In Vitro Evaluation of Chemically Analyzed Hypericum Triquetrifolium Extract Efficacy in Apoptosis Induction and Cell Cycle Arrest of the HCT-116 Colon Cancer Cell Line
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Plant Extracts
2.3. Silylation Derivatization
2.4. Gas Chromatography-Mass Spectrometry Analysis
2.5. Identification of Components
2.6. Cell Culture
2.7. MTT (Cell Viability)
2.8. Lactate Dehydrogenase
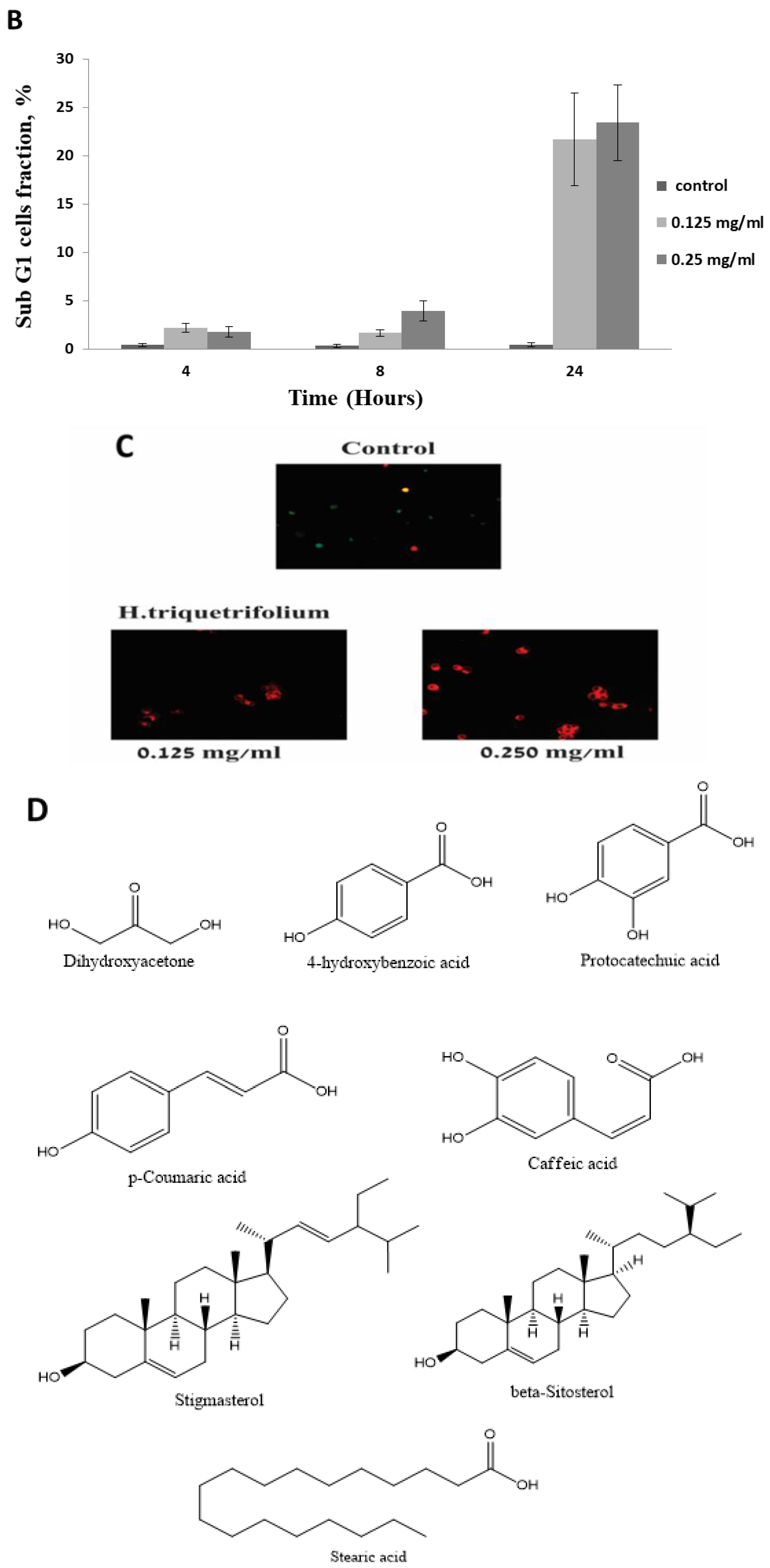
2.9. Apoptosis Detection
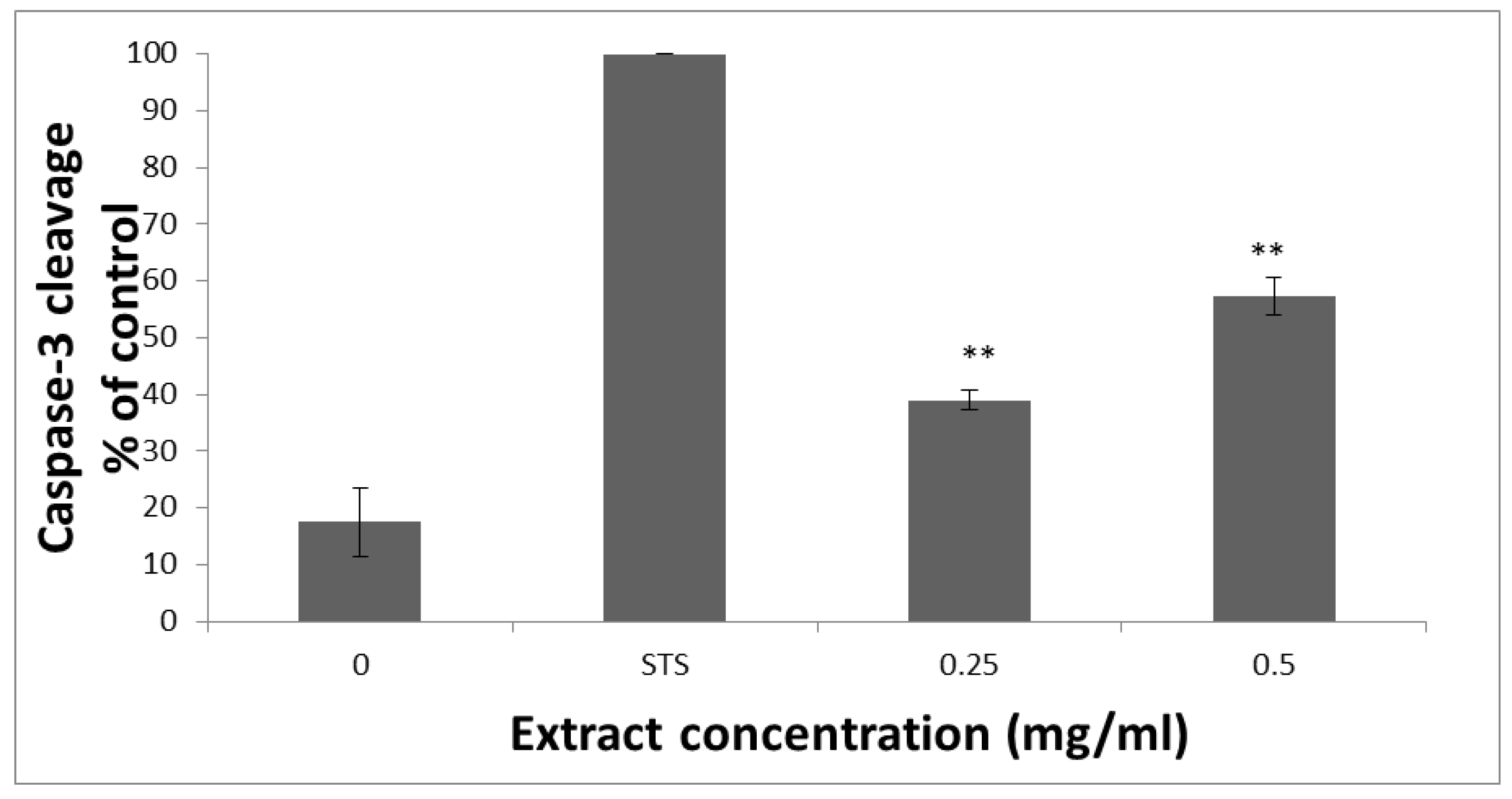
2.10. Caspase-3 Activity Assay
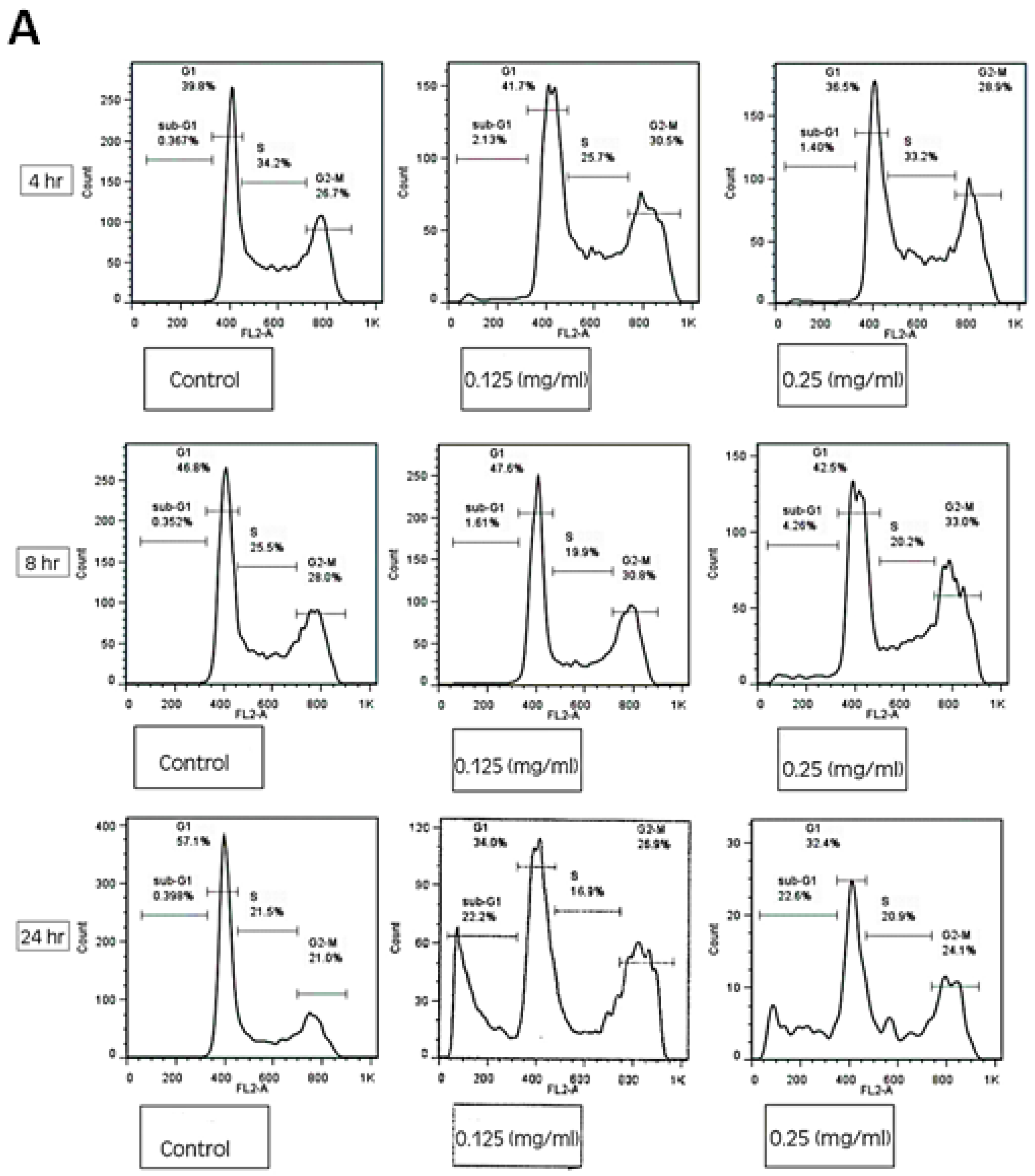
2.11. Cell Cycle Assay
2.12. FACS Analysis
2.13. Total RNA Isolation and cDNA Synthesis
- Apaf-1 for-AACCAGGATGGGTCACCATA
- Apaf-1 rev-ACTGAAACCCAATGCACTCC
- NOXA for-CAGAGCTGGAAGTCGAGTG
- NOXA rev-CAGGTTCCTGAGCAGAAGAG
2.14. Statistical Analysis
3. Results Sections in Wrong Order—Experimental Is Last-Renumber Everythi8ngn
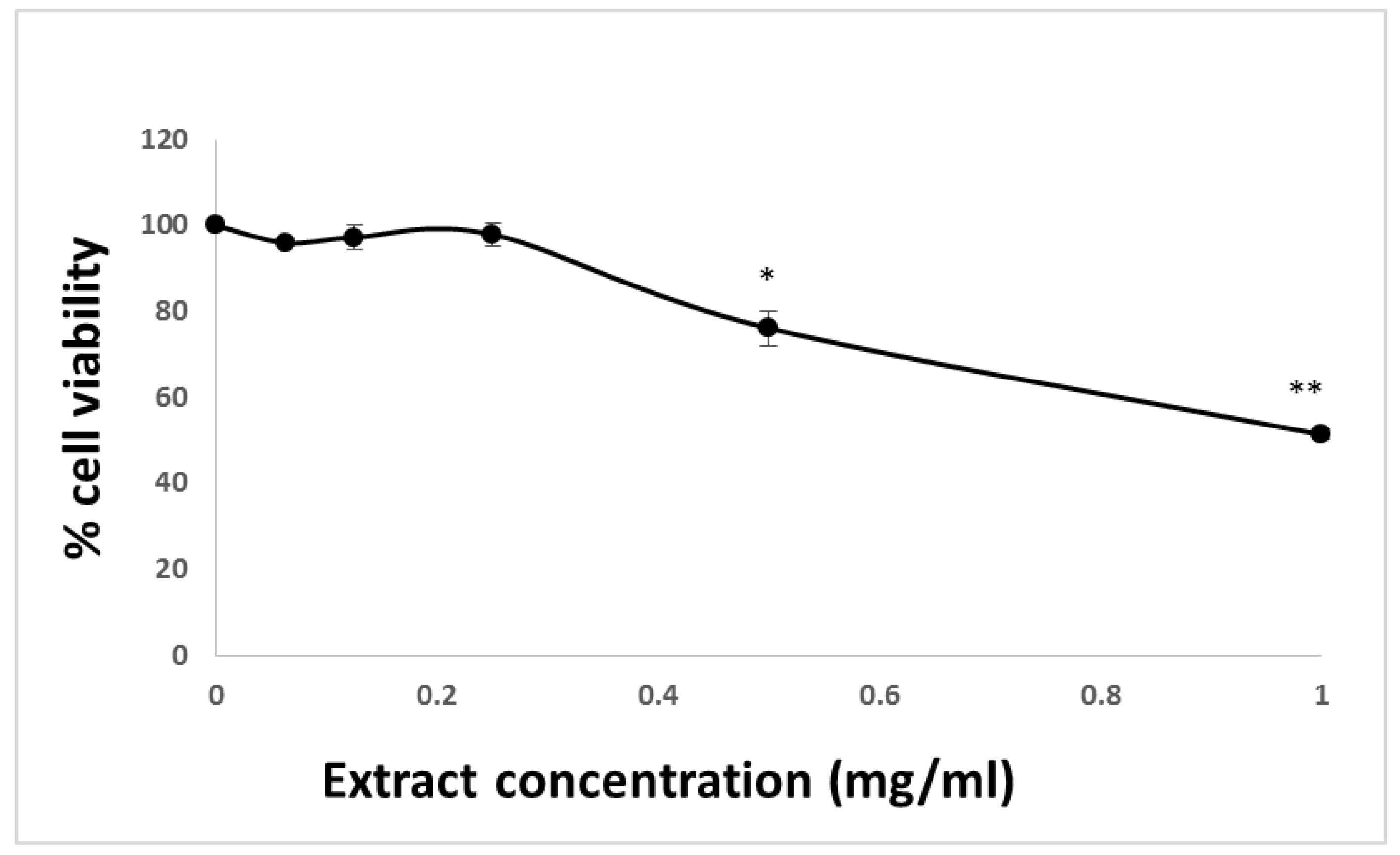
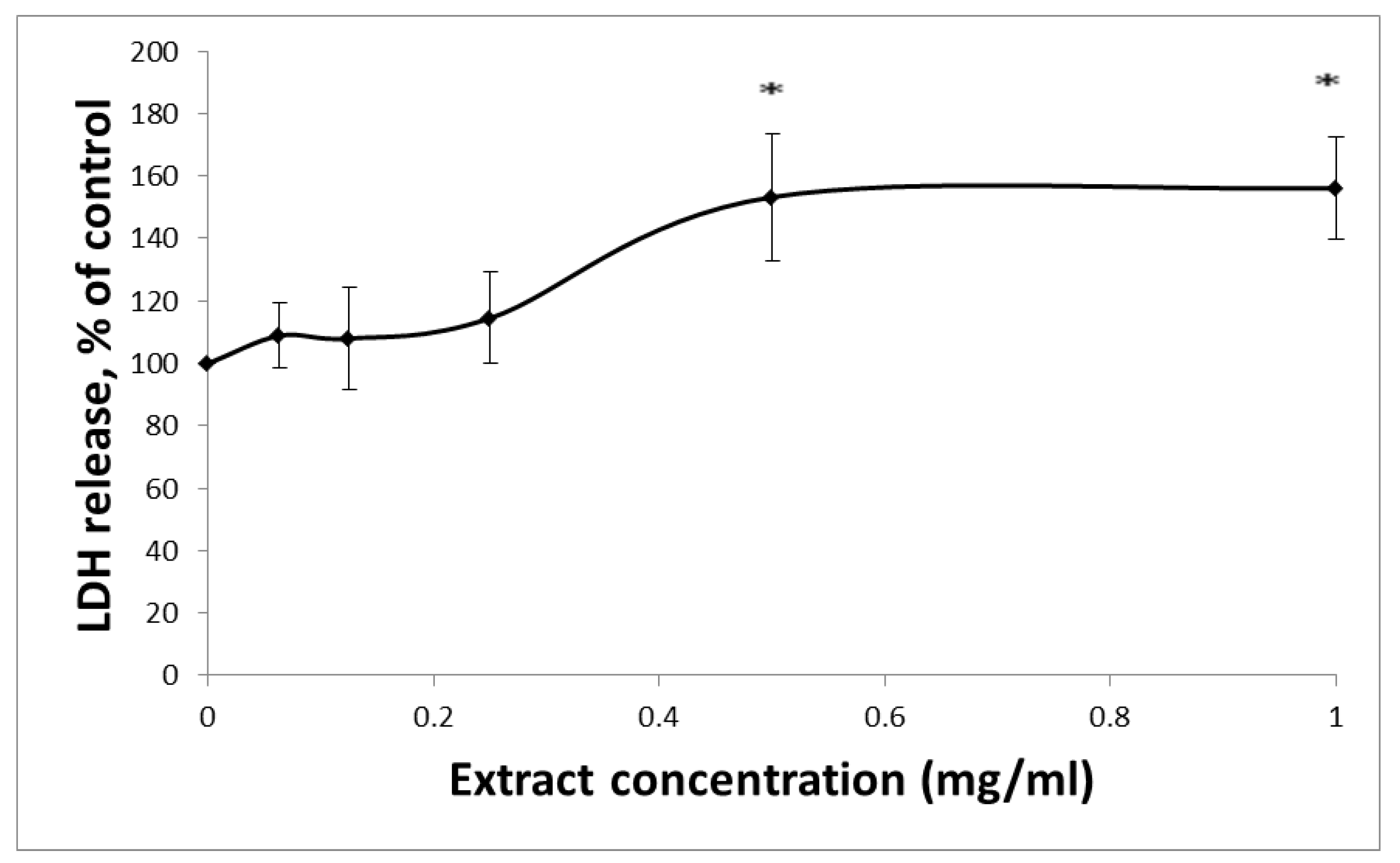
3.1. The Affects HCT-116 Cell Viability
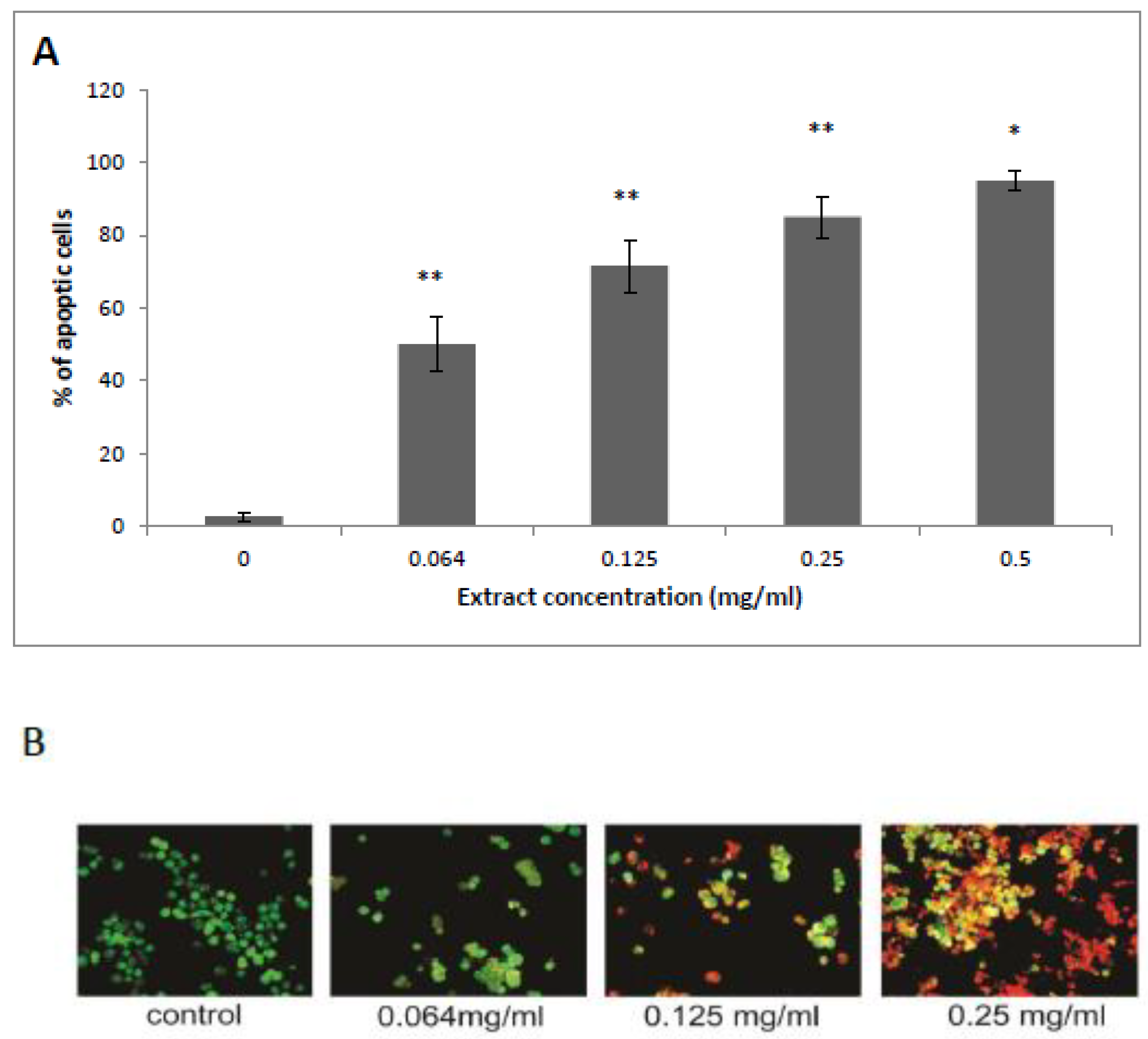
3.2. HTE Induces Apoptosis in HCT-116 Cells
3.3. mRNA Levels of Apaf-1 and NOXA
3.4. HTE Modulates the Cell Cycle
3.5. GC-MS Analysis of the HTE
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mullan, F. Seasons of survival: Reflections of a physician with cancer. N. Engl. J. Med. 1985, 313, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, N.; Chen, J.; Shen, J. Emerging glycolysis targeting and drug discovery from chinese medicine in cancer therapy. Evid.-Based Complement. Altern. Med. 2012, 2012, 873175. [Google Scholar] [CrossRef] [PubMed]
- Costea, T.; Hudita, A.; Ciolac, O.A.; Galateanu, B.; Ginghina, O.; Costache, M.; Ganea, C.; Mocanu, M.M. Chemoprevention of Colorectal Cancer by Dietary Compounds. Int. J. Mol. Sci. 2018, 19, 3787. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Sui, H.; Wang, Q.; He, N.; Duan, C.; Han, L.; Li, Q.; Lu, M.; Lv, S. A Chinese herbal formula, Yi-Qi-Fu-Sheng, inhibits migration/invasion of colorectal cancer by down-regulating MMP-2/9 via inhibiting the activation of ERK/MAPK signaling pathways. BMC Complement. Altern. Med. 2013, 13, 65. [Google Scholar] [CrossRef] [PubMed]
- Satia, J.A.; Littman, A.; Slatore, C.G.; Galanko, J.A.; White, E. Associations of herbal and specialty supplements with lung and colorectal cancer risk in the VITamins and Lifestyle study. Cancer Epidemiol. Biomark. Prev. 2009, 18, 1419–1428. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.N.; Sharma, S.K.; Ramsey, T.M.; Jiang, L.; Martin, M.S.; Baker, K.; Adams, P.D.; Bair, K.W.; Kaelin, W.G., Jr. Selective killing of transformed cells by cyclin/cyclin-dependent kinase 2 antagonists. Proc. Natl. Acad. Sci. USA 1999, 96, 4325–4329. [Google Scholar] [CrossRef]
- Mahadevan, D.; Plummer, R.; Squires, M.S.; Rensvold, D.; Kurtin, S.; Pretzinger, C.; Dragovich, T.; Adams, J.; Lock, V.; Smith, D.M.; et al. A phase I pharmacokinetic and pharmacodynamic study of AT7519, a cyclin-dependent kinase inhibitor in patients with refractory solid tumors. Ann. Oncol. 2011, 22, 2137–2143. [Google Scholar] [CrossRef]
- Schoffski, P. Polo-like kinase (PLK) inhibitors in preclinical and early clinical development in oncology. Oncologist 2009, 14, 559–570. [Google Scholar] [CrossRef]
- Maiti, B.; Li, J.; de Bruin, A.; Gordon, F.; Timmers, C.; Opavsky, R.; Patil, K.; Tuttle, J.; Cleghorn, W.; Leone, G. Cloning and characterization of mouse E2F8, a novel mammalian E2F family member capable of blocking cellular proliferation. J. Biol. Chem. 2005, 280, 18211–18220. [Google Scholar] [CrossRef]
