Development of Bioepoxy Resin Microencapsulated Ammonium-Polyphosphate for Flame Retardancy of Polylactic Acid
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of the Microcapsules
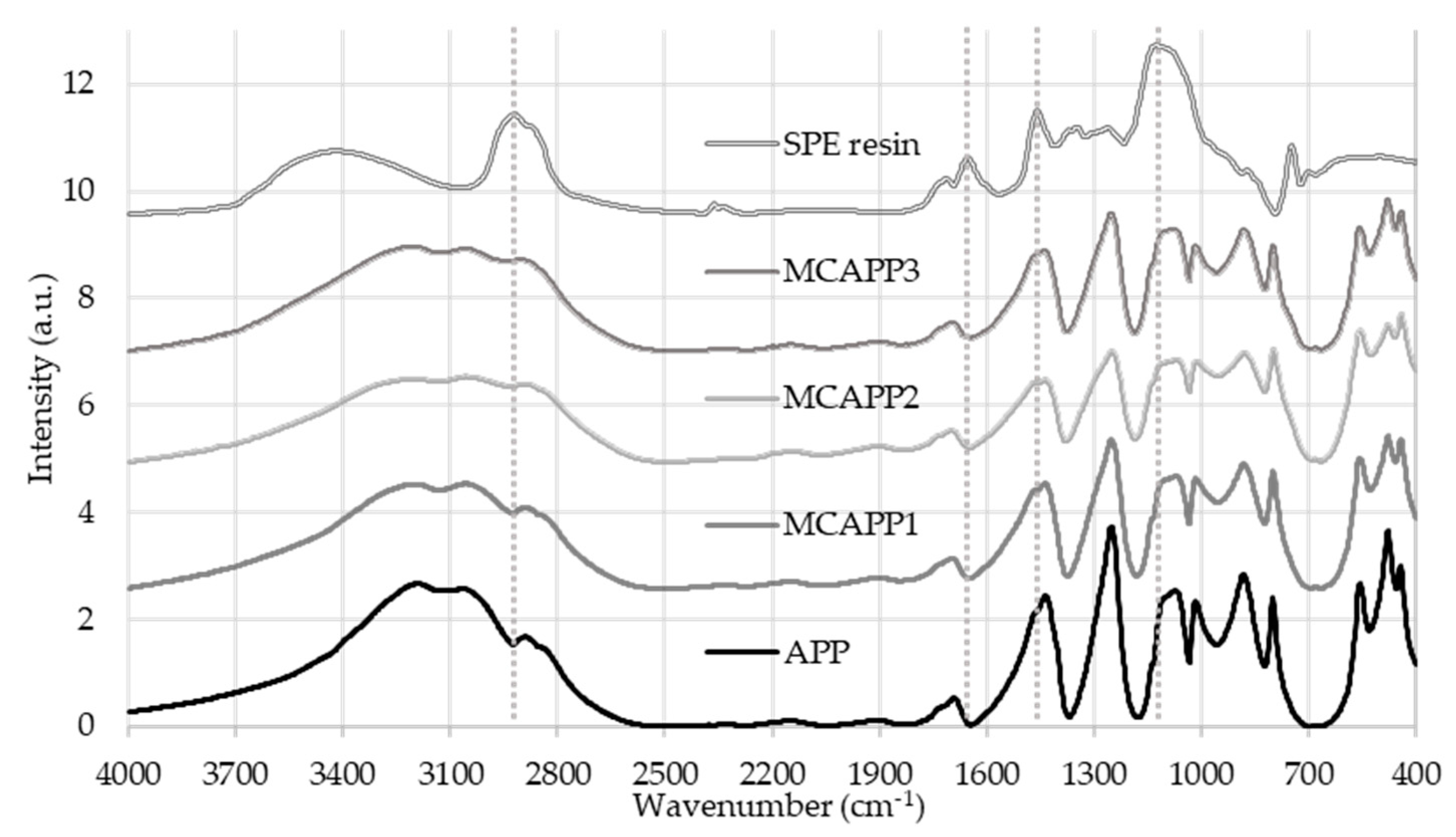
2.1.1. Fourier Transform Infrared (FTIR) Spectroscopy
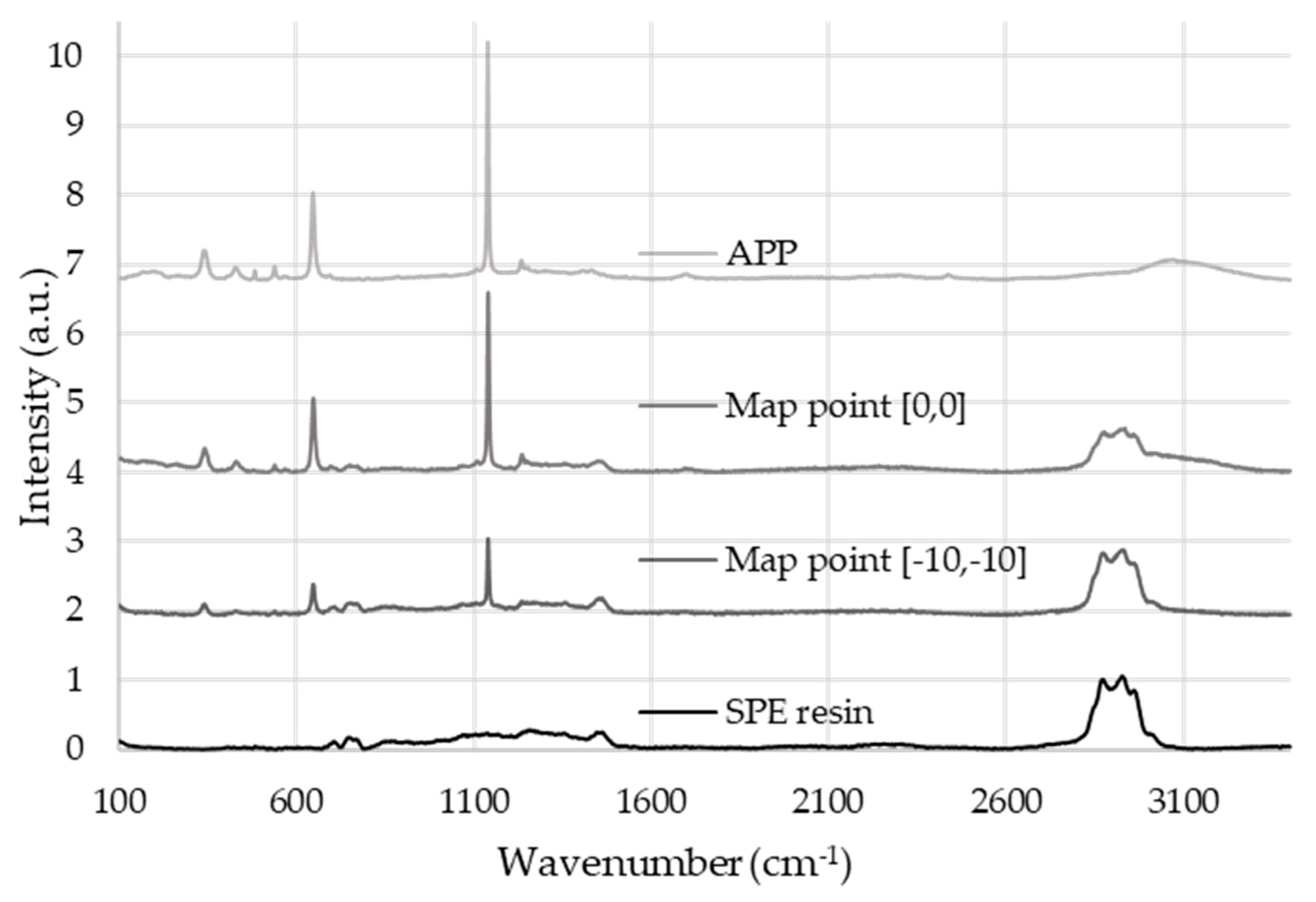
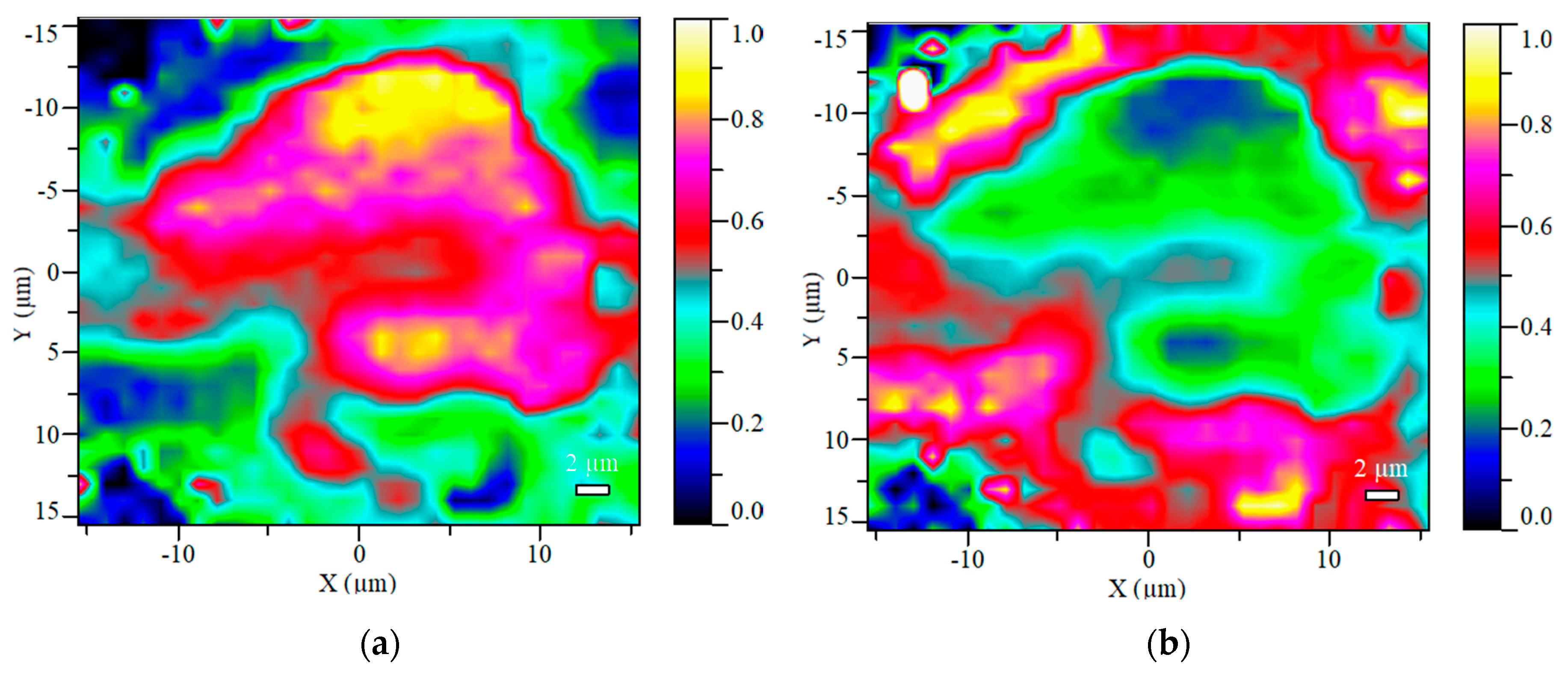
2.1.2. Raman Spectroscopy
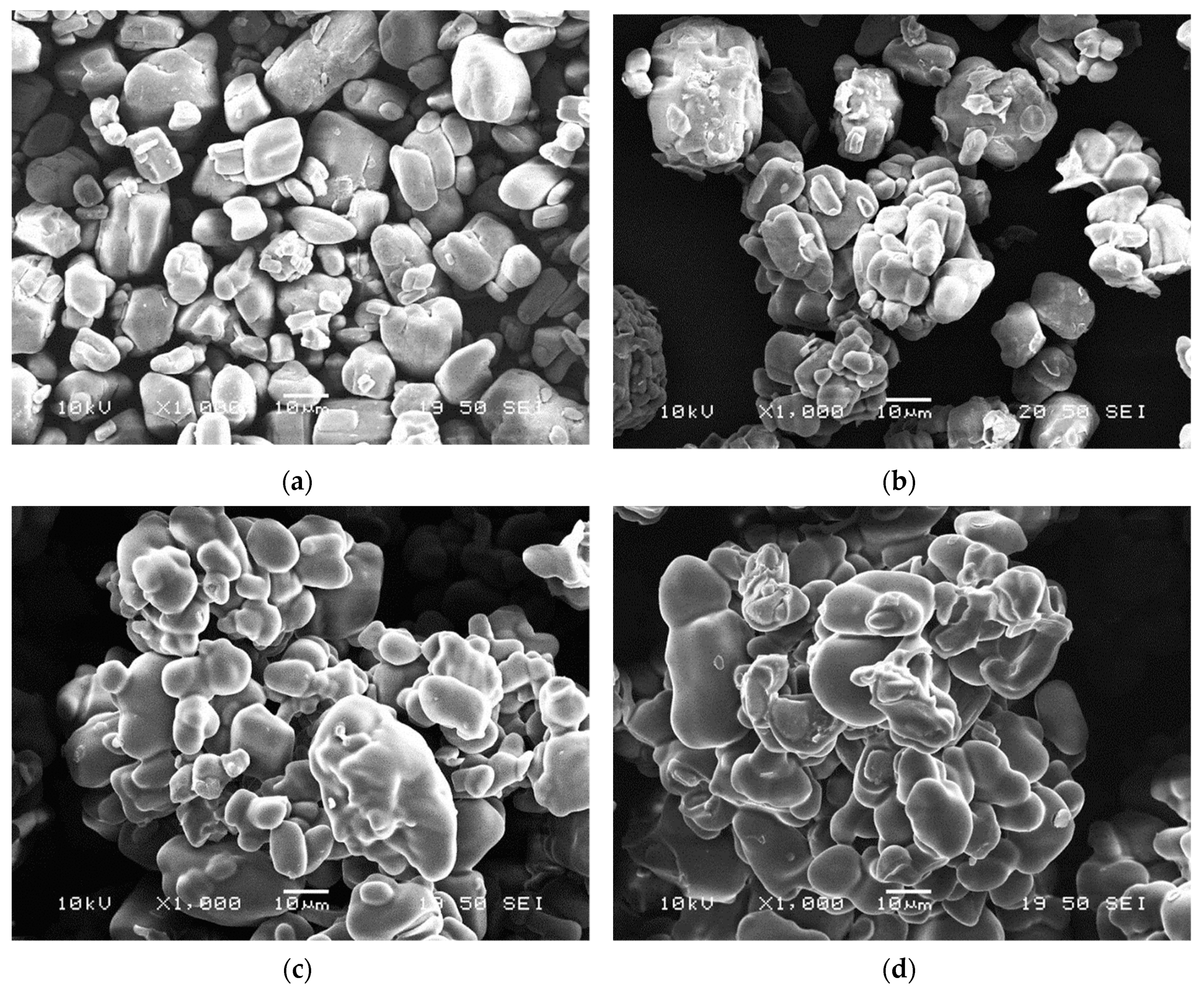
2.1.3. Scanning Electron Microscopy (SEM)
2.1.4. Determination of the Particle Size and Distribution
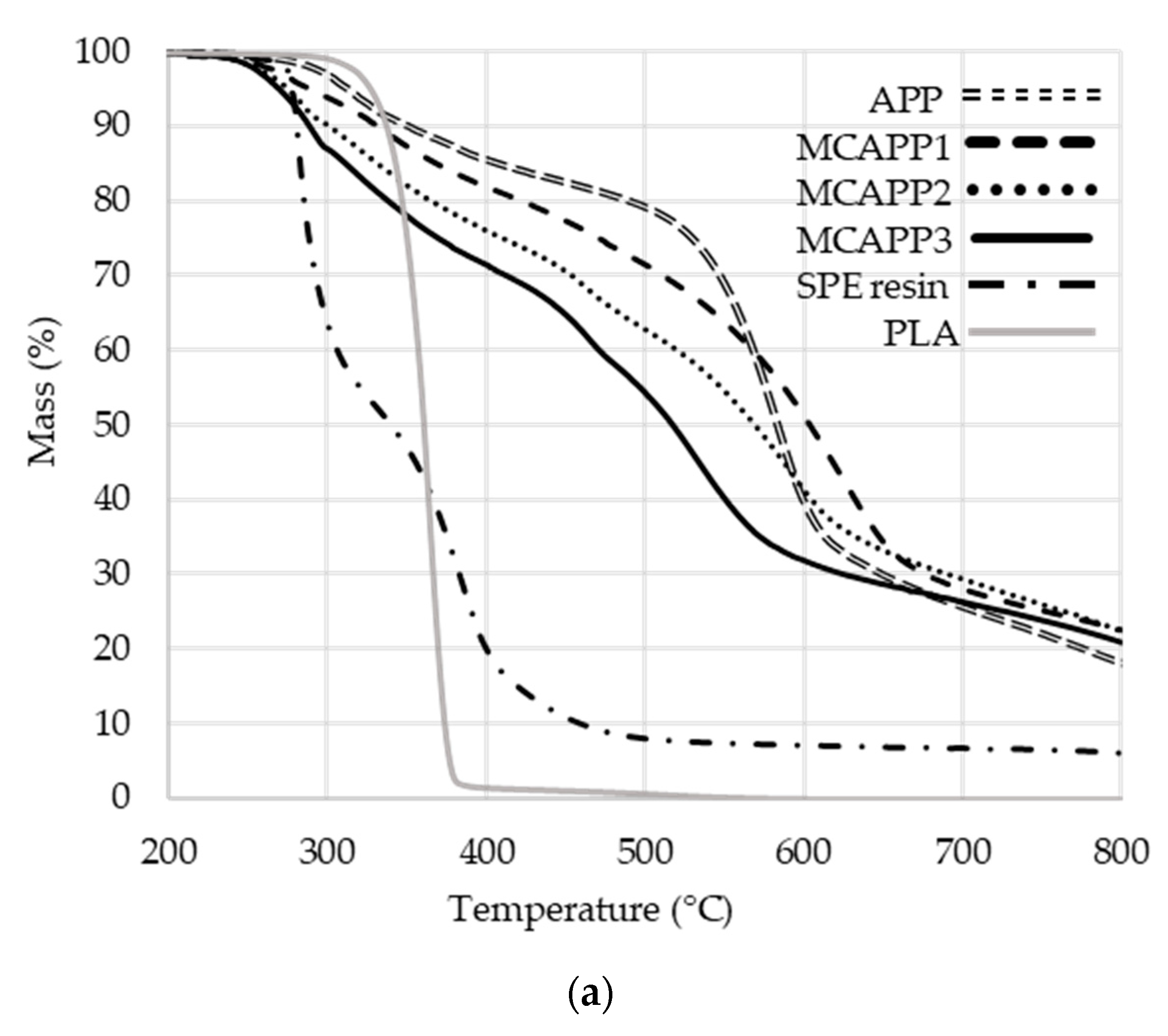
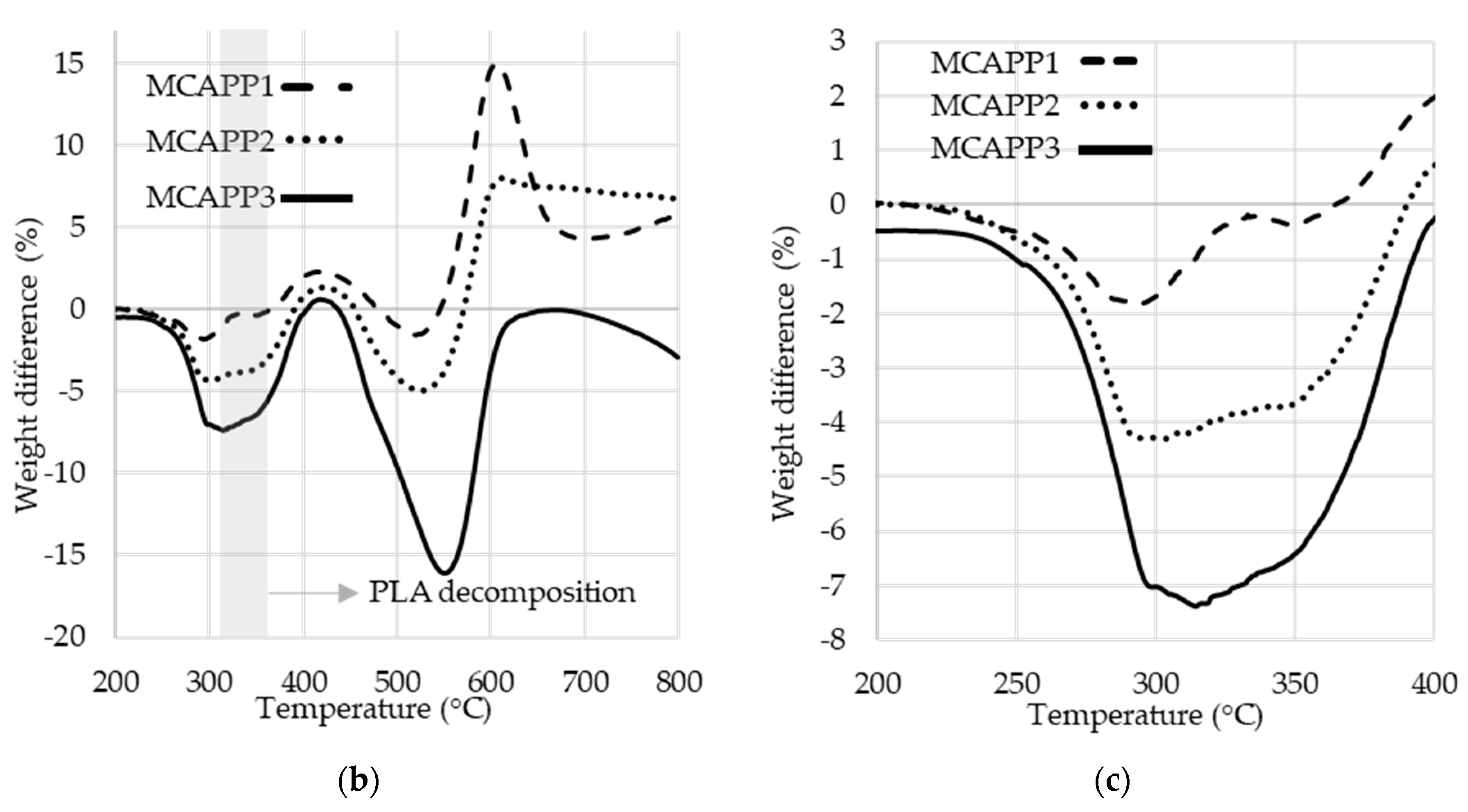
2.1.5. Thermogravimetric Analysis (TGA)
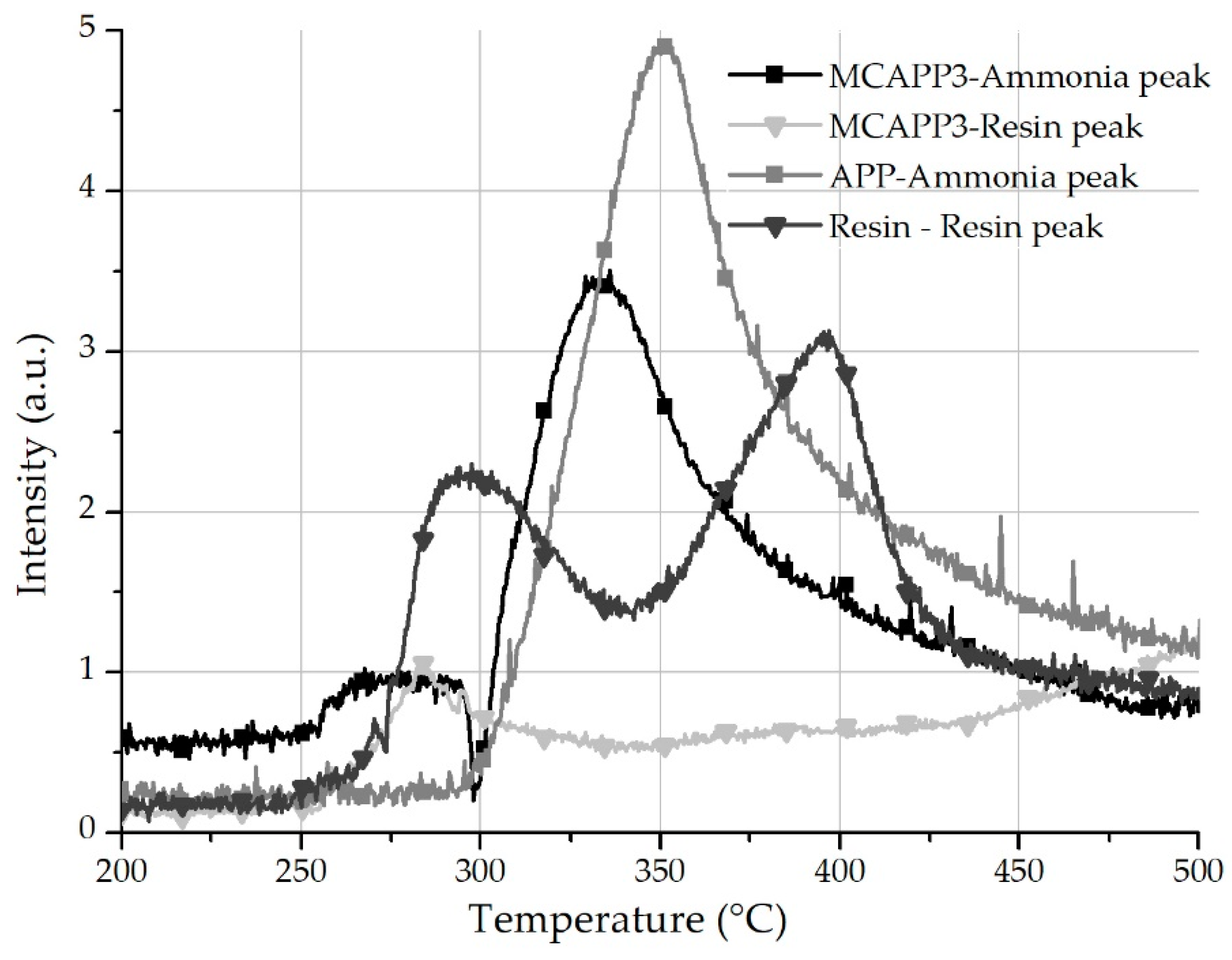
2.1.6. Thermogravimetry-FTIR Spectrometry
2.2. Characterization of Flame Retarded Polylactic Acid (PLA) Composites
2.2.1. Scanning Electron Microscopy (SEM)
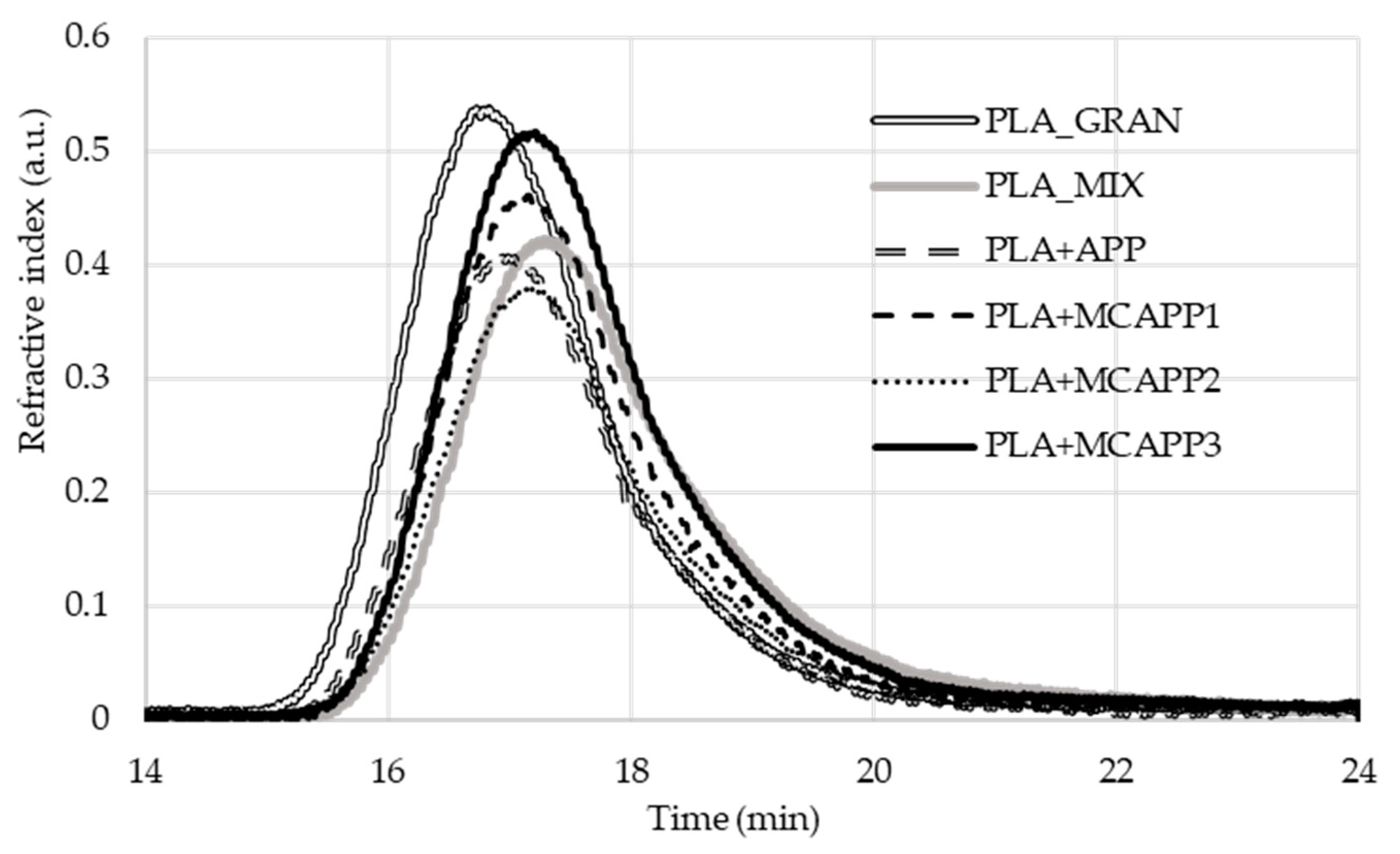
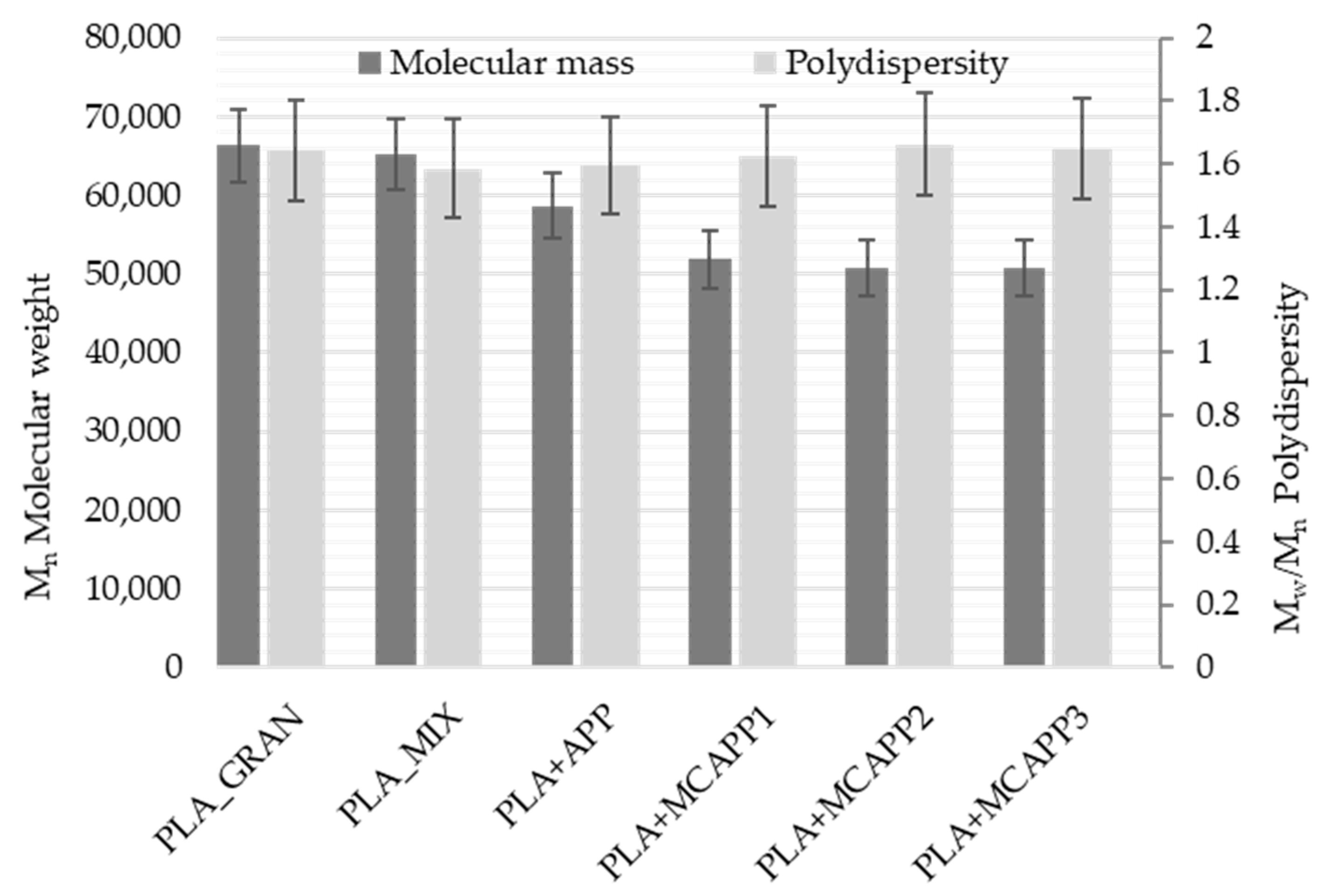
2.2.2. Gel Permeation Chromatography (GPC)
2.2.3. Differential Scanning Calorimetry (DSC)
2.2.4. Thermal Degradation
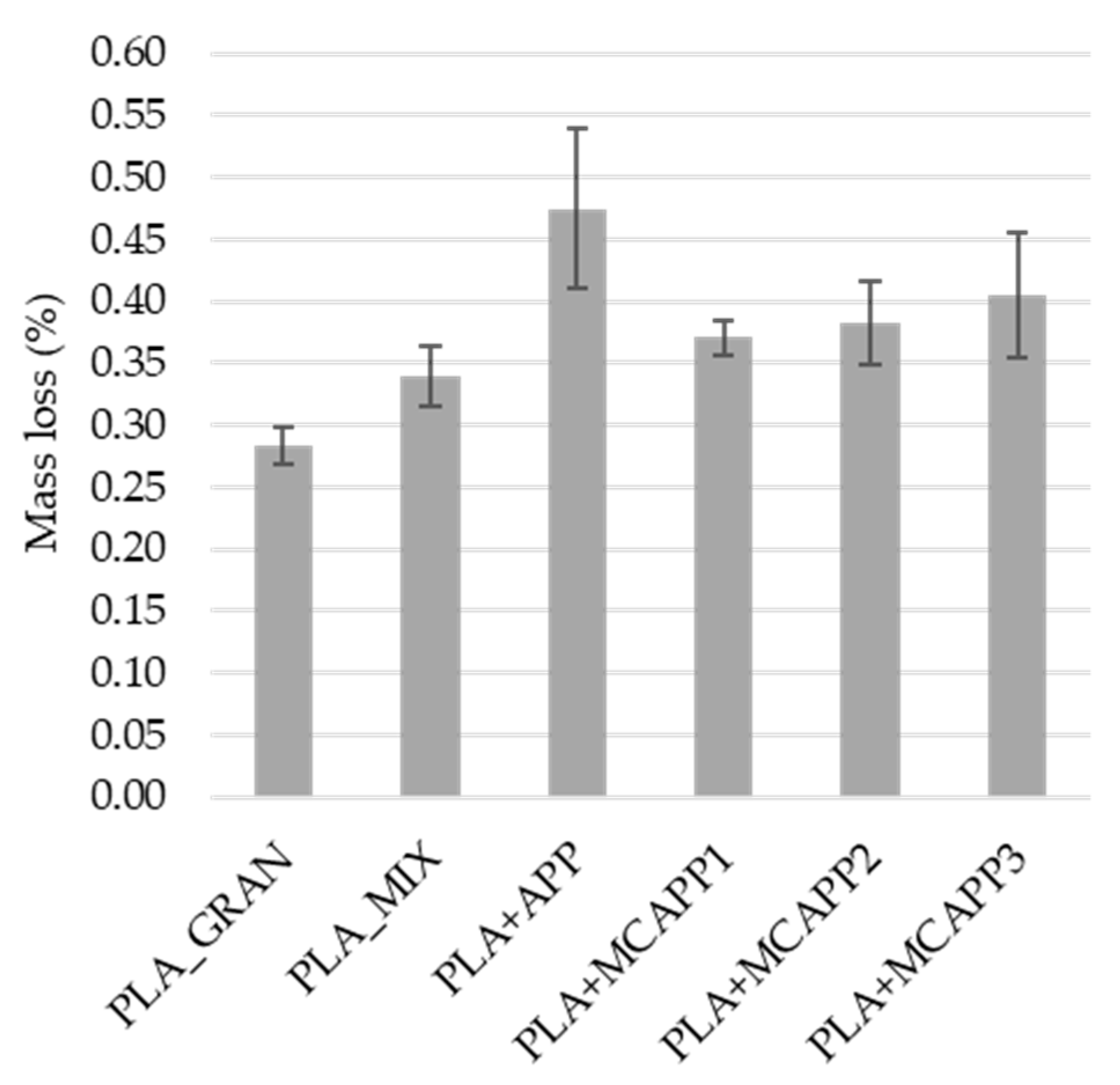
2.2.5. Water-Resistance Tests
2.2.6. Tensile Tests
2.2.7. Flame Retardancy Tests
3. Materials and Methods
3.1. Materials
3.2. Preparation of Microencapsulated Ammonium-Polyphosphate (APP)
3.3. Preparation of Flame Retarded PLA Composites
3.3.1. Kneading
3.3.2. Moulding
3.4. Characterization Methods
3.4.1. Fourier Transform Infrared Spectroscopy
3.4.2. Raman Spectroscopy
3.4.3. Scanning Electron Microscopy
3.4.4. Calculation of Particle Size Distribution
3.4.5. Differential Scanning Calorimetry
3.4.6. Thermogravimetric Analysis
3.4.7. Thermogravimetry-FTIR Spectrometry
3.4.8. Gel Permeation Chromatography
3.4.9. Water-Resistance of Flame Retarded (FR) PLA Composites
3.4.10. Tensile Tests
3.4.11. Limiting Oxygen Index
3.4.12. UL-94
3.4.13. Mass Loss Calorimetry
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shumao, L.; Jie, R.; Hua, Y.; Tao, Y.; Weizhong, Y. Influence of ammonium polyphosphate on the flame retardancy and mechanical propreties of ramie fiber-reinforced poly(lactic acid) biocomposites. Polym. Int. 2010, 59, 242–248. [Google Scholar] [CrossRef]
