Improving Water Permeability of Hydrophilic PVDF Membrane Prepared via Blending with Organic and Inorganic Additives for Humic Acid Separation
Abstract
:1. Introduction
2. Experimental
2.1. Material
2.2. Membrane Preparation
2.3. Analysis of Membrane Morphology
2.4. Water Contact Angle
2.5. Chemical Composition
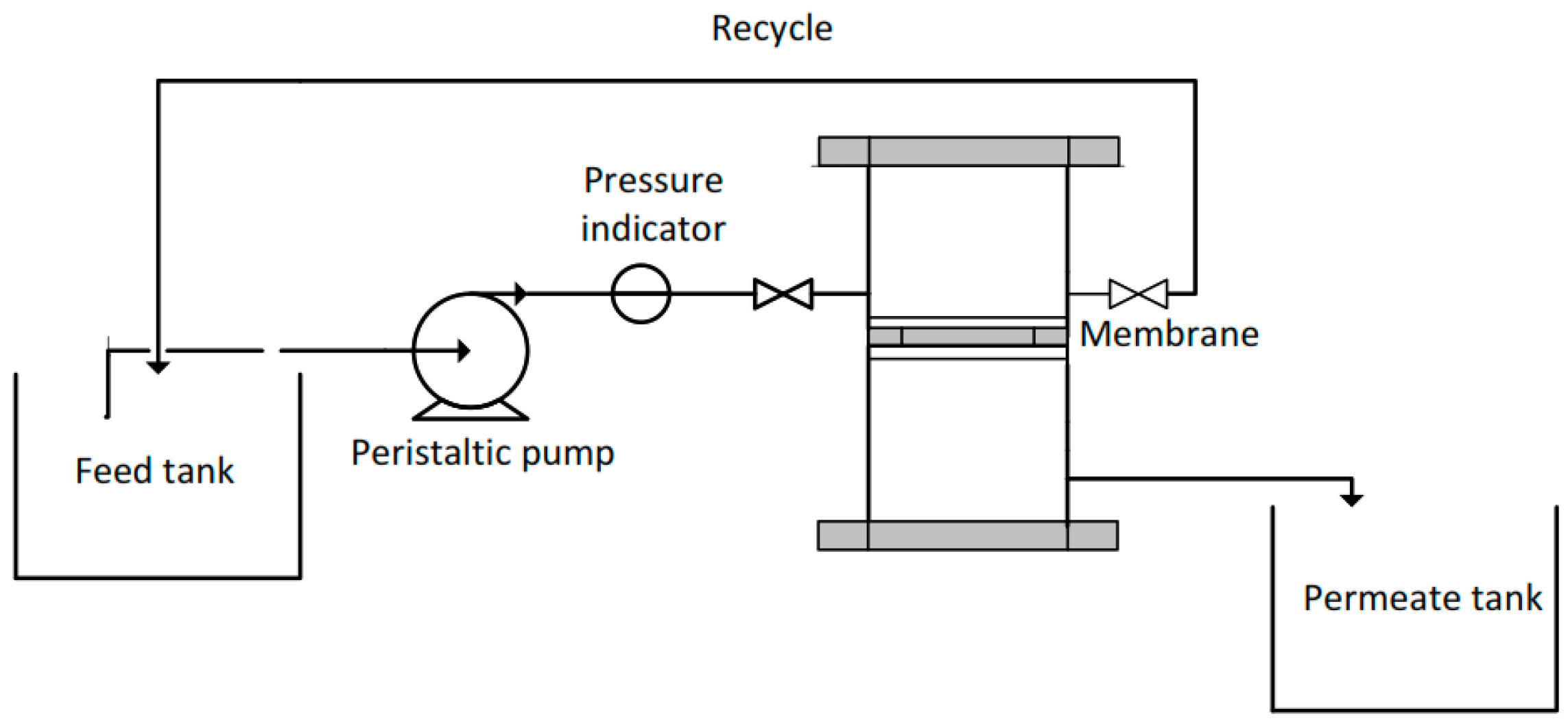
2.6. Filtration Performance
3. Result and Discussion
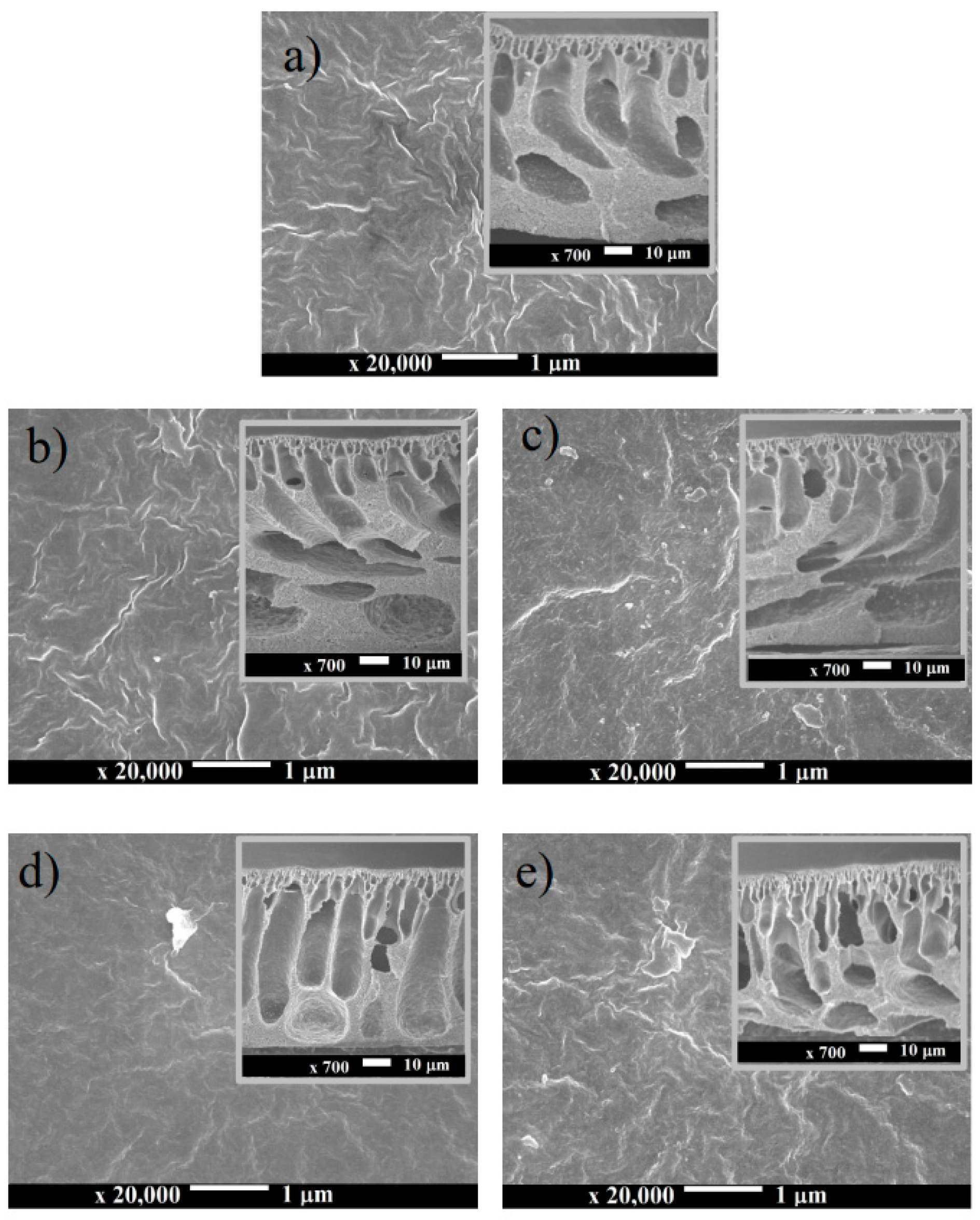
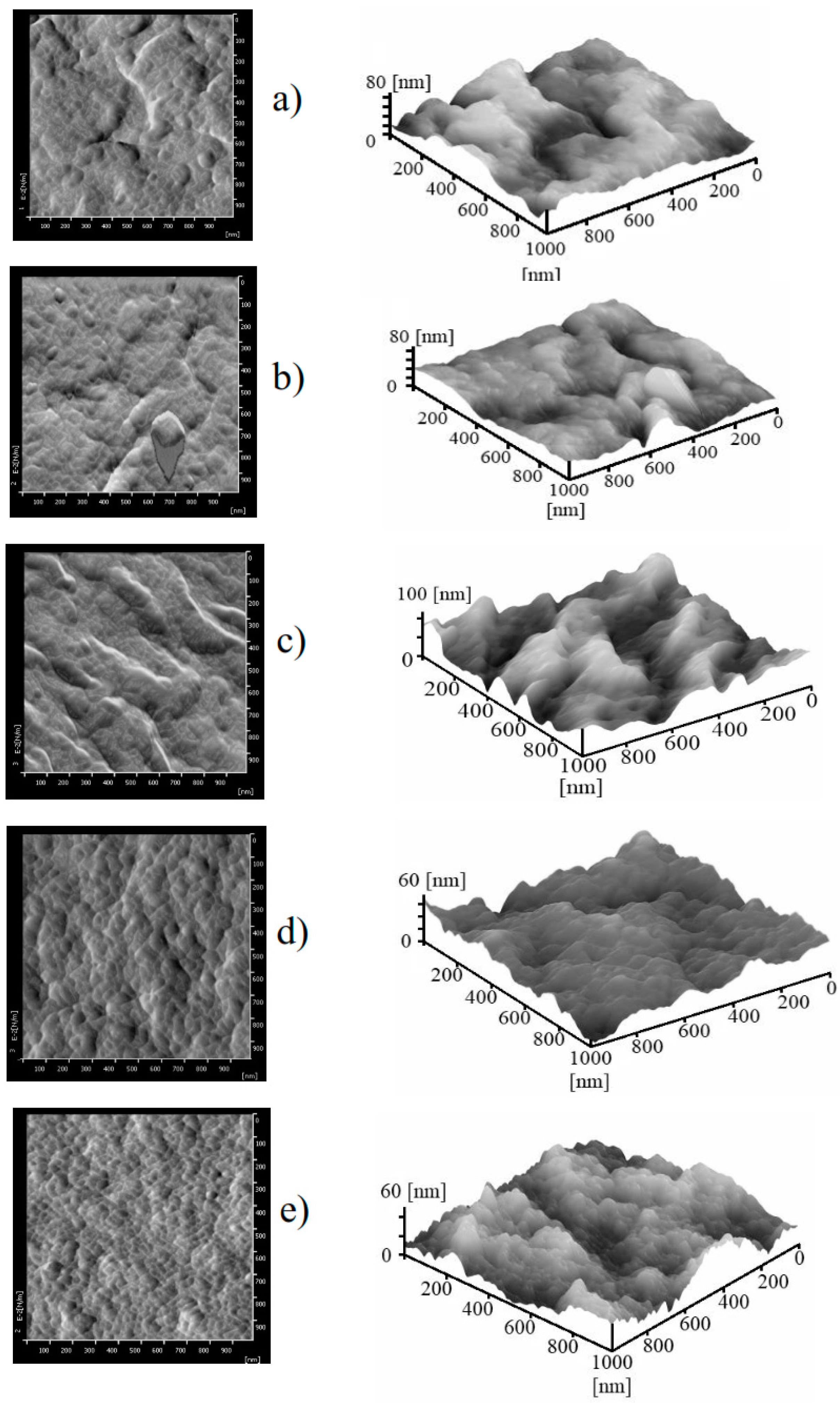
3.1. Membrane Morphology
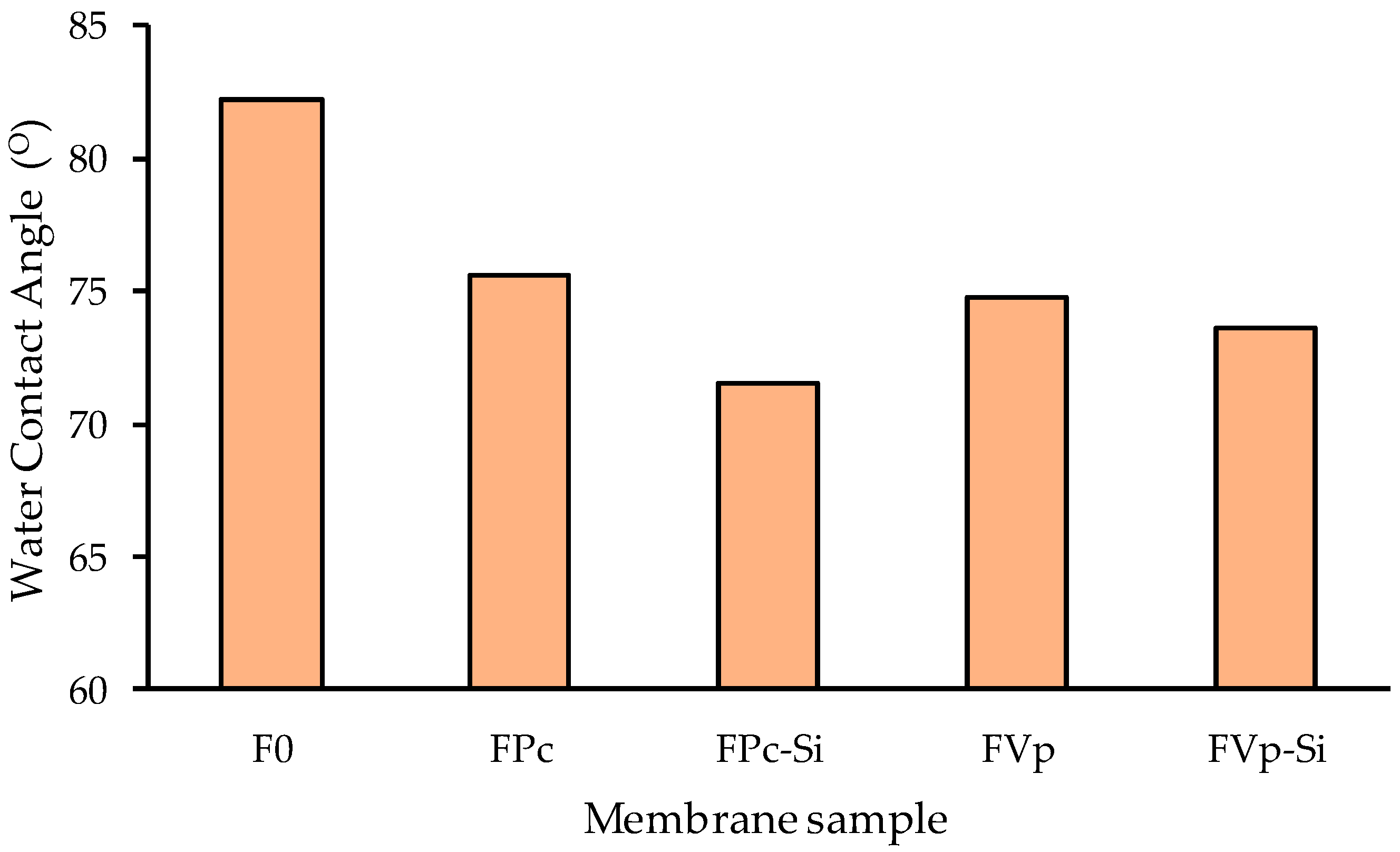
3.2. Hydrophilicity
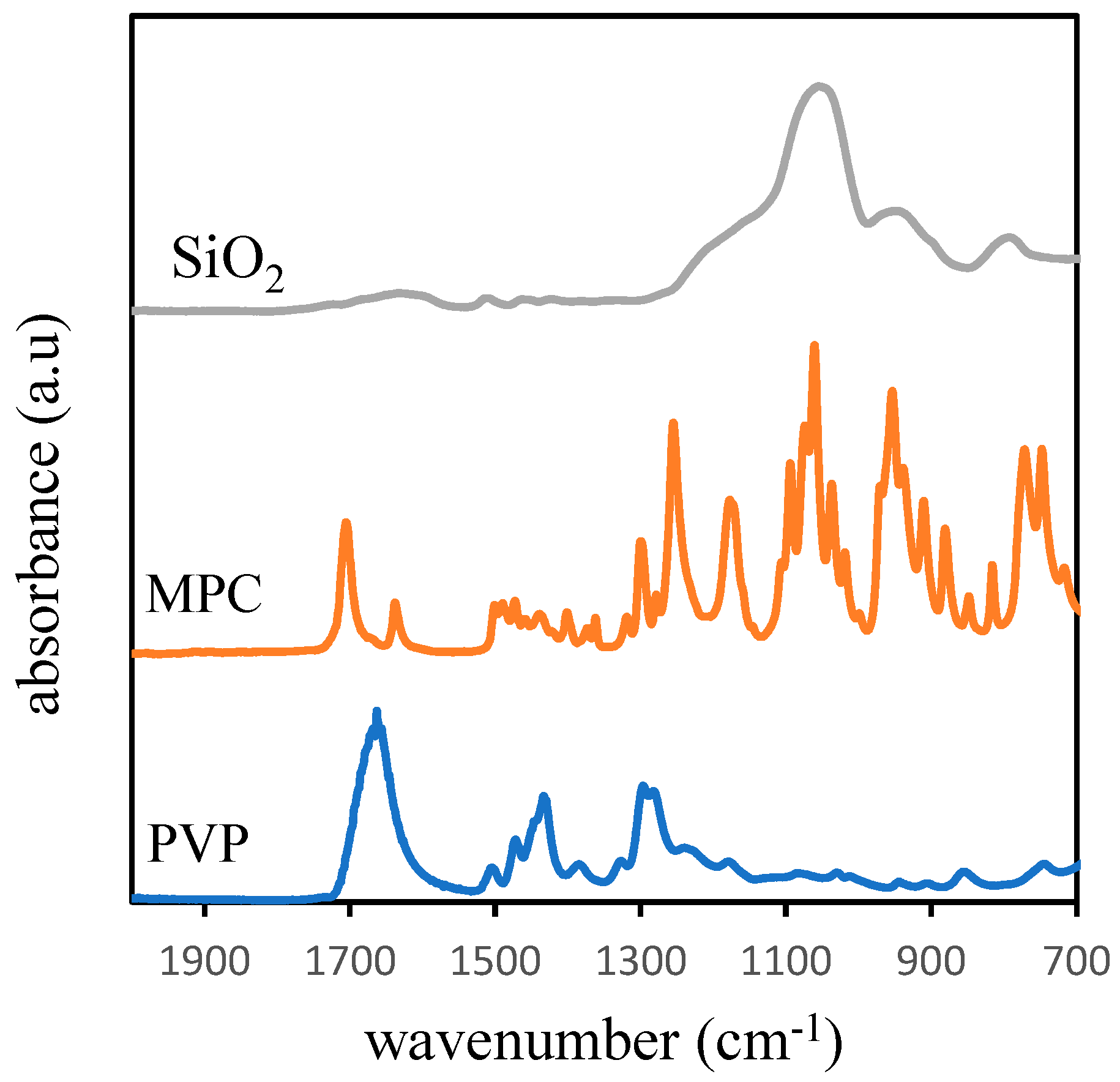
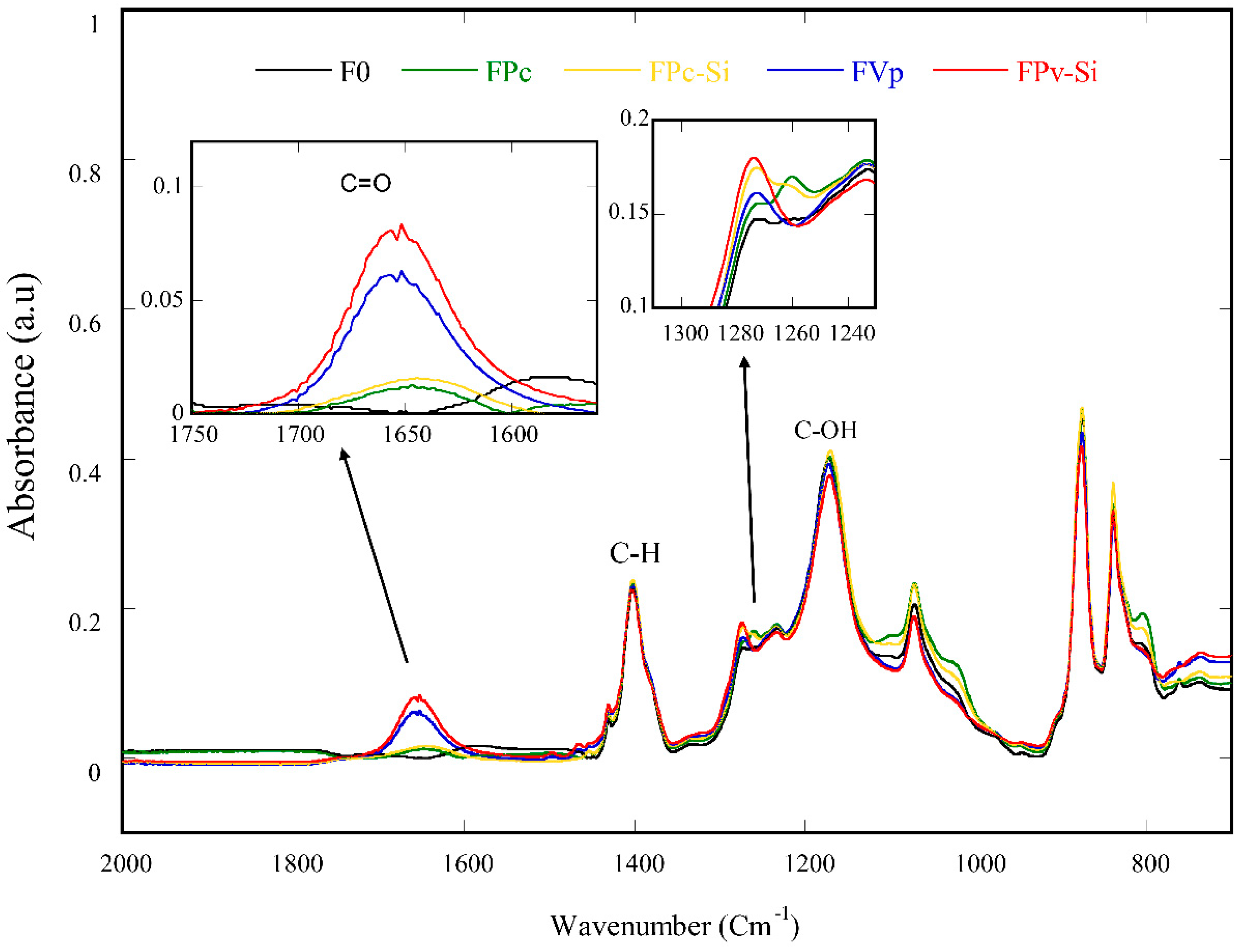
3.3. Membrane Surface Chemistry
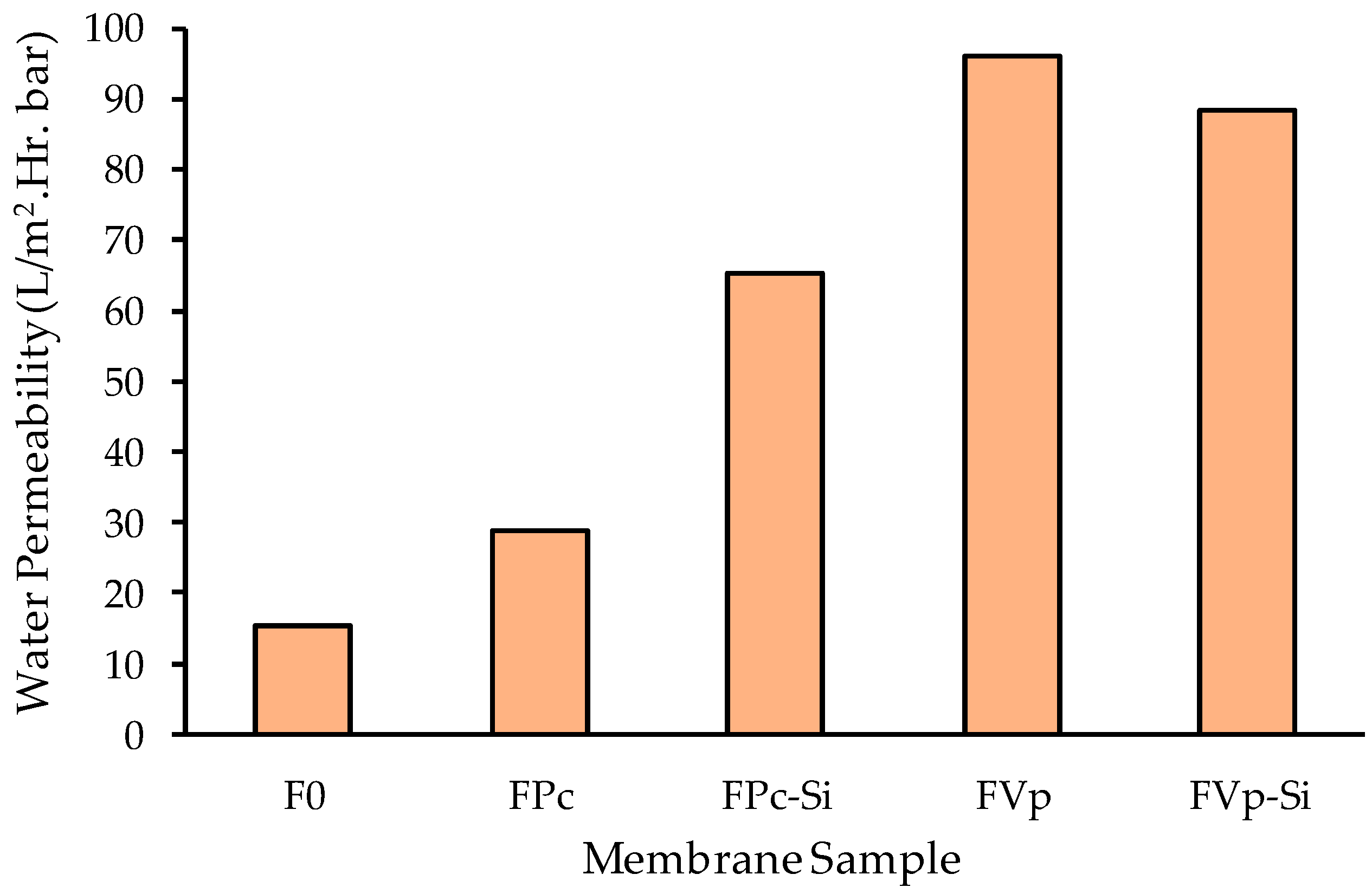
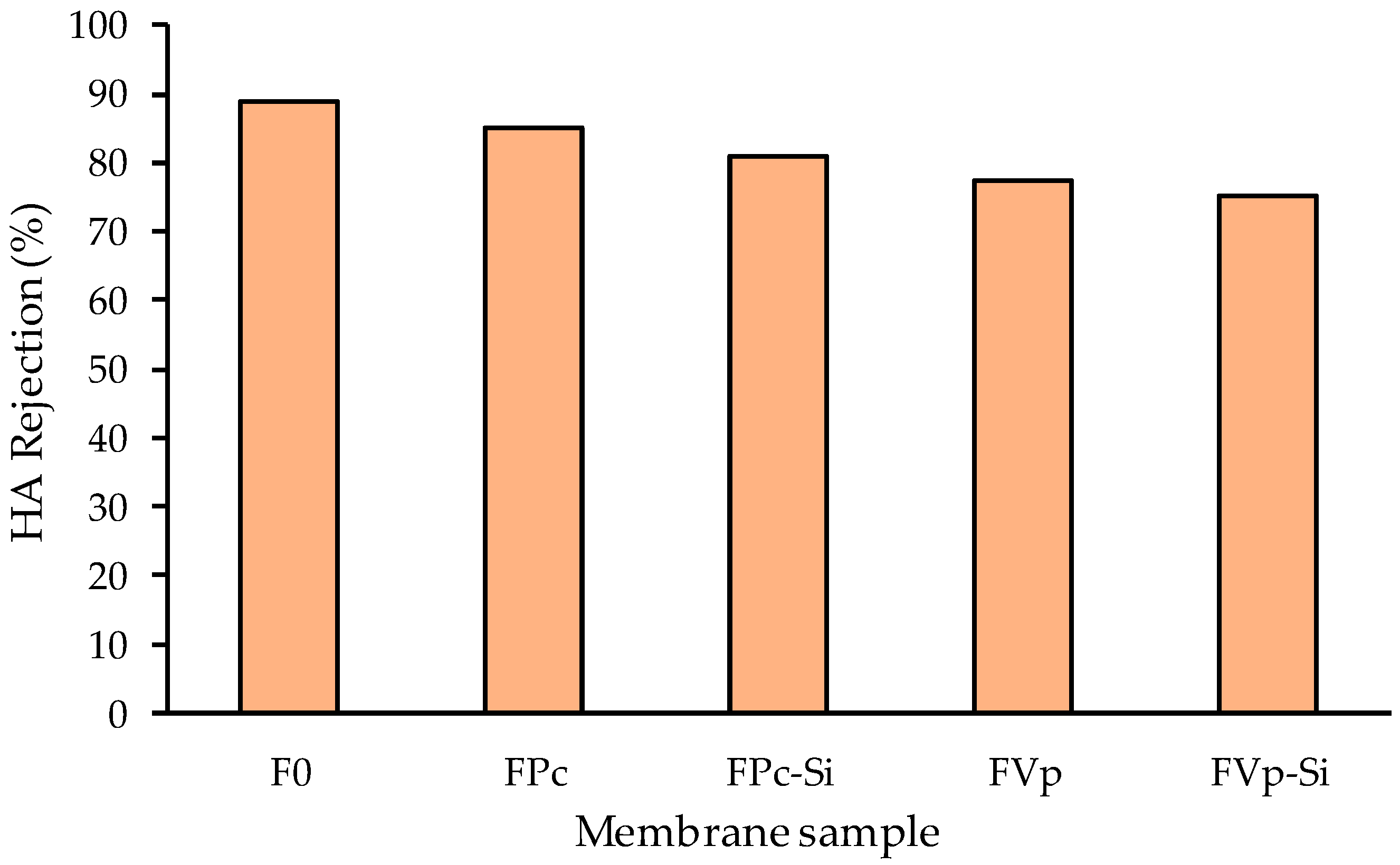
3.4. Filtration Profile
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ulbricht, M. Advanced functional polymer membranes. Polymer 2006, 47, 2217–2262. [Google Scholar] [CrossRef]
- Shen, J.N.; Ruan, H.M.; Wu, L.G.; Gao, C.J. Preparation and characterization of PES-SiO2 organic-inorganic composite ultrafiltration membrane for raw water pretreatment. Chem. Eng. J. 2011, 168, 1272–1278. [Google Scholar] [CrossRef]
- Kumar, M.; Ulbricht, M. Low fouling negatively charged hybrid ultrafiltration membranes for protein separation from sulfonated poly (arylene ether sulfone) block copolymer and functionalized multiwalled carbon nanotubes. Sep. Purif. Technol. 2014, 127, 181–191. [Google Scholar] [CrossRef]
