Rapid Analysis of the Chemical Compositions in Semiliquidambar cathayensis Roots by Ultra High-Performance Liquid Chromatography and Quadrupole Time-of-Flight Tandem Mass Spectrometry
Abstract
:1. Introduction
2. Results and Discussion
2.1. Optimization of Chromatographic Separation
2.2. Identification of Main Constituents in S. Cathayensis Extract
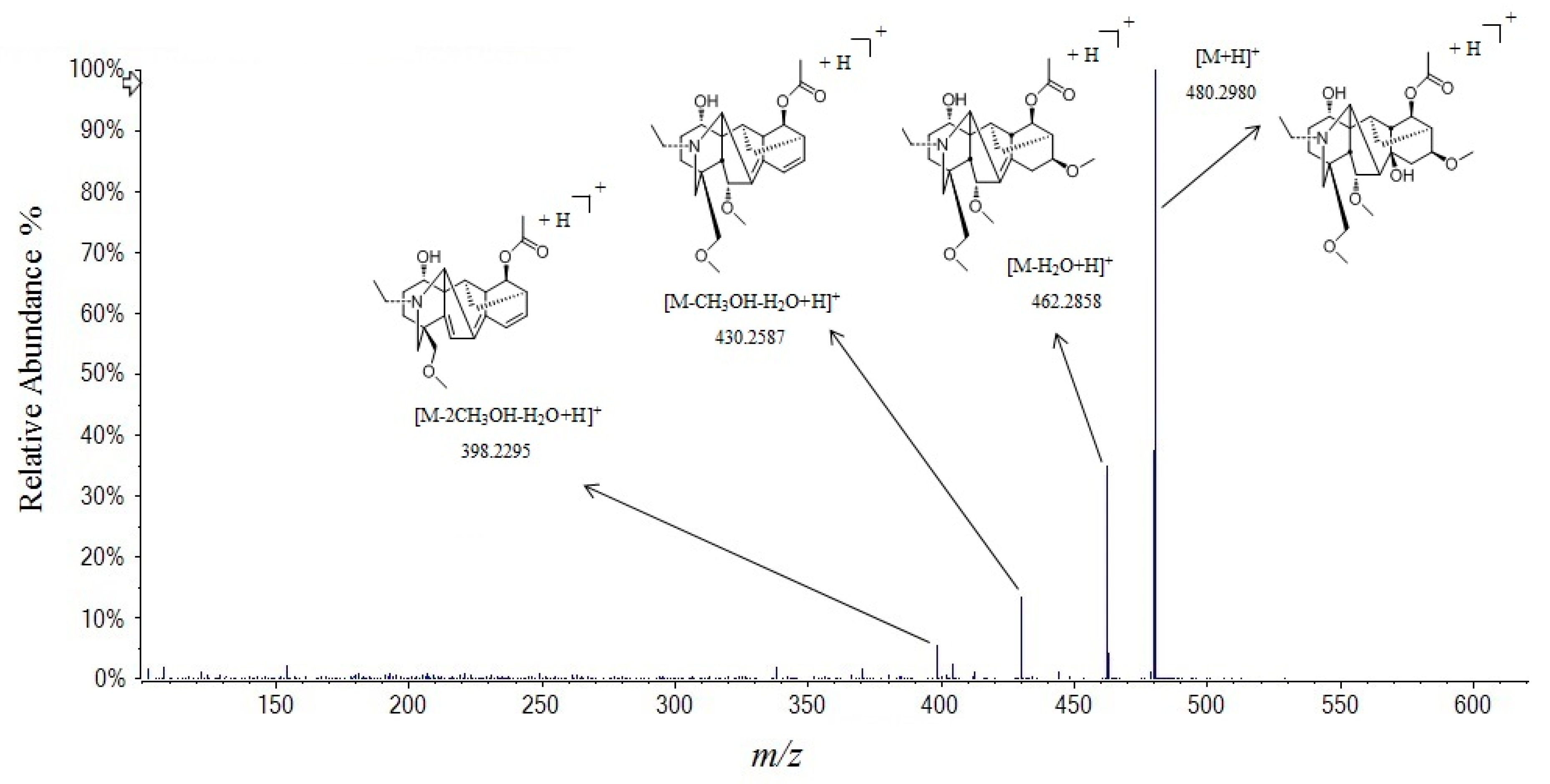
2.2.1. Alkaloids
2.2.2. Flavonoids
2.2.3. Terpenoids
2.2.4. Phenylpropanoids
2.2.5. Fatty Acids
2.2.6. Cyclic Peptides
2.2.7. Others
3. Experimental Section
3.1. Chemicals and Reagents
3.2. Plant Material and Extraction
3.3. UHPLC-Q-TOF-MS/MS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Chen, S.K.; Chen, B.Y.; Li, H. Zhongguo Zhiwu Zhi (Flora of China); VCH: Beijing, China, 1979; Volume 35, p. 58. [Google Scholar]
- Zhonghua Bencao Editorial Committee. State Administration of Traditional Chinese Medicine (Zhonghua). Zhonghua Bencao (The Chinese Herbal); Shanghai Scientific and Technical Press: Shanghai, China, 1999; pp. 129–231. [Google Scholar]
- Yang, L.; Wang, Y.Q.; Liu, S.C.; He, J.W. Research progress of chemical constituents and pharmacological activities from three commonly used Ban-feng-he medicinal plants. Chin. J. Exp. Tradit. Med. Form. 2016, 22, 191–196. [Google Scholar]
- Yang, W.L.; Yao, Z.S.; Luo, X.Q.; Ouyang, Y.W.; Li, X.Y.; Huang, Q.R.; Peng, W.J.; Tu, C.C. Analgesic and Anti-inflammatory Effects of the Ethanol Extracts of Jinlu Ban Fenghe (Semiliquidambar cathayensis) Root. Jiangxi Sci. 1999, 17, 176–179. [Google Scholar]
- Sun, J.; Zheng, X.L.; Cui, X.Z.; Meng, L. Study of inhibition effects on hepatitis B virus of Semi-cathayensis in vitro. Lishizhen Med. Mater. Med. Res. 2014, 25, 2391–2393. [Google Scholar]
- Liang, W.J.; Zeng, M.; Yang, Z.H.; Huang, M.L. Effect of Extract of Semiliquidambar cathayensis on blood stasis model rats. Chin. J. Chin. Mater. Med. 2015, 35, 366–369. [Google Scholar]
- Zhou, G.X.; Yang, Y.C.; Shi, J.G.; Yang, W.L. Studies on chemical constituents from Semil iquidambar cathayensis. Chin. Tradit. Herb. Drugs 2002, 33, 589–591. [Google Scholar]
- Lu, H.X.; Wu, Z.L.; Liang, W.J.; Chen, M.L.; Huang, B.B.; Wei, Q.Q. Chemical constituents from Semiliquidambar cathayensis roots. Chin. Med. Mat. 2015, 38, 2543–2546. [Google Scholar]
- He, J.W.; Huang, X.Y.; Wang, Y.Q.; Liang, J.; Liu, R.H.; Zhong, G.Y.; Yang, L. A new flavonol glycoside from the flowers of Hosta plantaginea with cyclooxygenases-1/2 inhibitory and antioxidant activities. Nat. Prod. Res. 2019, 33, 1599–1604. [Google Scholar] [CrossRef]
- He, J.W.; Yang, L.; Mu, Z.Q.; Zhu, Y.Y.; Zhong, G.Y.; Liu, Z.Y.; Zhou, Q.G.; Cheng, F. Anti-inflammatory and antioxidant activities of flavonoids from the flowers of Hosta plantaginea. RSC Adv. 2018, 8, 18175–18179. [Google Scholar] [CrossRef]
- Yang, L.; Jiang, S.T.; Zhou, Q.G.; Zhong, G.Y.; He, J.W. Chemical constituents from the flower of Hosta plantaginea with cyclooxygenases inhibition and antioxidant activities and their chemotaxonomic significance. Molecules 2017, 22, 1825. [Google Scholar] [CrossRef]
- He, J.W.; Xu, H.S.; Yang, L.; He, W.W.; Wang, C.X.; Lin, F.; Lian, Y.Y.; Sun, H.B.; Zhong, G.Y. New isocoumarins and related metabolites from Talaromyces flavus. Nat. Prod. Commun. 2016, 11, 805–808. [Google Scholar] [CrossRef]
