Microbial Conversion of Toxic Resin Acids
Abstract
1. Introduction
2. Distribution and Toxicity of RAs
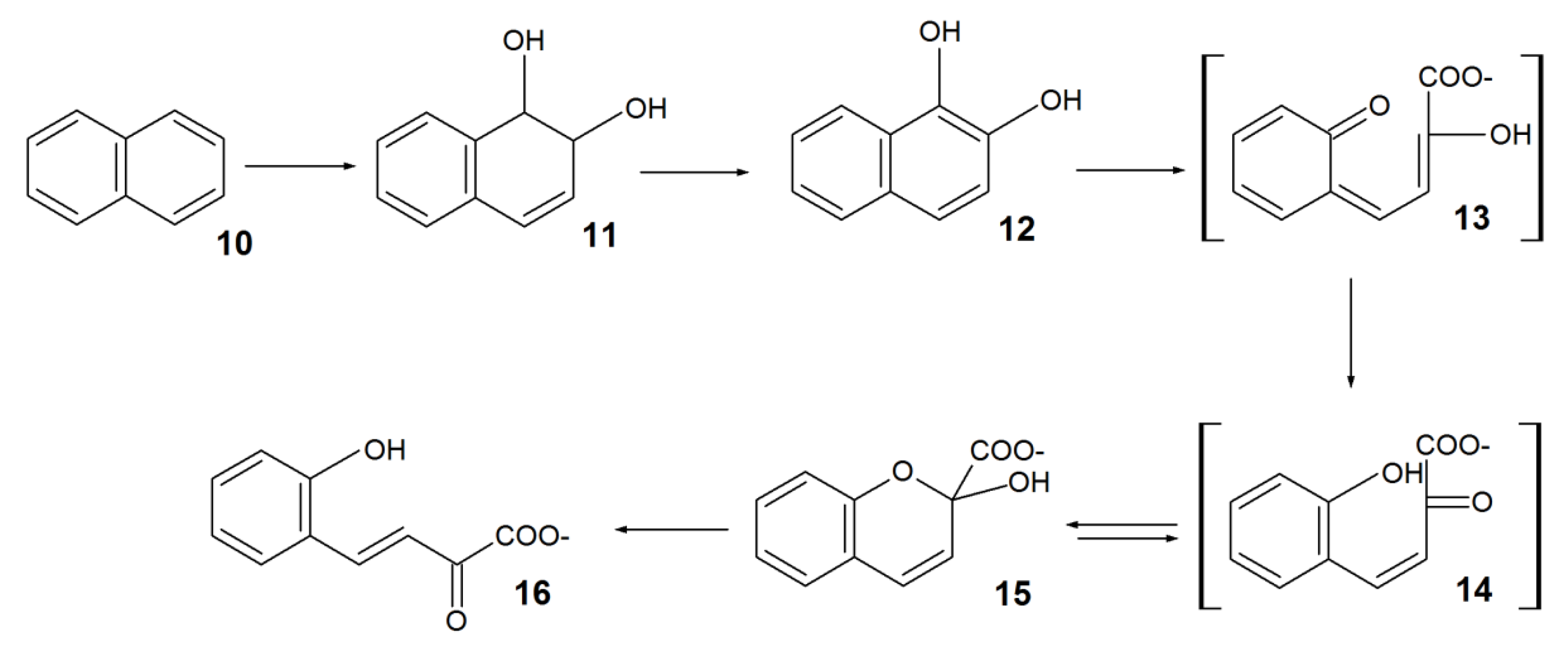
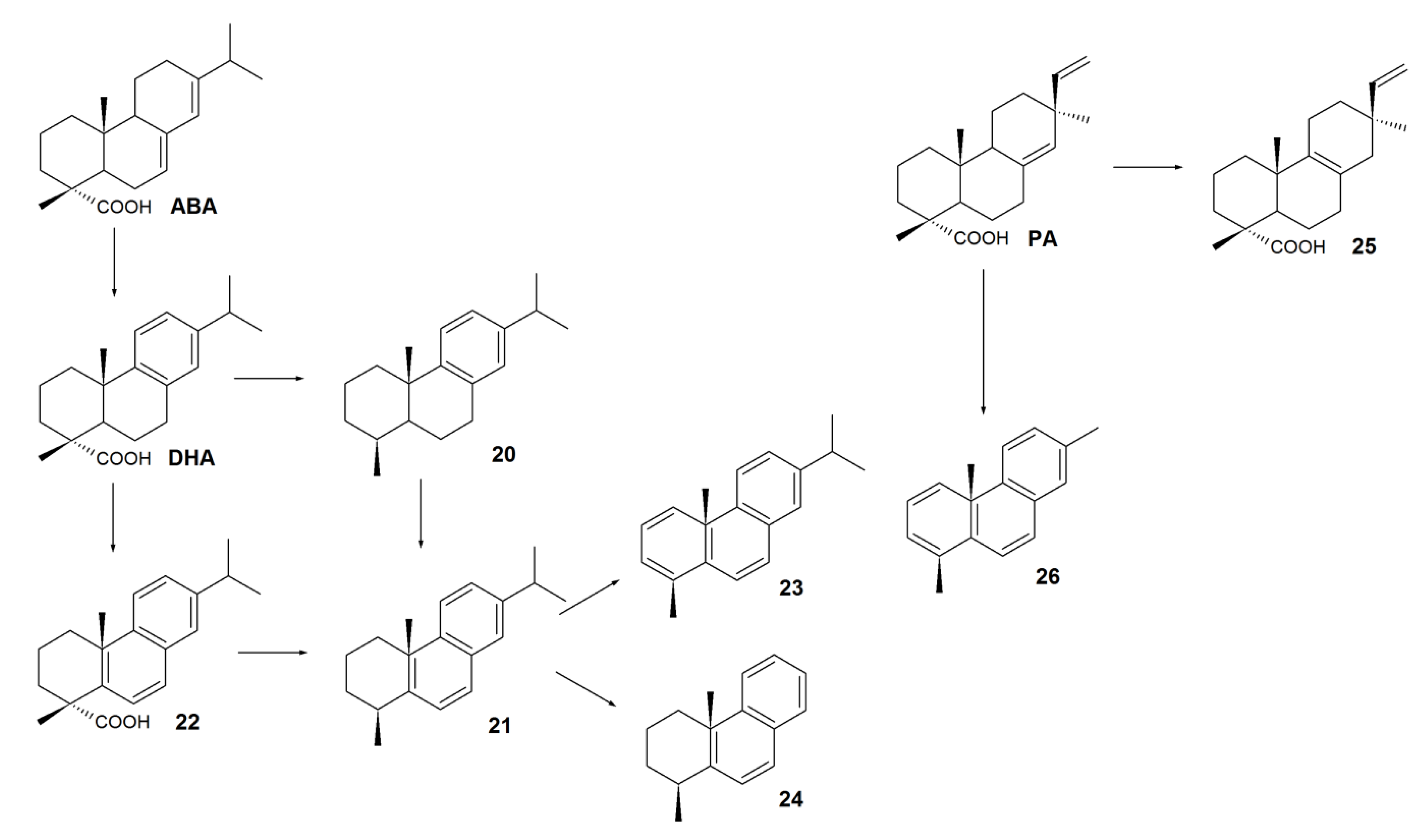
3. Biodegradation of RAs
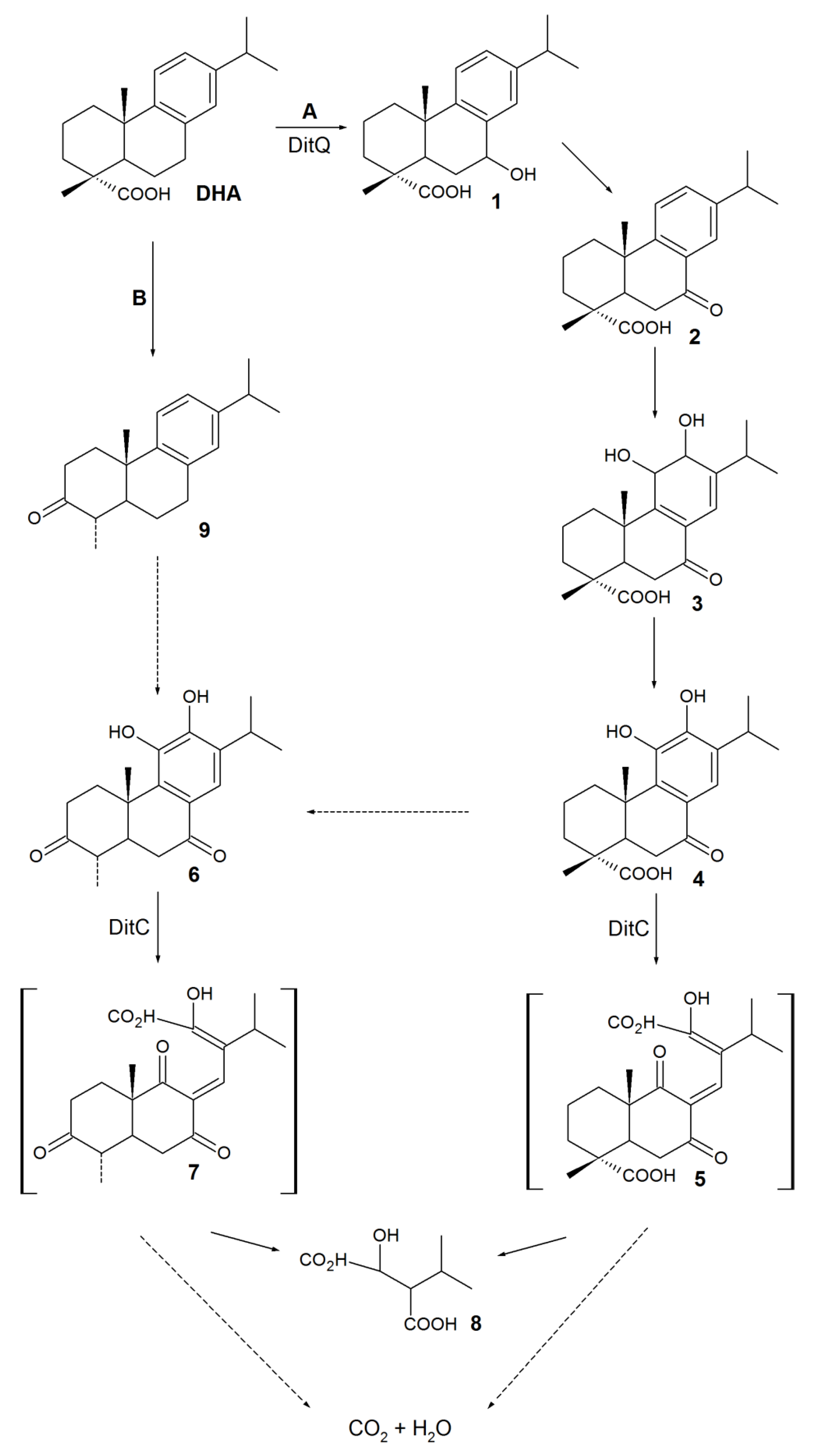
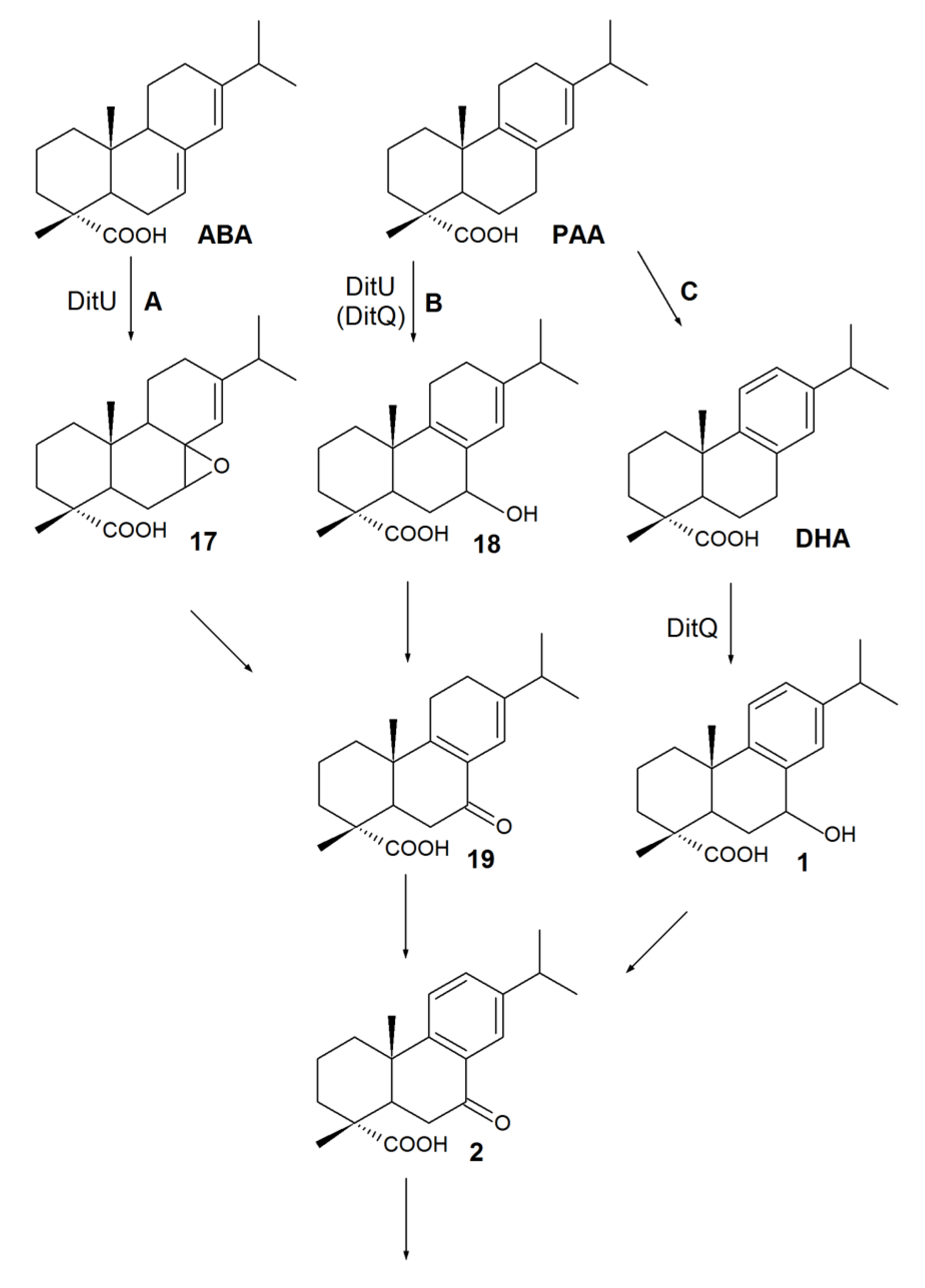
4. Biotransformation of RAs to Bioactive Compounds
5. Conclusions
Funding
Conflicts of Interest
References
- Food and Agriculture Organization of The United Nations, (FAO). Forestry Production and Trade (the Database). Available online: http://www.fao.org/faostat/en/#data/FO/visualize (accessed on 9 July 2019).