- Yu, T.; Ren, J.; Li, S.; Yuan, H.; Li, Y. Effect of fiber surface-treatments on the properties of poly(lactic acid)/ramie composites. Compos. Part A Appl. Sci. Manuf. 2010, 41, 499–505. [Google Scholar] [CrossRef]
- Rasal, R.M.; Janorkar, A.V.; Hirt, D.E. Poly(lactic acid) modifications. Prog. Polym. Sci. 2010, 35, 338–356. [Google Scholar] [CrossRef]
- Iwata, T. Biodegradable and Bio-Based Polymers: Future Prospects of Eco-Friendly Plastics. Angew. Chem. Int. Ed. 2015, 54, 3210–3215. [Google Scholar] [CrossRef]
- Jain, R.; Johnson, B.K.; Hess, H.L. Performance of line protection and supervisory elements for doubly fed wind turbines. In Proceedings of the 2015 IEEE Power & Energy Society General Meeting, Denver, CO, USA, 26–30 July 2015; pp. 1–5. [Google Scholar]
- Solarski, S.; Ferreira, M.; Devaux, E. Characterization of the thermal properties of PLA fibers by modulated differential scanning calorimetry. Polymer 2005, 46, 11187–11192. [Google Scholar] [CrossRef]
- Tokiwa, Y.; Calabia, B.P. Biodegradability and biodegradation of poly(lactide). Appl. Microbiol. Biotechnol. 2006, 72, 244–251. [Google Scholar] [CrossRef]
- Brzeziński, M.; Socka, M.; Kost, B. Microfluidics for producing polylactide nanoparticles and microparticles and their drug delivery application. Polym. Int. 2019, 68, 997–1014. [Google Scholar] [CrossRef]
- Senatov, F.S.; Niaza, K.V.; Stepashkin, A.A.; Kaloshkin, S.D. Low-cycle fatigue behavior of 3d-printed PLA-based porous scaffolds. Compos. Part B Eng. 2016, 97, 193–200. [Google Scholar] [CrossRef]
- Qi, X.; Ren, Y.; Wang, X. New advances in the biodegradation of Poly(lactic) acid. Int. Biodeterior. Biodegrad. 2017, 117, 215–223. [Google Scholar] [CrossRef]
- Jiang, Y.; Yan, P.; Wang, Y.; Zhou, C.; Lei, J. Form-stable phase change materials with enhanced thermal stability and fire resistance via the incorporation of phosphorus and silicon. Mater. Des. 2018, 160, 763–771. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Z.; Pan, Y.-T.; Yáñez, A.P.; Hu, S.; Zhang, X.-Q.; Wang, R.; Wang, D.-Y. Polydopamine induced natural fiber surface functionalization: A way towards flame retardancy of flax/poly(lactic acid) biocomposites. Compos. Part B Eng. 2018, 154, 56–63. [Google Scholar] [CrossRef]