- Mulyati, S.; Armando, M.A.; Mawardi, H.; Fahrina, A.; Malahayati, N.; Syawaliah, S. Fabrication of Hydrophilic and Strong Pet-Based Membrane from Wasted Plastic Bottle. Rasayan J. Chem. 2018, 11, 1609–1617. [Google Scholar] [CrossRef]
- Yuliwati, E.; Ismail, A.F.; Matsuura, T.; Kassim, M.A.; Abdullah, M.S. Effect of modified pvdf hollow fiber submerged ultrafiltration membrane for refinery wastewater treatment. Desalination 2011, 283, 214–220. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Xiao, P.; Li, J.; Tian, E.; Zhao, Y.; Ren, Y. High water permeable free-standing cellulose triacetate/graphene oxide membrane with enhanced antibiofouling and mechanical properties for forward osmosis. Colloids Surf. A Physicochem. Eng. Asp. 2016, 508, 327–335. [Google Scholar] [CrossRef]
- Ahmad, A.L.; Abdulkarim, A.A.; Ooi, B.S.; Ismail, S. Recent development in additives modifications of polyethersulfone membrane for flux enhancement. Chem. Eng. J. 2013, 223. [Google Scholar] [CrossRef]
- Nikooe, N.; Saljoughi, E. Preparation and characterization of novel PVDF nanofiltration membranes with hydrophilic property for filtration of dye aqueous solution. Appl. Surf. Sci. 2017, 413, 41–49. [Google Scholar] [CrossRef]
- Muchtar, S.; Wahab, M.Y.; Fang, L.; Jeon, S.; Rajabzadeh, S.; Takagi, R.; Mulyati, S.; Arahman, N.; Riza, M. Polydopamine-coated poly (vinylidene fl uoride) membranes with high ultraviolet resistance and antifouling properties for a photocatalytic membrane reactor. J. Appl. Polym. Sci. 2018, 47312, 1–9. [Google Scholar]