- Singh, P.; Srivastava, N.; Jagadish, R.; Upadhyay, A. Effect of toxic pollutants from pulp and paper mill on water and soil quality and its remediation. Int. J. Lakes Rivers 2019, 12, 1–20. [Google Scholar]
- Ottavioli, J.; Paoli, M.; Casanova, J.; Tomi, F.; Bighelli, A. Identification and quantitative determination of resin acids from corsican Pinus pinaster Aiton oleoresin using 13C-NMR spectroscopy. Chem. Biodivers. 2019, 16, e1800482. [Google Scholar] [CrossRef]
- Hernández, V.; Silva, M.; Gavilán, J.; Jiménez, B.; Barra, R.; Becerra, J. Resin acids in bile samples from fish inhabiting marine waters affected by pulp mill effluents. J. Chil. Chem. Soc. 2008, 53, 1718–1721. [Google Scholar] [CrossRef]
- Zheng, J.; Nicholson, R. Action of resin acids in nerve ending fractions isolated from fish central nervous system. Environ. Toxicol. Chem. 1998, 17, 1852–1859. [Google Scholar] [CrossRef]
- Rissanen, E.; Krumschnabel, G.; Nikinmaa, M. Dehydroabietic acid, a major component of wood industry effluents, interferes with cellular energetics in rainbow trout hepatocytes. Aquat. Toxicol. 2003, 62, 45–53. [Google Scholar] [CrossRef]
- Söderberg, T.A.; Johansson, A.; Gref, R. Toxic effects of some conifer resin acids and tea tree oil on human epithelial and fibroblast cells. Toxicology 1996, 107, 99–109. [Google Scholar] [CrossRef]
- Sunzel, B.; Söderberg, T.A.; Johansson, A.; Hallmans, G.; Gref, R. The protective effect of zinc on rosin and resin acid toxicity in human polymorphonuclear leukocytes and human gingival fibroblasts in vitro. J. Biomed. Mater. Res. 1997, 37, 20–28. [Google Scholar] [CrossRef]
- Ohmori, K.; Kawamura, Y. Cell transformation activities of abietic acid and dehydroabietic acid: Safety assessment of possible contaminants in paper and paperboard for food contact use. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2009, 26, 568–573. [Google Scholar] [CrossRef]
- Nestmann, E.R.; Lee, E.G.; Mueller, J.C.; Douglas, G.R. Mutagenicity of resin acids identified in pulp and paper mill effluents using the salmonella/mammalian-microsome assay. Environ. Mutagen. 1979, 1, 361–369. [Google Scholar] [CrossRef]
- Kinae, N.; Hashizume, T.; Makita, T.; Tomita, I.; Kimura, I.; Kanamori, H. Studies on the toxicity of pulp and paper mill effluents—I. Mutagenicity of the sediment samples derived from kraft paper mills. Water Res. 1981, 15, 17–24. [Google Scholar] [CrossRef]
- Burčová, Z.; Kreps, F.; Greifová, M.; Jablonský, M.; Ház, A.; Schmidt, Š.; Šurina, I. Antibacterial and antifungal activity of phytosterols and methyl dehydroabietate of Norway spruce bark extracts. J. Biotechnol. 2018, 282, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Miyajima, Y.; Saito, Y.; Takeya, M.; Goto, M.; Nakagawa-Goto, K. Synthesis of 4-epi-parviflorons A, C, and E: Structure-activity relationship study of antiproliferative abietane derivatives. J. Org. Chem. 2019, 84, 3239–3248. [Google Scholar] [CrossRef] [PubMed]
- Liss, S.N.; Bicho, P.A.; Saddler, J.N. Microbiology and biodegradation of resin acids in pulp mill effluents: A minireview. Can. J. Microbiol. 1997, 75, 599–611. [Google Scholar] [CrossRef]
- Martin, V.J.J.; Yu, Z.; Mohn, W.W. Recent advances in understanding resin acid biodegradation: Microbial diversity and metabolism. Arch. Microbiol. 1999, 172, 131–138. [Google Scholar] [CrossRef]
- Keeling, C.I.; Bohlmann, J. Diterpene resin acids in conifers. Phytochemistry 2006, 67, 2415–2423. [Google Scholar] [CrossRef]
- Rogachev, A.D.; Salakhutdinov, N.F. Chemical composition of Pinus sibirica (Pinaceae). Chem. Biodivers. 2015, 12, 1–53. [Google Scholar] [CrossRef]
- Gonçalves, M.D.; Bortoleti, B.T.S.; Tomiotto-Pellissier, F.; Miranda-Sapla, M.M.; Assolini, J.P.; Carloto, A.C.M.; Carvalho, P.G.C.; Tudisco, E.T.; Urbano, A.; Ambrósio, S.R.; et al. Dehydroabietic acid isolated from Pinus elliottii exerts in vitro antileishmanial action by pro-oxidant effect, inducing ROS production in promastigote and downregulating Nrf2/ferritin expression in amastigote forms of Leishmania amazonensis. Fitoterapia 2018, 128, 224–232. [Google Scholar] [CrossRef]
- Simoneit, B.R.T.; Cox, R.E.; Oros, D.R.; Otto, A. Terpenoid compositions of resins from Callitris species (Cupressaceae). Molecules 2018, 23, 3384. [Google Scholar] [CrossRef]
- Schicchi, R.; Geraci, A.; Rosselli, S.; Spinella, A.; Maggio, A.; Bruno, M. Phytochemical investigation of the needles of Abies nebrodensis (Lojac.) Mattei. Nat. Prod. Res. 2019, 1–6. [Google Scholar] [CrossRef]