- Zhang, J.-F.; Sun, X. Mechanical Properties of Poly(lactic acid)/Starch Composites Compatibilized by Maleic Anhydride. Biomacromolecules 2004, 5, 1446–1451. [Google Scholar] [CrossRef] [PubMed]
- Li, B.-H.; Yang, M.-C. Improvement of thermal and mechanical properties of poly(L-lactic acid) with 4,4-methylene diphenyl diisocyanate. Polym. Adv. Technol. 2006, 17, 439–443. [Google Scholar] [CrossRef]
- Garlotta, D. A Literature Review of Poly(Lactic Acid). J. Polym. Environ. 2001, 9, 63–84. [Google Scholar] [CrossRef]
- Carrasco, F.; Pagès, P.; Gámez-Pérez, J.; Santana, O.O.; Maspoch, M.L. Processing of poly(lactic acid): Characterization of chemical structure, thermal stability and mechanical properties. Polym. Degrad. Stab. 2010, 95, 116–125. [Google Scholar] [CrossRef]
- Wang, J.; Ren, Q.; Zheng, W.; Zhai, W. Improved Flame-Retardant Properties of Poly(lactic acid) Foams Using Starch as a Natural Charring Agent. Ind. Eng. Chem. Res. 2014, 53, 1422–1430. [Google Scholar] [CrossRef]
- Zhang, K.; An, Z.; Cai, D.; Guo, Z.; Xiao, J. Key Role of Desert–Oasis Transitional Area in Avoiding Oasis Land Degradation from Aeolian Desertification in Dunhuang, Northwest China. Land Degrad. Dev. 2017, 28, 142–150. [Google Scholar] [CrossRef]
- Jang, J.Y.; Jeong, T.K.; Oh, H.J.; Youn, J.R.; Song, Y.S. Thermal stability and flammability of coconut fiber reinforced poly(lactic acid) composites. Compos. Part B Eng. 2012, 43, 2434–2438. [Google Scholar] [CrossRef]
- Li, S.; Yuan, H.; Yu, T.; Yuan, W.; Ren, J. Flame-retardancy and anti-dripping effects of intumescent flame retardant incorporating montmorillonite on poly(lactic acid). Polym. Adv. Technol. 2009, 20, 1114–1120. [Google Scholar] [CrossRef]
- Duquesne, S.; Magnet, S.; Jama, C.; Delobel, R. Intumescent paints: Fire protective coatings for metallic substrates. Surf. Coat. Technol. 2004, 180–181, 302–307. [Google Scholar] [CrossRef]
