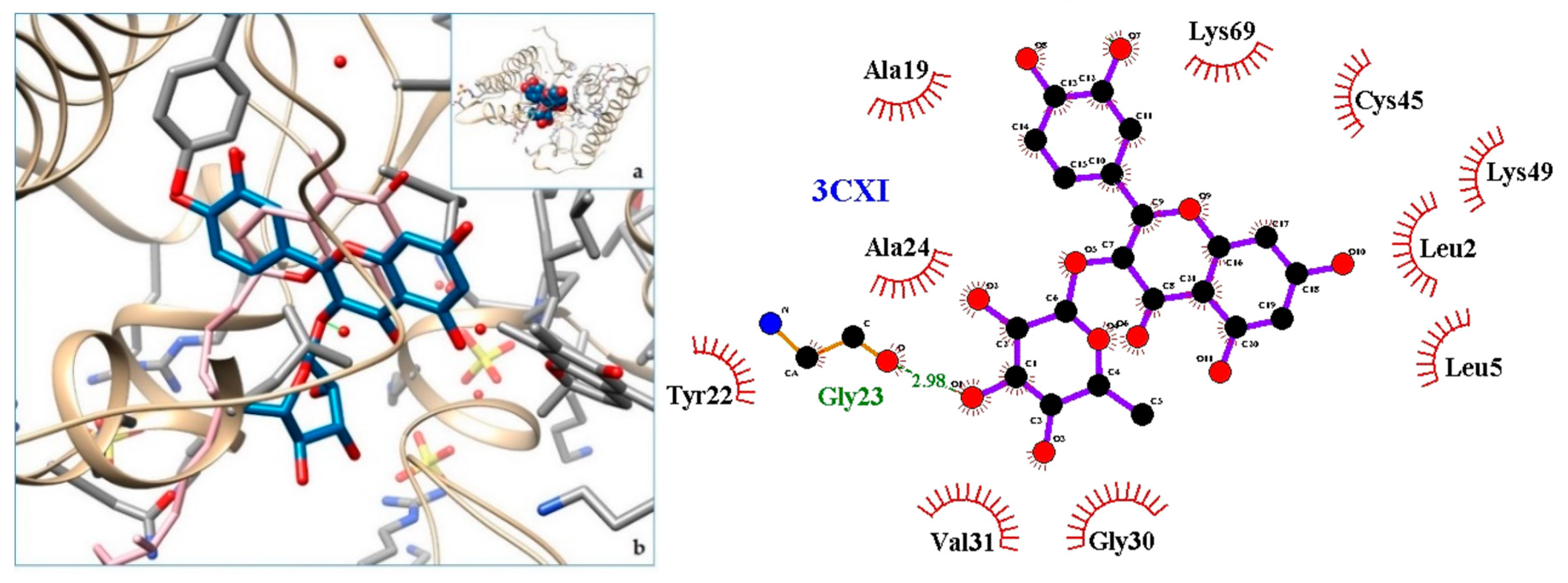
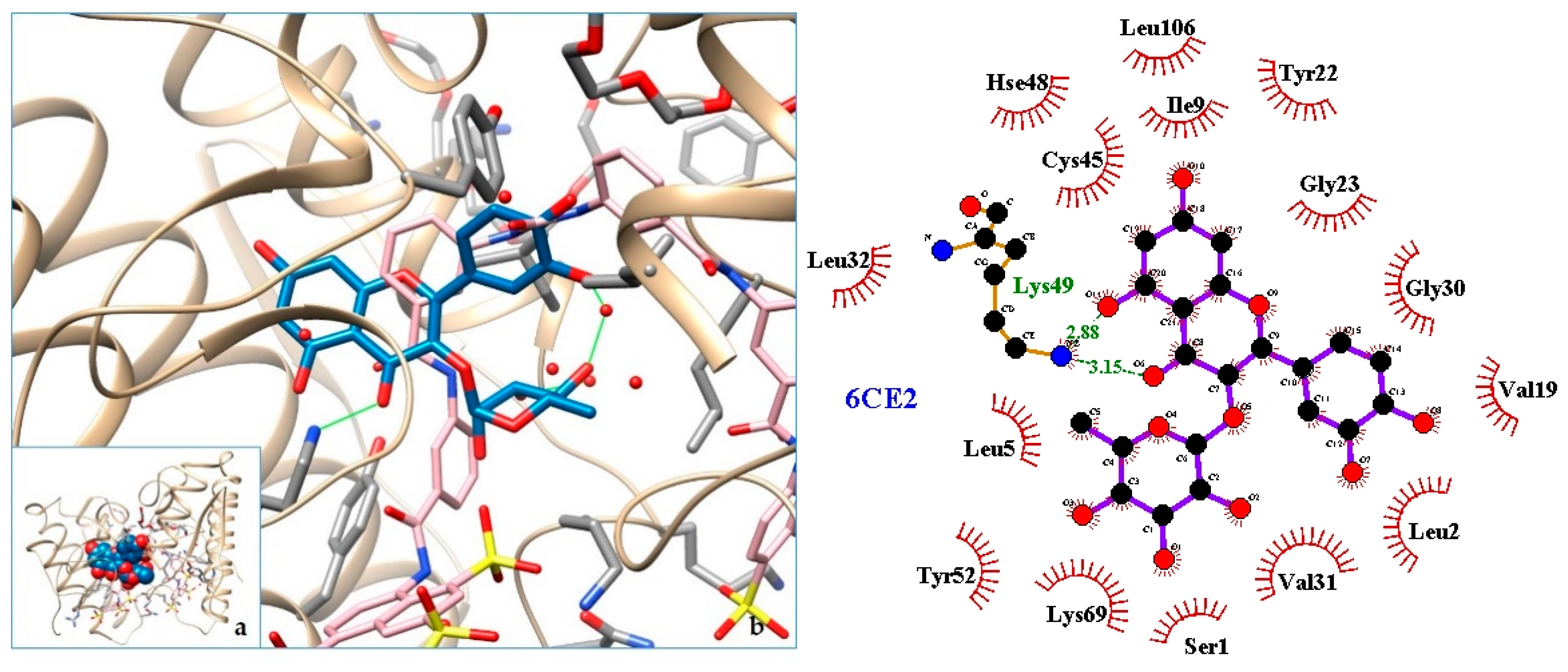
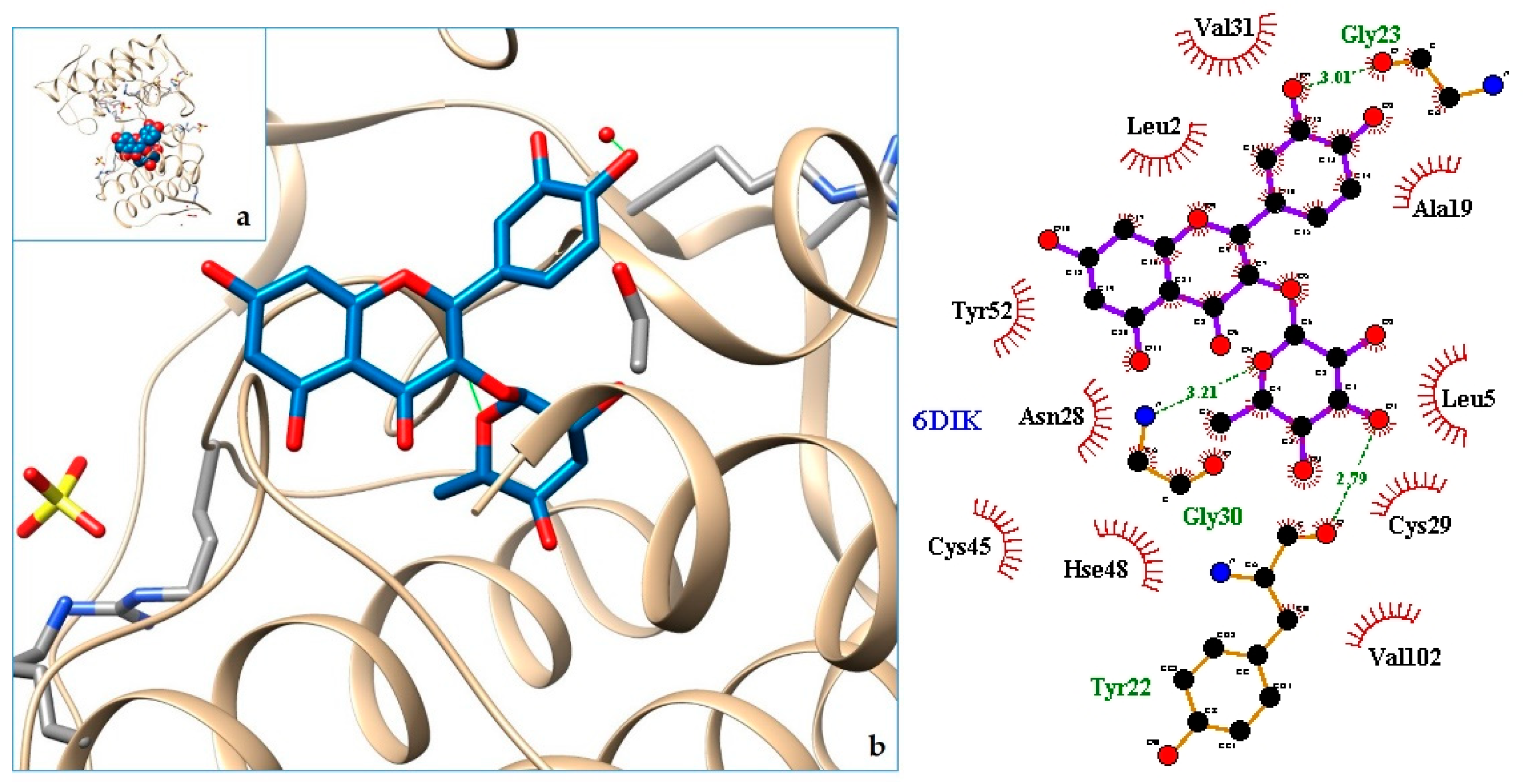
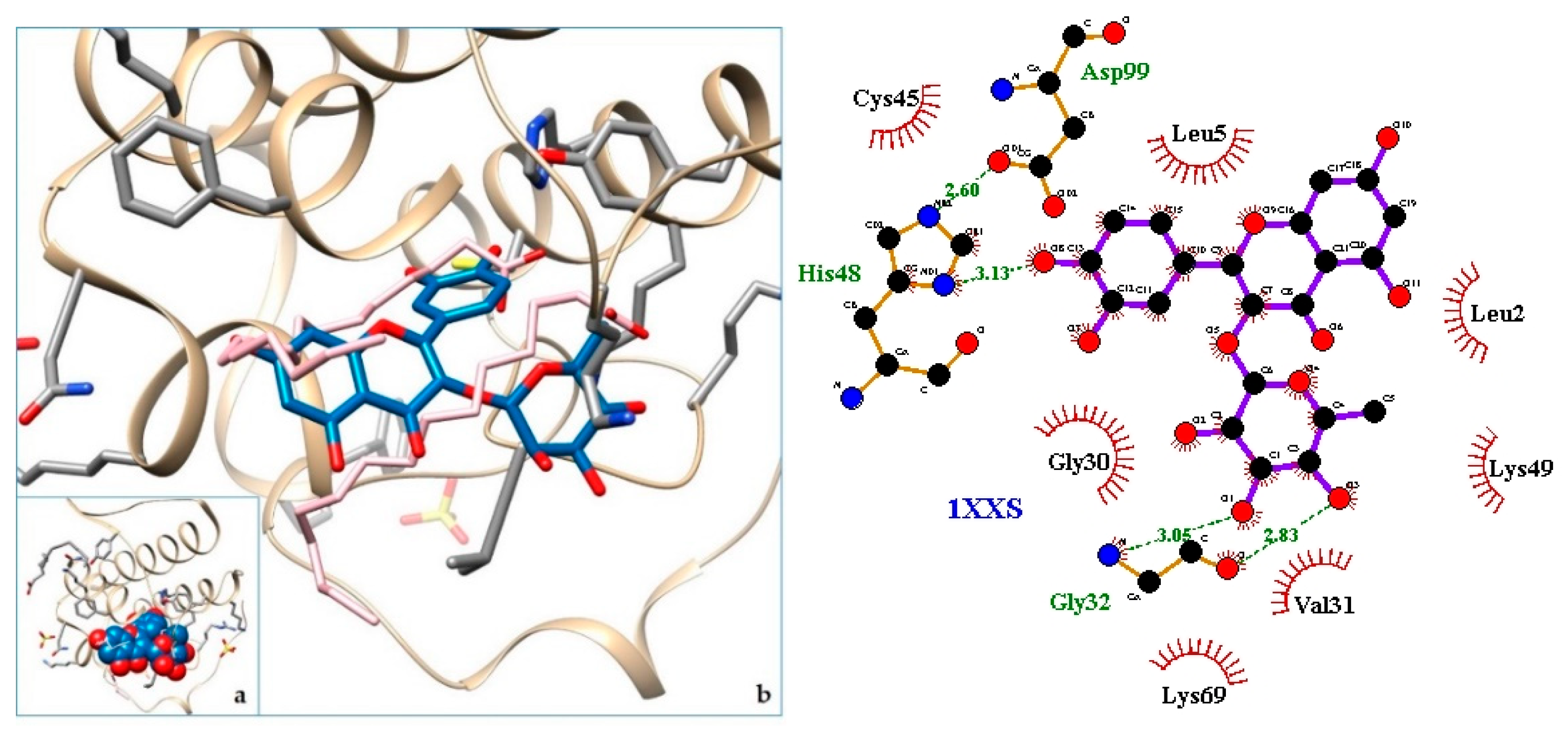
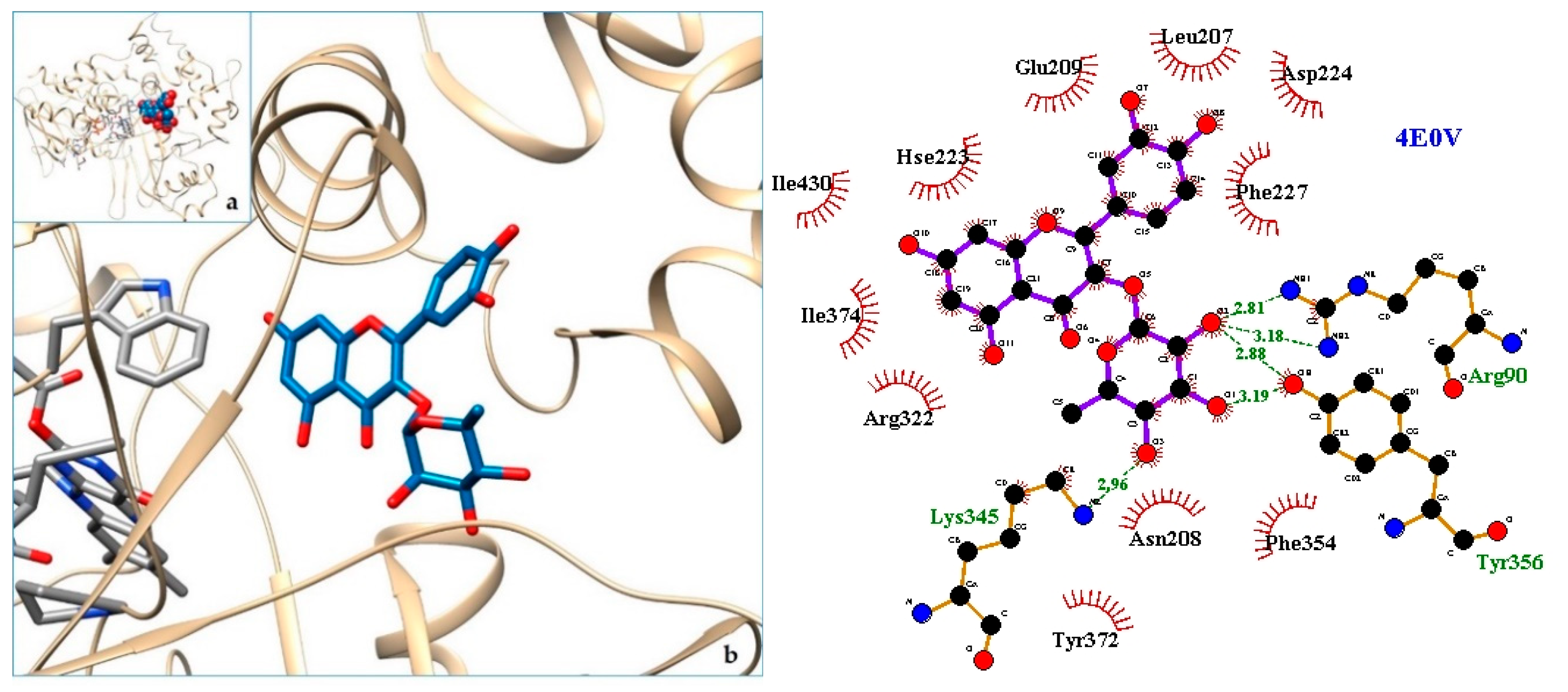
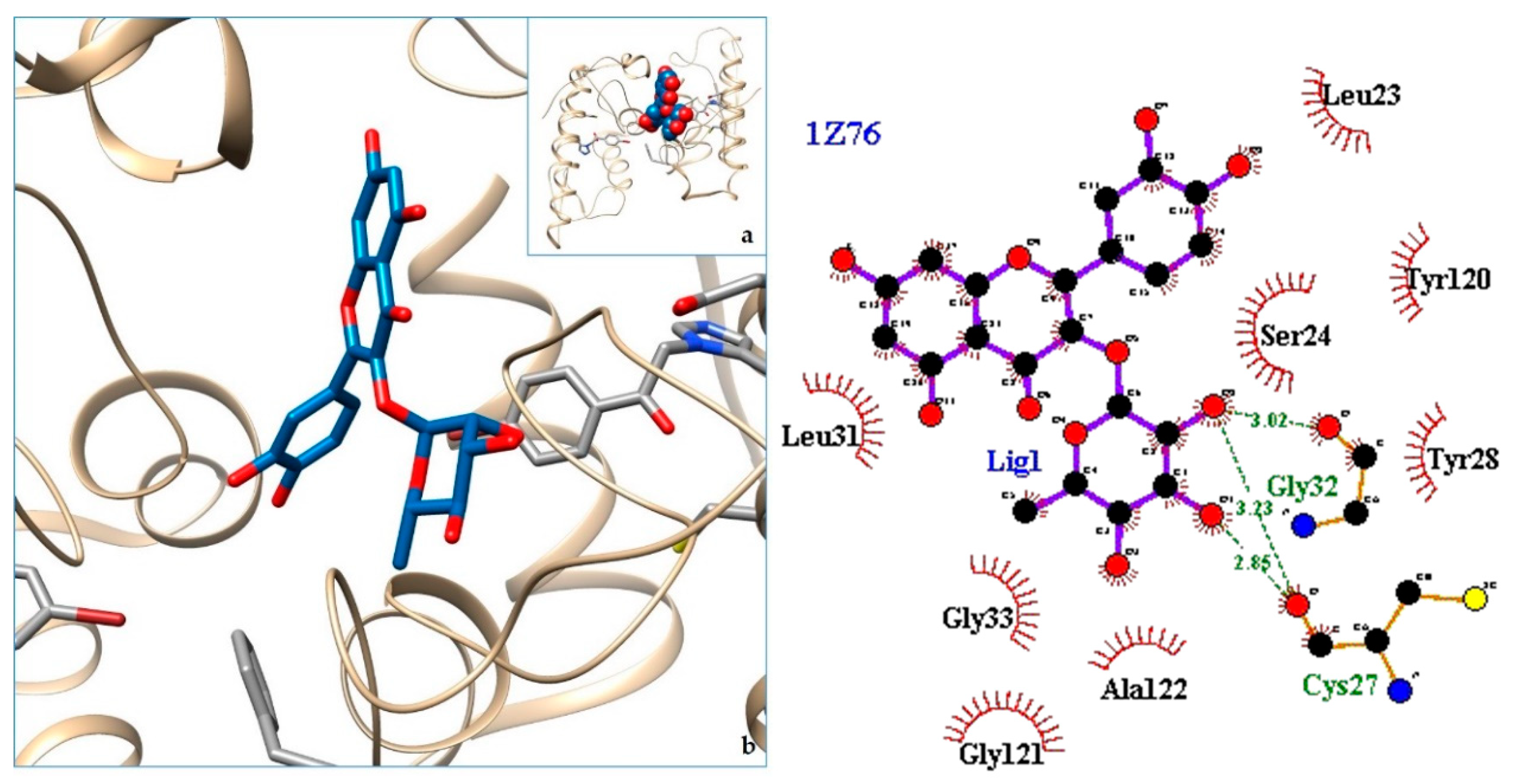
2.3. Docking
3. Discussion
4. Materials and Methods
4.1. Ethnobotanical Survey
4.2. Chemical and Activity Prospection: Bibliographic Review
4.3. Docking
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| PDB ID | Toxin |
| 4GUE | N-terminal kinase domain of RSK2 |
| 5A4W | AtGSTF2 from Arabidopsis thaliana |
| 3CYL | PLA2—Phospholipase A2: Piratoxin I (myotoxic Lys49-PLA2) from Bothrops pirajai |
| 5TFV | MT-I—Basic phospholipase a2 myotoxin iii |
| 5TS5 | LAAO—L-amino acid oxidase from Bothrops atrox |
| 3CXI | PLA2—Phospholipase A2: BthTX-I—Bothropstoxin I from Bothrops jararacussu venom/ |
| 6CE2 | PLA2—Phospholipase A2: Myotoxin (MjTX-I) from Bothrops moojeni |
| 6DIK | PLA2—Phospholipase A2: Bothropstoxin I (BthTX-I) |
| 1XXS | svPLA2—Phospholipase A2: myotoxin II from Bothrops moojeni |
| 4E0V | LAAO—L-amino acid oxidasefrom the Bothrops jararacussu venom |
| 1Z76 | svPLA2—Acidic phospholipase A2 (BthA-I) from Bothrops jararacussu |
| 2QOG | VRV-PL-V—Crotoxin B, the basic PLA2 from Crotalus durissus terrificus |
| 1QLL | PLA2—Piratoxin-II (Prtx-II) - a K49 PLA2 from Bothrops pirajai |
| 3DSL | Bothropasin, the Main Hemorrhagic Factor from Bothrops jararaca venom. |
| 2W12 | SVMP—P-I snake venom metalloproteinase BaP1 |
| 5Z2G | NNH1—L-amino acid oxidase from venom of Naja atra |
| 3KVE | LAAO—L-amino acid oxidase from Vipera ammodytes venom |
| 5GZ4 | PDE I—Phosphodiesterase (PDE) from Taiwan cobra (Naja atra atra) venom |
| 3V9M | VRV-PL-V—Phospholipase ACII4 from Australian King Brown Snake (Pseudechis australis) |
| 1PSH | NN-PL-I—Phospholipase A2 from indian cobra (Naja naja) |
| 1REO | LAAO—L-amino acid oxidase from Agkistrodon Halys Pallas (Gloydius halys) |
| 5GZ5 | NNH1—Phosphodiesterase (PDE) from Taiwan cobra (Naja atra atra) |
| 1A3D | PLA2—Phospholipase A2 (Pla2) from Naja naja |
Appendix A
| 4GUE (305) | 5A4W (212) | 1QLL (121) | 1XXS (122) | 1Z76 (123) | 2QOG (122) | 2W12 (202) | 3CXI (121) | 3CYL (121) | 3DSL (419) | 4E0V (497) | 5TFV (122) | 5TS5 (484) | 6CE2 (121) | 6DIK (121) | |
| 4GUE (305) | 100% | 2.95% (9) 5.25% (16) | 1.64% (5) 2.30% (7) | 1.64 % (5) 2.95% (9) | 1.97 % (6) 1.97 % (6) | 1.31% (4) 1.31% (4) | 8.20% (25) 14.10% (43) | 1.97% (6) 2.95% (9) | 1.64% (5) 2.30 % (7) | 5.90% (18) 8.20% (25) | 8.52% (26) 16.07 % (49) | 1.97% (6) 3.28 % (10) | 6.23% (19) 13.77% (42) | 1.31 % (4) 1.64 % (5) | 1.97 % (6) 2.95 % (9) |
| 5A4W (212) | 4.25% (9) 7.55% (16) | 100 | 2.36% (5) 2.83% (6) | 2.83% (6) 4.72% (10) | 2.83% (6) 4.25 % (9) | 3.77% (8) 4.25% (9) | 6.60% (14) 9.91% (21) | 2.36% (5) 2.83% (6) | 2.62% (13) 3.43% (17) | 4.25% (9) 8.49% (18) | 19.81% (42) 35.38% (75) | 2.36% (5) 2.83% (6) | 19.81% (42) 35.38% (75) | 5.19% (11) 6.60% (14) | 2.36% (5) 2.83% (6) |
| 1QLL (121) | 4.13% (5) 5.79 % (7) | 4.13 % (5) 4.96 % (6) | 100% | 93.39% (113) 95.04% (115) | 48.76% (59) 70.25% (85) | 48.76% (59) 62.81% (76) | 4.96% (6) 7.44% (9) | 98.35% (119) 99.17% (120) | 99.17% (120) 99.17% (120) | 14.05% (17) 21.49% (26) | 19.83 % (24) 35.54 % (43) | 60.33% (73) 71.07% (86) | 5.79 % (7) 9.09 % (11) | 86.78% (105) 89.26% (108) | 99.17% (120) 99.17% (120) |
| 1XXS (122) | 4.10% (5) 7.38% (9) | 4.92% (6) 8.20% (10) | 92.62% (113) 94.26% (115) | 100% | 50.82% (62) 69.67% (85) | 49.18% (60) 63.11% (77) | 4.92% (6) 7.38% (9) | 94.26% (115) 95.08% (116) | 92.62% (113) 94.26% (115) | 13.93% (17) 21.31% (26) | 9.84% (12) 16.39% (20) | 59.84% (73) 69.67% (85) | 4.10% (5) 5.74% (7) | 82.79% (101) 85.25% (104) | 93.44% (114) 95.08% (116) |