- Wajs-Bonikowska, A.; Smeds, A.; Willför, S. Chemical composition and content of lipophilic seed extractives of some Abies and Picea species. Chem. Biodivers. 2016, 13, 1194–1201. [Google Scholar] [CrossRef]
- Choudhary, M.I.; Atif, M.; Shan, S.A.A.; Sultan, S.; Erum, S.; Khan, S.N. Atta-Ur-Rahman Biotransformation of dehydroabietic acid with microbial cell cultures and α-glucosidase inhibitory activity of resulting metabolites. Int. J. Pharm. Pharm. Sci. 2014, 6, 375–378. [Google Scholar]
- González, M.A.; Perez-Guaita, D.; Correa-Royero, J.; Zapata, B.; Agudelo, L.; Mesa-Arango, A.; Betancur-Galvis, L. Synthesis and biological evaluation of dehydroabietic acid derivatives. Eur. J. Med. Chem. 2010, 45, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.S.; Hirai, S.; Goto, T.; Kuroyanagi, K.; Lee, J.Y.; Uemura, T.; Ezaki, Y.; Takahashi, N.; Kawada, T. Dehydroabietic acid, a phytochemical, acts as ligand for PPARs in macrophages and adipocytes to regulate inflammation. Biochem. Biophys. Res. Commun. 2008, 369, 333–338. [Google Scholar] [CrossRef]
- Janocha, S. Umsatz von Harzsäuren Durch die Bakteriellen Cytochrome CYP105A1 und CYP106A2—Strukturelle Grundlagen und Potentielle Anwendungen. Ph.D. Thesis, Univarsität des Saarlandes, Saarbrücken, Germany, 2013. [Google Scholar]
- Plemenkov, V.V.; Appolonova, S.A.; Kirlitsa, D.A. To the question of the native content of resin acids in the galipot of conifers. Chem. Comput. Simulation. Butl. Commun. 2004, 5, 30–32. [Google Scholar]
- Peng, G.; Roberts, J.C. Solubility and toxicity of resin acids. Wat. Res. 2000, 34, 2779–2785. [Google Scholar] [CrossRef]
- Zanella, E. Effect of pH on acute toxicity of dehydroabietic acid and chlorinated dehydroabietic acid to fish and Daphnia. Bull. Environ. Contam. Toxicol. 1983, 30, 133–140. [Google Scholar] [CrossRef]
- Volkman, J.K.; Holdsworth, D.G.; Richardson, D.E. Determination of resin acids by gas chromatography and high-perfomance liquid chromatography in paper mill effluent, river waters and sediments from the upper Derwent Estuary, Tasmania. J. Chromatogr. 1993, 643, 209–219. [Google Scholar] [CrossRef]
- Leppanen, H.; Marttinen, S.; Oikari, A. The use of fish bile metabolite analyses as exposure biomarkers to pulp and paper mill effluents. Chemosphere 1998, 36, 2621–2634. [Google Scholar] [CrossRef]
- Oikari, A.; Holmbom, B.; Bister, H. Uptake of resin acids into tissues of trout (Salmo gairdneri Richardson). Ann. Zool. Fenn. 1982, 19, 61–64. [Google Scholar]
- Lindesjöö, E.; Adolfsson-Erici, M.; Ericson, G.; Förlin, L. Biomarker responses and resin acids in fish chronically exposed to effluents from a total chlorine-free pulp mill during regular production. Ecotoxicol. Environ. Saf. 2002, 53, 238–247. [Google Scholar] [CrossRef]
- Teschke, K.; Demers, P.A.; Davies, H.W.; Kennedy, S.M.; Marion, S.A.; Leung, V. Determinants of exposure to inhalable particulate, wood dust, resin acids, and monoterpenes in a lumber mill environment. Ann. Occup. Hyg. 1999, 43, 247–255. [Google Scholar] [CrossRef]
- Simoneit, B.R.T.; Rogge, W.F.; Lang, Q.; Ja, R. Molecular characterization of smoke from campfire burning of pine wood (Pinus elliottii). Chemosphere 2000, 2, 107–122. [Google Scholar] [CrossRef]
- Ozaki, A.; Ooshima, T.; Mori, Y. Migration of dehydroabietic and abietic acids from paper and paperboard food packaging into food-simulating solvents and Tenax TA. Food Addit. Contam. 2006, 23, 854–860. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhang, S.; Geng, Z.; Wang, D.; Liu, F.; Zhang, M. Analysis of abietic acid and dehydroabietic acid residues in raw ducks and cooked ducks. Poult. Sci. 2014, 93, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhang, S.; Geng, Z.; Wang, D.; Liu, F.; Zhang, M.; Bian, H.; Xu, W. Simultaneous determination of abietic acid and dehydroabietic acid residues in duck meat by HPLC-PAD-FLD. Food Anal. Methods 2014, 7, 1627–1633. [Google Scholar] [CrossRef]
- Tarabanko, V.E.; Ulyanova, O.A.; Kalacheva, G.S. Investigation of dynamics of the content of terpene compounds in pine bark-based composts and their growth-promoting activity. Russ. J. Bioorganic Chem. 2010, 1, 121–126. [Google Scholar]
- Ayars, G.H.; Altman, L.C.; Frazier, E.; Chi, E.Y. The toxicity of constituents of cedar and pine woods pulmonary epitheii. J. Allergy Clin. Immunol. 1989, 83, 610–618. [Google Scholar] [CrossRef]
- Suh, S.J.; Kwak, C.H.; Chung, T.W.; Park, S.J.; Cheeeei, M.; Park, S.S.; Seo, C.S.; Son, J.K.; Chang, Y.C.; Park, Y.G.; et al. Pimaric acid from Aralia cordata has an inhibitory effect on TNF-α-induced MMP-9 production and HASMC migration via down-regulated NF-κB and AP-1. Chem. Biol. Interact. 2012, 199, 112–119. [Google Scholar] [CrossRef]