- Wahab, M.Y.; Muchtar, S.; Jeon, S.; Fang, L.; Rajabzadeh, S.; Takagi, R.; Arahman, N.; Mulyati, S.; Riza, M.; Matsuyama, H. Synergistic effects of organic and inorganic additives in preparation of composite poly (vinylidene fl uoride) antifouling ultra fi ltration membranes. J. Appl. Polym. Sci. 2019, 47737, 1–10. [Google Scholar]
- Liu, F.; Hashim, N.A.; Liu, Y.; Abed, M.R.M.; Li, K. Progress in the production and modification of PVDF membranes. J. Memb. Sci. 2011, 375, 1–27. [Google Scholar] [CrossRef]
- Ishihara, K.; Ueda, T.; Nakabayashi, N. Preparation of Phospholipid Polymers and Their Properties as Polymer Hydrogel Membranes. Polym. J. 1990, 22, 355–360. [Google Scholar] [CrossRef]
- Ishihara, K.; Iwasaki, Y.; Ebihara, S.; Shindo, Y.; Nakabayashi, N. Photoinduced graft polymerization of 2-methacryloyloxyethyl phosphorylcholine on polyethylene membrane surface for obtaining blood cell adhesion resistance. Colloids Surf. B Biointerfaces 2000, 18, 325–335. [Google Scholar] [CrossRef]
- Xu, Y.; Takai, M.; Ishihara, K. Protein adsorption and cell adhesion on cationic, neutral, and anionic 2-methacryloyloxyethyl phosphorylcholine copolymer surfaces. Biomaterials 2009, 30, 4930–4938. [Google Scholar] [CrossRef]
- Arahman, N.; Satria, S.; Razi, F.; Bilad, M.R. The Effect of Ca and Mg Ions on the Filtration Profile of Sodium Alginate Solution in a Polyethersulfone-2-(methacryloyloxy) ethyl phosphorylchloline membrane. Water 2018, 10, 1207. [Google Scholar] [CrossRef]