| 1Z76 (122) | 4.92% (6) 4.92% (6) | 4.92% (6) 7.38% (9) | 48.36% (59) 69.67% (85) | 50.82% (62) 69.67% (85) | 100% | 56.56% (69) 66.39% (81) | 7.38% (9) 9.02% (11) | 50.0 % (61) 70.49% (86) | 48.36% (59) 69.67% (85) | 18.85% (23) 28.69% (35) | 8.20% (19) 9.02% (11) | 56.56% (69) 71.31% (87) | 8.20% (10) 9.84% (12) | 50.00% (61) 65.57% (80) | 49.18% (60) 70.49% (86) |
| 2QOG (122) | 3.28% (4) 3.28% (4) | 6.56% (8) 7.38% (9) | 48.36% (59) 62.30% (76) | 49.18% (60) 63.11% (77) | 56.56% (69) 66.39% (81) | 100% | 7.38% (9) 10.66% (13) | 47.54% (58) 62.30% (76) | 48.36% (59) 62.30% (76) | 22.13% (27) 28.69% (35) | 9.84% (12) 18.03% (22) | 63.93% (78) 76.23% (93) | 9.84% (12) 18.03% (22) | 47.54% (58) 57.38% (70) | 48.36% (59) 62.30% (76) |
| 2W12 (202) | 12.38% (25) 21.29% (43) | 6.93% (14) 10.40% (21) | 2.97 % (6) 4.46 % (9) | 2.97% (6) 4.46% (9) | 4.46% (9) 5.45% (11) | 4.46% (9) 6.44% (13) | 100% | 2.97% (6) 4.46% (9) 9 | 2.97% (6) 4.46% (9) | 52.48% (106) 67.82% (137) | 3.47% (7) 5.45% (11) | 1.98% (4) 2.97% (6) | 3.47% (7) 5.45% (11) | 7.92% (16) 12.87% (26) | 2.97% (6) 4.46 % (9) |
| 3CXI (121) | 4.96% (6) 7.44% (9) | 4.13% (5) 4.96% (6) | 98.35% (119) 99.17 % (120) | 95.04% (115) 95.87% (116) | 50.41% (61) 71.07% (86) | 47.93% (58) 62.81% (76) | 4.96% (6) 7.44% (9) | 100% | 98.35% (119) 99.17% (120) | 14.05% (17) 21.49% (26) | 19.83% (24) 34.71% (42) | 59.50% (72) 70.25% (85) | 5.79% (7) 9.09% (11) | 85.95% (104) 89.26% (108) | 99.17% (120) 100% (122) |
| 3CYL (121) | 4.13% (5) 5.79% (7) | 10.74% (13) 14.05% (17t) | 99.17% (120) 99.17% (120) | 93.39% (113) 95.04% (115) | 48.76% (59) 70.25% (85) | 48.76% (59) 62.81 % (76) | 4.96% (6) 7.44% (9) | 98.35% (119) 99.17% (120) | 100% | 14.05% (17) 21.49% (26) | 19.83% (24) 35.54% (43) | 59.50% (72) 70.25% (85) | 5.79% (7) 9.09% (11) | 87.60% (106) 90.08% (109) | 99.17% (120) 99.17% (120) |
| 3DSL (479) | 4.30% (18) 5.97% (25) | 2.15% (9) 4.30% (18) | 4.06% (17) 6.21% (26) | 4.06% (26) 6.21% (26) | 5.49% (23) 8.35% (35) | 6.44% (35) 8.35% (35) | 25.30% (106) 32.70% (137) | 4.06% (17) 6.21% (26) | 4.06% (17) 6.21% (26 | 100% | 2.39% (10) 3.34 % (14) | 4.06% (17) 5.97% (25) | 2.39% (10) 3.58% (15) | 4.06% (17) 6.68% (28) | 4.06% (17) 6.21% (26) |
| 4E0V (497) | 5.23% (26) 9.86% (49) | 8.45% (42) 15.09% (75) | 4.83% (24) 8.65% (43) | 2.41% (12) 4.02% (20) | 2.01% (19) 2.21% (11) | 2.41% (12) 4.43% (22) | 1.41% (7) 2.21% (11) | 4.83% (24) 8.45% (42 | 4.83% (24) 8.65% (43) | 2.01% (10) 2.82 % (14) | 100% | 3.22% (16) 5.63% (28) | 95.37% (474) 95.98% (477) | 2.41% (12) 4.23% (21) | 4.83% (24) 8.65% (43) |
| 5TVF (122) | 4.92% (6) 8.20% (10) | 4.10% (5) 4.92% (6) | 59.84% (73) 70.49% (86) | 59.84% (73) 69.67% (85) | 56.56% (69) 71.31% (87) | 63.93% (78) 76.23% (93) | 3.28% (4) 4.92% (6) | 59.02% (72) 69.67% (85) | 59.02% (72) 69.67% (85) | 13.93% (17) 20.49% (25) | 13.11% (16) 22.95% (28) | 100% | 13.11% (16) 22.95% (28) | 56.56% (69) 66.39% (81) | 59.02% (72) 69.67% (85) |
| 5TS5 (484) | 3.93% (19) 8.68% (42) | 8.68% (42) 15.50% (75) | 1.45 % (7) 2.27 % (11) | 1.03% (5) 1.45% (7) | 2.07% (10) 2.48% (12) | 2.48% (12) 4.55% (22) | 1.45% (7) 2.27% (11) | 1.45% (7) 2.27% (11) | 1.45% (7) 2.27% (11) | 2.07% (10) 3.10% (15) | 97.93% (474) 98.55% (477) | 3.31% (16) 5.79% (28) | 100% | 2.48% (12) 4.34% (21) | 1.45% (7) 2.27% (11) |