- Kang, M.-S.; Hirai, S.; Goto, T.; Kuroyanagi, K.; Kim, Y.-I.; Ohyama, K.; Uemura, T.; Lee, J.-Y.; Sakamoto, T.; Ezaki, Y.; et al. Dehydroabietic acid, a diterpene, improves diabetes and hyperlipidemia in obese diabetic KK-Ay mice. BioFactors 2009, 35, 442–448. [Google Scholar] [CrossRef]
- Hjortness, M.K.; Riccardi, L.; Hongdusit, A.; Ruppe, A.; Zhao, M.; Kim, E.Y.; Zwart, P.H.; Sankaran, B.; Arthanari, H.; Sousa, M.C.; et al. Abietane-type diterpenoids inhibit protein tyrosine phosphatases by stabilizing an inactive enzyme conformation. Biochemistry 2018, 57, 5886–5896. [Google Scholar] [CrossRef]
- Huang, X.C.; Wang, M.; Pan, Y.M.; Yao, G.Y.; Wang, H.S.; Tian, X.Y.; Qin, J.K.; Zhang, Y. Synthesis and antitumor activities of novel thiourea α-aminophosphonates from dehydroabietic acid. Eur. J. Med. Chem. 2013, 69, 508–520. [Google Scholar] [CrossRef]
- Thummuri, D.; Guntuku, L.; Challa, V.S.; Ramavat, R.N.; Naidu, V.G.M. Abietic acid attenuates RANKL induced osteoclastogenesis and inflammation associated osteolysis by inhibiting the NF-KB and MAPK signaling. J. Cell. Physiol. 2018, 234, 443–453. [Google Scholar] [CrossRef]
- Cao, Q.; Wang, S.X.; Cheng, Y.X. Abietane diterpenoids with potent cytotoxic activities from the resins of Populus euphratica. Nat. Prod. Commun. 2019, 14, 1–5. [Google Scholar] [CrossRef]
- Fallarero, A.; Skogman, M.; Kujala, J.; Rajaratnam, M.; Moreira, V.M.; Yli-Kauhaluoma, J.; Vuorela, P. (+)-Dehydroabietic acid, an abietane-type diterpene, inhibits Staphylococcus aureus biofilms in vitro. Int. J. Mol. Sci. 2013, 14, 12054–12072. [Google Scholar] [CrossRef]
- Kostamo, A.; Kukkonen, J.V.K. Removal of resin acids and sterols from pulp mill effluents by activated sludge treatment. Water Res. 2003, 37, 2813–2820. [Google Scholar] [CrossRef]
- Stuthridge, T.R.; Campin, D.N.; Langdon, A.G.; Mackie, K.L.; McFarlane, P.N.; Wilkins, A.L. Treatability of bleached kraft pulp and paper mill wastewaters in a New Zealand aerated lagoon treatment system. Water Sci. Technol. 1991, 24, 309–317. [Google Scholar] [CrossRef]
- Belmonte, M.; Xavier, C.; Decap, J.; Martinez, M.; Sierra-Alvarez, R.; Vidal, G. Improved aerobic biodegradation of abietic acid in ECF bleached kraft mill effluent due to biomass adaptation. J. Hazard. Mater. 2006, 135, 256–263. [Google Scholar] [CrossRef]
- Werker, A.G.; Hall, E.R. Limitations for biological removal of resin acids from pulp mill effluent. Water Sci. Technol. 1999, 40, 281–288. [Google Scholar] [CrossRef]
- Cheremnykh, K.M.; Luchnikova, N.A.; Grishko, V.V.; Ivshina, I.B. Bioconversion of ecotoxic dehydroabietic acid using Rhodococcus actinobacteria. J. Hazard. Mater. 2018, 346, 103–112. [Google Scholar] [CrossRef]
- Bicho, P.A.; Martin, V.; Saddler, J.N. Growth, induction, and substrate-specificity of dehydroabietic acid-degrading bacteria isolated from a kraft mill effluent enrichment. Appl. Environ. Microbiol. 1995, 61, 3245–3250. [Google Scholar]
- Morgan, C.A.; Wyndham, R.C. Isolation and characterization of resin acid degrading bacteria found in effluent from a bleached kraft pulp mill. Can. J. Microbiol. 1996, 42, 423–430. [Google Scholar] [CrossRef]
- Mohn, W.W.; Wilson, A.E.; Bicho, P.; Moore, E.R. Physiological and phylogenetic diversity of bacteria growing on resin acids. Syst. Appl. Microbiol. 1999, 22, 68–78. [Google Scholar] [CrossRef]
- Yu, Z.; Stewart, G.R.; Mohn, W.W. Apparent contradiction: Psychrotolerant bacteria from hydrocarbon-contaminated Arctic tundra soils that degrade diterpenoids synthesized by trees. Appl. Environ. Microbiol. 2000, 66, 5148–5154. [Google Scholar] [CrossRef]
- Cheremnykh, K.M.; Grishko, V.V.; Ivshina, I.B. Bacterial degradation of ecotoxic dehydroabietic acid. Catal. Ind. 2017, 9, 331–338. [Google Scholar] [CrossRef]
- Yu, Z.; Mohn, W.W. Isolation and characterization of thermophilic bacteria capable of degrading dehydroabietic acid. Can. J. Microbiol. 1999, 45, 513–519. [Google Scholar] [CrossRef]
- Lindberg, L.E.; Holmbom, B.R.; Väisänen, O.M.; Weber, A.M.-L.; Salkinoja-Salonen, M.S. Degradation of paper mill water components in laboratory tests with pure cultures of bacteria. Biodegradation 2001, 12, 141–148. [Google Scholar] [CrossRef]
- Mohn, W.W.; Yu, Z.; Moore, E.R.B.; Muttray, A.F. Lessons learned from Sphingomonas species that degrade abietane triterpenoids. J. Ind. Microbiol. Biotechnol. 1999, 23, 374–379. [Google Scholar] [CrossRef]