- He, J.W.; Wang, C.X.; Yang, L.; Chen, G.D.; Hu, D.; Guo, L.D.; Yao, X.S.; Gao, H. A pair of new polyketide enantiomers from three endolichenic fungal strains Nigrospora sphaerica, Alternaria alternata, and Phialophora sp. Nat. Prod. Commun. 2016, 11, 829–831. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.L.; Sang, M.G.; Liu, E.W.; Banahene, P.O.; Zhang, Y.; Wang, T.; Han, L.F.; Gao, X.M. Rapid profiling and pharmacokinetic studies of major compounds in crude extract from Polygonum multiflorum by UHPLC-Q-TOF-MS and UPLC-MS/MS. J. Pharm. Biomed. Anal. 2017, 140, 45–61. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Li, S.Z.; Pi, Z.F.; Song, F.R.; Lin, N.; Liu, S.; Liu, Z.Q. Chemical profiling of Wu-tou decoction by UPLC-Q-TOF-MS. Talanta 2014, 118, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Li, S.Z.; Liu, S.; Pi, Z.F.; Song, F.R.; Liu, Z.Q. Chemical profiling of Fufang-Xialian-Capsule by UHPLC-Q-TOF-MS and its antioxidant activity evaluated by in vitro method. J. Pharm. Biomed. Anal. 2017, 138, 289–301. [Google Scholar] [CrossRef]
- Li, Y.J.; Wei, H.L.; Qi, L.W.; Chen, J.; Ren, M.T.; Li, P. Characterization and identification of saponins in Achyranthes bidentata by rapid-resolution liquid chromatography with electrospray ionization quadrupole time-of-flight tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2010, 24, 2975–2985. [Google Scholar] [CrossRef]
- Kale, A.; Gaikwad, S.; Mundhe, K.; Deshpande, N.; Salvekar, J. Quantification of phenolics and flavonoids by spectrophotometer from-Juglans regia. Int. J. Pharma Bio Sci. 2010, 1, 1–4. [Google Scholar]
- Wu, W.; Yan, C.Y.; Li, L.; Liu, Z.Q.; Liu, S.Y. Studies on the flavones using liquid chromatography-electrospray ionization tandem mass spectrometry. J. Chromatogr. A 2004, 1047, 213–220. [Google Scholar] [CrossRef]
- Yan, M.M.; Chen, M.; Zhou, F.; Cai, D.S.; Bai, H.; Wang, P.L.; Lei, H.L.; Ma, Q. Separation and analysis of flavonoid chemical constituents in flowers of Juglans regia L. by ultra-high-performance liquid chromatography-hybrid quadrupole time-of-flight mass spectrometry. J. Pharm. Biomed. Anal. 2019, 164, 734–741. [Google Scholar] [CrossRef]
- Chen, H.X.; Shen, S.L.; Han, F.M.; Chen, Y. HPLC-ESI/MS analysis of stachydrine and its metabolites in rat urine. Acta Pharm. Sin. 2006, 41, 467–470. [Google Scholar]
- Wu, X.H.; Tang, S.H.; Jin, Y.; Wang, S.S.; Wang, X.J.; Hattori, M.; Zhang, H.; Wang, Z.G. Determination of the metabolic profile of gentianine after oral administration to rats by high performance liquid chromatography/electrospray ionization-trap mass spectrometry. J. Chromatogr. B. 2015, 989, 98–103. [Google Scholar] [CrossRef]
- Sun, H.; Wang, M.; Zhang, A.H.; Ni, B.; Dong, H.; Wang, X.J. UPLC–Q-TOF–HDMS analysis of constituents in the root of two kinds of aconitum using a metabolomics approach. Phytochem. Anal. 2013, 24, 263–276. [Google Scholar] [CrossRef]