| 6CE2 (121) | 3.31% (4) 4.13% (5) | 9.09% (11) 11.57% (14) | 86.78% (105) 89.26% (108) | 83.47% (101) 85.95% (104) | 50.41% (61) 66.12% (80) | 47.93% (58) 57.85% (70) | 13.22% (16) 21.49% (26) | 85.95% (104) 89.26% (108) | 87.60% (106) 90.08% (109) | 14.05% (17) 23.14% (28) | 9.92% (12) 17.36% (21) | 57.02% (69) 66.94% (81) | 9.92% (12) 17.36% (21) | 100% | 86.78% (105) 89.26% (108) |
| 6DIK (121) | 4.96 % (6) 7.44 % (9) | 4.13% (5) 4.96% (6) | 99.17% (120) 99.17% (120) | 94.21% (114) 95.87% (116) | 49.59% (60) 71.07% (86) | 48.76% (59) 62.81% (76) | 4.96% (6) 7.44 % (9) | 99.17% (120) 100% (122) | 99.17% (120) 99.17% (120) | 14.05% (17) 21.49% (26) | 19.83% (43) 35.54% (43) | 59.50% (72) 70.25% (85) | 5.79% (7) 9.09% (11) | 86.78% (105) 89.26% (108) | 100% |
References
- Thirupathi, K.; Kumar, S.S.; Raju, V.S.; Ravikumar, B.; Krishna, D.R.; Mohan, G.K. A review of medicinal plants of the genus Cordia: Their chemistry and pharmacological uses. J. Nat. Remedies 2008, 8, 1–10. [Google Scholar]
- Matias, E.F.F.; Alves, E.F.; do Nascimento Silva, M.K.; de Alencar Carvalho, V.R.; Coutinh, H.D.M.; da Costa, J.G.M. The genus Cordia: Botanists, ethno, chemical and pharmacological aspects. Brazilian J. Pharmacogn. 2015, 25, 542–552. [Google Scholar] [CrossRef]
- Moir, M.; Thomson, R.H.; Hausen, B.M.; Simatupang, M.H. Cordiachromes: A new group of terpenoid quinones from Cordia spp. J. Chem. Soc. Chem. Commun. 1972, 6, 363–364. [Google Scholar] [CrossRef]
- Moir, M.; Thomson, R.H. Naturally occurring quinones. Part XXII. Terpenoid quinones in Cordia spp. J. Chem. Soc. Perkin Trans. 1 1973, 1352–1357. [Google Scholar] [CrossRef]
- Kaur, S.; Singh, V.; Kumar, G.; Kad, G.L.; Singh, J. A short and facile synthesis of 2-(1Z)-(3-hydroxy-3,7-dimethylocta-1,6-dienyl)-1,4-benzenediol and 1-(3′-methoxypropanoyl)-2,4,5-trimethoxybenzene isolated from Cordia alliodora. Nat. Prod. Res. 2010, 24, 440–447. [Google Scholar] [CrossRef]
- Sinha, A.K.; Joshi, B.P.; Sharma, A.; Kumar, J.K.; Kaul, V.K. Microwave-assisted rapid synthesis of methyl 2,4,5-trimethoxyphenylpropionate, a metabolite of Cordia alliodora. Nat. Prod. Res. 2003, 17, 419–422. [Google Scholar] [CrossRef]
- Stevens, K.L.; Jurd, L.; Manners, G. Alliodorin, a phenolic terpenoid from Cordia alliodora. Tetrahedron Lett. 1973, 14, 2955–2958. [Google Scholar] [CrossRef]
- Chen, T.K.; Ales, D.C.; Baenziger, N.C.; Wiemer, D.F. Ant-Repellent Triterpenoids from Cordia alliodora. J. Org. Chem. 1983, 48, 3525–3531. [Google Scholar] [CrossRef]
- Kahn, P.H.; Cossy, J. A short synthesis of cordiachromene. Tetrahedron Lett. 1999, 40, 8113–8114. [Google Scholar] [CrossRef]
- Bouzbouz, S.; Goujon, J.-Y.; Deplanne, J.; Kirschleger, B. Enantioselective Synthesis of Cordiachromene. Eur. J. Org. Chem. 2000, 2000, 3223–3228. [Google Scholar] [CrossRef]
- Gary Manners, B.D.; Jurd, L.; Southwell, C.K.; Bultman, J.D.; Moir, M.; Thomson, R.H.; Perkin, J. The Hydroquinone Terpenoids of Cordia alliodora. J. Chem. Soc. Perkin Trans. 1976, 1, 405–410. [Google Scholar] [CrossRef]
- Ioset, J.R.; Marston, A.; Gupta, M.P.; Hostettmann, K. Antifungal and larvicidal compounds from the root bark of Cordia alliodora. J. Nat. Prod. 2000, 63, 424–426. [Google Scholar] [CrossRef]
- Fouseki, M.M.; Damianakos, H.; Karikas, G.A.; Roussakis, C.; Gupta, M.P.; Chinou, I. Chemical constituents from Cordia alliodora and C. colloccoca (Boraginaceae) and their biological activities. Fitoterapia 2016, 15, 9–14. [Google Scholar] [CrossRef]