- Smith, D.J.; Martin, V.J.J.; Mohn, W.W. A cytochrome P450 involved in the metabolism of abietane diterpenoids by Pseudomonas abietaniphila BKME-9. J. Bacteriol. 2004, 186, 3631–3639. [Google Scholar] [CrossRef]
- Smith, D.J.; Park, J.; Tiedje, J.M.; Mohn, W.W. A large gene cluster in Burkholderia xenovorans encoding abietane diterpenoid catabolism. J. Bacteriol. 2007, 189, 6195–6204. [Google Scholar] [CrossRef]
- Smith, D.J.; Patrauchan, M.A.; Florizone, C.; Eltis, L.D.; Mohn, W.W. Distinct roles for two CYP226 family cytochromes P450 in abietane diterpenoid catabolism by Burkholderia xenovorans LB400. J. Bacteriol. 2008, 190, 1575–1583. [Google Scholar] [CrossRef]
- Muttray, A.F.; Yu, Z.; Mohn, W.W. Population dynamics and metabolic activity of Pseudomonas abietaniphila BKME-9 within pulp mill wastewater microbial communities assayed by competitive PCR and RT-PCR. FEMS Microbiol. Ecol. 2001, 38, 21–31. [Google Scholar] [CrossRef][Green Version]
- Burnes, T.A.; Blanchette, R.A.; Farrell, R.L. Bacterial biodegradation of extractives and patterns of bordered pit membrane attack in pine wood. Appl. Environ. Microbiol. 2000, 66, 5201–5205. [Google Scholar] [CrossRef]
- Kallioinen, A.; Vaari, A.; Rättö, M.; Konn, J.; Siika-Aho, M.; Viikari, L. Effects of bacterial treatments on wood extractives. J. Biotechnol. 2003, 103, 67–76. [Google Scholar] [CrossRef]
- Cámara, B.; Strömpl, C.; Verbarg, S.; Spröer, C.; Pieper, D.H.; Tindall, B.J. Pseudomonas reinekei sp. nov., Pseudomonas moorei sp. nov. and Pseudomonas mohnii sp. nov., novel species capable of degrading chlorosalicylates or isopimaric acid. Int. J. Syst. Evol. Microbiol. 2007, 57, 923–931. [Google Scholar] [CrossRef]
- Wilson, A.E.; Moore, E.R.; Mohn, W.W. Isolation and characterization of isopimaric acid-degrading bacteria from a sequencing batch reactor. Appl. Environ. Microbiol. 1996, 62, 3146–3151. [Google Scholar]
- Mohn, W.W. Bacteria obtained from a sequencing batch reactor that are capable of growth on dehydroabietic acid. Appl. Environ. Microbiol. 1995, 61, 2145–2150. [Google Scholar]
- Côté, R.; Otis, C. Étude de la biodégradation de l’acide dehydroabietique par Bacillus psychrophilus. Rev. Des Sci. L’eau 1989, 2, 313–324. [Google Scholar] [CrossRef][Green Version]
- Martin, V.J.J.; Mohn, W.W. A novel aromatic-ring-hydroxylating dioxygenase from the diterpenoid-degrading bacterium Pseudomonas abietaniphila BKME-9. J. Bacteriol. 1999, 181, 2675–2682. [Google Scholar]
- Martin, V.J.J.; Mohn, W.W. Genetic investigation of the catabolic pathway for degradation of abietane diterpenoids by Pseudomonas abietaniphila BKME-9. J. Bacteriol. 2000, 182, 3784–3793. [Google Scholar] [CrossRef][Green Version]
- Morgan, C.A.; Wyndham, R.C. Characterization of tdt genes for the degradation of tricyclic diterpenes by Pseudomonas diterpeniphila A19-6a. Can. J. Microbiol. 2002, 48, 49–59. [Google Scholar] [CrossRef]
- Eaton, R.W.; Chapman, P.J. Bacterial metabolism of naphthalene: Construction and use of recombinant bacteria to stody ring cleavage os 1,2-dihydroxynaphthalene and subsequent reactions. J. Bacteriol. 1992, 174, 7542–7554. [Google Scholar] [CrossRef]
- Tavendale, M.H.; McFarlane, P.N.; Mackie, K.L.; Wilkins, A.L.; Langdon, A.G. The fate of resin acids-1. The biotransformation and degradation of deuterium labelled dehydroabietic acid in anaerobic sediments. Chemosphere 1997, 35, 2137–2151. [Google Scholar] [CrossRef]
- Tavendale, M.H.; McFarlane, P.N.; Mackie, K.L.; Wilkins, A.L.; Langdon, A.G. The fate of resin acids-2. The fate of resin acids and resin acid derived neutral compounds in anaerobic sediments. Chemosphere 1997, 35, 2153–2166. [Google Scholar] [CrossRef]
- Meyer, T.; Yang, M.I.; Tran, H.N.; Allen, D.G.; Edwards, E.A. Impact of resin and fatty acids on full-scale anaerobic treatment of pulp and paper mill effluents. Environ. Eng. Sci. 2016, 33, 394–403. [Google Scholar] [CrossRef]
- Aguirre, M.; Vuorenmaa, J.; Valkonen, E.; Kettunen, H.; Callens, C.; Haesebrouck, F.; Ducatelle, R.; Van Immerseel, F.; Goossens, E. In-feed resin acids reduce matrix metalloproteinase activity in the ileal mucosa of healthy broilers without inducing major effects on the gut microbiota. Vet. Res. 2019, 50, 1–14. [Google Scholar] [CrossRef]
- Savluchinske-Feio, S.; Curto, M.J.M.; Gigante, B.; Roseiro, J.C. Antimicrobial activity of resin acid derivatives. Appl. Microbiol. Biotechnol. 2006, 72, 430–436. [Google Scholar] [CrossRef]
- Savluchinske-Feio, S.; Nunes, L.; Tavares, P.; Silva, A.M.; Roseiro, J.C.; Gigante, B.; João, M.; Curto, M. Activity of dehydroabietic acid derivatives against wood contaminant fungi. J. Microbiol. Methods 2007, 70, 465–470. [Google Scholar] [CrossRef]