- Tan, G.G.; Jing, J.; Zhu, Z.Y.; Lou, Z.Y.; Li, W.H.; Zhao, L.; Zhang, G.Q.; Chai, Y.F. Detection and identification of diterpenoid alkaloids, isoflavonoids and saponins in Qifu decoction and rat plasma by liquid chromatography-time-of-flight mass spectrometry. Biomed. Chromatogr. 2012, 26, 178–191. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.J.; Liu, Z.Q.; Song, F.R.; Yu, Z.; Li, H.L.; Liu, S.Y. Structural analysis of monoterpene glycosides extracted from Paeonia lactiflora Pall. using electrospray ionization Fourier transform ion cyclotron resonance mass spectrometry and high-performance liquid chromatography/electrospray ionization tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2007, 21, 3193–3199. [Google Scholar] [PubMed]
- Chen, L.L.; Qi, J.; Chang, Y.X.; Zhu, D.N.; Yu, B.Y. Identification and determination of the major constituents in Traditional Chinese Medicinal formula Danggui-Shaoyao-San by HPLC-DAD-ESI-MS/MS. J. Pharm. Biomed. Anal. 2009, 50, 127–137. [Google Scholar] [CrossRef]
- Huang, X.F.; Ouyang, H.; Li, J.M.; Lu, Y.J.; Li, W.; Gong, Q.F. Identification of characteristic constituents in Atractylodis Macrocephalae Rhizoma from different regions by UPLC-Q-TOF-MS/MS. Chin. J. Exp. Tradit. Med. Form. 2017, 23, 27–33. [Google Scholar]
- Xiong, Y.K.; Liu, X.; Wan, Y.T.; Yan, Z.H.; Liu, Z.Y. UPLC-ESI/MS determination of five active ingredients in Zangyinchen capsule. Res. Explo. Lab. 2017, 36, 40–42. [Google Scholar]
- Zhang, Q.; Qi, M.D.; Kang, Y.; Wang, F.F.; Qu, J.X.; Sun, Z.R.; Guo, Y.Z.; Wang, C.G.; Liu, Y. Rapid identification of glycosides and aglycones in pericarp of Sapindus mukorossi Gaertn. Using UHPLC-LTQ Orbitrap MS. J. Chin. Mass Spectrom. Soc. 2018, 39, 224–239. [Google Scholar]
- Xue, Q.; Li, R.; Song, S.W.; Miao, W.J.; Liu, J.; Chen, H.B.; Guo, D.A. A targeted strategy to analyze untargeted mass spectral data: Rapid chemical profiling of Scutellaria baicalensis using ultra-highperformance liquid chromatography coupled with hybrid quadrupole orbitrap mass spectrometry and key ion filtering. J. Chromatog. A 2016, 1441, 83–95. [Google Scholar]
- Chen, Y.J.; Wu, H.; Wei, Z.Y.; Chen, L.M.; Dong, J.J.; Liu, J.; Jia, Z.X.; Xiao, H.B. Identification of chemical constituents in Aster tataricus by UHPLC-Q-TOF-MS/MS. Acta Pharm. Sin. 2019, 54, 1645–1654. [Google Scholar]
- Li, J.M.; He, M.Z.; Ouyang, H.; Tan, T.; Li, Y.; Feng, Y.L.; Yang, S.L. Rapid identification of chemical constituents of Folium hibisci mutabilis by UHPLC-Q-TOF-MS/MS. Chin. Pharm. J. 2016, 51, 1162–1168. [Google Scholar]
- Zhou, D.Y.; Xu, Q.; Xue, X.Y.; Zhang, F.F.; Liang, X.M. Analysis of flavonoid glycosides in Fructus Aurantii by high performance liquid chromatography-electrospray ionization mass spectrometry. Chinese J. Anal. Chem. 2006, 34, 31–35. [Google Scholar]
- Liu, G.Q.; Dong, J.; Wang, H.; Wan, L.R.; Duan, Y.S.; Chen, S.Z. ESI fragmentation studies of four tea catechins. Chem. J. Chin. Univ. 2009, 30, 1566–1570. [Google Scholar]
- Tong, C.Y.; Peng, M.J.; Shi, S.Y. Rapid identification of flavonoid compounds in Pericarpium Citri Reticulatae by online extraction-high performance liquid chromatography- diode array detection-quadrupole time-of-flight mass spectrometry. Chin. J. Chromatogr. 2018, 36, 278–284. [Google Scholar]
- Wang, Y.M.; Liu, Q.; Fan, S.J.; Yang, X.T.; Ming, L.L.; Wang, H.M.; Liu, J.H. Rapid analysis and characterization of multiple constituents of corn silk aqueous extract using ultra-high-performance liquid chromatography combined with quadrupole time-of-flight mass spectrometry. J. Sep. Sci. 2019, 42, 3054–3066. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.L.; Qi, W.; Wu, X.D.; He, Z.M. Fragmentation mechanisms of phenolic acids from Danshen in an ion trap by electrospray ionization multi-stage tandem mass spectrometry. J. Chin. Mass Spectr. Soc. 2008, 29, 129–136. [Google Scholar]