- Matias, E.F.F.; Alves, E.F.; Silva, M.K.N.; Carvalho, V.R.A.; Figueredo, F.G.; Ferreira, J.V.A.; Coutinho, H.D.M.; Silva, J.M.F.L.; Ribeiro-Filho, J.; Costa, J.G.M. Seasonal variation, chemical composition and biological activity of the essential oil of Cordia verbenacea DC (Boraginaceae) and the sabinene. Ind. Crops Prod. 2016, 87, 45–53. [Google Scholar] [CrossRef]
- De Alencar Carvalho, V.R.; do Nascimento Silva, M.K.; Aguiar, J.J.S.; de Carvalho Nilo Bitu, V.; da Costa, J.G.M.; Ribeiro-Filho, J.; Coutinho, H.D.M.; Pinho, A.I.; Fagner Ferreira Matias, E. Antibiotic-Modifying Activity and Chemical Profile of the Essential Oil from the Leaves of Cordia verbenacea DC. J. Essent. Oil Bear. Plants 2017, 20. [Google Scholar] [CrossRef]
- Matias, E.F.F.; Alves, E.F.; Silva, M.K.N.; Carvalho, V.R.A.; Medeiros, C.R.; Santos, F.A.V.; Bitu, V.C.N.; Souza, C.E.S.; Figueredo, F.G.; Boligon, A.A.; et al. Potentiation of antibiotic activity of aminoglycosides by natural products from Cordia verbenacea DC. Microb. Pathog. 2016, 95, 111–116. [Google Scholar] [CrossRef]
- Costa De Oliveira, D.M.; Luchini, A.C.; Seito, L.N.; Gomes, J.C.; Crespo-López, M.E.; Di Stasi, L.C. Cordia verbenacea and secretion of mast cells in different animal species. J. Ethnopharmacol. 2011, 135, 463–468. [Google Scholar] [CrossRef]
- Parisotto, E.B.; Michielin, E.M.Z.; Biscaro, F.; Ferreira, S.R.S.; Filho, D.W.; Pedrosa, R.C. The antitumor activity of extracts from Cordia verbenacea D.C. obtained by supercritical fluid extraction. J. Supercrit. Fluids 2012. [Google Scholar] [CrossRef]
- Ticli, F.K.; Hage, L.I.S.; Cambraia, R.S.; Pereira, P.S.; Magro, Â.J.; Fontes, M.R.M.; Stábeli, R.G.; Giglio, J.R.; França, S.C.; Soares, A.M.; et al. Rosmarinic acid, a new snake venom phospholipase A2 inhibitor from Cordia verbenacea (Boraginaceae): Antiserum action potentiation and molecular interaction. Toxicon 2005, 46, 318–327. [Google Scholar] [CrossRef]
- Missouri Botanical Garden Tropicos Database. 2018. Available online: http://tropicos.org (accessed on 20 September 2019).
- Luzuriaga-Quichimbo, C.X. Estudio Etnobotánico en Comunidades Kichwas Amazónicas de Pastaza, Ecuador. Doctoral Thesis, Universidad de Extremadura, Badajoz, España, 2017. Available online: http://dehesa.unex.es/handle/10662/6419 (accessed on 10 September 2018).
- Dos Santos, R.F.E.P.; Silva Silva, I.S.D.M.; d’Costa, L.R.; Mendonça Barbosa, A.; Santos Silva, K.; Ribeiro Amorim, M.; Mendonça Diz, F.; Honório Lins, T.; Santos Sales Verissimo, R.C.; Ferreira Padilha, F.; et al. Study of antimicrobial potential and cytotoxic of Cordia nodosa species. BMC Proc. 2014, 8, P69. [Google Scholar] [CrossRef]
- De La Torre, L.; Navarrete, H.; Muriel, M.P.; Macía, M.J.; Balslev, H. Enciclopedia de las Plantas Útiles del Ecuador; Herbario QCA de la Escuela de Ciencias Biológicas de la Pontificia Universidad Católica del Ecuador & Herbario AAU del Departamento de Ciencias Biológicas de la Universidad de Aarhus: Aarhus, Denmark, 2008; ISBN 978-9978-77-135-8. [Google Scholar]
- OMS Organización Mundial de la Salud Datos y Cifras Situación Mundial Desafíos a la Producción de Antídotos. Available online: https://www.who.int/es/news-room/fact-sheets/detail/snakebite-envenoming (accessed on 10 September 2019).
- Convention on Biological Diversity. 2010 CoP10 Decisions. 2010, pp. 1–7. Available online: https://www.cbd.int/decisions/cop/?m=cop-10 (accessed on 10 September 2019).
- De Filipps, R.; Maina, S.L.; Crepin, J. Medicinal plants of the Guianas (Guyana, Surinam, French Guiana); Department of Botany, National Museum of Natural History, Smithsonian Institution: Washington, DC, USA, 2004. [Google Scholar]