- Yu, B.; Lane, M.E.; Wadler, S. SU9516, a cyclin-dependent kinase 2 inhibitor, promotes accumulation of high molecular weight E2F complexes in human colon carcinoma cells. Biochem. Pharmacol. 2002, 64, 1091–1100. [Google Scholar] [CrossRef]
- Banerjee, D.; Gorlick, R.; Liefshitz, A.; Danenberg, K.; Danenberg, P.C.; Danenberg, P.V.; Klimstra, D.; Jhanwar, S.; Cordon-Cardo, C.; Fong, Y.; et al. Levels of E2F-1 expression are higher in lung metastasis of colon cancer as compared with hepatic metastasis and correlate with levels of thymidylate synthase. Cancer Res. 2000, 60, 2365–2367. [Google Scholar] [PubMed]
- Kaur, M.; Singh, R.P.; Gu, M.; Agarwal, R.; Agarwal, C. Grape seed extract inhibits in vitro and in vivo growth of human colorectal carcinoma cells. Clin. Cancer Res. 2006, 12, 6194–6202. [Google Scholar] [CrossRef] [PubMed]
- Yasui, W.; Fujimoto, J.; Suzuki, T.; Ono, S.; Naka, K.; Yokozaki, H.; Tahara, E. Expression of cell-cycle-regulating transcription factor E2F-1 in colorectal carcinomas. Pathobiology 1999, 67, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.Y.; Istfan, N.W. Docosahexaenoic acid is a potent inducer of apoptosis in HT-29 colon cancer cells. Prostaglandins Leukot. Essent. Fatty Acids 2000, 63, 301–308. [Google Scholar] [CrossRef]
- Otto, T.; Sicinski, P. Cell cycle proteins as promising targets in cancer therapy. Nat. Rev. Cancer 2017, 17, 93–115. [Google Scholar] [CrossRef]
- Fearon, E.R.; Vogelstein, B. A genetic model for colorectal tumorigenesis. Cell 1990, 61, 759–767. [Google Scholar] [CrossRef]
- Zaid, H.; Silbermann, M.; Ben-Arye, E.; Saad, B. Greco-arab and islamic herbal-derived anticancer modalities: From tradition to molecular mechanisms. Evid.-Based Complement. Altern. Med. 2012, 2012, 349040. [Google Scholar] [CrossRef]
- Chandra, S.A.; Nolan, M.W.; Malarkey, D.E. Chemical carcinogenesis of the gastrointestinal tract in rodents: An overview with emphasis on NTP carcinogenesis bioassays. Toxicol. Pathol. 2010, 38, 188–197. [Google Scholar] [CrossRef]
- Volanis, D.; Kadiyska, T.; Galanis, A.; Delakas, D.; Logotheti, S.; Zoumpourlis, V. Environmental factors and genetic susceptibility promote urinary bladder cancer. Toxicol. Lett. 2010, 193, 131–137. [Google Scholar] [CrossRef]
- Rouis, Z.; Abid, N.; Koudja, S.; Yangui, T.; Elaissi, A.; Cioni, P.L.; Flamini, G.; Aouni, M. Evaluation of the cytotoxic effect and antibacterial, antifungal, and antiviral activities of Hypericum triquetrifolium Turra essential oils from Tunisia. BMC Complement. Altern. Med. 2013, 13, 24. [Google Scholar] [CrossRef]
- Li, X.M.; Luo, X.G.; He, J.F.; Wang, N.; Zhou, H.; Yang, P.L.; Zhang, T.C. Induction of apoptosis in human cervical carcinoma HeLa cells by active compounds from Hypericum ascyron L. Oncol. Lett. 2018, 15, 3944–3950. [Google Scholar] [PubMed]
- Qiu, D.; Zhou, M.; Lin, T.; Chen, J.; Wang, G.; Huang, Y.; Jiang, X.; Tian, W.; Chen, H. Cytotoxic Components from Hypericum elodeoides Targeting RXRalpha and Inducing HeLa Cell Apoptosis through Caspase-8 Activation and PARP Cleavage. J. Nat. Prod. 2019, 82, 1072–1080. [Google Scholar] [CrossRef] [PubMed]
- Mirmalek, S.A.; Azizi, M.A.; Jangholi, E.; Yadollah-Damavandi, S.; Javidi, M.A.; Parsa, Y.; Parsa, T.; Salimi-Tabatabaee, S.A.; Ghasemzadeh Kolagar, H.; Alizadeh-Navaei, R. Cytotoxic and apoptogenic effect of hypericin, the bioactive component of Hypericum perforatum on the MCF-7 human breast cancer cell line. Cancer Cell Int. 2016, 16, 3. [Google Scholar] [CrossRef] [PubMed]