- Wan, M.Q.; Zhang, Y.B.; Yang, Y.F.; Liu, X.Y.; Jia, L.Y.; Jia, L.Y.; Yang, X.W. Analysis of the chemical composition of Angelicae Pubescentis Radix by ultra-performance liquid chromatography and quadrupole time-of-flighttandem mass spectrometry. J. Chin. Pharm. Sci. 2019, 28, 145–159. [Google Scholar]
- Lasano, N.F.; Hamid, A.H.; Karim, R.; Dek, M.S.P.; Shukri, R.; Ramli, N.S. Nutritional composition, anti-diabetic properties and identification of active compounds using UHPLC-ESI-orbitrap-MS/MS in Mangifera odorata L. peel and seed kernel. Molecules 2019, 24, 320. [Google Scholar] [CrossRef]
- Chen, Y.L.; Li, N.; Yao, Y.Y.; Liu, X.; Guo, Y.L.; Zhang, X.M.; Liang, X.M. Rapid analysis of chemical composition of Acalypha australis based on UHPLC-Q-TOF-MS/MS. Chin. Med. Mat. 2018, 41, 1352–1358. [Google Scholar]
- Wang, T.H.; Zhang, J.; Qiu, X.H.; Bai, J.Q.; Gao, Y.H.; Xu, W. Application of ultra-high-performance liquid chromatography coupled with LTQ-orbitrap mass spectrometry for the qualitative and quantitative analysis of Polygonum multiflorum Thumb. and its processed products. Molecules 2016, 21, 40. [Google Scholar] [CrossRef]
- Miranda, V.; Maycock, C.D.; Ventura, M.R. A Stereoselective Synthesis of (+)-Piscidic Acid and Cimicifugic Acid L. Eur. J. Org. Chem. 2015, 34, 7529–7533. [Google Scholar] [CrossRef]
- Liu, M.; He, M.Z.; Gao, H.W.; Guo, S.; Jia, J.; Ouyang, H.; Feng, Y.L.; Yang, S.L. Strategy for rapid screening of antioxidant and anti-inflammatory active ingredients in Gynura procumbens (Lour.) Merr. based on UHPLC-Q-TOF-MS/MS and characteristic ion filtration. Biomed. Chromatogr. 2019, 33, e4635. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.L.; Liang, X.R.; Xie, Y.Y. Qualitative and quantitative analysis on the chemical constituents in Orthosiphon stamineus Benth. using ultra high-performance liquid chromatography coupled with electrospray ionization tandem mass spectrometry. J. Pharm. Biomed. Anal. 2019, 164, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Ji, D.; Huang, Z.Y.; Fei, C.H.; Xue, W.W.; Lu, T.L. Comprehensive profiling and characterization of chemical constituents of rhizome of Anemarrhena asphodeloides Bge. J. Chromatogr. B 2017, 1060, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.P.; Chen, Q.H.; Liu, X.Y. Diterpenoid alkaloids. Nat. Prod. Rep. 2010, 27, 529–570. [Google Scholar] [CrossRef]
- He, F.; Wang, C.J.; Xie, Y.; Cheng, C.S.; Liu, Z.Q.; Liu, L.; Zhou, H. Simultaneous quantification of nine aconitum alkaloids in Aconiti Lateralis Radix Praeparata and related products using UHPLC-QQQ-MS/MS. Sci Rep.-UK 2017, 7, 13023. [Google Scholar] [CrossRef]
- Song, L.; Zhang, H.; Liu, X.; Zhao, Z.L.; Chen, S.L.; Wang, Z.T.; Xu, H.X. Rapid determination of yunaconitine and related alkaloids in aconites and aconite-containing drugs by ultra high-performance liquid chromatography–tandem mass spectrometry. Biomed. Chromatogr. 2012, 26, 1567–1574. [Google Scholar] [CrossRef]