- Valadeau, C.; Castillo, J.A.; Sauvain, M.; Lores, A.F.; Bourdy, G. The rainbow hurts my skin: Medicinal concepts and plants uses among the Yanesha (Amuesha), an Amazonian Peruvian ethnic group. J. Ethnopharmacol. 2010, 127, 175–192. [Google Scholar] [CrossRef]
- Guimaraes, C.; Moreira-Dill, L.; Fernandes, R.; Costa, T.; Hage-Melim, L.; Marcussi, S.; Carvalho, B.; Silva, S.; Zuliani, J.; Fernandes, C.; et al. Biodiversity as a Source of Bioactive Compounds Against Snakebites. Curr. Med. Chem. 2014. [Google Scholar] [CrossRef] [PubMed]
- Salvador, G.H.; Dos Santos, J.I.; Lomonte, B.; Fontes, M.R. Crystal structure of a phospholipase A2 from Bothrops asper venom: Insights into a new putative “myotoxic cluster”. Biochimie 2017, 133, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Feliciano, P.R.; Rustiguel, J.K.; Soares, R.O.; Sampaio, S.V.; Cristina Nonato, M. Crystal structure and molecular dynamics studies of L-amino acid oxidase from Bothrops atrox. Toxicon 2017, 128, 50–59. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, J.I.; Soares, A.M.; Fontes, M.R. Comparative structural studies on Lys49-phospholipases A(2) from Bothrops genus reveal their myotoxic site. J. Struct. Biol. 2009, 167, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Salvador, G.H.M.; Dreyer, T.R.; Gomes, A.A.S.; Cavalcante, W.L.G.; Dos Santos, J.I.; Gandin, C.A.; de Oliveira Neto, M.; Gallacci, M.; Fontes, M.R.M. Structural and functional characterization of suramin-bound MjTX-I from Bothrops moojeni suggests a particular myotoxic mechanism. Sci. Rep. 2018, 8, 10317. [Google Scholar] [CrossRef]
- Cardoso, F.F.; Borges, R.J.; Dreyer, T.R.; Salvador, G.H.M.; Cavalcante, W.L.G.; Pai, M.D.; Gallacci, M.; Fontes, M.R.M. Structural basis of phospholipase A2-like myotoxin inhibition by chicoric acid, a novel potent inhibitor of ophidian toxins. Biochim Biophys Acta Gen. Subj 2018, 1862, 2728–2737. [Google Scholar] [CrossRef]
- Watanabe, L.; Soares, A.M.; Ward, R.J.; Fontes, M.R.; Arni, R.K. Structural insights for fatty acid binding in a Lys49-phospholipase A (2): Crystal structure of myotoxin II from Bothrops moojeni complexed with stearic acid. Biochimie 2005, 87, 161–167. [Google Scholar] [CrossRef]
- Ullah, A.; Souza, T.A.; Abrego, J.R.; Betzel, C.; Murakami, M.T.; Arni, R.K. Structural insights into selectivity and cofactor binding in snake venom L-amino acid oxidases. Biochem.Biophys.Res.Commun. 2012, 421, 124–128. [Google Scholar] [CrossRef]
- Magro, A.J.; Takeda, A.A.; Soares, A.M.; Fontes, M.R. Structure of BthA-I complexed with p-bromophenacyl bromide: Possible correlations with lack of pharmacological activity. Acta Crystallogr. Sect. D 2005, 61, 1670–1677. [Google Scholar] [CrossRef]
- Marchi-Salvador, D.P.; Correa, L.C.; Magro, A.J.; Oliveira, C.Z.; Soares, A.M.; Fontes, M.R. Insights into the role of oligomeric state on the biological activities of crotoxin: Crystal structure of a tetrameric phospholipase A2 formed by two isoforms of crotoxin B from Crotalus durissus terrificus venom. Proteins 2008, 72, 883–891. [Google Scholar] [CrossRef]
- Lee, W.H.; Da Silva Giotto, M.T.; Marangoni, S.; Toyama, M.H.; Polikarpov, I.; Garratt, R.C. Structural Basis for Low Catalytic Activity in Lys49 Phospholipases A2-A Hypothesis: The Crystal Structure of Piratoxin II Complexed to Fatty Acid. Biochemistry 2001, 40, 28. [Google Scholar] [CrossRef] [PubMed]