- Zheng, Z.; Ren, L.; Zhai, Z.; Wang, Y.; Hang, F. Surface modi fi cation on polyethylene terephthalate fi lms with 2-methacryloyloxyethyl phosphorylcholine. Mater. Sci. Eng. C 2013, 33, 3041–3046. [Google Scholar] [CrossRef]
- Loh, C.H.; Wang, R.; Shi, L.; Fane, A.G. Fabrication of high performance polyethersulfone UF hollow fiber membranes using amphiphilic Pluronic block copolymers as pore-forming additives. J. Memb. Sci. 2011, 380, 114–123. [Google Scholar] [CrossRef]
- Arahman, N.; Mulyati, S.; Lubis, M.R.; Razi, F.; Takagi, R.; Matsuyama, H. Modification of polyethersulfone hollow fiber membrane with different polymeric additives. Membr. Water Treat. 2016, 7, 355–365. [Google Scholar] [CrossRef]
- Shockravi, A.; Vatanpour, V.; Najjar, Z.; Bahadori, S.; Javadi, A. A new high performance polyamide as an effective additive for modification of antifouling properties and morphology of asymmetric PES blend ultrafiltration membranes. Microporous Mesoporous Mater. 2017, 246, 24–36. [Google Scholar] [CrossRef]
- Susanto, H.; Ulbricht, M. Characteristics, performance and stability of polyethersulfone ultrafiltration membranes prepared by phase separation method using different macromolecular additives. J. Memb. Sci. 2009, 327, 125–135. [Google Scholar] [CrossRef]