- Sarimahmut, M.; Balikci, N.; Celikler, S.; Ari, F.; Ulukaya, E.; Guleryuz, G.; Ozel, M.Z. Evaluation of genotoxic and apoptotic potential of Hypericum adenotrichum Spach. in vitro. Regul. Toxicol. Pharmacol. 2016, 74, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Hamzeloo-Moghadam, M.; Khalaj, A.; Malekmohammadi, M. Cytotoxic Activity and Apoptosis Induction of Hypericum scabrum L. Iran. Red Crescent Med. J. 2015, 17, e19453. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Q.; Li, J.; Chen, Y.; Lin, J.; Lai, F.; Chen, X.; Lin, X.; Peng, J. Ethyl acetate extract of Hypericum japonicum induces apoptosis via the mitochondria-dependent pathway in vivo and in vitro. Mol. Med. Rep. 2015, 12, 4851–4858. [Google Scholar] [CrossRef] [PubMed]
- Kadan, S.; Sasson, Y.; Saad, B.; Zaid, H. Gundelia tournefortii Antidiabetic Efficacy: Chemical Composition and GLUT4 Translocation. Evid.-Based Complement. Altern. Med. 2018, 2018, 8294320. [Google Scholar] [CrossRef]
- Saad, B.; Azaizeh, H.; Said, O. Tradition and perspectives of arab herbal medicine: A review. Evid.-Based Complement. Altern. Med. 2005, 2, 475–479. [Google Scholar] [CrossRef]
- Mintz-Oron, S.; Mandel, T.; Rogachev, I.; Feldberg, L.; Lotan, O.; Yativ, M.; Wang, Z.; Jetter, R.; Venger, I.; Adato, A.; et al. Gene expression and metabolism in tomato fruit surface tissues. Plant Physiol. 2008, 147, 823–851. [Google Scholar] [CrossRef]
- Kadan, S.; Saad, B.; Sasson, Y.; Zaid, H. In vitro evaluation of anti-diabetic activity and cytotoxicity of chemically analysed Ocimum basilicum extracts. Food Chem. 2016, 196, 1066–1074. [Google Scholar] [CrossRef]
- Kadan, S.; Saad, B.; Sasson, Y.; Zaid, H. In Vitro Evaluations of Cytotoxicity of Eight Antidiabetic Medicinal Plants and Their Effect on GLUT4 Translocation. Evid.-Based Complement. Altern. Med. 2013, 2013, 549345. [Google Scholar] [CrossRef] [PubMed]
- Saydam, O.; Glauser, D.L.; Heid, I.; Turkeri, G.; Hilbe, M.; Jacobs, A.H.; Ackermann, M.; Fraefel, C. Herpes simplex virus 1 amplicon vector-mediated siRNA targeting epidermal growth factor receptor inhibits growth of human glioma cells in vivo. Mol. Ther. 2005, 12, 803–812. [Google Scholar] [CrossRef] [PubMed]
- Masters, S.C.; Fu, H. 14-3-3 proteins mediate an essential anti-apoptotic signal. J. Biol. Chem. 2001, 276, 45193–45200. [Google Scholar] [CrossRef] [PubMed]
- Napoli, E.; Wong, S.; Hung, C.; Ross-Inta, C.; Bomdica, P.; Giulivi, C. Defective mitochondrial disulfide relay system, altered mitochondrial morphology and function in Huntington’s disease. Hum. Mol. Genet. 2013, 22, 989–1004. [Google Scholar] [CrossRef]
- Hershko, T.; Ginsberg, D. Up-regulation of Bcl-2 homology 3 (BH3)-only proteins by E2F1 mediates apoptosis. J. Biol. Chem. 2004, 279, 8627–8634. [Google Scholar] [CrossRef]
- Mukherjee, A.; Adhikari, N.; Jha, T. A pentanoic acid derivative targeting matrix metalloproteinase-2 (MMP-2) induces apoptosis in a chronic myeloid leukemia cell line. Eur. J. Med. Chem. 2017, 141, 37–50. [Google Scholar] [CrossRef]
- Narayanan, A.; Baskaran, S.A.; Amalaradjou, M.A.; Venkitanarayanan, K. Anticarcinogenic properties of medium chain fatty acids on human colorectal, skin and breast cancer cells in vitro. Int. J. Mol. Sci. 2015, 16, 5014–5027. [Google Scholar] [CrossRef]
- Carvalho, C.M.; Menezes, P.F.; Letenski, G.C.; Praes, C.E.; Feferman, I.H.; Lorencini, M. In vitro induction of apoptosis, necrosis and genotoxicity by cosmetic preservatives: Application of flow cytometry as a complementary analysis by NRU. Int. J. Cosmet. Sci. 2012, 34, 176–182. [Google Scholar] [CrossRef]
- Smith, K.R.; Granberry, M.; Tan, M.C.B.; Daniel, C.L.; Gassman, N.R. Dihydroxyacetone induces G2/M arrest and apoptotic cell death in A375P melanoma cells. Environ. Toxicol. 2018, 33, 333–342. [Google Scholar] [CrossRef]