- Zhang, C.M.; He, Y.; Li, T.T.; Miao, X.L.; Song, M.T.; Qian, C.M.; Li, R.M. Analysis of chemical components in herbal formula Qi Bai Granule by UPLC-ESI-Q-TOF-MS. Nat. Prod. Res. 2019, 33, 2271–2275. [Google Scholar] [CrossRef]
- Cecilia, J.S.; Jesús, L.S.; Celia, R.P.; Antonio, S.C.; Alberto, F.G. Comprehensive, untargeted, and qualitative RP-HPLC-ESIQTOF/MS2 metabolite profiling of green asparagus (Asparagus officinalis). J. Food Compos. Anal. 2016, 46, 78–87. [Google Scholar]
- Wang, S.F.; Chen, P.H.; Jiang, W.; Wu, L.H.; Chen, L.L. Identification of the effective constituents for anti-inflammatory activity of Ju-Zhi-Jiang-Tang: An ancient traditional Chinese medicine formula. J. Chromatogr. A 2014, 1348, 105–124. [Google Scholar] [CrossRef]
- Matsumoto, T.; Shishido, A.; Morita, H.; Itokawa, H.; Takeya, K. Cyclolinopeptides F-I,cyclic peptides from linseed. Phytochemistry 2001, 57, 251–260. [Google Scholar] [CrossRef]
Sample Availability: Not available. |
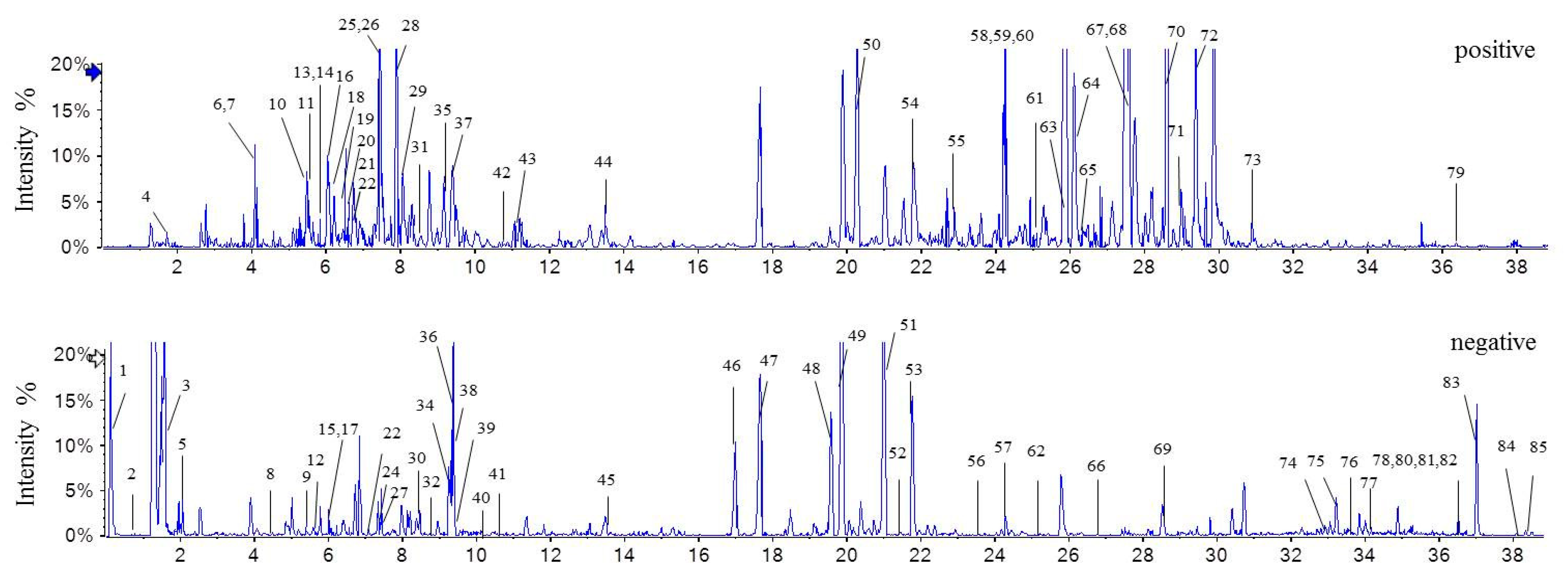
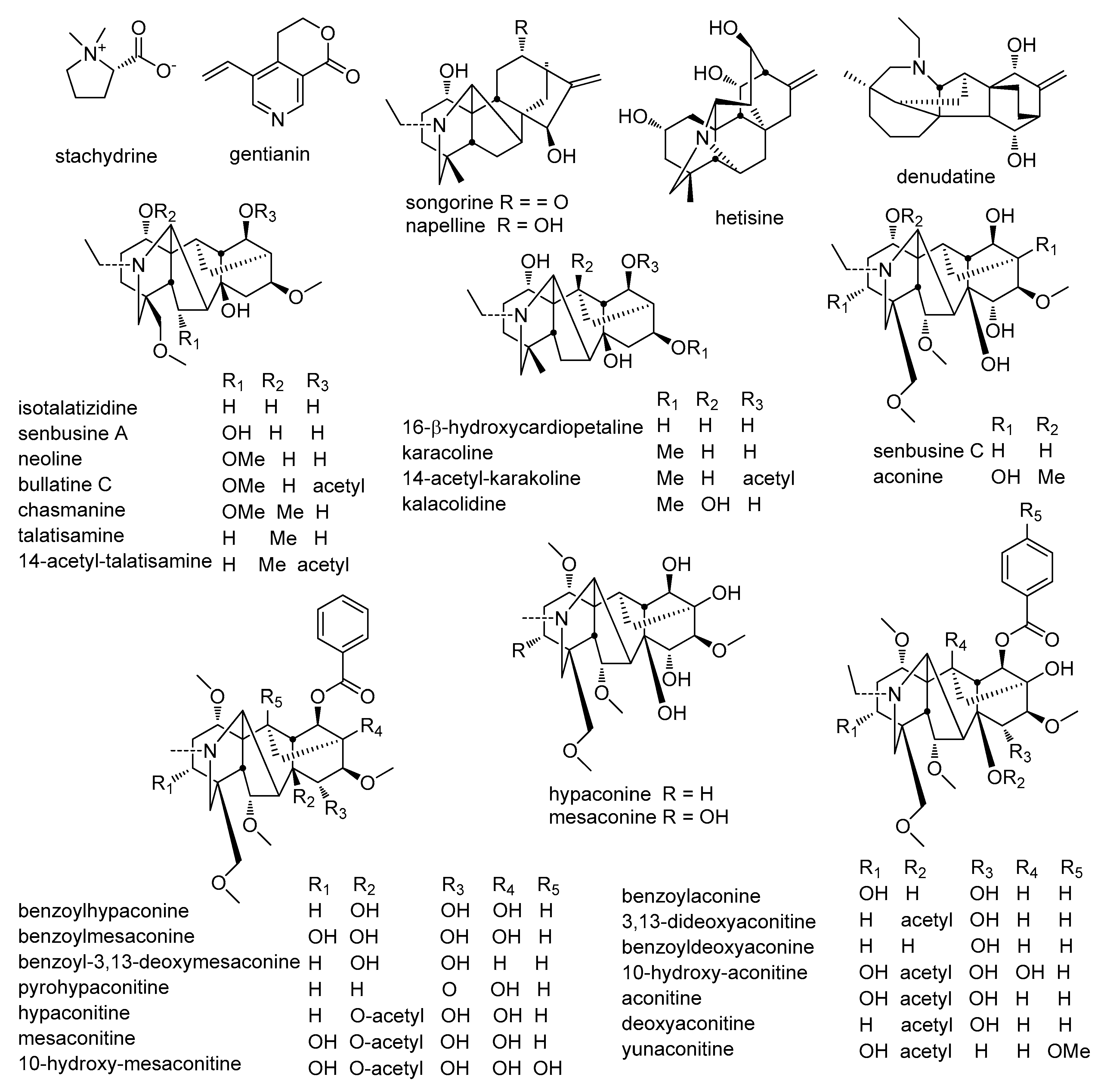

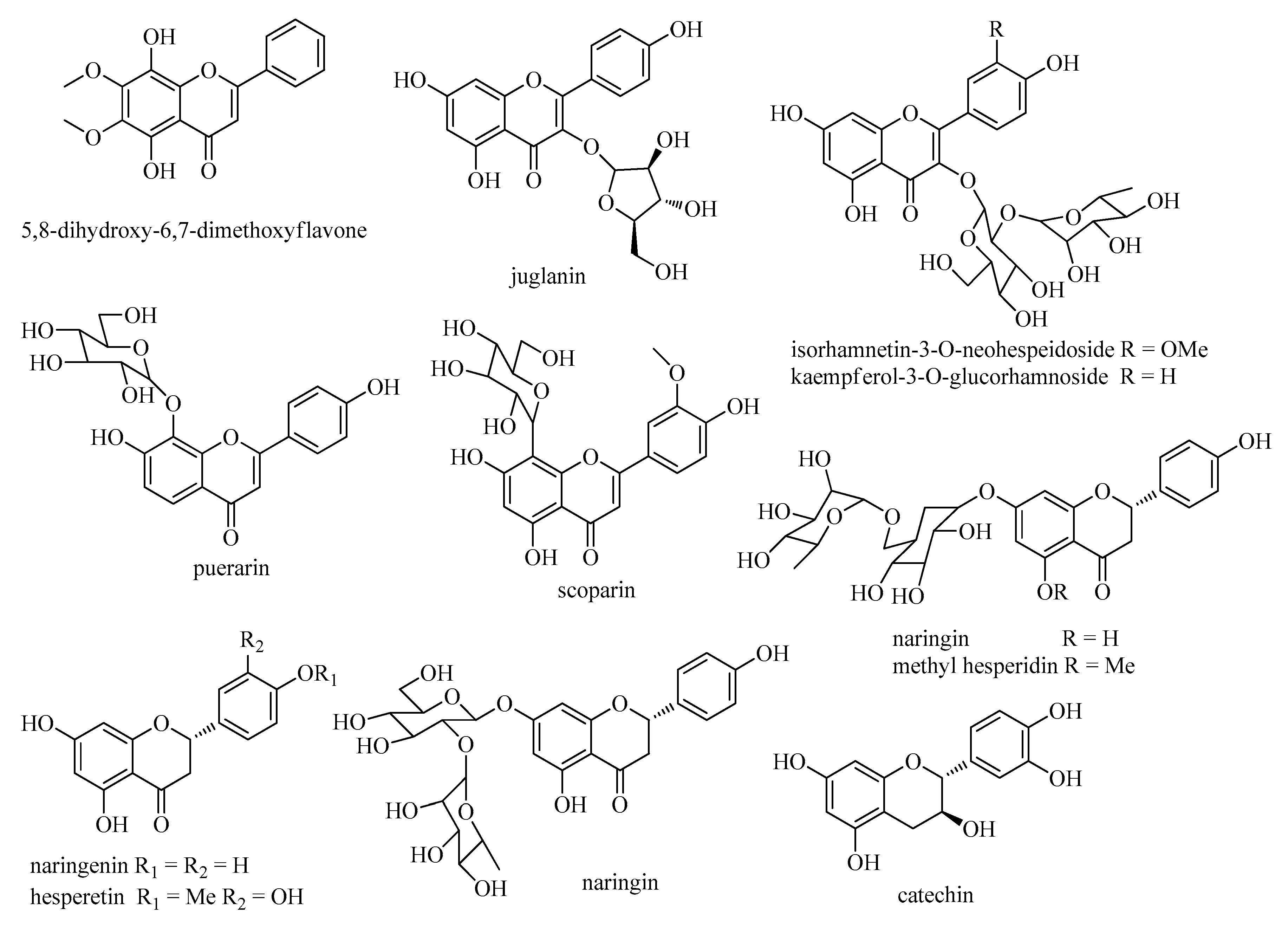
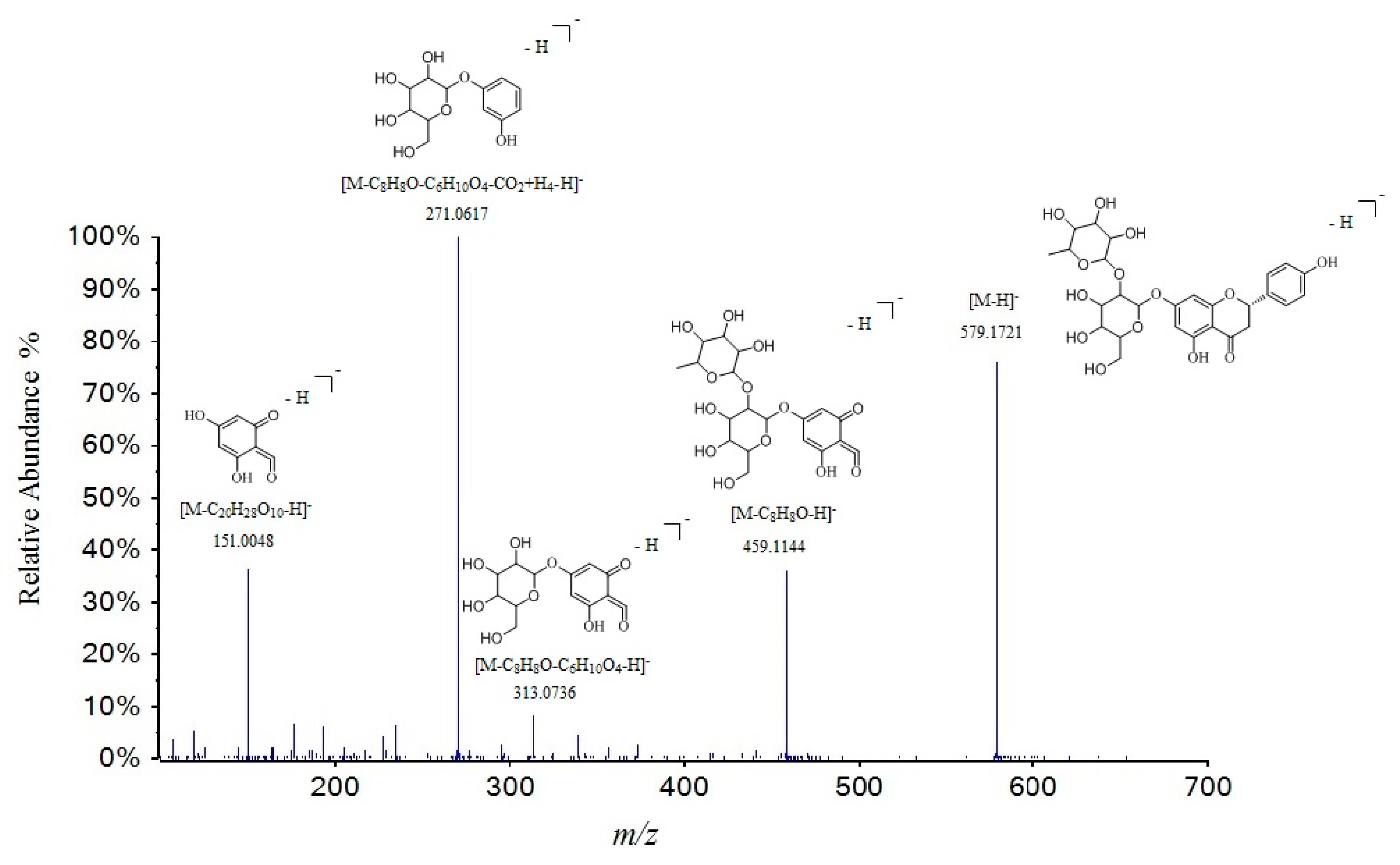
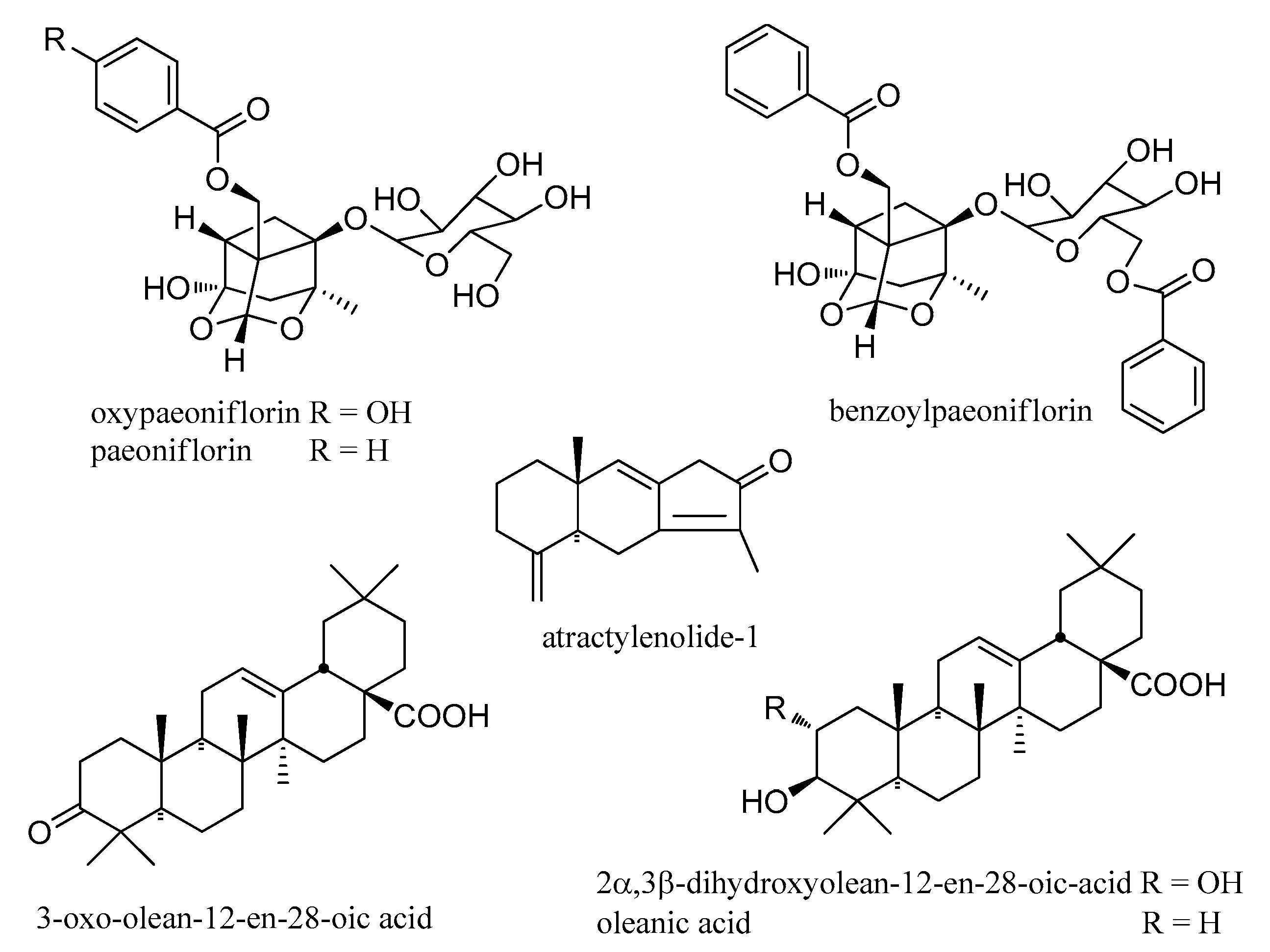
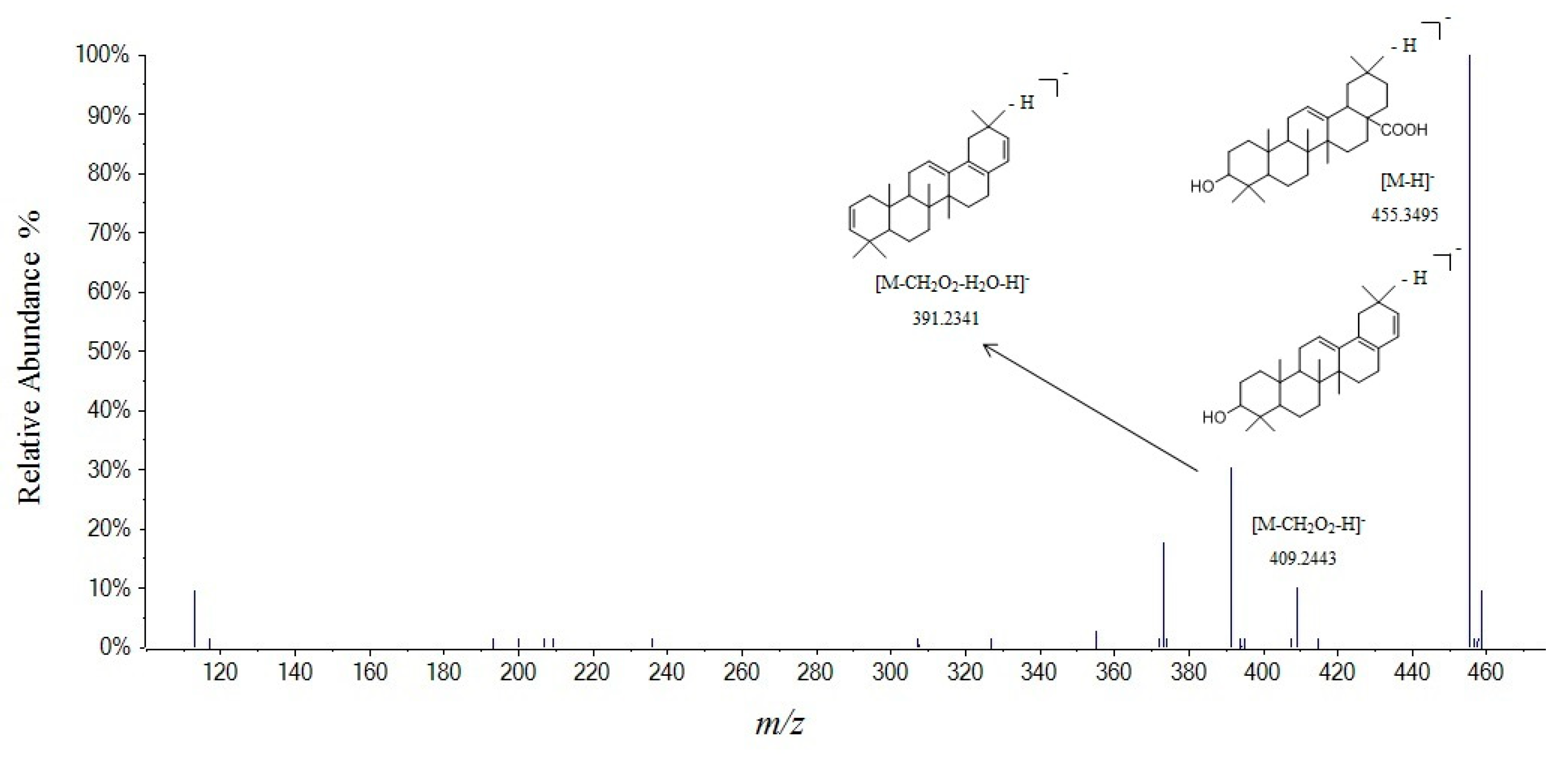
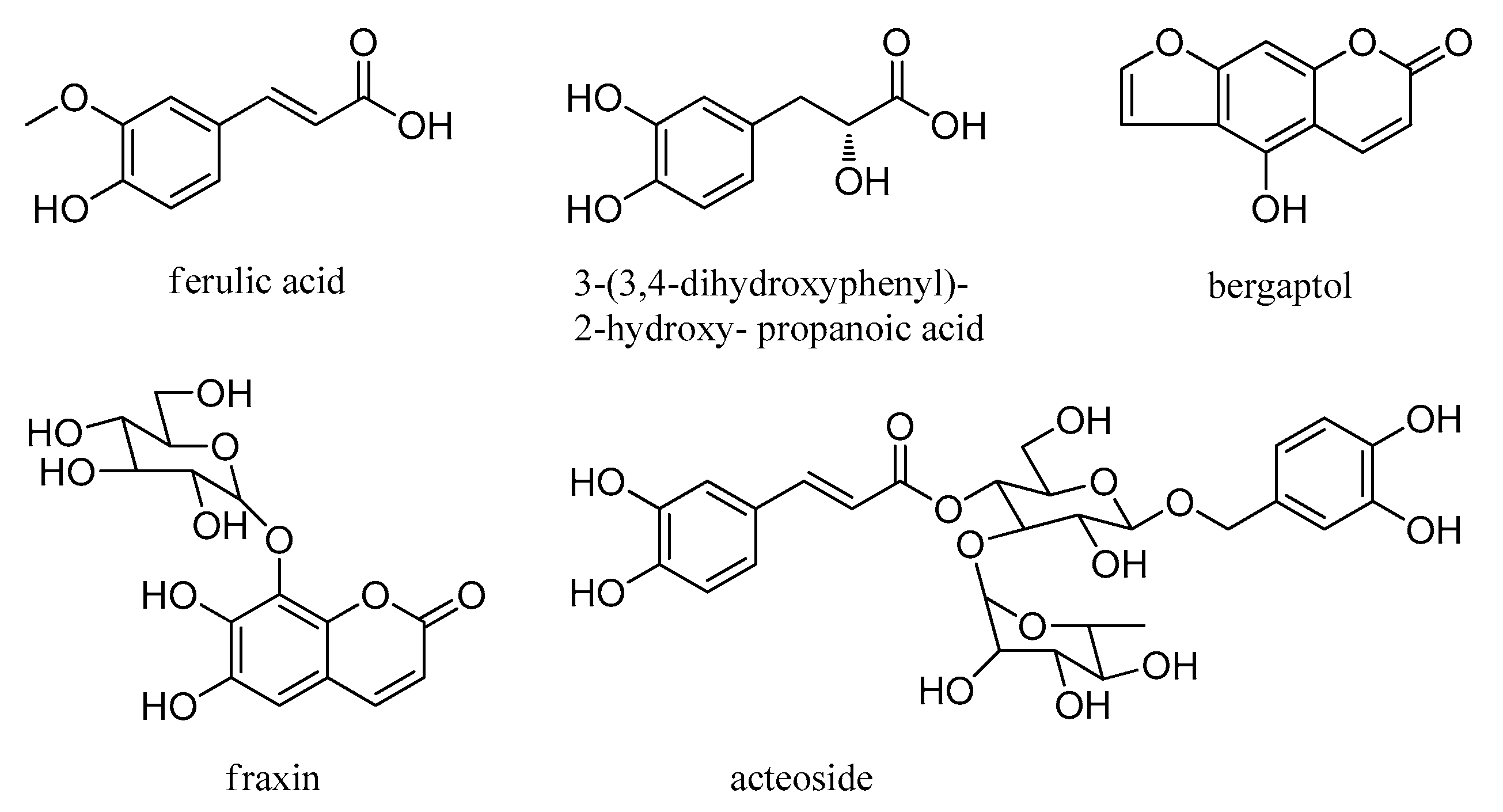
| No. | tR (min) | Compounds | Molecular Formula | Molecular Weight | Measured Mass [M + H] | Error (ppm) | MS2 | Ref. |
|---|---|---|---|---|---|---|---|---|
| Alkaloids | ||||||||
| 4 | 1.74 | stachydrine | C7H13NO2 | 143.0946 | 144.1020 | 0.8 | 144.1004 a, 128.0647, 102.0585 | [21] |
| 6 | 4.06 | gentianin | C10H9NO2 | 175.0633 | 176.0706 | −0.2 | 176.0714 a, 148.0763, 133.0524, 130.0660, 103.0560, 120.0820, 117.0350 | [22] |
| 7 | 4.10 | kalacolidine | C22H35NO5 | 393.2515 | 394.2593 | 1.4 | 394.2588, 376.2485 a | [23] |
| 10 | 5.49 | mesaconine | C24H39NO9 | 485.2625 | 486.2702 | 1.0 | 486.2697 a, 436.2332, 404.2070 | [23] |
| 11 | 5.54 | 16-β-hydroxycardiopetaline | C21H33NO4 | 363.2410 | 364.2486 | 1.0 | 364.2488 a, 346.2382, 328.2275 | [24] |
| 13 | 5.84 | senbusine A | C23H37NO6 | 423.2621 | 424.2699 | 1.3 | 424.2701 a, 406.2591, 388.2487 | [23] |
| 16 | 6.05 | carmichaeline | C22H35NO4 | 377.2566 | 378.2645 | 1.6 | 378.2640, 360.2532 a, 328.2271 | [23] |
| 18 | 6.10 | isotalatizidine | C23H37NO5 | 407.2672 | 408.2749 | 1.2 | 390.2642 a, 358.2378 | [23] |
| 19 | 6.46 | aconine | C25H41NO9 | 499.2781 | 500.2861 | 1.3 | 500.2863 a, 450.2495 | [15] |
| 20 | 6.56 | songorine | C22H31NO3 | 357.2304 | 358.2381 | 1.2 | 358.2375, 340.2270 a | [23] |
| 21 | 6.6 | napelline | C22H33NO3 | 359.2460 | 360.2536 | 0.7 | 360.2536, 342.2430 a | [15] |
| 22 | 6.74 | hetisine | C20H27NO3 | 329.1991 | 330.2069 | 1.6 | 330.2062 a, 312.1954 | [23] |
| 25 | 7.38 | hypaconine | C24H39NO8 | 469.2676 | 470.2751 | 0.6 | 470.2764 a, 438.2498, 406.2216 | [24] |
| 26 | 7.44 | senbusine C | C24H39NO7 | 453.2727 | 454.2805 | 1.3 | 454.2786 a, 404.2427 | [23] |
| 28 | 7.91 | neoline | C24H39NO6 | 437.2777 | 438.2854 | 0.8 | 438.2841 a, 420.2738, 388.2480, 356.2222, 154.1223 | [23] |
| 29 | 8.08 | 14-acetyl-karakoline | C24H37NO5 | 419.2672 | 420.2749 | 1.0 | 420.2757 a, 402.2653 | [24] |
| 35 | 9.18 | talatisamine | C24H39NO5 | 421.2828 | 422.2906 | 1.2 | 390.2642 a, 358.2379 | [24] |
| 37 | 9.41 | denudatine | C22H33NO2 | 343.2511 | 344.2589 | 1.4 | 344.2587 a, 326.2480 | [23] |