- Simone, S.; Figoli, A.; Criscuoli, A.; Carnevale, M.C.; Rosselli, A.; Drioli, E. Preparation of hollow fibre membranes from PVDF/PVP blends and their application in VMD. J. Memb. Sci. 2010, 364, 219–232. [Google Scholar] [CrossRef]
- Marbelia, L.; Bilad, M.R.; Vankelecom, I.F.J. Gradual PVP leaching from PVDF/PVP blend membranes and its effects on membrane fouling in membrane bioreactors. Sep. Purif. Technol. 2019, 213, 276–282. [Google Scholar] [CrossRef]
- Sali, S.; Mackey, H.R. Effect of Graphene Oxide Synthesis Method on Properties and Performance of Polysulfone-Graphene Oxide Mixed Matrix Membranes. Nanomaterials 2019, 9, 769. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhu, L.; Shan, B.; Xie, C.; Liu, C.; Cui, F.; Li, G. Preparation and characterization of SLS-CNT/PES ultra fi ltration membrane with antifouling and antibacterial properties. J. Memb. Sci. 2018, 548, 459–469. [Google Scholar] [CrossRef]
- Esfahani, M.R.; Firouzjaei, M.D.; Aktij, S.A.; Dabaghian, Z.; Abolhassani, M.; Rahimpour, A.; Eke, J.; Escobar, I.C.; Koutahzadeh, N.; Greenlee, L.F.; et al. Nanocomposite membranes for water separation and purification: Fabrication, modification, and applications. Sep. Purif. Technol. 2019, 213, 465–499. [Google Scholar] [CrossRef]
- Lin, J.; Ye, W.; Zhong, K.; Shen, J.; Jullok, N.; Sotto, A.; Bruggen, B. Van Der Enhancement of polyethersulfone (PES) membrane doped by monodisperse Stöber silica for water treatment. Chem. Eng. Process. Process Intensif. 2016, 107, 194–205. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, H.; Liu, J.; Zhang, Y. Preparation and antifouling property of polyethersulfone ultrafi ltration hybrid membrane containing halloysite nanotubes grafted with MPC via RATRP method. Desalination 2014, 344, 313–320. [Google Scholar] [CrossRef]