- Gao, M.; Wu, W.; Yan, Y. Thermal degradation and flame retardancy of epoxy resins containing intumescent flame retardant. J. Therm. Anal. Calorim. 2009, 95, 605–608. [Google Scholar] [CrossRef]
- Gao, M.; Yang, S. A novel intumescent flame-retardant epoxy resins system. J. Appl. Polym. Sci. 2010, 115, 2346–2351. [Google Scholar] [CrossRef]
- Nie, S.; Song, L.; Guo, Y.; Wu, K.; Xing, W.; Lu, H.; Hu, Y. Intumescent Flame Retardation of Starch Containing Polypropylene Semibiocomposites: Flame Retardancy and Thermal Degradation. Ind. Eng. Chem. Res. 2009, 48, 10751–10758. [Google Scholar] [CrossRef]
- Li, B.; Xu, M. Effect of a novel charring–foaming agent on flame retardancy and thermal degradation of intumescent flame retardant polypropylene. Polym. Degrad. Stab. 2006, 91, 1380–1386. [Google Scholar] [CrossRef]
- Wang, X.; Hu, Y.; Song, L.; Xuan, S.; Xing, W.; Bai, Z.; Lu, H. Flame retardancy and thermal degradation of intumescent flame retardant poly(lactic acid)/starch biocomposites. Ind. Eng. Chem. Res. 2011, 50, 713–720. [Google Scholar] [CrossRef]
- Bourbigot, S.; Le Bras, M.; Duquesne, S.; Rochery, M. Recent Advances for Intumescent Polymers. Macromol. Mater. Eng. 2004, 289, 499–511. [Google Scholar] [CrossRef]
- Almeras, X.; Le Bras, M.; Hornsby, P.; Bourbigot, S.; Marosi, G.; Keszei, S.; Poutch, F. Effect of fillers on the fire retardancy of intumescent polypropylene compounds. Polym. Degrad. Stab. 2003, 82, 325–331. [Google Scholar] [CrossRef]
- Almeras, X.; Le Bras, M.; Poutch, F.; Bourbigot, S.; Marosi, G.; Anna, P. Effect of fillers on fire retardancy of intumescent polypropylene blends. Macromol. Symp. 2003, 198, 435–448. [Google Scholar] [CrossRef]
- Réti, C.; Casetta, M.; Duquesne, S.; Bourbigot, S.; Delobel, R. Flammability properties of intumescent PLA including starch and lignin. Polym. Adv. Technol. 2008, 19, 628–635. [Google Scholar] [CrossRef]
- Bourbigot, S.; Le Bras, M.; Delobel, R.; Decressain, R.; Amoureux, J.-P. Synergistic effect of zeolite in an intumescence process: Study of the carbonaceous structures using solid-state NMR. J. Chem. Soc. Faraday Trans. 1996, 92, 149–158. [Google Scholar] [CrossRef]