| 42 | 10.76 | bullatine C | C26H41NO7 | 479.2883 | 480.2962 | 1.2 | 480.2980 a, 462.2858, 430.2587, 398.2295 | [23] |
| 43 | 11.22 | chasmanine | C25H41NO6 | 451.2934 | 452.3012 | 1.2 | 452.3016 a, 420.2755, 388.2490 | [23] |
| 44 | 13.41 | 14-acetyl-talatisamine | C26H41NO6 | 463.2934 | 464.3011 | 1.0 | 464.3011 a, 432.2746 | [23] |
| 50 | 20.28 | benzoylmesaconine | C31H43NO10 | 589.2887 | 590.2966 | 1.1 | 590.2941 a, 540.2579, 508.2323, 105.0341 | [15] |
| 54 | 21.85 | benzoylaconine | C32H45NO10 | 603.3044 | 604.3122 | 0.9 | 604.3110 a, 554.2748 | [15] |
| 55 | 22.91 | benzoylhypaconine | C31H43NO9 | 573.2938 | 574.3015 | 0.8 | 574.3017 a, 542.2755, 510.2495 | [15] |
| 58 | 24.20 | benzoyl-3,13-deoxymesaconine | C31H43NO8 | 557.2989 | 558.3068 | 1.1 | 558.3055 a, 540.2955, 508.2697 | [15] |
| 59 | 24.25 | 10-hydroxy-mesaconitine | C33H45NO12 | 647.2942 | 648.3024 | 1.4 | 648.3002 a, 588.2791, 556.2540, 370.1653 | [23] |
| 60 | 24.27 | benzoyldeoxyaconine | C32H45NO9 | 587.3094 | 588.3172 | 0.8 | 588.3174 a, 556.2918 | [15] |
| 61 | 25.21 | pyrohypaconitine | C31H41NO8 | 555.2832 | 556.2910 | 0.9 | 556.2915 a, 524.2647, 492.2414, 452.2072, 402.2285, 238.1807, 192.1383 | [15] |
| 63 | 25.87 | mesaconitine | C33H45NO11 | 631.2993 | 632.3070 | 0.7 | 632.3032 a, 572.2826, 540.2566, 512.2622, 508.2315, 354.1685, 105.0342 | [15] |
| 64 | 26.12 | 10-hydroxy-aconitine | C34H47NO12 | 661.3098 | 662.3180 | 1.4 | 662.3168 a, 602.2957, 570.2702, 384.1809 | [15] |
| 67 | 27.49 | hypaconitine | C33H45NO10 | 615.3044 | 616.3125 | 1.5 | 616.3089 a, 556.2876, 524.2618, 496.2638, 342.2055, 338.1739, 105.0340 | [15] |
| 68 | 27.58 | aconitine | C34H47NO11 | 645.3149 | 646.3232 | 1.5 | 646.3199 a, 586.2985, 554.2735, 526.2783, 522.2487, 368.1850, 105.0342 | [23] |
| 70 | 28.60 | deoxyaconitine | C34H47NO10 | 629.3200 | 630.3281 | 1.3 | 630.3256 a, 570.3047, 538.2787, 510.2830, 478.2575, 356.2219, 352.1905 | [15] |
| 71 | 28.98 | yunaconitine | C35H49NO11 | 659.3306 | 660.3386 | 1.2 | 660.3396 a, 572.2866, 540.2591, 354.1735 | [24] |
| 72 | 29.38 | 3,13-dideoxyaconitine | C34H47NO9 | 613.3251 | 614.3326 | 0.4 | 614.3302 a, 554.3091, 522.2835, 494.2880, 462.2620, 105.0345 | [23] |
| Terpenoids | ||||||||
| 14 | 5.94 | oxypaeoniflorin | C23H28O12 | 496.1581 | 497.1657 | 0.7 | 497.2676, 349.1575, 197.0831, 133.0687, 121.0297 a | [25] |
| 31 | 8.56 | paeoniflorin | C23H28O11 | 480.1632 | 481.1711 | 1.4 | 319.1245, 197.0808, 179.0691,151.0750, 133.0650, 105.0342 a | [26] |
| 65 | 26.21 | benzoylpaeoniflorin | C30H32O12 | 584.1894 | 585.1970 | 0.5 | 585.3271, 319.1195, 267.0885, 249.0785, 197.0807, 179.0705, 121.0666,105.0349 a | [26] |
| 73 | 30.98 | atractylenolide-1 | C15H18O2 | 230.1307 | 231.1380 | 0.0 | 213.1257, 163.0778, 155.0848, 143.0931, 128.0610, 115.0541, 105.0712 a | [27] |
| 79 | 36.18 | 3-oxo-olean-12-en-28-oic acid | C30H46O3 | 454.3447 | 455.3516 | −0.8 | 455.3539, 437.3426, 247.1668, 233.1531, 229.1584, 197.1332, 189.1615 a | [28,29] |
| No. | tR (min) | Compounds | Molecular Formula | Molecular Weight | Measured Mass [M − H]− | Error (ppm) | MS2 | Ref. |
|---|---|---|---|---|---|---|---|---|
| Flavonoids | ||||||||
| 24 | 7.33 | puerarin | C21H20O9 | 416.1107 | 415.1015 | −4.6 | 415.0970, 295.0614, 277.0490, 267.0653 a | [30] |
| 33 | 8.85 | scoparin | C22H22O11 | 462.1162 | 461.1069 | −4.4 | 415.0991 a, 252.0361 | [30] |