- Shen, J.; Zhang, Q.; Yin, Q.; Cui, Z.; Li, W.; Xing, W. Fabrication and characterization of amphiphilic PVDF copolymer ultra fi ltration membrane with high anti-fouling property. J. Memb. Sci. 2017, 521, 95–103. [Google Scholar] [CrossRef]
- Mavukkandy, M.O.; Bilad, M.R.; Kujawa, J.; Al-Gharabli, S.; Arafat, H.A. On the effect of fumed silica particles on the structure, properties and application of PVDF membranes. Sep. Purif. Technol. 2017, 187, 365–373. [Google Scholar] [CrossRef]
- Discart, V.; Bilad, M.R.; Moorkens, R.; Arafat, H.; Vankelecom, I.F.J. Decreasing membrane fouling during Chlorella vulgaris broth fi ltration via membrane development and coagulant assisted fi ltration. ALGAL 2015, 9, 55–64. [Google Scholar] [CrossRef]
- Meringolo, C.; Mastropietro, T.F.; Poerio, T.; Fontananova, E.; De Filpo, G.; Curcio, E.; Di, G. Tailoring PVDF Membranes Surface Topography and Hydrophobicity by a Sustainable Two-Steps Phase Separation Process. Sustain. Chem. Eng. 2018, 6, 10069–10077. [Google Scholar] [CrossRef]
- Izati, N.; Nawi, M.; Bilad, M.R.; Zolkhiflee, N. Development of A Novel Corrugated Polyvinylidene difluoride Membrane via Improved Imprinting Technique for Membrane Distillation. Polymer 2019, 11, 1–13. [Google Scholar]