- Yilmaz, B.; Karabay, A.Z. Food Additive Sodium Benzoate (NaB) Activates NFkappaB and Induces Apoptosis in HCT116 Cells. Molecules 2018, 23, 723. [Google Scholar] [CrossRef]
- Forsey, R.J.; Shahidullah, H.; Sands, C.; McVittie, E.; Aldridge, R.D.; Hunter, J.A.; Howie, S.E. Epidermal Langerhans cell apoptosis is induced in vivo by nonanoic acid but not by sodium lauryl sulphate. Br. J. Dermatol. 1998, 139, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Jeong, M.; Kim, H.M.; Kim, H.J.; Choi, J.H.; Jang, D.S. Kudsuphilactone B, a nortriterpenoid isolated from Schisandra chinensis fruit, induces caspase-dependent apoptosis in human ovarian cancer A2780 cells. Arch. Pharm. Res. 2017, 40, 500–508. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.K.; Roh, H.S.; Yu, J.S.; Baek, J.; Lee, S.; Ra, M.; Kim, S.Y.; Baek, K.H.; Kim, K.H. Pinecone of Pinus koraiensis Inducing Apoptosis in Human Lung Cancer Cells by Activating Caspase-3 and its Chemical Constituents. Chem. Biodivers. 2017, 14. [Google Scholar] [CrossRef] [PubMed]
- Sitarek, P.; Skala, E.; Toma, M.; Wielanek, M.; Szemraj, J.; Skorski, T.; Bialas, A.J.; Sakowicz, T.; Kowalczyk, T.; Radek, M.; et al. Transformed Root Extract of Leonurus sibiricus Induces Apoptosis through Intrinsic and Extrinsic Pathways in Various Grades of Human Glioma Cells. Pathol. Oncol. Res. 2017, 23, 679–687. [Google Scholar] [CrossRef]
- Wang, X.N.; Wang, K.Y.; Zhang, X.S.; Yang, C.; Li, X.Y. 4-Hydroxybenzoic acid (4-HBA) enhances the sensitivity of human breast cancer cells to adriamycin as a specific HDAC6 inhibitor by promoting HIPK2/p53 pathway. Biochem. Biophys. Res. Commun. 2018, 504, 812–819. [Google Scholar] [CrossRef]
- Jin, Z.; Wang, W.F.; Huang, J.P.; Wang, H.M.; Ju, H.X.; Chang, Y. Dryocrassin ABBA Induces Apoptosis in Human Hepatocellular Carcinoma HepG2 Cells Through a Caspase-Dependent Mitochondrial Pathway. Asian Pac. J. Cancer Prev. 2016, 17, 1823–1828. [Google Scholar] [CrossRef]
- Lee, H.A.; Lee, J.H.; Han, J.S. 2,7″-Phloroglucinol-6,6′-bieckol protects INS-1 cells against high glucose-induced apoptosis. Biomed. Pharmacother. 2018, 103, 1473–1481. [Google Scholar] [CrossRef]
- Park, C.; Cha, H.J.; Hong, S.H.; Kim, G.Y.; Kim, S.; Kim, H.S.; Kim, B.W.; Jeon, Y.J.; Choi, Y.H. Protective Effect of Phloroglucinol on Oxidative Stress-Induced DNA Damage and Apoptosis through Activation of the Nrf2/HO-1 Signaling Pathway in HaCaT Human Keratinocytes. Mar. Drugs 2019, 17, 225. [Google Scholar] [CrossRef]
- Chang, L.C.; Tsai, T.R.; Wang, J.J.; Lin, C.N.; Kuo, K.W. The rhamnose moiety of solamargine plays a crucial role in triggering cell death by apoptosis. Biochem. Biophys. Res. Commun. 1998, 242, 21–25. [Google Scholar] [CrossRef]
- Gong, J.; Zhou, S.; Yang, S. Vanillic Acid Suppresses HIF-1alpha Expression via Inhibition of mTOR/p70S6K/4E-BP1 and Raf/MEK/ERK Pathways in Human Colon Cancer HCT116 Cells. Int. J. Mol. Sci. 2019, 20, 465. [Google Scholar] [CrossRef]
- Kakkar, S.; Bais, S. A review on protocatechuic Acid and its pharmacological potential. ISRN Pharmacol. 2014, 2014, 952943. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.C.; Ou, T.T.; Chang, C.H.; Chan, K.C.; Wang, C.J. Correction to Protocatechuic Acid Inhibits Oleic Acid-Induced Vascular Smooth Muscle Cell Proliferation through Activation of AMP-Activated Protein Kinase and Cell Cycle Arrest in G0/G1 Phase. J. Agric. Food Chem. 2015, 63, 4003. [Google Scholar] [CrossRef] [PubMed]
- Tsao, S.M.; Hsia, T.C.; Yin, M.C. Protocatechuic acid inhibits lung cancer cells by modulating FAK, MAPK, and NF-kappaB pathways. Nutr. Cancer 2014, 66, 1331–1341. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Guo, Z.; Wang, Y.; Lei, J.; Yu, J. Protocatechuic acid inhibits the growth of ovarian cancer cells by inducing apoptosis and autophagy. Phytother. Res. 2018, 32, 2256–2263. [Google Scholar] [CrossRef] [PubMed]