- Le Bras, M.; Bourbigot, S.; Le Tallec, Y.; Laureyns, J. Synergy in intumescence—Application to β-cyclodextrin carbonisation agent in intumescent additives for fire retardant polyethylene formulations. Polym. Degrad. Stab. 1997, 56, 11–21. [Google Scholar] [CrossRef]
- Lim, K.-S.; Bee, S.-T.; Sin, L.T.; Tee, T.-T.; Ratnam, C.T.; Hui, D.; Rahmat, A.R. A review of application of ammonium polyphosphate as intumescent flame retardant in thermoplastic composites. Compos. Part B Eng. 2016, 84, 155–174. [Google Scholar] [CrossRef]
- Wang, J.-S.; Wang, D.-Y.; Liu, Y.; Ge, X.-G.; Wang, Y.-Z. Polyamide-enhanced flame retardancy of ammonium polyphosphate on epoxy resin. J. Appl. Polym. Sci. 2008, 108, 2644–2653. [Google Scholar] [CrossRef]
- Li, R.-M.; Deng, C.; Deng, C.-L.; Dong, L.-P.; Di, H.-W.; Wang, Y.-Z. An efficient method to improve simultaneously the water resistance, flame retardancy and mechanical properties of POE intumescent flame-retardant systems. RSC Adv. 2015, 5, 16328–16339. [Google Scholar] [CrossRef]
- Chen, M.-J.; Lin, Y.-C.; Wang, X.-N.; Zhong, L.; Li, Q.-L.; Liu, Z.-G. Influence of Cuprous Oxide on Enhancing the Flame Retardancy and Smoke Suppression of Epoxy Resins Containing Microencapsulated Ammonium Polyphosphate. Ind. Eng. Chem. Res. 2015, 54, 12705–12713. [Google Scholar] [CrossRef]
- Yang, Y.; Xue, Y.; Yu, Y.; Ma, N.; Shao, Y. Experimental study on flexural performance of partially precast steel reinforced concrete beams. J. Constr. Steel Res. 2017, 133, 192–201. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, Y.; Li, L.; Wang, Z. Microencapsulated ammonium polyphosphate with epoxy resin shell: Preparation, characterization, and application in EP system. Polym. Adv. Technol. 2011, 22, 2403–2408. [Google Scholar] [CrossRef]
- Chen, X.; Jiao, C. Study on flame retardance of co-microencapsulated ammonium polyphosphate and pentaerythritol in polypropylene. J. Fire Sci. 2010, 28, 509–521. [Google Scholar] [CrossRef]