- Nawi, N.I.M.; Bilad, M.R.; Nordin, N.A.H.M.; Mavukkandy, M.O.; Putra, Z.A.; Wirzal, M.D.H.; Jaafar, J.; Khan, A.L. Exploiting the Interplay between Liquid-Liquid Demixing and Crystallization of the PVDF Membrane for Membrane Distillation. Int. J. Polym. Sci. 2018, 2018, 1525014. [Google Scholar] [CrossRef]
- Sharma, N.; Purkait, M.K. Preparation of hydrophilic polysulfone membrane using polyacrylic acid with polyvinyl pyrrolidone. J. Appl. Polym. Sci. 2015, 132, 1–13. [Google Scholar] [CrossRef]
- Rafizah, W.A.W.; Ismail, A.F. Effect of carbon molecular sieve sizing with poly (vinyl pyrrolidone) K-15 on carbon molecular sieve–polysulfone mixed matrix membrane. J. Memb. Sci. 2008, 307, 53–61. [Google Scholar] [CrossRef]
- Han, M.; Nam, S. Thermodynamic and rheological variation in polysulfone solution by PVP and its effect in the preparation of phase inversion membrane. J. Memb. Sci. 2002, 202, 55–61. [Google Scholar] [CrossRef]
- Liang, S.; Kang, Y.; Tiraferri, A.; Giannelis, E.P.; Huang, X.; Elimelech, M. Highly Hydrophilic Polyvinylidene Fluoride (PVDF) Ultra fi ltration Membranes via Postfabrication Grafting of Surface-Tailored Silica Nanoparticles. Appl. Mater. Interface 2013, 5, 6694–6703. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
| Code | Polymer Composition (wt%) | ||||
|---|---|---|---|---|---|
| PVDF | MPC | PVP | SiO2 | DMSO | |
| F0 | 20 | 80 | |||
| FPc | 20 | 2 | 78 | ||
| FPc-Si | 20 | 2 | 0.5 | 77.5 | |
| FVp | 20 | 2 | 78 | ||