- Yip, E.C.; Chan, A.S.; Pang, H.; Tam, Y.K.; Wong, Y.H. Protocatechuic acid induces cell death in HepG2 hepatocellular carcinoma cells through a c-Jun N-terminal kinase-dependent mechanism. Cell Biol. Toxicol. 2006, 22, 293–302. [Google Scholar] [CrossRef]
- Kianmehr, Z.; Khorsandi, K.; Mohammadi, M.; Hosseinzadeh, R. Low-level laser irradiation potentiates anticancer activity of p-coumaric acid against human malignant melanoma cells. Melanoma Res. 2019. [Google Scholar] [CrossRef]
- Pei, K.; Ou, J.; Huang, J.; Ou, S. p-Coumaric acid and its conjugates: Dietary sources, pharmacokinetic properties and biological activities. J. Sci. Food Agric. 2016, 96, 2952–2962. [Google Scholar] [CrossRef]
- Sharma, S.H.; Rajamanickam, V.; Nagarajan, S. Supplementation of p-coumaric acid exhibits chemopreventive effect via induction of Nrf2 in a short-term preclinical model of colon cancer. Eur. J. Cancer Prev. 2019, 28, 472–482. [Google Scholar] [CrossRef]
- Liang, Z.; Yuan, Z.; Guo, J.; Wu, J.; Yi, J.; Deng, J.; Shan, Y. Ganoderma lucidum Polysaccharides Prevent Palmitic Acid-Evoked Apoptosis and Autophagy in Intestinal Porcine Epithelial Cell Line via Restoration of Mitochondrial Function and Regulation of MAPK and AMPK/Akt/mTOR Signaling Pathway. Int. J. Mol. Sci. 2019, 20, 478. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, J.; Liu, W.; Yao, G.; Xu, F.; Hayashi, T.; Onodera, S.; Ikejima, T. Attenuating effect of silibinin on palmitic acid-induced apoptosis and mitochondrial dysfunction in pancreatic beta-cells is mediated by estrogen receptor alpha. Mol. Cell. Biochem. 2019, 460, 81–92. [Google Scholar] [CrossRef]
- Zou, Y.; Kong, M. Tetrahydroxy stilbene glucoside alleviates palmitic acid-induced inflammation and apoptosis in cardiomyocytes by regulating miR-129-3p/Smad3 signaling. Cell. Mol. Biol. Lett. 2019, 24, 5. [Google Scholar] [CrossRef] [PubMed]
- Meng, P.H.; Raynaud, C.; Tcherkez, G.; Blanchet, S.; Massoud, K.; Domenichini, S.; Henry, Y.; Soubigou-Taconnat, L.; Lelarge-Trouverie, C.; Saindrenan, P.; et al. Crosstalks between myo-inositol metabolism, programmed cell death and basal immunity in Arabidopsis. PLoS ONE 2009, 4, e7364. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Feng, G.; Wu, L.; Zhong, S.; Gao, X.; Tong, Y.; Cui, W.; Qin, Y.; Xu, W.; Xiao, X.; et al. Caffeic acid phenethyl ester suppressed growth and metastasis of nasopharyngeal carcinoma cells by inactivating the NF-kappaB pathway. Drug Des. Dev. Ther. 2019, 13, 1335–1345. [Google Scholar] [CrossRef] [PubMed]
- Motawi, T.K.; Abdelazim, S.A.; Darwish, H.A.; Elbaz, E.M.; Shouman, S.A. Modulation of Tamoxifen Cytotoxicity by Caffeic Acid Phenethyl Ester in MCF-7 Breast Cancer Cells. Oxid. Med. Cell. Longev. 2016, 2016, 3017108. [Google Scholar] [CrossRef]
- Zeng, N.; Hongbo, T.; Xu, Y.; Wu, M.; Wu, Y. Anticancer activity of caffeic acid nbutyl ester against A431 skin carcinoma cell line occurs via induction of apoptosis and inhibition of the mTOR/PI3K/AKT signaling pathway. Mol. Med. Rep. 2018, 17, 5652–5657. [Google Scholar]
- Khan, A.A.; Alanazi, A.M.; Jabeen, M.; Chauhan, A.; Abdelhameed, A.S. Design, synthesis and in vitro anticancer evaluation of a stearic acid-based ester conjugate. Anticancer Res. 2013, 33, 2517–2524. [Google Scholar]
- Liu, Y.; Cheng, Y.; Li, J.; Wang, Y.; Liu, Y. Epoxy Stearic Acid, an Oxidative Product Derived from Oleic Acid, Induces Cytotoxicity, Oxidative Stress, and Apoptosis in HepG2 Cells. J. Agric. Food Chem. 2018, 66, 5237–5246. [Google Scholar] [CrossRef]