| 34 | 9.17 | isorhamnetin-3-O-neohespeidoside | C28H32O16 | 624.1690 | 623.1602 | −2.5 | 623.1609, 461.1022 a, 417.1203, 315.0710, 153.0226, 145.0338 | [31] |
| 36 | 9.38 | kaempferol-3-O-glucorhamnoside | C27H30O15 | 594.1585 | 593.1485 | −4.6 | 593.1010, 547.1243, 430.0954, 275.0347,112.9889 a | [32] |
| 39 | 9.44 | methyl hesperidin | C29H36O15 | 624.2054 | 623.1963 | −2.9 | 623.0455, 577.0923, 534.0988, 461.1451, 410.0366, 315.1067, 145.0319 a | [33] |
| 17 | 6.06 | catechin | C15H14O6 | 290.0790 | 289.0715 | −1.1 | 221.0899, 205.0532, 203.0700 a, 187.0372, 159.0452, 125.0280, 123.0486 | [34] |
| 46 | 17.01 | naringin | C27H32O14 | 580.1792 | 579.1716 | −0.6 | 579.1721, 459.1144, 313.0736, 271.0617 a, 177.0209, 151.0048 | [33] |
| 48 | 19.58 | hesperidin | C28H34O15 | 610.1898 | 609.1815 | −1.6 | 609.1826, 301.0706 a, 286.0481, 242.0583 | [35] |
| 56 | 23.57 | 5,8-dihydroxy-6,7-dimethoxyflavone | C17H14O6 | 314.0790 | 313.0709 | −2.9 | 297.0313, 283.0226, 266.0197, 255.0309 a, 227.0318, 211.0393, 185.0235, 183.0456 | [33] |
| 57 | 24.10 | juglanin | C20H18O10 | 418.0900 | 417.0814 | −3.1 | 161.0578, 135.0527, 129.0226 a | [20] |
| 62 | 25.32 | naringenin | C15H12O5 | 272.0685 | 271.0609 | −1.1 | 151.0030, 119.0509 a, 117.0421 | [33] |
| 66 | 26.53 | hesperetin | C16H14O6 | 302.0790 | 301.0713 | −1.7 | 258.0578 a, 134.0383 | [33] |
| Terpenoids | ||||||||
| 76 | 33.74 | 2α,3β-dihydroxyolean-12-en-28-oic-acid | C30H48O4 | 472.3553 | 471.3466 | −3.0 | 471.3494 a, 451.0162, 411.0302, 389.2158, 330.9965, 264.9917 | [28,29] |
| 81 | 36.45 | oleanic acid | C30H48O3 | 456.3604 | 455.3512 | −4.2 | 455.3495 a, 409.2443, 391.2341, 373.2227, 355.2079 | [28,29] |
| Phenylpropanoids | ||||||||
| 15 | 6.01 | ferulic acid | C10H10O4 | 194.0579 | 193.0510 | 2.0 | 134.0379 a | [36] |
| 22 | 7.10 | fraxin | C16H18O10 | 370.0900 | 369.0814 | −3.5 | 223.0462 a, 205.0350, 129.0210, 125.0241 | [32] |
| 32 | 8.76 | 3-(3,4-dihydroxyphenyl)-2-hydroxy-propanoic acid | C9H10O5 | 198.0528 | 197.0456 | 0.2 | 162.8375 a, 160.8401, 138.0358, 123.0085 | [37] |
| 38 | 9.44 | acteoside | C29H36O15 | 624.2054 | 623.1963 | −2.9 | 623.0455, 577.0923, 461.1451, 315.1067, 145.0319 a | [30] |
| 52 | 21.42 | bergaptol | C11H6O4 | 202.0266 | 201.0192 | −0.8 | 228.9172 a, 166.8855, 147.8874, 117.0436 | [38] |
| Fatty Acids | ||||||||
| 5 | 2.05 | citric acid | C6H8O7 | 192.0270 | 191.0199 | 0.7 | 146.9074, 111.0110 a | [36] |
| 69 | 28.24 | trihydroxy-octadecaenoic acid | C18H34O5 | 330.2406 | 329.2326 | −1.5 | 329.2354, 229.1443, 211.1346, 183.1371, 171.1026 a, 139.1137 | [27] |
| 74 | 32.9 | dihydroxy-octadecatrienoic acid | C18H30O4 | 310.2144 | 309.2069 | −0.9 | 291.1995 a, 199.8548, 179.1442, 110.0373 | [27] |
| 77 | 34.13 | hydroxy-octadecatrienoic acid | C18H30O3 | 294.2195 | 293.2121 | −0.4 | 293.2080 a, 199.8526, 149.0939, 125.1018 | [28] |
| 78 | 36.16 | linolenic acid | C18H30O2 | 278.2246 | 277.2171 | −0.7 | 134.8951 a | [28] |
| 80 | 36.22 | stearic acid | C18H36O2 | 284.2715 | 283.2640 | −0.9 | 283.2633, 199.8512 a | [28] |
| 83 | 37.05 | linoleic acid | C18H32O2 | 280.2402 | 279.2329 | −0.2 | 279.2319 a, 261.2194 | [28] |
| 84 | 38.09 | palmitic acid | C16H32O2 | 256.2402 | 255.2332 | 0.9 | 256.2333, 255.2327 a, 114.9333 | [28] |