| FVp-Si | 20 | 2 | 0.5 | 77.5 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arahman, N.; Mulyati, S.; Fahrina, A.; Muchtar, S.; Yusuf, M.; Takagi, R.; Matsuyama, H.; Nordin, N.A.H.; Bilad, M.R. Improving Water Permeability of Hydrophilic PVDF Membrane Prepared via Blending with Organic and Inorganic Additives for Humic Acid Separation. Molecules 2019, 24, 4099. https://doi.org/10.3390/molecules24224099
Arahman N, Mulyati S, Fahrina A, Muchtar S, Yusuf M, Takagi R, Matsuyama H, Nordin NAH, Bilad MR. Improving Water Permeability of Hydrophilic PVDF Membrane Prepared via Blending with Organic and Inorganic Additives for Humic Acid Separation. Molecules. 2019; 24(22):4099. https://doi.org/10.3390/molecules24224099
Chicago/Turabian StyleArahman, Nasrul, Sri Mulyati, Afrillia Fahrina, Syawaliah Muchtar, Mukramah Yusuf, Ryosuke Takagi, Hideto Matsuyama, Nik Abdul Hadi Nordin, and Muhammad Roil Bilad. 2019. "Improving Water Permeability of Hydrophilic PVDF Membrane Prepared via Blending with Organic and Inorganic Additives for Humic Acid Separation" Molecules 24, no. 22: 4099. https://doi.org/10.3390/molecules24224099
APA StyleArahman, N., Mulyati, S., Fahrina, A., Muchtar, S., Yusuf, M., Takagi, R., Matsuyama, H., Nordin, N. A. H., & Bilad, M. R. (2019). Improving Water Permeability of Hydrophilic PVDF Membrane Prepared via Blending with Organic and Inorganic Additives for Humic Acid Separation. Molecules, 24(22), 4099. https://doi.org/10.3390/molecules24224099