- Rusmini, P.; Cortese, K.; Crippa, V.; Cristofani, R.; Cicardi, M.E.; Ferrari, V.; Vezzoli, G.; Tedesco, B.; Meroni, M.; Messi, E.; et al. Trehalose induces autophagy via lysosomal-mediated TFEB activation in models of motoneuron degeneration. Autophagy 2019, 15, 631–651. [Google Scholar] [CrossRef]
- Alvarez-Sala, A.; Attanzio, A.; Tesoriere, L.; Garcia-Llatas, G.; Barbera, R.; Cilla, A. Apoptotic effect of a phytosterol-ingredient and its main phytosterol (beta-sitosterol) in human cancer cell lines. Int. J. Food Sci. Nutr. 2019, 70, 323–334. [Google Scholar] [CrossRef]
- Cao, Z.Q.; Wang, X.X.; Lu, L.; Xu, J.W.; Li, X.B.; Zhang, G.R.; Ma, Z.J.; Shi, A.C.; Wang, Y.; Song, Y.J. β-Sitosterol and Gemcitabine Exhibit Synergistic Anti-pancreatic Cancer Activity by Modulating Apoptosis and Inhibiting Epithelial-Mesenchymal Transition by Deactivating Akt/GSK-3beta Signaling. Front. Pharmacol. 2018, 9, 1525. [Google Scholar] [CrossRef]
- Shin, E.J.; Choi, H.K.; Sung, M.J.; Park, J.H.; Chung, M.Y.; Chung, S.; Hwang, J.T. Anti-tumour effects of beta-sitosterol are mediated by AMPK/PTEN/HSP90 axis in AGS human gastric adenocarcinoma cells and xenograft mouse models. Biochem. Pharmacol. 2018, 152, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Yuan, D.; Yan, R.; Meng, L.; Zhang, Y.; Zhu, K. Stigmasterol exhibits potent antitumor effects in human gastric cancer cells mediated via inhibition of cell migration, cell cycle arrest, mitochondrial mediated apoptosis and inhibition of JAK/STAT signalling pathway. J. BUON 2018, 23, 1420–1425. [Google Scholar] [PubMed]
- Fadeel, B.; Ottosson, A.; Pervaiz, S. Big wheel keeps on turning: Apoptosome regulation and its role in chemoresistance. Cell Death Differ. 2008, 15, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Oda, E.; Ohki, R.; Murasawa, H.; Nemoto, J.; Shibue, T.; Yamashita, T.; Tokino, T.; Taniguchi, T.; Tanaka, N. Noxa, a BH3-only member of the Bcl-2 family and candidate mediator of p53-induced apoptosis. Science 2000, 288, 1053–1058. [Google Scholar] [CrossRef] [PubMed]
- Vogel, C.; Marcotte, E.M. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat. Rev. Genet. 2012, 13, 227–232. [Google Scholar] [CrossRef]
- Adebayo, I.A.; Gagman, H.A.; Balogun, W.G.; Adam, M.A.A.; Abas, R.; Hakeem, K.R.; Nik Him, N.; Samian, M.R.B.; Arsad, H. Detarium microcarpum, Guiera senegalensis, and Cassia siamea Induce Apoptosis and Cell Cycle Arrest and Inhibit Metastasis on MCF7 Breast Cancer Cells. Evid.-Based Complement. Altern. Med. 2019, 2019, 6104574. [Google Scholar]
- Intisar, A.; Zhang, L.; Luo, H.; Kiazolu, J.B.; Zhang, R.; Zhang, W. Anticancer constituents and cytotoxic activity of methanol-water extract of Polygonum bistorta L. Afr. J. Tradit. Complement. Altern. Med. 2012, 10, 53–59. [Google Scholar] [CrossRef][Green Version]
- Cevik, D.; Kan, Y.; Kirmizibekmez, H. Mechanisms of action of cytotoxic phenolic compounds from Glycyrrhiza iconica roots. Phytomedicine 2019, 58, 152872. [Google Scholar] [CrossRef]
- Owen, R.W.; Giacosa, A.; Hull, W.E.; Haubner, R.; Spiegelhalder, B.; Bartsch, H. The antioxidant/anticancer potential of phenolic compounds isolated from olive oil. Eur. J. Cancer 2000, 36, 1235–1247. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
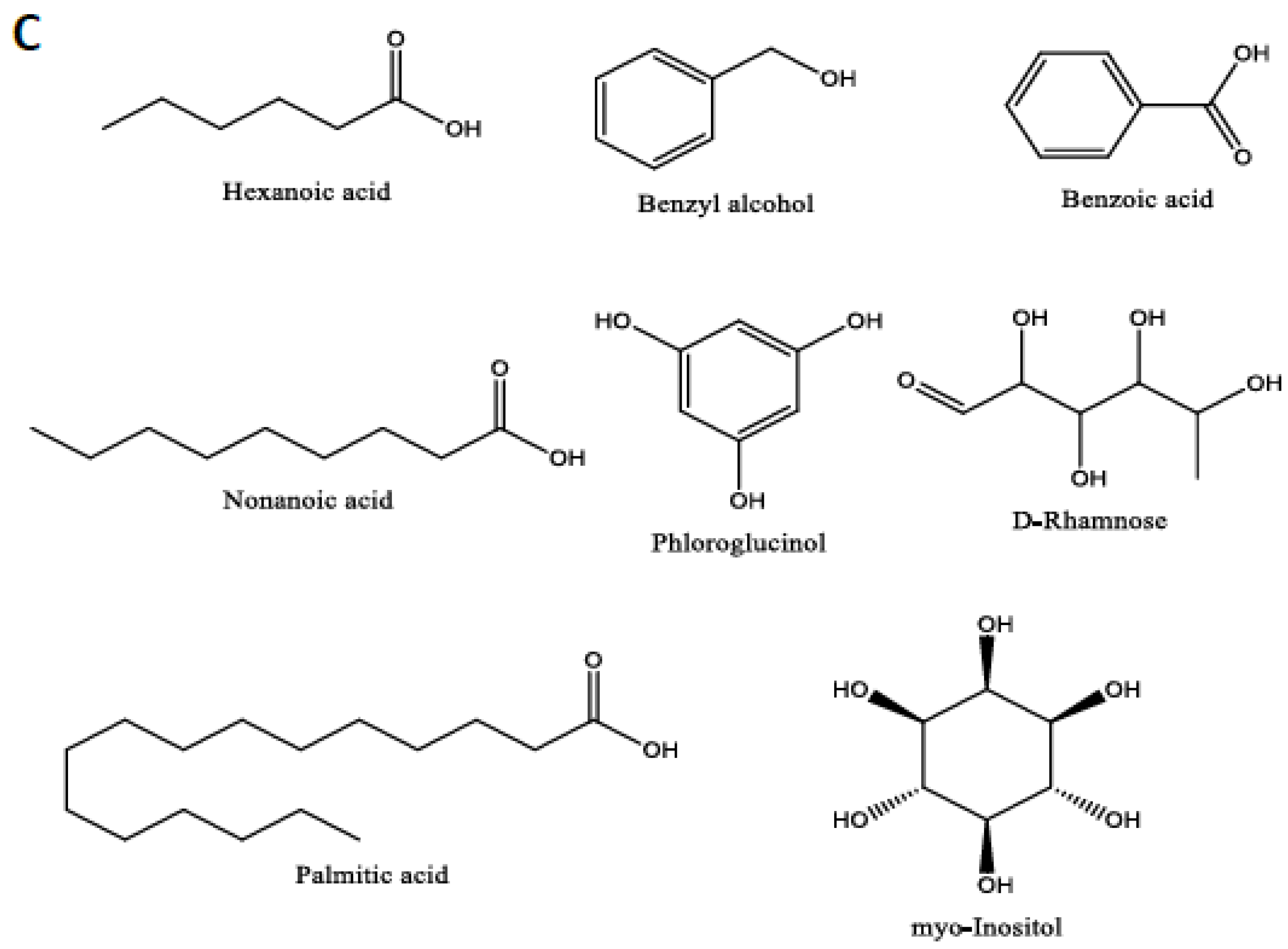
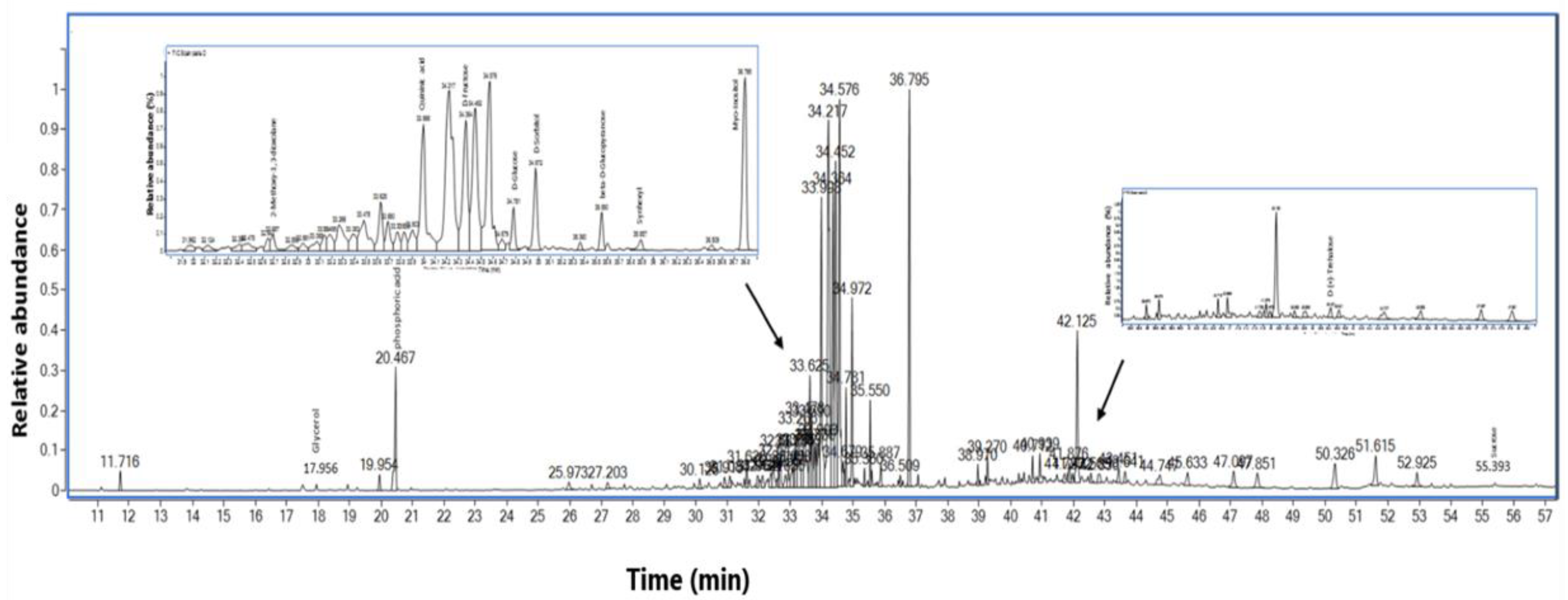
| Peak | Name | Rt | % Area | DW mg/g | Match Factor | Relation to Apoptosis | References |
|---|---|---|---|---|---|---|---|
| 1 | Pentanoic acid | 10.791 | 0.01 | 0.054207 | 89 | Its derivatives are used to induce apoptosis in cell lines. | [36] |