- Tolmacheva, I.A.; Tarantin, A.V.; Boteva, A.A.; Anikina, L.V.; Vikharev, Y.; Grishko, V.V.; Tolstikov, A.G. Synthesis and biological activity of nitrogen-containing derivatives of methyl dehydroabietate. Pharm. Chem. J. 2006, 40, 489–493. [Google Scholar] [CrossRef]
- Xu, D.D.; Yan, Y.; Jiang, C.X.; Liang, J.J.; Li, H.F.; Wu, Q.X.; Zhu, Y. Sesquiterpenes and diterpenes with cytotoxic activities from the aerial parts of Carpesium humile. Fitoterapia 2018, 128, 50–56. [Google Scholar] [CrossRef]
- Lee, T.K.; Park, J.Y.; Yu, J.S.; Jang, T.S.; Oh, S.T.; Pang, C.; Ko, Y.J.; Kang, K.S.; Kim, K.H. 7α,15-Dihydroxydehydroabietic acid from Pinus koraiensis inhibits the promotion of angiogenesis through downregulation of VEGF, p-Akt and p-ERK in HUVECs. Bioorganic Med. Chem. Lett. 2018, 28, 1084–1089. [Google Scholar] [CrossRef]
- González, M. Aromatic abietane diterpenoids: Their biological activity and synthesis. Nat. Prod. Rep. 2015, 684–704. [Google Scholar] [CrossRef]
- Parimal, K.; Khale, A.; Pramod, K. Resins from herbal origin and a focus on their applications. Int. J. Pharm. Sci. Res. 2011, 2, 1077–1085. [Google Scholar]
- Yadav, B.K.; Gidwani, B.; Vyas, A. Rosin: Recent advances and potential applications in novel drug delivery system. J. Bioact. Compat. Polym. 2015, 1–16. [Google Scholar] [CrossRef]
- Gonzalez, M.A. Aromatic abietane diterpenoids: Total syntheses and synthetic studies. Tetrahedron 2015, 71, 1883–1908. [Google Scholar] [CrossRef]
- Kugler, S.; Ossowicz, P.; Malarczyk-Matusiak, K.; Wierzbicka, E. Advances in rosin-based chemicals: The latest recipes, applications and future trends. Molecules 2019, 24, 1651. [Google Scholar] [CrossRef]
- Loughlin, W.A. Biotransformations in organic synthesis. Bioresour. Technol. 2000, 74, 49–62. [Google Scholar] [CrossRef]
- Huang, F.X.; Yang, W.Z.; Ye, F.; Tian, J.Y.; Hu, H.B.; Feng, L.M.; Guo, D.A.; Ye, M. Microbial transformation of ursolic acid by Syncephalastrum racemosum (Cohn) Schroter AS 3.264. Phytochemistry 2012, 82, 56–60. [Google Scholar] [CrossRef]
- Gouiric, S.C.; Feresin, G.E.; Tapia, A.A.; Rossomando, P.C.; Schmeda-Hirschmann, G.; Bustos, D.A. 1b,7b-Dihydroxydehydroabietic acid, a new biotransformation product of dehydroabietic acid by Aspergillus niger. World J. Microbiol. Biotechol. 2004, 20, 281–284. [Google Scholar] [CrossRef]
- Özşen, Ö.; Kıran, İ.; Dağ, İ.; Atlı, Ö.; Çiftçi, G.A.; Demirci, F. Biotransformation of abietic acid by fungi and biological evaluation of its metabolites. Process Biochem. 2017, 52, 130–140. [Google Scholar] [CrossRef]
- Tapia, A.A.; Vallejo, M.D.; Gouiric, S.C.; Feresin, G.E.; Rossomando, P.C.; Bustos, D.A. Hydroxylation of dehydroabietic acid by Fusarium species. Phytochemistry 1997, 46, 131–133. [Google Scholar] [CrossRef]
- Van Beek, T.A.; Claassen, F.W.; Dorado, J.; Godejohann, M.; Sierra-Alvarez, R.; Wijnberg, J.B.P.A. Fungal biotransformation products of dehydroabietic acid. J. Nat. Prod. 2007, 70, 154–159. [Google Scholar] [CrossRef]
- Yang, N.-Y.; Liu, L.; Tao, W.-W.; Duan, J.-A.; Tian, L.-J. Phytochemistry diterpenoids from Pinus massoniana resin and their cytotoxicity against A431 and A549 cells. Phytochemistry 2010, 71, 1528–1533. [Google Scholar] [CrossRef]
- Witzig, R.; Aly, H.A.H.; Strömpl, C.; Wray, V.; Junca, H.; Pieper, D.H. Molecular detection and diversity of novel diterpenoid dioxygenase DitA1 genes from proteobacterial strains and soil samples. Environ. Microbiol. 2007, 9, 1202–1218. [Google Scholar] [CrossRef]
- Bleif, S.; Hannemann, F.; Lisurek, M.; von Kries, J.P.; Zapp, J.; Dietzen, M.; Antes, I.; Bernhardt, R. Identification of CYP106A2 as a regioselective allylic bacterial diterpene hydroxylase. ChemBioChem 2011, 12, 576–582. [Google Scholar] [CrossRef]
- Janocha, S.; Zapp, J.; Hutter, M.; Kleser, M.; Bohlmann, J.; Bernhardt, R. Resin acid conversion with CYP105A1: An enzyme with potential for the production of pharmaceutically relevant diterpenoids. ChemBioChem 2013, 14, 467–473. [Google Scholar] [CrossRef]



| Acid | Scots Pine P. silvestris | Ordinary Spruce P. excelsa | Maritime Pine P. pinaster |
|---|---|---|---|
| Abietic | 7.86 | 13.95 | 16.10 |
| Dehydroabietic | 64.58 | 50.08 | 23.50 |
| Pimaric | 10.86 | 7.57 | 10.80 |
| Isopimaric | 8.26 | 18.83 | 6.90 |
| Unidentified | 8.43 | 9.55 | – |
| RA | Solubility, mg/L | Acute Toxicity (LD50), mg/L | ||||
|---|---|---|---|---|---|---|
| Daphnia Daphnia magna, 48 h | Rainbow Trout Oncorhynchus mykiss, 96 h | Red Salmon O. nerka, 96 h | Silver Salmon O. kisutch, 96 h | Fathead Minnow Pimephales promelas, 96 h | ||