| 85 | 38.20 | oleic acid | C18H34O2 | 282.2559 | 281.2484 | −0.9 | 281.2489 a | [28] |
| Cyclic Peptides | ||||||||
| 27 | 7.59 | cyclo trileucyl (or isoleucyl) | C18H33N3O3 | 339.2522 | 384.2488 b | −1.3 | 135.0456 a | [28] |
| 40 | 10.07 | cyclo tetraleucyl (or isoleucyl) | C24H44N4O4 | 452.3363 | 497.3328 b | −1.1 | 497.1555, 451.3294 a, 433.3159, 337.2669, 224.1758, 137.0247 | [28] |
| 45 | 13.48 | cyclo pentaleucyl (or isoleucyl) | C30H55N5O5 | 565.4203 | 610.4168 b | −1.0 | 564.4112 a, 546.4021, 225.1592 | [28] |
| 47 | 17.66 | cyclo hexaleucyl (or isoleucyl) | C36H66N6O6 | 678.5044 | 723.5020 b | 0.7 | 677.4961 a | [28] |
| 49 | 19.86 | cyclo hetaleucyl (or isoleucyl) | C42H77N7O7 | 791.5885 | 836.5862 b | 0.8 | 790.5791 a | [28] |
| 51 | 21.0 | cyclo octaleucyl (or isoleucyl) | C48H88N8O8 | 904.6725 | 949.6704 b | 0.9 | 946.6691, 903.6636 a | [28] |
| 53 | 21.78 | cyclo nonaleucyl (or isoleucyl) | C54H99N9O9 | 1017.7566 | 1062.7546 b | 0.9 | 1062.7547, 1016.7472 a | [28] |
| Others | ||||||||
| 1 | 0.35 | glucogallin | C13H16O10 | 332.0744 | 331.0662 | −2.7 | 169.0124, 125.0257 a | [39] |
| 2 | 0.69 | gallic acid | C7H6O5 | 170.0215 | 169.0149 | 3.6 | 124.0178 a | [40] |
| 3 | 1.46 | sucrose | C12H22O11 | 342.1162 | 341.1078 | −3.5 | 221.0641, 179.0592, 161.0419, 119.0379, 113.0253 a | [41] |
| 8 | 4.29 | piscidic acid | C11H12O7 | 256.0583 | 255.0508 | −0.7 | 218.8641, 180.9830, 165.0550 a, 118.9815 | [42] |
| 9 | 5.37 | vanillin | C8H8O3 | 152.0473 | 151.0410 | 4.1 | 105.0368 a | [43] |
| 12 | 5.59 | protocatechuic acid pentoside | C12H14O8 | 286.0689 | 285.0611 | −1.6 | 152.0117, 108.0243 a | [44] |
| 30 | 8.37 | vanillic acid | C8H8O4 | 168.0423 | 167.0354 | 2.2 | 108.0206 a | [36] |
| 41 | 10.65 | paeonol | C9H10O3 | 166.0630 | 165.0564 | 3.8 | 147.0476, 119.0505, 117.0379 a, 103.0575, 101.0392 | [31] |
| 75 | 33.10 | dibutyl phthalate | C16H22O4 | 278.1518 | 277.1444 | −0.3 | 147.0072 a | [45] |
| 82 | 36.52 | dimethisterone | C23H32O2 | 340.2402 | 339.2326 | −1.1 | 339.2317, 163.1140 a | [45] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.; Liu, R.-H.; He, J.-W. Rapid Analysis of the Chemical Compositions in Semiliquidambar cathayensis Roots by Ultra High-Performance Liquid Chromatography and Quadrupole Time-of-Flight Tandem Mass Spectrometry. Molecules 2019, 24, 4098. https://doi.org/10.3390/molecules24224098
Yang L, Liu R-H, He J-W. Rapid Analysis of the Chemical Compositions in Semiliquidambar cathayensis Roots by Ultra High-Performance Liquid Chromatography and Quadrupole Time-of-Flight Tandem Mass Spectrometry. Molecules. 2019; 24(22):4098. https://doi.org/10.3390/molecules24224098
Chicago/Turabian StyleYang, Li, Rong-Hua Liu, and Jun-Wei He. 2019. "Rapid Analysis of the Chemical Compositions in Semiliquidambar cathayensis Roots by Ultra High-Performance Liquid Chromatography and Quadrupole Time-of-Flight Tandem Mass Spectrometry" Molecules 24, no. 22: 4098. https://doi.org/10.3390/molecules24224098
APA StyleYang, L., Liu, R.-H., & He, J.-W. (2019). Rapid Analysis of the Chemical Compositions in Semiliquidambar cathayensis Roots by Ultra High-Performance Liquid Chromatography and Quadrupole Time-of-Flight Tandem Mass Spectrometry. Molecules, 24(22), 4098. https://doi.org/10.3390/molecules24224098