- Yang, L.; Cheng, W.; Zhou, J.; Li, H.; Wang, X.; Chen, X.; Zhang, Z. Effects of microencapsulated APP-II on the microstructure and flame retardancy of PP/APP-II/PER composites. Polym. Degrad. Stab. 2014, 105, 150–159. [Google Scholar] [CrossRef]
- Wang, B.; Qian, X.; Shi, Y.; Yu, B.; Hong, N.; Song, L.; Hu, Y. Cyclodextrin microencapsulated ammonium polyphosphate: Preparation and its performance on the thermal, flame retardancy and mechanical properties of ethylene vinyl acetate copolymer. Compos. Part B Eng. 2015, 69, 22–30. [Google Scholar] [CrossRef]
- Shi, X.; Ju, Y.; Zhang, M.; Wang, X. The intumescent flame-retardant biocomposites of poly(lactic acid) containing surface-coated ammonium polyphosphate and distiller’s dried grains with solubles (DDGS). Fire Mater. 2018, 42, 190–197. [Google Scholar] [CrossRef]
- Ran, G.; Liu, X.; Guo, J.; Sun, J.; Li, H.; Gu, X.; Zhang, S. Improving the flame retardancy and water resistance of polylactic acid by introducing polyborosiloxane microencapsulated ammonium polyphosphate. Compos. Part B Eng. 2019, 173, 106772. [Google Scholar] [CrossRef]
- Bourbigot, S.; Duquesne, S.; Fontaine, G.; Bellayer, S.; Turf, T.; Samyn, F. Characterization and reaction to fire of polymer nanocomposites with and without conventional flame retardants. Mol. Cryst. Liq. Cryst. 2008, 486. [Google Scholar] [CrossRef]
- Bodzay, B.; Bocz, K.; Bárkai, Z.; Marosi, G. Influence of rheological additives on char formation and fire resistance of intumescent coatings. Polym. Degrad. Stab. 2011, 96, 355–362. [Google Scholar] [CrossRef]
- Fischer, E.W.; Sterzel, H.J.; Wegner, G. Investigation of the structure of solution grown crystals of lactide copolymers by means of chemical reactions. Kolloid-Z. Z. Für Polym. 1973, 251, 980–990. [Google Scholar] [CrossRef]
- Fontaine, G.G.; Bourbigot, S. Intumescent Polylactide: A Nonflammable Material. J. Appl. Polym. Sci. 2009, 113, 3860–3865. [Google Scholar] [CrossRef]
- Kowalski, A.; Duda, A.; Penczek, S. Polymerization of L,L-Lactide Initiated by Aluminum Isopropoxide Trimer or Tetramer. Macromolecules 1998, 31, 2114–2122. [Google Scholar] [CrossRef]