- Muniz, J.R.; Ambrosio, A.L.; Selistre-de-Araujo, H.S.; Cominetti, M.R.; Moura-da-Silva, A.M.; Oliva, G.; Garratt, R.C.; Souza, D.H. The three-dimensional structure of bothropasin, the main hemorrhagic factor from Bothrops jararaca venom: Insights for a new classification of snake venom metalloprotease subgroups. Toxicon 2008, 52, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Lingott, T.J.; Schleberger, C.; Gutierrez, J.M.; Merfort, I. High-Resolution Crystal Structure of the Snake Venom Metalloproteinase Bap1 Complexed with a Peptidomimetic: Insight Into Inhibitor Binding. Biochemistry 2009, 48, 6166. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.V.; Chien, K.Y.; Lin, C.C.; Chiang, L.C.; Lin, T.H.; Wu, W.G. Crystal Structure of L-Amino Acid Oxidase from Naja Atra (Taiwan Cobra). Available online: https://www.rcsb.org/structure/5Z2G (accessed on 13 November 2019). [CrossRef]
- Gergiova, D.; Murakami, M.T.; Perbandt, M.; Arni, R.K.; Betzel, C. Structure of Native L-amino Acid Oxidase from Vipera ammodytes Ammodytes: Stabilization of the Quaternary Structure by Divalent Ions and Structural Changes in the Dynamic Active Site. Available online: https://www.rcsb.org/structure/3KVE (accessed on 13 November 2019).
- Lin, C.C.; Wu, B.S.; Wu, W.G. Crystal Structure of Snake Venom Phosphodiesterase (PDE) from Taiwan Cobra (Naja atra atra). Available online: http://dx.doi.org/10.2210/PDB5GZ4/PDB (accessed on 13 November 2019).
- Trabi, M.; Millers, E.-K.; Richards, R.; Snelling, H.; Keegan, R.; Lavin, M.F.; de Jersey, J.; Guddat, L.W.; Masci, P. Mechanistic Studies on the Anticoagulant Activity of a Phospholipase A2 from the Venom of the Australian King Brown Snake (Pseudechis australis). Available online: https://www.rcsb.org/structure/3V9M (accessed on 13 November 2019).
- Fremont, D.H.; Anderson, D.H.; Wilson, I.A.; Dennis, E.A.; Xuong, N.H. Crystal structure of phospholipase A2 from Indian cobra reveals a trimeric association. Proc. Natl. Acad. Sci. USA 1993, 90, 342–346. [Google Scholar] [CrossRef]
- Zhang, H.; Teng, M.; Niu, L.; Wang, Y.; Wang, Y.; Liu, Q.; Huang, Q.; Hao, Q.; Dong, Y.; Liu, P. Purification, partial characterization, crystallization and structural determination of AHP-LAAO, a novel L-amino-acid oxidase with cell apoptosis-inducing activity from Agkistrodon halys pallas venom. Acta Crystallogr. Sect. D Biol. Crystallogr. 2004, 60, 974–977. [Google Scholar] [CrossRef]
- Lin, C.C.; Wu, B.S.; Wu, W.G. Crystal Structure of Snake Venom Phosphodiesterase (PDE) from Taiwan Cobra (Naja atra atra) in Complex with AMP. Available online: https://www.rcsb.org/structure/5GZ5 (accessed on 13 November 2019).
- Segelke, B.W.; Nguyen, D.; Chee, R.; Xuong, N.H.; Dennis, E.A. Structures of two novel crystal forms of Naja naja naja phospholipase A2 lacking Ca2+ reveal trimeric packing. J. Mol. Biol. 1998, 279, 223–232. [Google Scholar] [CrossRef]
- Mendes, M.M.; Vieira, S.A.P.B.; Gomes, M.S.R.; Paula, V.F.; Alcântara, T.M.; Homsi-Brandeburgo, M.I.; Dos Santos, J.I.; Magro, A.J.; Fontes, M.R.M.; Rodrigues, V.M. Triacontyl p-coumarate: An inhibitor of snake venom metalloproteinases. Phytochemistry 2013. [Google Scholar] [CrossRef] [PubMed]
- Laskowski, R.A.; Swindells, M.B. LigPlot+: Multiple ligand-protein interaction diagrams for drug discovery. J. Chem. Inf. Model. 2011. [Google Scholar] [CrossRef] [PubMed]
- Alcaraz, M.J.; Hoult, J.R. Effects of hypolaetin-8-glucoside and related flavonoids on soybean lipoxygenase and snake venom phospholipase A2. Arch. Int. Pharmacodyn. Ther. 1985, 278, 4–12. [Google Scholar] [PubMed]
- Girish, K.S.; Kemparaju, K. Inhibition of Naja naja venom hyaluronidase: Role in the management of poisonous bite. Life Sci. 2006, 78, 1433–1440. [Google Scholar] [CrossRef]