| 2 | 2,3-Butanediol | 12.863 | 0.05 | 0.286145 | 98 | N/A | |
| 3 | 1,3-Propanediol | 13.570 | 0.01 | 0.05732 | 88 | N/A | |
| 4 | Lactic Acid | 13.819 | 0.14 | 0.891773 | 93 | N/A | |
| 5 | Hexanoic acid | 14.002 | 0.04 | 0.276932 | 96 | Also known as caproic acid. Reported to induce apoptosis in human colorectal, skin, and breast cancer. Moreover, it “could potentially be used to prevent and/or treat these cancers”. | [37] |
| 6 | Succinimide | 15.525 | 0.01 | 0.060801 | 88 | N/A | |
| 7 | Benzyl alcohol | 16.447 | 0.11 | 0.667908 | 91 | 1% benzyl alcohol was reported to induce high apoptosis and necrosis in human dermal fibroblasts. | [38] |
| 8 | 4-Hydroxybutanoic acid | 16.96 | 0.07 | 0.465751 | 86 | N/A | |
| 9 | Glycerol | 17.956 | 6.27 | 39.74729 | 99 | N/A | |
| 10 | 3-Hydroxybutanoic acid | 18.065 | 0.01 | 0.075168 | 92 | N/A | |
| 11 | Dihydroxyacetone | 18.768 | 0.03 | 0.184817 | 78 | Reported to induce G2/M arrest and apoptotic cell death in melanoma A375P cell line. | [39] |
| 12 | Benzoic Acid | 19.244 | 0.19 | 1.22039 | 98 | Sodium benzoate was reported to activate NFκB and induce apoptosis in HCT116 cells. | [40] |
| 13 | Octanoic acid | 19.808 | 0.02 | 0.143862 | 83 | N/A | |
| 14 | phosphoric acid | 20.467 | 3.85 | 24.42644 | 86 | N/A | |
| 15 | 1,2,3-Butanetriol | 20.958 | 0.30 | 1.88296 | 98 | N/A | |
| 16 | Nonanoic acid | 22.371 | 0.11 | 0.679532 | 87 | Reported to induce apoptosis in vivo in epidermal Langerhans. | [41] |
| 17 | (E)-Erythrono-1,4-lacton | 22.971 | 0.04 | 0.230517 | 89 | N/A | |
| 18 | Pyroglutamic acid | 25.973 | 0.69 | 4.36759 | 94 | N/A | |
| 19 | L-Threitol | 26.515 | 0.09 | 0.581057 | 93 | N/A | |
| 20 | meso-Erythritol | 26.698 | 0.33 | 2.11151 | 95 | N/A | |
| 21 | 3-Hydroxybenzoic acid | 27.584 | 0.11 | 0.674429 | 85 | N/A | |
| 22 | 4-Hydroxybenzoic acid | 29.078 | 0.36 | 2.265056 | 81 | Detected in Schisandra chinensis fruit extract, which is known to induce caspase-dependent apoptosis in human ovarian cancer A2780 cells. It was also detected in pinecones of Pinus koraiensis extract and exhibited cytotoxic activity, with IC50 value around 1 mg/mL in four human lung cancer cell lines, A549, H1264, H1299, and Calu-6. Similarly, Sitarek and colleagues reported antiproliferative and cell cycle arrest activity on glioma cells for the same compound in the root extract of Leonurus sibiricus L. It increased Bax, Bcl-2, p53, Caspases in glioma and breast cancer cell lines. | [42,43,44,45] |
| 23 | Arabinonic acid, γ-lactone | 29.429 | 0.06 | 0.402076 | 89 | N/A | |
| 24 | Phloroglucinol | 29.59 | 0.09 | 0.590668 | 92 | Reported to induce apoptosis in distinct cancer cell lines. | [46,47,48] |
| 25 | Levoglucosan | 31.084 | 0.66 | 4.189809 | 95 | N/A | |
| 26 | d-(-)-Rhamnose | 31.545 | 0.31 | 1.948759 | 94 | Apoptosis inducer and anti-cancer agent, especially in human breast cancer. | [49] |
| 27 | Vanillic Acid | 31.962 | 0.71 | 4.500047 | 77 | An antioxidant and has some anti-cancer benefits. | [50] |
| 28 | 2-Methoxy-1,3-dioxolane | 32.687 | 2.80 | 17.73789 | 91 | N/A | |
| 29 | Methyl α-d-glucofuranoside | 32.951 | 0.52 | 3.281267 | 86 | Close derivates are used for tumor treatment. | |
| 30 | Protocatechuic acid | 33.068 | 0.25 | 1.559802 | 84 | Known to induce apoptosis in human ovarian, breast, lung, liver, cervix, and prostate cancer cells, as well as others, by modulating FAK, MAPK, c-June, and NF-κB pathways.It was also reported to lead to cell cycle arrest. | [51,52,53,54,55] |
| 31 | Shikimic acid | 33.068 | 0.46 | 2.892089 | 87 | N/A | |
| 32 | Quininic acid | 33.998 | 13.41 | 85.04314 | 88 | N/A | |
| 33 | D-Fructose | 34.364 | 18.78 | 119.1289 | 91 | N/A | |