| DHA | 5.11 | 1.28–6.35 | 0.77–1.32 | 0.50–2.10 | 0.75–1.85 | 2.10–3.20 |
| ABA | 2.75 | 0.68 | 0.72–1.53 | 0.20 | 0.40 | 2.38 |
| LPA | 2.54 | 0.50 | 0.61–1.00 | – | – | – |
| NAA | 2.31 | 0.35 | 0.63–0.71 | – | – | 1.30–1.70 |
| PA | 2.17 | 0.26 | 0.74–1.23 | – | 0.32 | – |
| SPA | 1.82 | 0.13 | – | – | 0.36 | – |
| IPA | 1.70 | 0.07 | 0.40–1.00 | 0.70 | 0.20 | – |
| Study Object | Concentration | Conditions | Reference |
|---|---|---|---|
| Fine flounder Paralychthys adspersus Small-eyes flounder P. microps | |||
| Bile | 30.5–41.9 µg/g, total RA content | Caught near the PPM effluent discharge site | [4] |
| Rainbow trout O. mykiss | |||
| Bile | <200 µg/g DHA | After 57 days of exposure to PPM effluents | [32] |
| Blood plasma | 155–318 µg/g DHA | After 4 days of exposure to DHA (1.2 mg/L) in water | [31] |
| Liver | 98–103 µg/g DHA | After 4 days of exposure to DHA (1.2 mg/L) | |
| 202–351 µg/g, total RA content | After 2 days of exposure to a mixture of RAs (1.4 mg/L) in water | ||
| Kidney | 47–114 µg/g DHA | After 4 days of exposure to DHA (1.2 mg/L) | |
| 72–115 µg/g, total RA content | After 2 days of exposure to a mixture of RAs (1.4 mg/L) in water | ||
| Strain | Substrate | Reference |
|---|---|---|
| Gram-negative | ||
| Alcaligenes sp. D11-13 | DHA | [53] |
| Betaproteobacterium sp. DhA-71, DhA-73 | DHA | [57] |
| Burkholderia cepacia F45L5 | DHA, ABA, IPA | [58] |
| Burkholderia sp. DhA-54 | DHA | [59] |
| Burkholderia sp. IpA-51 | IPA | [59] |
| B. xenovorans LB400 | DHA, ABA, PA | [60,61,62] |
| Pseudomonas abietaniphila BKME-9 | DHA, ABA | [52,63] |
| P. fluorescens NRRL B21432 | Mixture of RAs | [64] |
| P. marginalis E-001624 | Mixture of RAs | [65] |
| P. mohnii IpA-2T, P. moorei RW10T | IPA | [66] |
| “Pseudomonas multiresinivorans” * (P. nitroducent) IpA-1 * | IPA | [67] |
| P. reinekei Mt1 | IPA | [66] |
| Pseudomonas sp. A19-6a | ABA | [53] |
| Pseudomonas sp. DhA-92 | DHA | [55] |
| Pseudomonas sp. IpA-2 | IPA | [67] |
| Pseudomonas sp. IpA-93, IpA-95 | IPA | [55] |
| P. vancouverensis Dha-51 | DHA | [59] |
| Ralstonia sp. BKME-6 | DHA | [52] |
| Serratia marcescens NRRL B21429 | Mixture of RAs | [64] |
| Sphingomonas sp. DhA-33 | DHA | [54,68] |
| Sphingomonas sp. DhA-95 | DHA | [55] |
| Xanthomonas campestris NRRL B21430 | Mixture of RAs | [64] |
| Zoogloea ramigera DhA-35 | DHA | [68] |
| Gram-positive | ||
| Bacillus psychrophilus | DHA | [69] |
| Dietzia maris IEGM 55T | DHA | [56] |
| Gordonia rubripertincta IEGM 104, IEGM 105, IEGM 109 | DHA | [51] |
| G. terrae IEGM 150 | DHA | [51] |
| Mycobacterium sp. DhA-55 | DHA | [54] |
| Mycobacterium sp. IpA-13 | IPA | [67] |
| Rhodococcus erythropolis IEGM 267 | DHA | [51] |
| R. rhodochrous IEGM 107 | DHA | [51] |
| R. ruber IEGM 80 | DHA | [51] |
| Compound | Biological Activity | Biocatalyst | Reference |
|---|---|---|---|
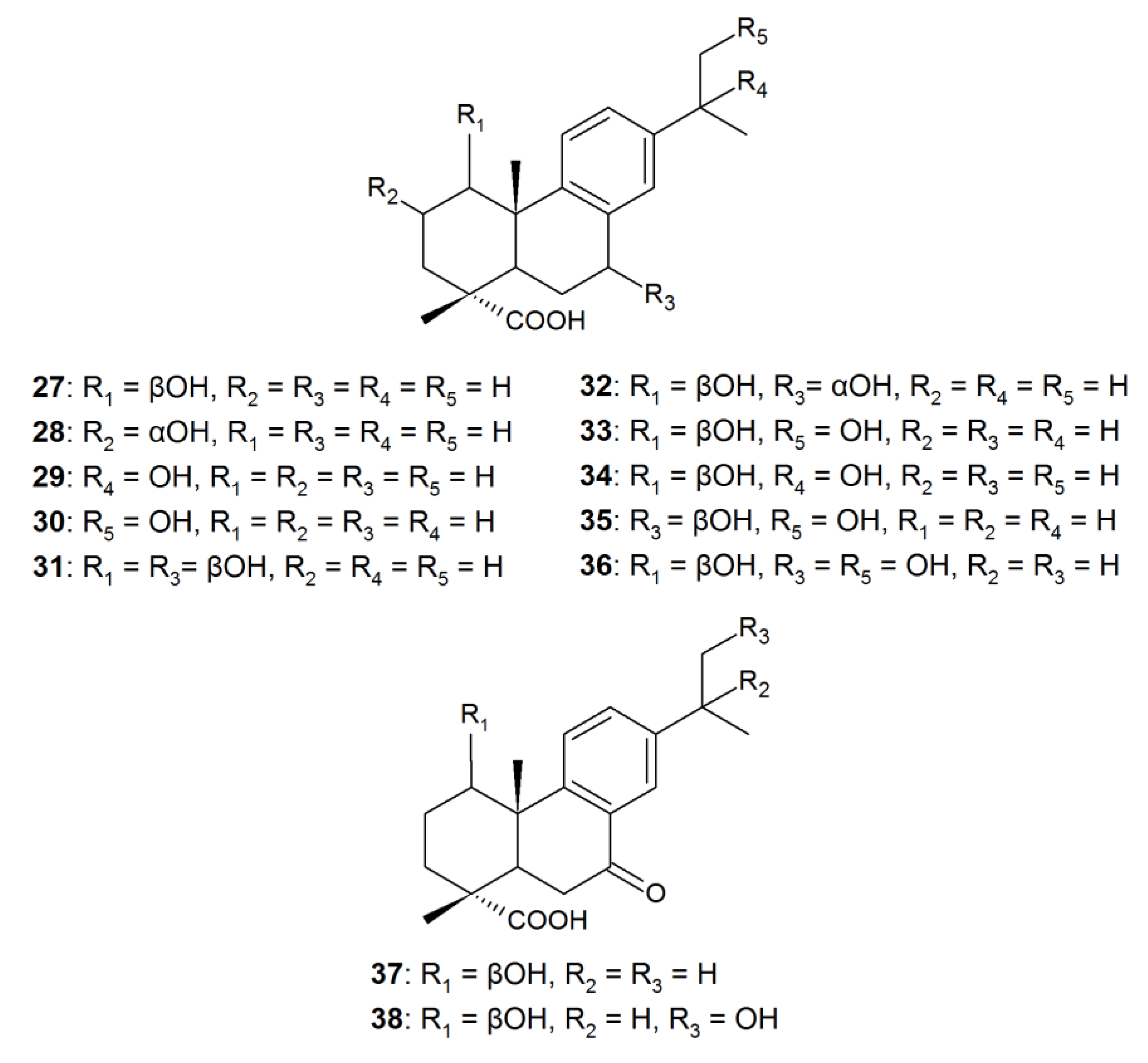
| 1β-hydroxy-DHA (27) | Antimicrobial, inhibitory activity against α-glucosidase | Aspergillus niger, Cephalosporium aphidicola, Cunninghamella elegans, Fusarium moniliforme, F. oxysporum, Gibberella fujikuroi, Neurospora crassa, Phlebiopsis gigantea, Rhizopus stolonifera | [22,90,91,92,93] |
| 2α-hydroxy-DHA (28) | Antimicrobial, selective antitumor | Mucor ramannianus | [91] |
| 7β-hydroxy-DHA (1) | Antimicrobial, fungicidal, antitumor | A. niger, N. crassa | [78,79,90,91,94] |
| 15-hydroxy-DHA (29) | Anti-inflammatory. An intermediate of antiviral and antitumor agent synthesis | C. aphidicola, C. elegans, G. fujkuroi, R. stolonifera | [22,83] |
| 16-hydroxy-DHA (30) | Antimicrobial | C. aphidicola, C. elegans, G. fujkuroi, R. stolonifera | [22] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luchnikova, N.A.; Ivanova, K.M.; Tarasova, E.V.; Grishko, V.V.; Ivshina, I.B. Microbial Conversion of Toxic Resin Acids. Molecules 2019, 24, 4121. https://doi.org/10.3390/molecules24224121
Luchnikova NA, Ivanova KM, Tarasova EV, Grishko VV, Ivshina IB. Microbial Conversion of Toxic Resin Acids. Molecules. 2019; 24(22):4121. https://doi.org/10.3390/molecules24224121
Chicago/Turabian StyleLuchnikova, Natalia A., Kseniya M. Ivanova, Ekaterina V. Tarasova, Victoria V. Grishko, and Irina B. Ivshina. 2019. "Microbial Conversion of Toxic Resin Acids" Molecules 24, no. 22: 4121. https://doi.org/10.3390/molecules24224121
APA StyleLuchnikova, N. A., Ivanova, K. M., Tarasova, E. V., Grishko, V. V., & Ivshina, I. B. (2019). Microbial Conversion of Toxic Resin Acids. Molecules, 24(22), 4121. https://doi.org/10.3390/molecules24224121