- Nishijima, C.M.; Rodrigues, C.M.; Silva, M.A.; Lopes-Ferreira, M.; Vilegas, W.; Hiruma-Lima, C.A. Anti-hemorrhagic activity of four brazilian vegetable species against Bothrops jararaca venom. Molecules 2009, 14, 172–180. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int. J. Surg. 2010. [Google Scholar] [CrossRef]
- Tu, M.; Cheng, S.; Lu, W.; Du, M. Advancement and prospects of bioinformatics analysis for studying bioactive peptides from food-derived protein: Sequence, structure, and functions. TrACTrends Anal. Chem. 2018. [Google Scholar] [CrossRef]
- Tu, M.; Wang, C.; Chen, C.; Zhang, R.; Liu, H.; Lu, W.; Jiang, L.; Du, M. Identification of a novel ACE-inhibitory peptide from casein and evaluation of the inhibitory mechanisms. Food Chem. 2018. [Google Scholar] [CrossRef]




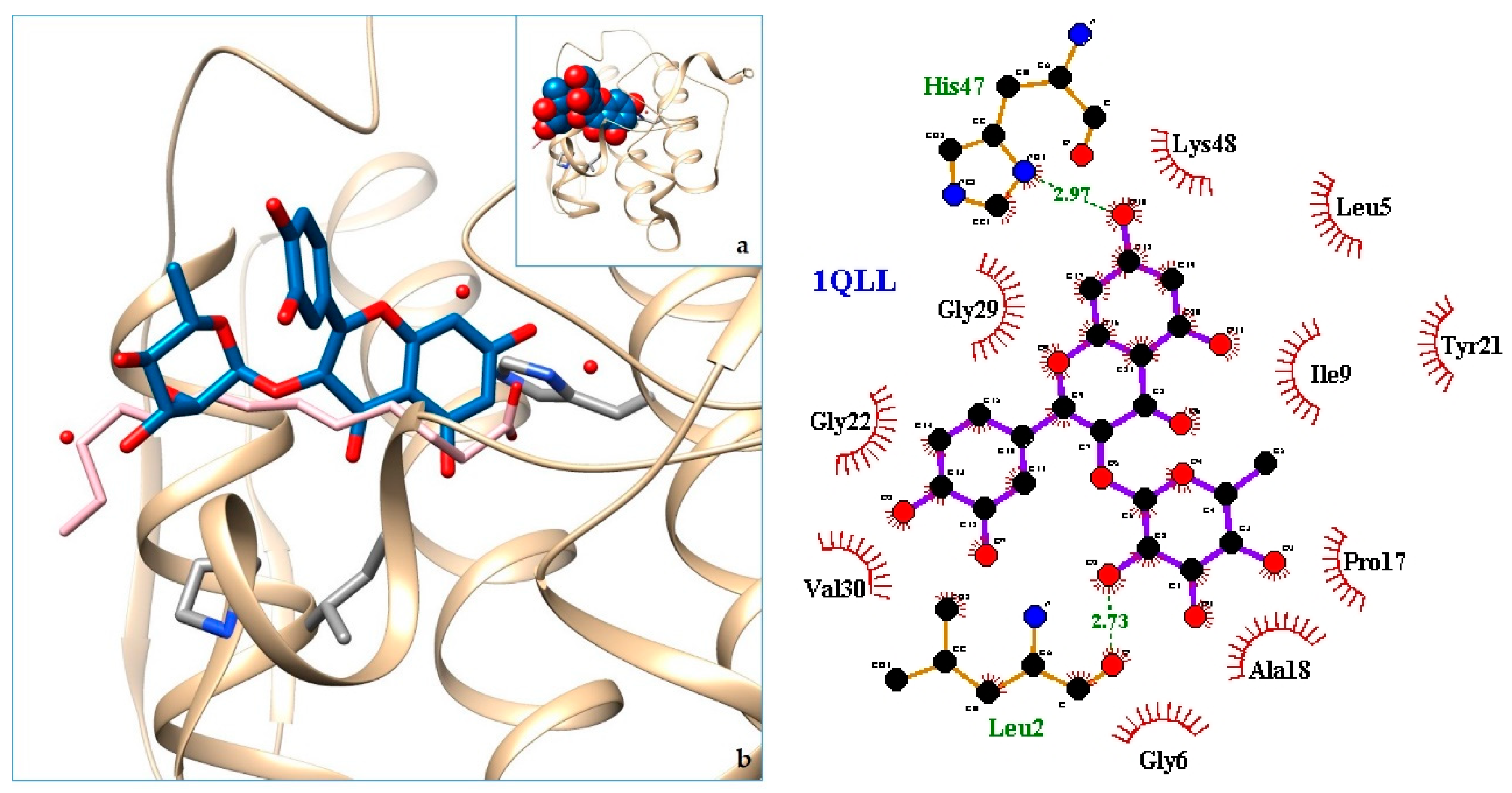
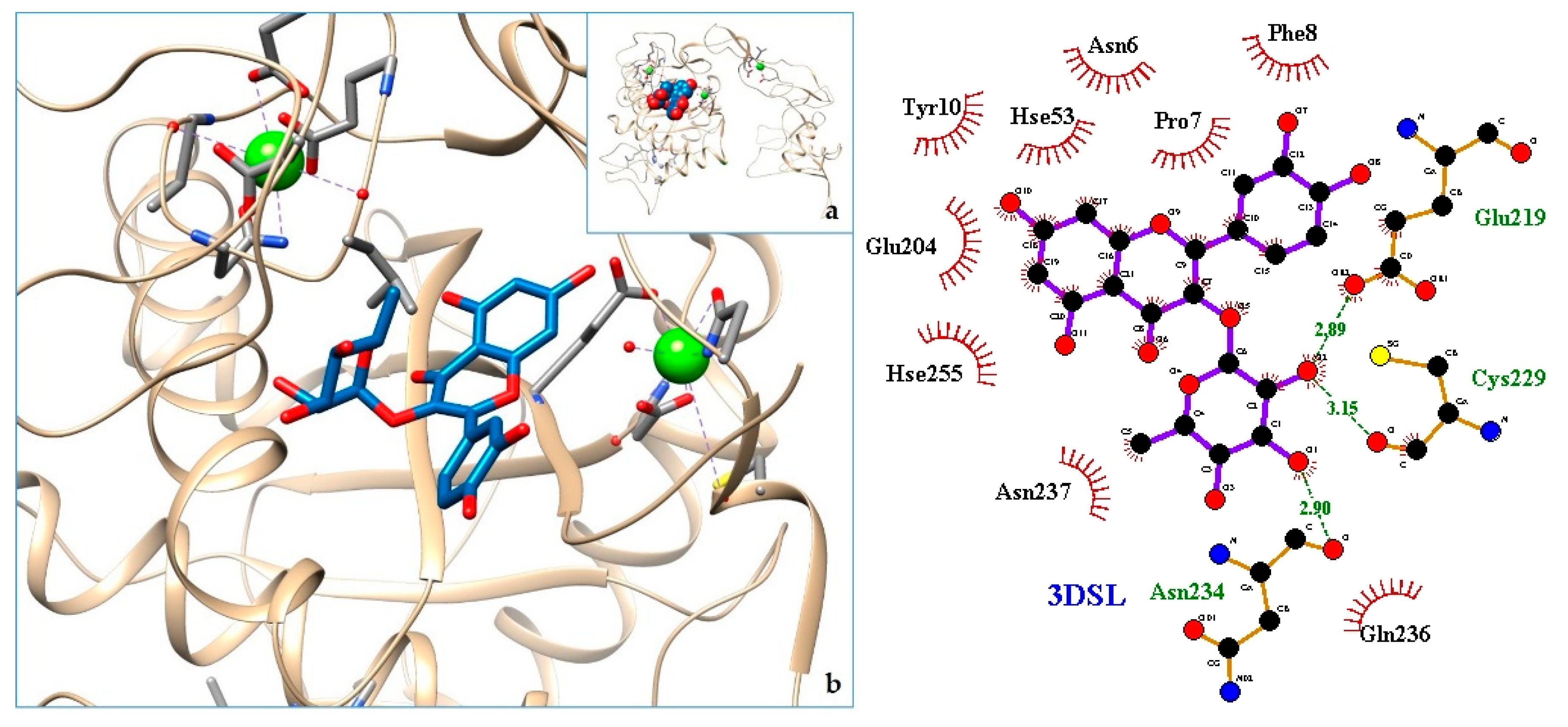
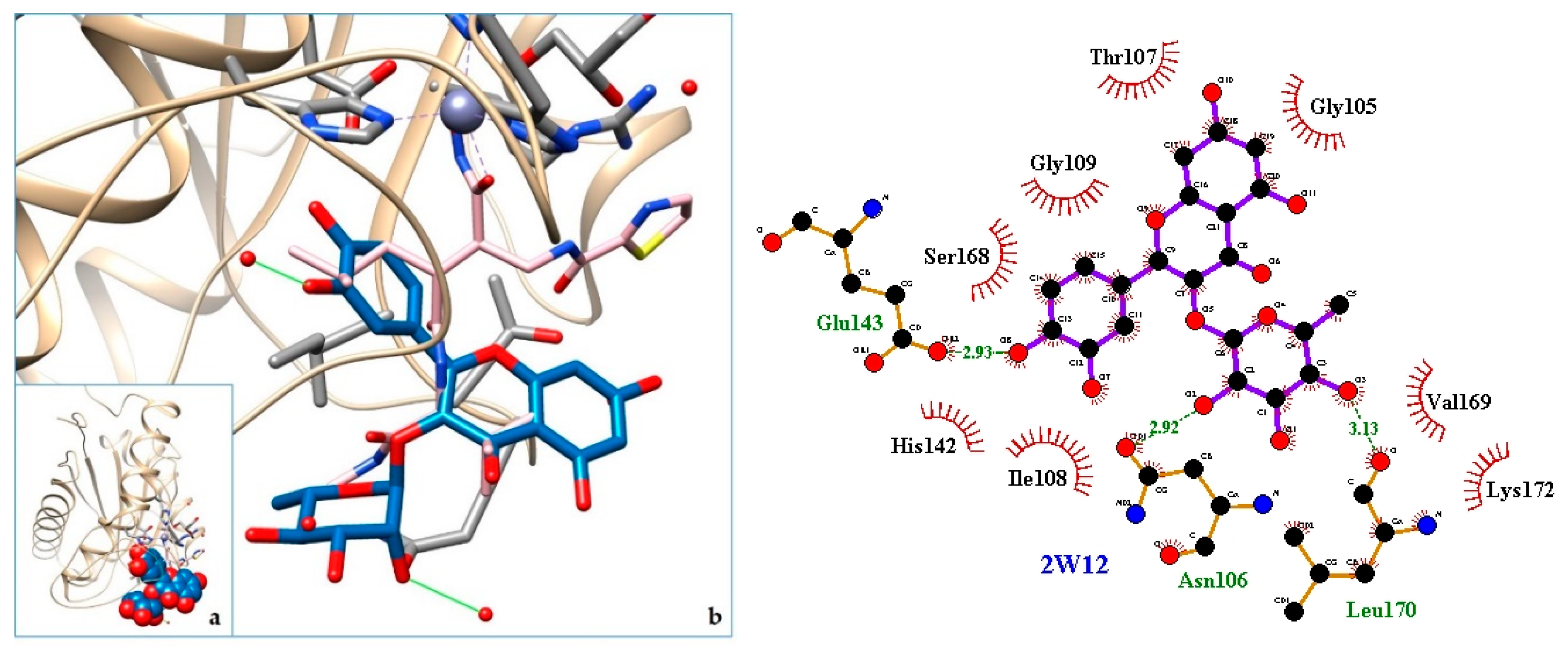
| Organ/System | Part Used | Formulation | Traditional Knowledge | Ethnic Group-Province (Country) | Reference |
|---|---|---|---|---|---|
| Circulatory system | leaves | decoction | hypertension | Amerindian NorthWest District (Guiana) | [26] |
| Digestive system | gases | Siona-Sucumbíos (Ecuador) | [23] | ||
| Respiratory system | bark | cooking | treat cough | Secoya-Sucumbíos (Ecuador) | [23] |
| stem | |||||
| inner bark | finely grate and decoction | cold and shortness of breath | Amerindian (French Guiana) | [26] | |
| leaves | decoction | whooping cough | Amerindian NorthWest District (Guiana) | [26] | |
| fruit | suck | snot in babies | Amerindian NorthWest District (Guiana) | [26] | |
| Musculature and skeleton | leaves | crush the leaves and rub the body with them | rheumatism, sprains, muscle aches, bruises | Amerindian NorthWest District (Guiana) | [26] |
| Nervous system and mental illness | leaves | baths with the decoction of the leaves | madness and psychiatric disorders | Yanesha (Perú) | [27] |
| decoction | headache | Amerindian NorthWest District (Guiana) | [26] | ||
| Symptoms and states of undefined origin | bark | indeterminate conditions | Secoya-Sucumbíos (Ecuador) | [23] | |
| flowers | Kichwa del Oriente-Orellana (Ecuador) | ||||
| fruit | energizing | Wao-Orellana (Ecuador) | |||
| leaves | infusion | dizziness | ethnicity not specified-Napo (Ecuador) | ||
| decoction | fever | Amerindian NorthWest District (Guiana) | [26] | ||
| Poisoning | leaves | apply directly in the affected place | spider bite, to decrease inflammation and prevent gangrene | East Kichwa-Napo and Orellana (Ecuador) | [23] |
| fruit | |||||
| plant | cooking | ||||
| bark | cooking | ||||
| root | cooking | ||||
| bark | scraped and in water | snake bites, to decrease inflammation and prevent gangrene | East Kichwa, Shuar-Napo, Orellana, Pastaza, Sucumbíos (Ecuador), Piaroa (Venezuela) | [23,26] | |
| infusion | |||||
| root | infusion | ||||
| stem | juice | ||||
| fruit | juice | ||||
| young leaves | chewed | ||||
| leaves | apply directly in the affected place |
| Toxin | PDB ID | Reference |
|---|---|---|
| 1. MT-I—Basic phospholipase a2 myotoxin iii | 5TFV | [29] |
| 2. LAAO—L-amino acid oxidase from Bothrops atrox | 5TS5 | [30] |
| 3. PLA2—Phospholipase A2: Piratoxin I (myotoxic Lys49-PLA2) from Bothrops pirajai | 3CYL | [31] |
| 4. PLA2—Phospholipase A2: BthTX-I—Bothropstoxin I from Bothrops jararacussu venom/ | 3CXI | [31] |
| 5. PLA2—Phospholipase A2: Myotoxin (MjTX-I) from Bothrops moojeni | 6CE2 | [32] |
| 6. PLA2—Phospholipase A2: Bothropstoxin I (BthTX-I) | 6DIK | [33] |
| 7. svPLA2—Phospholipase A2: myotoxin II from Bothrops moojeni | 1XXS | [34] |
| 8. LAAO—L-amino acid oxidasefrom the B. jararacussu venom | 4E0V | [35] |