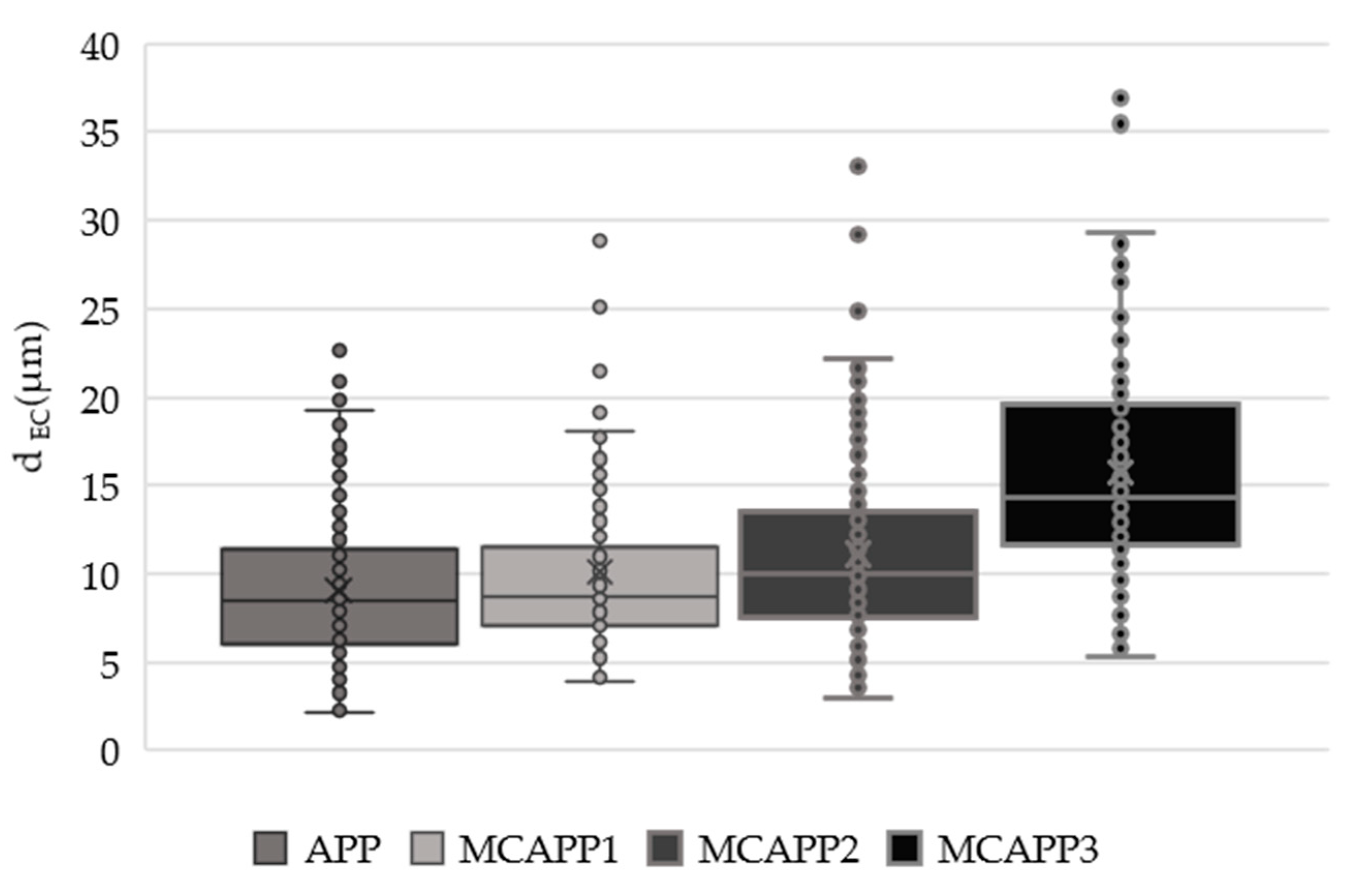
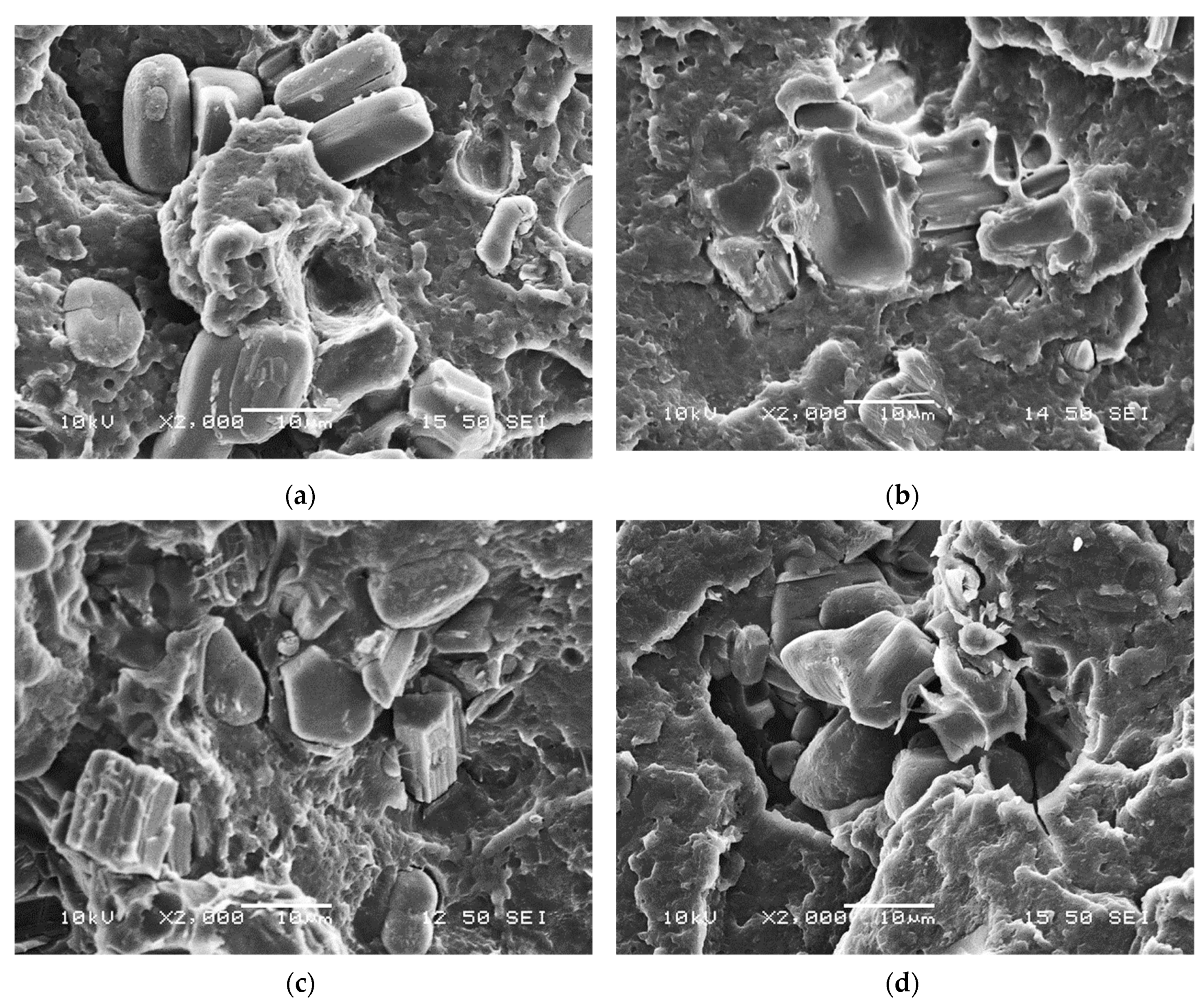

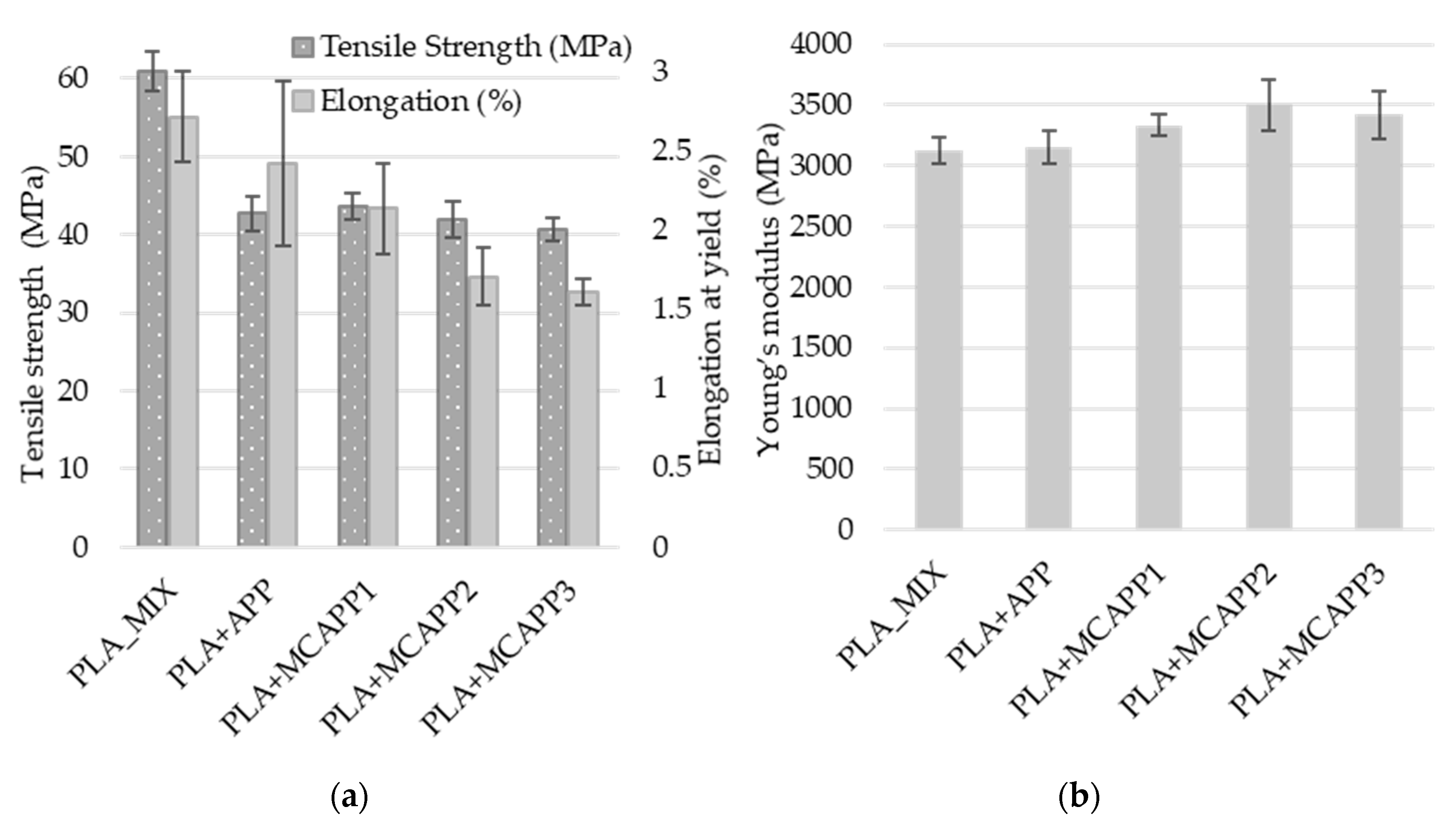
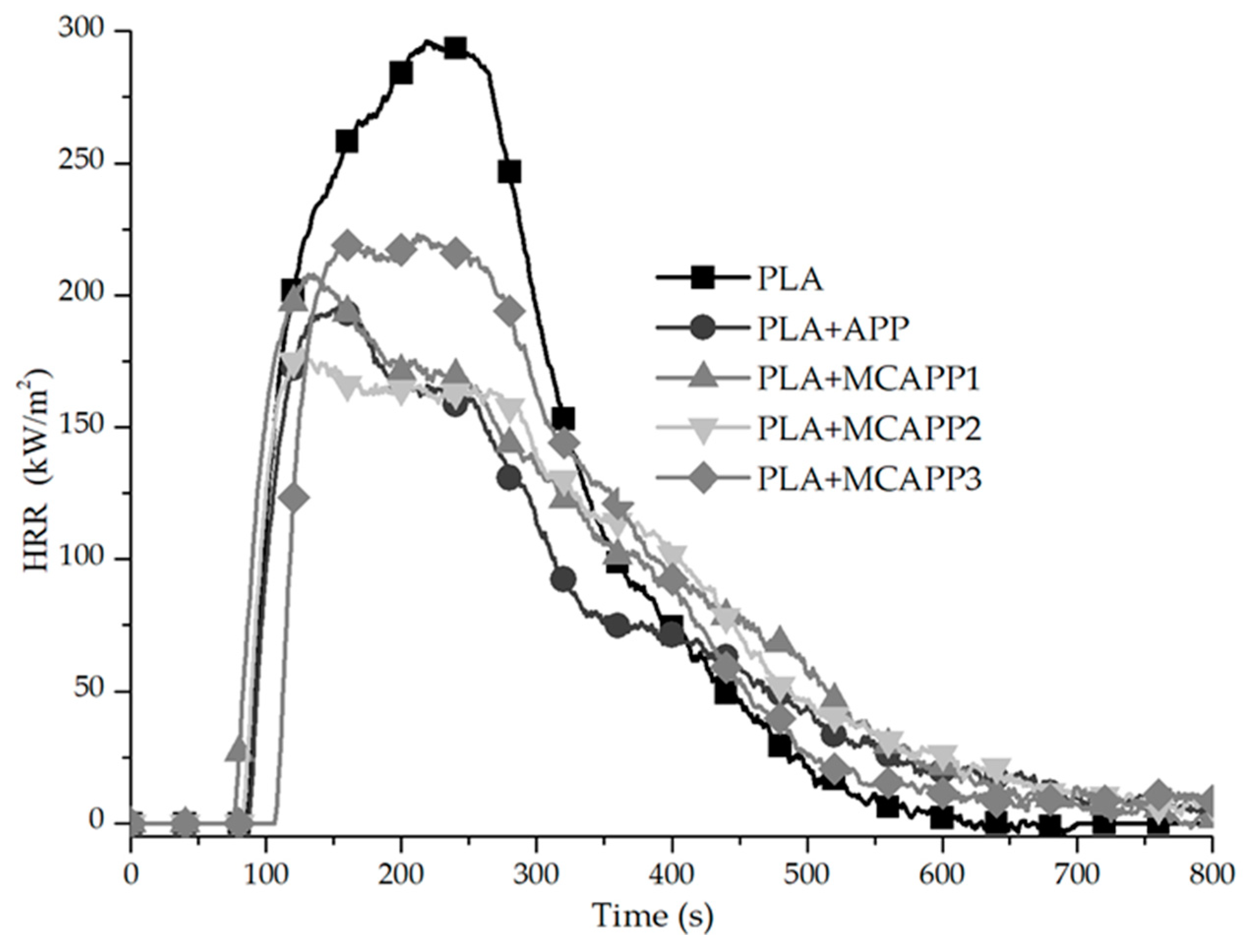
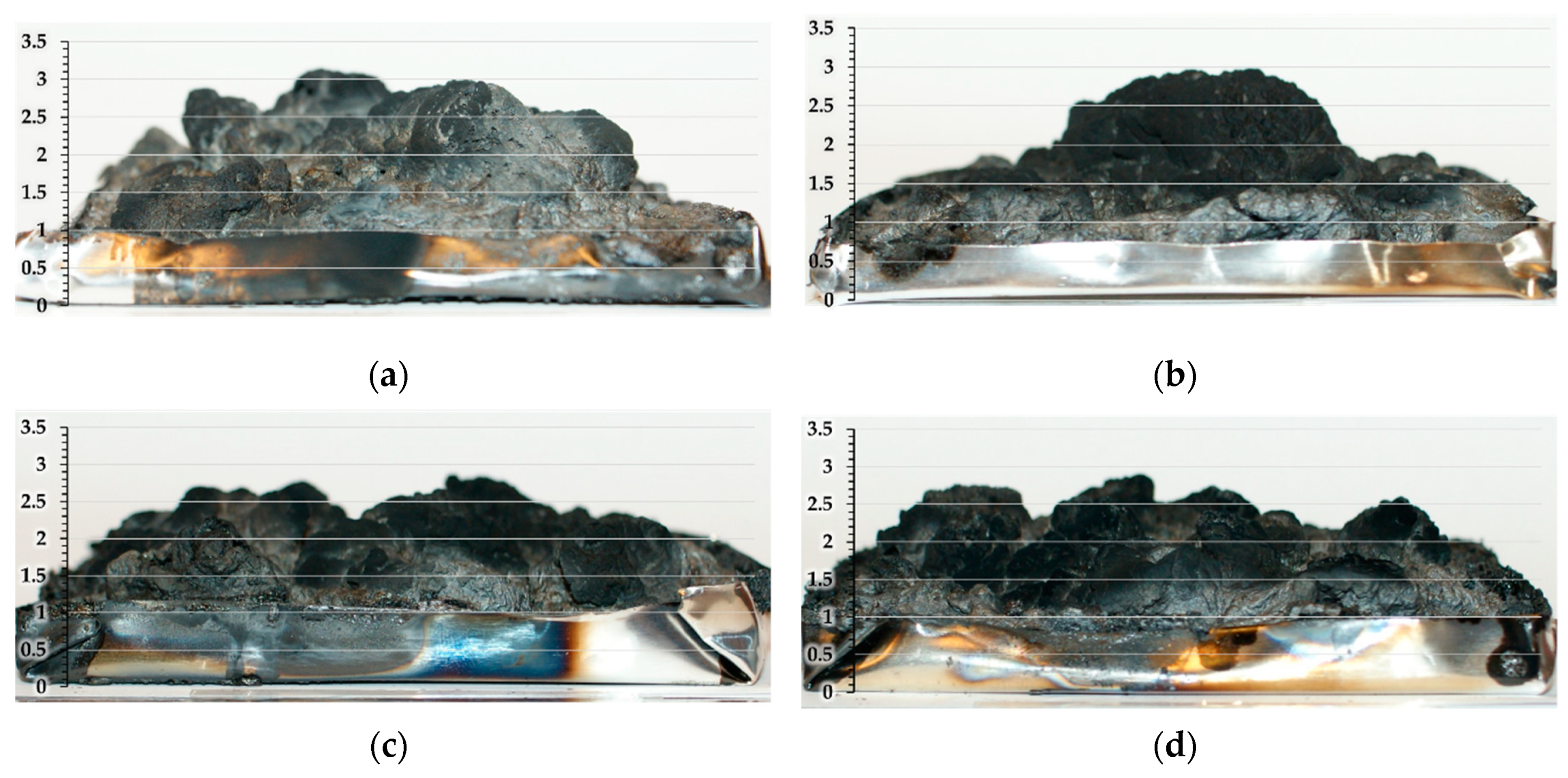
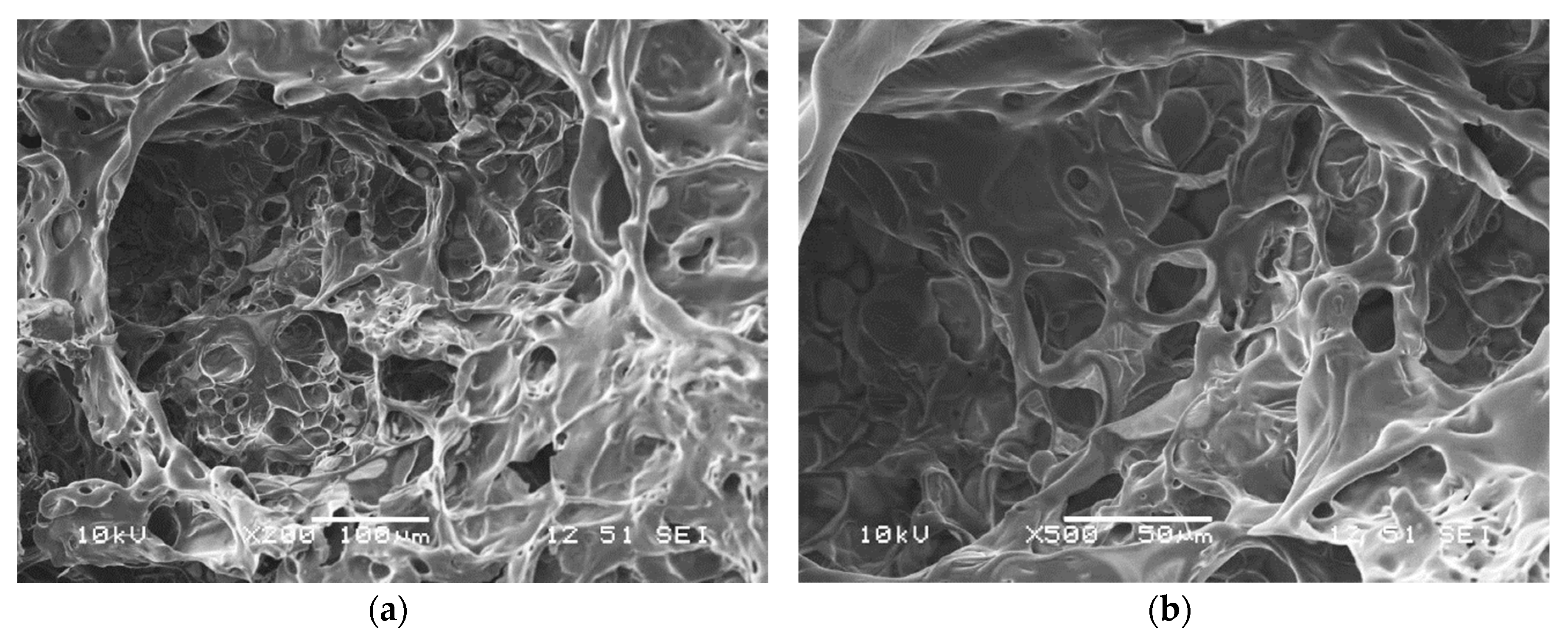
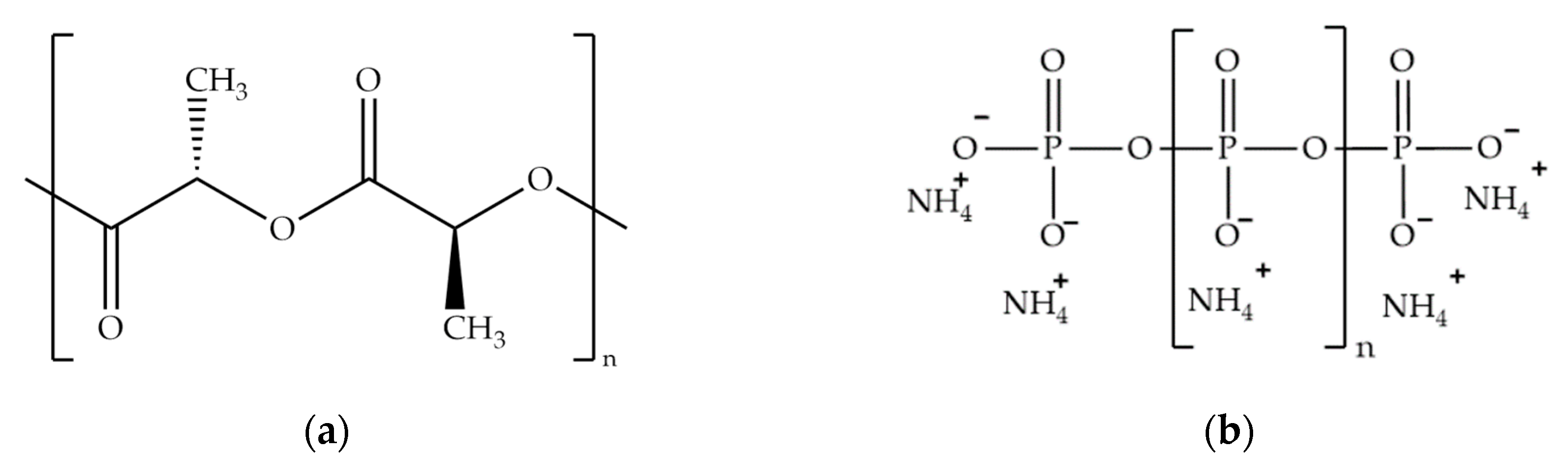
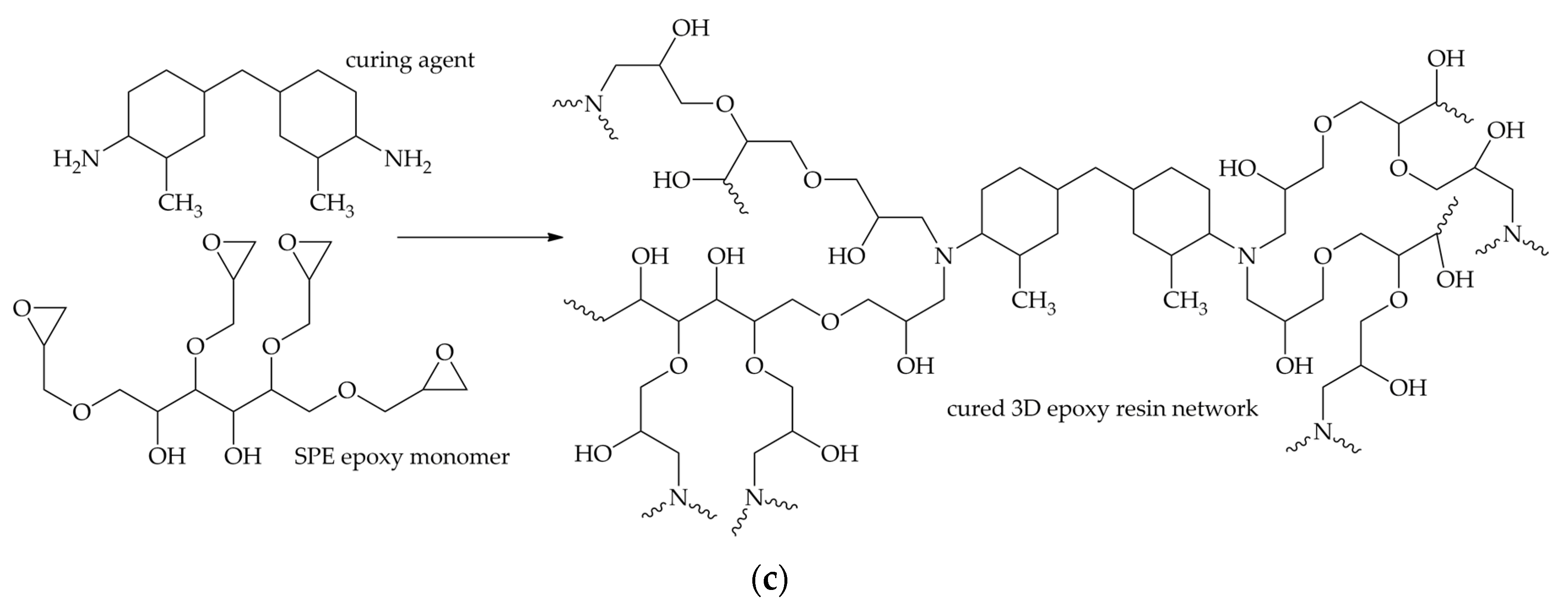
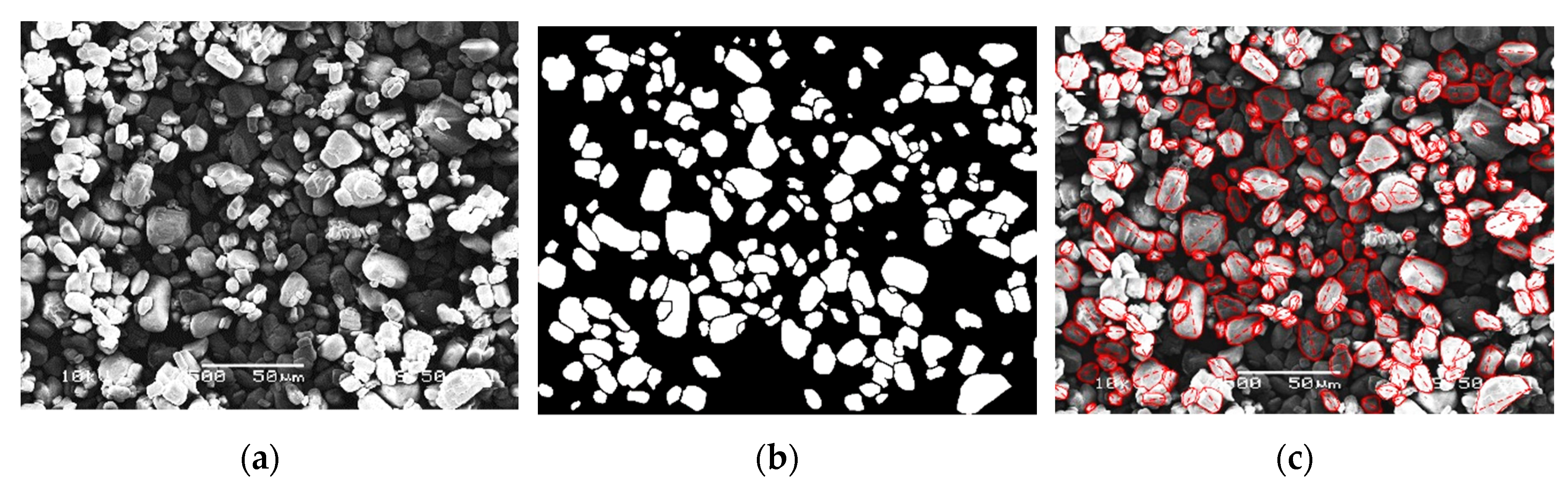
| Computed Parameters | APP | MCAPP1 | MCAPP2 | MCAPP3 |
|---|---|---|---|---|
| Average dEC (µm) | 9.0 ± 3.9 | 10.1 ± 4.7 | 11.1 ± 5.1 | 15.7 ± 6.1 |
| Average dM (µm) | 11.5 ± 5.1 | 12.8 ± 6.2 | 13.5 ± 6.4 | 19.4 ± 7.4 |
| Thermal Properties | Symbol and unit | PLA MIX | PLA + APP | PLA + MCAPP1 | PLA + MCAPP2 | PLA + MCAPP3 |
|---|---|---|---|---|---|---|
| Recrystallisation enthalpy 1 | ΔHm (J/g) | 4.27 | 3.47 | 3.47 | 3.30 | 4.24 |
| Melting enthalpy | ΔHm (J/g) | 44.82 | 38.63 | 39.92 | 40.48 | 43.10 |
| Crystallinity 1 | (%) | 51.3 | 44.5 | 46.1 | 47.0 | 49.2 |
| Cooling crystallization heat 2 | ΔHc (J/g) | 2.91 | 34.02 | 37.69 | 39.14 | 40.13 |
| Cooling crystallization peak 2 | Tc (°C) | 109.7 | 105.6 | 112.4 | 116.6 | 112.4 |
| Melting enthalpy 3 | ΔHm (J/g) | 13.60 | 38.65 | 42.23 | 43.05 | 41.59 |
| Crystallinity 3 | (%) | 17.1 | 48.5 | 53.0 | 54.1 | 52.2 |
| Formulation | Tmax (°C) 1 | T5% (°C) 2 | Weight at 800 °C (%) | Mass loss rate at Tmax (%/°C) |
|---|---|---|---|---|
| PLA_GRAN | 360 | 325 | 0.0 | 3.0 |
| PLA_MIX | 365 | 327 | 0.0 | 3.4 |
| PLA + APP | 363 | 327 | 4.7 | 2.4 |
| PLA + MCAPP1 | 363 | 322 | 5.8 | 2.4 |
| PLA + MCAPP2 | 366 | 315 | 6.3 | 2.2 |
| PLA + MCAPP3 | 368 | 310 | 8.4 | 2.0 |
| Sample | LOI (%) | UL-94 (3 mm) | ||
|---|---|---|---|---|
| t1/t2 (s) 1 | Cotton Ignition | Rating | ||
| PLA_GRAN | 21.5 | -/- | yes | N.R. 2 |
| PLA_MIX | 20.5 | 11/- | yes | N.R. 2 |
| PLA + APP | 28.0 | 4.8/1.4 | yes | V-2 |
| PLA + MCAPP1 | 28.5 | 1.3/1.4 | no | V-0 |
| PLA + MCAPP2 | 29.0 | 2.8/0.9 | no | V-0 |
| PLA + MCAPP3 | 28.0 | 2.2/0.6 | no | V-0 |
| Formulation | tign (s) | pHRR (kW/m2) | pHRR Decrease * (%) | THR (MJ/m2) | THR decrease * (%) | Residue (wt%) |
|---|---|---|---|---|---|---|