| 34 | p-Coumaric acid | 34.679 | 0.24 | 1.521807 | 89 | Reported to induced apoptosis and cell cycle arrest in several cell lines, including human colon cancer. | [56,57,58] |
| 35 | D-Glucose | 34.781 | 3.34 | 21.1588 | 96 | N/A | |
| 36 | D-Sorbitol | 34.972 | 6.79 | 43.05749 | 94 | N/A | |
| 37 | β-d-Glucopyranose | 35.55 | 2.72 | 17.22668 | 98 | N/A | |
| 38 | 3-Hexyl-7,8,9,10-tetrahydro-6,6,9-trimethyl-6H-dibenzo(b,d)pyran-1-ol, | 35.887 | 1.07 | 6.810763 | 79 | Also called Synhexyl | |
| 39 | Palmitic Acid | 35.887 | 1.07 | 6.810763 | 98 | Reported to induce apoptosis in dozens of cancer cell lines via MAPK and AMPK/Akt/mTOR, miR-129-3p/Smad3, and estrogen receptor alpha signaling pathways. | [59,60,61] |
| 40 | Myoinositol | 36.795 | 18.69 | 118.5457 | 97 | Reported to be involved in apoptosis induction in the Arabidopsis plant. | [62] |
| 41 | Caffeic acid | 37.066 | 0.50 | 3.155426 | 81 | Reported to induce apoptosis and cell cycle arrest in several cell lines, including human colon, breast, nasopharyngeal carcinoma, melanoma, lung, nasopharyngeal and others. It altered the mTOR/PI3K/AKT signaling pathway and inactivated NF-κB pathway. | [63,64,65] |
| 42 | L-Rhamnose | 37.74 | 0.26 | 1.662177 | 85 | N/A | |
| 43 | Stearic acid | 37.915 | 0.52 | 3.312062 | 91 | Reported to arrest the cell cycle and induce apoptosis in HepG2 and other cancer cell lines. | [66,67] |
| 44 | Glyceryl-glycoside | 38.97 | 0.72 | 4.565657 | 94 | N/A | |
| 45 | D-(+)-Trehalose | 43.451 | 1.31 | 8.317046 | 92 | Induced autophagy. | [68] |
| 46 | (2R)-(E)-Catechine | 44.747 | 0.55 | 3.465693 | 86 | N/A | |
| 47 | (2R-cis)-Catechine | 45.142 | 0.08 | 0.496909 | 84 | N/A | |
| 48 | β-Sitosterol | 53.782 | 0.19 | 1.17753 | 88 | Also named phytosterol. Reported to induce apoptosis and cell cycle arrest in HCT116, MCF-7, A549, and HeLa cell lines. Altered the PI3K/Akt signaling pathway and AMPK/PTEN/HSP90. | [69,70,71] |
| 49 | Stigmasterol | 53.782 | 0.18 | 1.15534 | 90 | Reported to lead to cell cycle arrest, mitochondrial-mediated apoptosis, and inhibition of JAK/STAT signaling pathway. It also inhibited cell migration in human gastric cancer cells. | [72] |
| 50 | Sucrose | 55.393 | 10.91285 | 69.21846 | 92 | N/A |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahajna, S.; Kadan, S.; Tietel, Z.; Saad, B.; Khasib, S.; Tumeh, A.; Ginsberg, D.; Zaid, H. In Vitro Evaluation of Chemically Analyzed Hypericum Triquetrifolium Extract Efficacy in Apoptosis Induction and Cell Cycle Arrest of the HCT-116 Colon Cancer Cell Line. Molecules 2019, 24, 4139. https://doi.org/10.3390/molecules24224139
Mahajna S, Kadan S, Tietel Z, Saad B, Khasib S, Tumeh A, Ginsberg D, Zaid H. In Vitro Evaluation of Chemically Analyzed Hypericum Triquetrifolium Extract Efficacy in Apoptosis Induction and Cell Cycle Arrest of the HCT-116 Colon Cancer Cell Line. Molecules. 2019; 24(22):4139. https://doi.org/10.3390/molecules24224139
Chicago/Turabian StyleMahajna, Shahinaz, Sleman Kadan, Zipora Tietel, Bashar Saad, Said Khasib, Aziz Tumeh, Doron Ginsberg, and Hilal Zaid. 2019. "In Vitro Evaluation of Chemically Analyzed Hypericum Triquetrifolium Extract Efficacy in Apoptosis Induction and Cell Cycle Arrest of the HCT-116 Colon Cancer Cell Line" Molecules 24, no. 22: 4139. https://doi.org/10.3390/molecules24224139
APA StyleMahajna, S., Kadan, S., Tietel, Z., Saad, B., Khasib, S., Tumeh, A., Ginsberg, D., & Zaid, H. (2019). In Vitro Evaluation of Chemically Analyzed Hypericum Triquetrifolium Extract Efficacy in Apoptosis Induction and Cell Cycle Arrest of the HCT-116 Colon Cancer Cell Line. Molecules, 24(22), 4139. https://doi.org/10.3390/molecules24224139