| 9. svPLA2—Acidic phospholipase A2 (BthA-I) from Bothrops jararacussu | 1Z76 | [36] |
| 10. VRV-PL-V—Crotoxin B, the basic PLA2 from Crotalus durissusterrificus | 2QOG | [37] |
| 11. PLA2—Piratoxin-II (Prtx-II) - a K49 PLA2 from Bothrops pirajai | 1QLL | [38] |
| 12. Bothropasin, the Main Hemorrhagic Factor from Bothrops jararaca venom | 3DSL | [39] |
| 13. SVMP—P-I snake venom metalloproteinase BaP1 | 2W12 | [40] |
| 14. NNH1—L-amino acid oxidase from venom of Naja atra | 5Z2G | [41] |
| 15. LAAO—L-amino acid oxidase from Vipera ammodytes venom | 3KVE | [42] |
| 16. PDE I—Phosphodiesterase (PDE) from Taiwan cobra (Naja atra atra) venom | 5GZ4 | [43] |
| 17. VRV-PL-V—Phospholipase ACII4 from Australian King Brown Snake (Pseudechis australis) | 3V9M | [44] |
| 18. NN-PL-I—Phospholipase A2 from indian cobra (Naja naja) | 1PSH | [45] |
| 19. LAAO—L-amino acid oxidase from Agkistrodon Halys Pallas (Gloydius halys) | 1REO | [46] |
| 20. NNH1—Phosphodiesterase (PDE) fromTaiwan cobra (Naja atra atra) | 5GZ5 | [47] |
| 21. PLA2—Phospholipase A2 (Pla2) from Naja naja | 1A3D | [48,49] |
| 1. 5TFV | −9.71 | MT-I—Basic Phospholipase a2 Myotoxin iii |
| 2. 5TS5 | −9.47 | LAAO—L-amino acid oxidase from Bothrops atrox |
| 3. 3CYL | −9.49 | PLA2—Phospholipase A2: Piratoxin I (myotoxic Lys49-PLA2) from Bothrops pirajai |
| 4. 3CXI | −9.37 | PLA2—Phospholipase A2: BthTX-I—Bothropstoxin I from Bothrops jararacussu venom/ |
| 5. 4GUE | −9.30 | N-terminal kinase domain of RSK2 with flavonoid glycoside quercetrin |
| 6. 6CE2 | −9.19 | PLA2—Phospholipase A2: Myotoxin (MjTX-I) from Bothrops moojeni |
| 7. 6DIK | −9.16 | PLA2—Phospholipase A2: Bothropstoxin I (BthTX-I) |
| 8. 1XXS | −9.01 | svPLA2—Phospholipase A2: myotoxin II from Bothrops moojeni |
| 9. 4E0V | −8.96 | LAAO—L-amino acid oxidasefrom the B. jararacussu venom |
| 10. 1Z76 | −8.56 | svPLA2—Acidic phospholipase A2 (BthA-I) from Bothrops jararacussu |
| 11. 2QOG | −8.32 | VRV-PL-V—Crotoxin B, the basic PLA2 from Crotalus durissusterrificus |
| 12. 5A4W | −8.28 | AtGSTF2 from Arabidopsis thaliana |
| 13. 1QLL | −8.23 | PLA2—Piratoxin-II (Prtx-II) - a K49 PLA2 from Bothrops pirajai |
| 14. 3DSL | −8.20 | Bothropasin, the Main Hemorrhagic Factor from Bothrops jararaca venom. |
| 15. 2W12 | −7.71 | SVMP—P-I snake venom metalloproteinase BaP1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luzuriaga-Quichimbo, C.X.; Blanco-Salas, J.; Muñoz-Centeno, L.M.; Peláez, R.; Cerón-Martínez, C.E.; Ruiz-Téllez, T. In Silico Molecular Studies of Antiophidic Properties of the Amazonian Tree Cordia nodosa Lam. Molecules 2019, 24, 4160. https://doi.org/10.3390/molecules24224160
Luzuriaga-Quichimbo CX, Blanco-Salas J, Muñoz-Centeno LM, Peláez R, Cerón-Martínez CE, Ruiz-Téllez T. In Silico Molecular Studies of Antiophidic Properties of the Amazonian Tree Cordia nodosa Lam. Molecules. 2019; 24(22):4160. https://doi.org/10.3390/molecules24224160
Chicago/Turabian StyleLuzuriaga-Quichimbo, Carmen X., José Blanco-Salas, Luz María Muñoz-Centeno, Rafael Peláez, Carlos E. Cerón-Martínez, and Trinidad Ruiz-Téllez. 2019. "In Silico Molecular Studies of Antiophidic Properties of the Amazonian Tree Cordia nodosa Lam." Molecules 24, no. 22: 4160. https://doi.org/10.3390/molecules24224160
APA StyleLuzuriaga-Quichimbo, C. X., Blanco-Salas, J., Muñoz-Centeno, L. M., Peláez, R., Cerón-Martínez, C. E., & Ruiz-Téllez, T. (2019). In Silico Molecular Studies of Antiophidic Properties of the Amazonian Tree Cordia nodosa Lam. Molecules, 24(22), 4160. https://doi.org/10.3390/molecules24224160