| PLA GRAN | 82 ± 2 | 284 ± 7 | 1.4 | 61.9 ± 1.7 | –0.3 | 1.7 ± 0.4 |
| PLA MIX | 87 ± 2 | 288 ± 18 | 0 | 62.1 ± 1.2 | 0 | 1.4 ± 0.2 |
| PLA + APP | 82 ± 4 | 189 ± 13 | 34.2 | 44.4 ± 2.0 | 28.2 | 26.6 ± 3.0 |
| PLA + MCAPP1 | 82 ± 3 | 175 ± 14 | 38.9 | 42.1 ± 3.8 | 32.0 | 28.0 ± 4.3 |
| PLA + MCAPP2 | 80 ± 3 | 154 ± 8 | 46.2 | 38.5 ± 3.5 | 37.9 | 28.8 ± 2.8 |
| PLA + MCAPP3 | 84 ± 4 | 167 ± 17 | 41.7 | 42.4 ± 2.2 | 31.5 | 26.2 ± 4.7 |
| Sample Code | APP (wt%) | SPE (wt%) | Curing Agent (wt%) |
|---|---|---|---|
| MCAPP1 | 88.24 | 8.83 | 2.93 |
| MCAPP2 | 78.90 | 15.80 | 5.30 |
| MCAPP3 | 71.43 | 21.43 | 7.14 |
| Sample code | PLA (wt%) | MCAPP (wt%) | |
|---|---|---|---|
| APP (wt%) | SPE Bioresin (wt%) | ||
| PLA_GRAN | 100 | 0 | 0 |
| PLA_MIX | 100 | 0 | 0 |
| PLA + APP | 85 | 15 | 0 |
| PLA + MCAPP1 | 85 | 13.4 | 1.6 |
| PLA + MCAPP2 | 85 | 11.8 | 3.2 |
| PLA + MCAPP3 | 85 | 10.7 | 4.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Decsov, K.; Bocz, K.; Szolnoki, B.; Bourbigot, S.; Fontaine, G.; Vadas, D.; Marosi, G. Development of Bioepoxy Resin Microencapsulated Ammonium-Polyphosphate for Flame Retardancy of Polylactic Acid. Molecules 2019, 24, 4123. https://doi.org/10.3390/molecules24224123
Decsov K, Bocz K, Szolnoki B, Bourbigot S, Fontaine G, Vadas D, Marosi G. Development of Bioepoxy Resin Microencapsulated Ammonium-Polyphosphate for Flame Retardancy of Polylactic Acid. Molecules. 2019; 24(22):4123. https://doi.org/10.3390/molecules24224123
Chicago/Turabian StyleDecsov, Kata, Katalin Bocz, Beáta Szolnoki, Serge Bourbigot, Gaëlle Fontaine, Dániel Vadas, and György Marosi. 2019. "Development of Bioepoxy Resin Microencapsulated Ammonium-Polyphosphate for Flame Retardancy of Polylactic Acid" Molecules 24, no. 22: 4123. https://doi.org/10.3390/molecules24224123
APA StyleDecsov, K., Bocz, K., Szolnoki, B., Bourbigot, S., Fontaine, G., Vadas, D., & Marosi, G. (2019). Development of Bioepoxy Resin Microencapsulated Ammonium-Polyphosphate for Flame Retardancy of Polylactic Acid. Molecules, 24(22), 4123. https://doi.org/10.3390/molecules24224123







