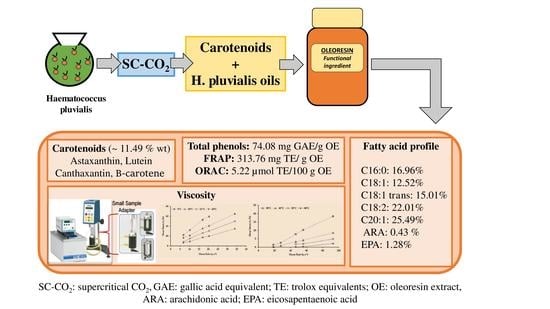
Determining the Potential of Haematococcus pluvialis Oleoresin as a Rich Source of Antioxidants
Abstract
1. Introduction
2. Results and Discussion
2.1. Carotenoid Content in Oleoresin Samples of Haematococcus Pluvialis
2.2. Antioxidant Capacity and Total Phenol Content
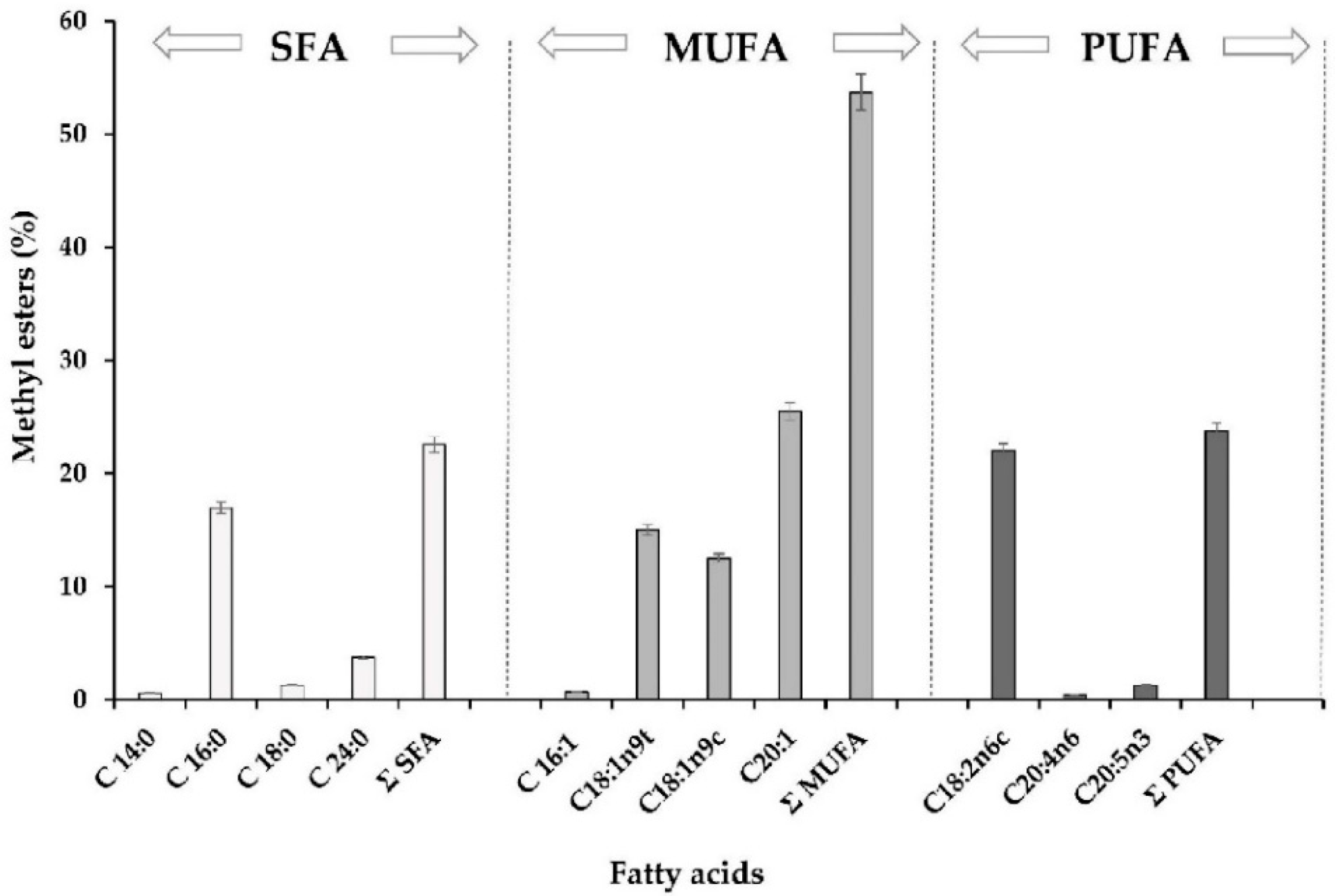
2.3. Fatty Acid Profile of Oleoresin Samples from H. pluvialis
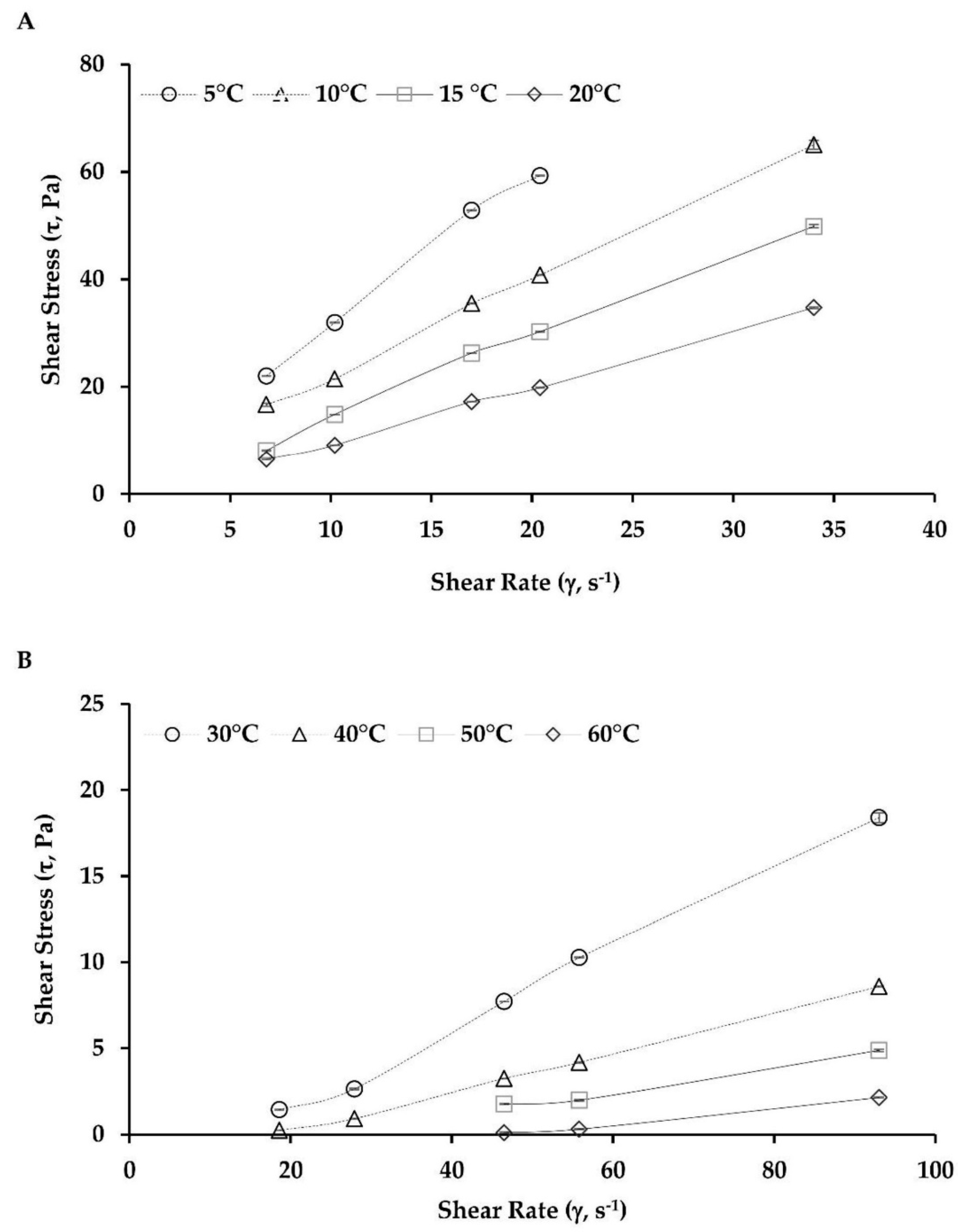
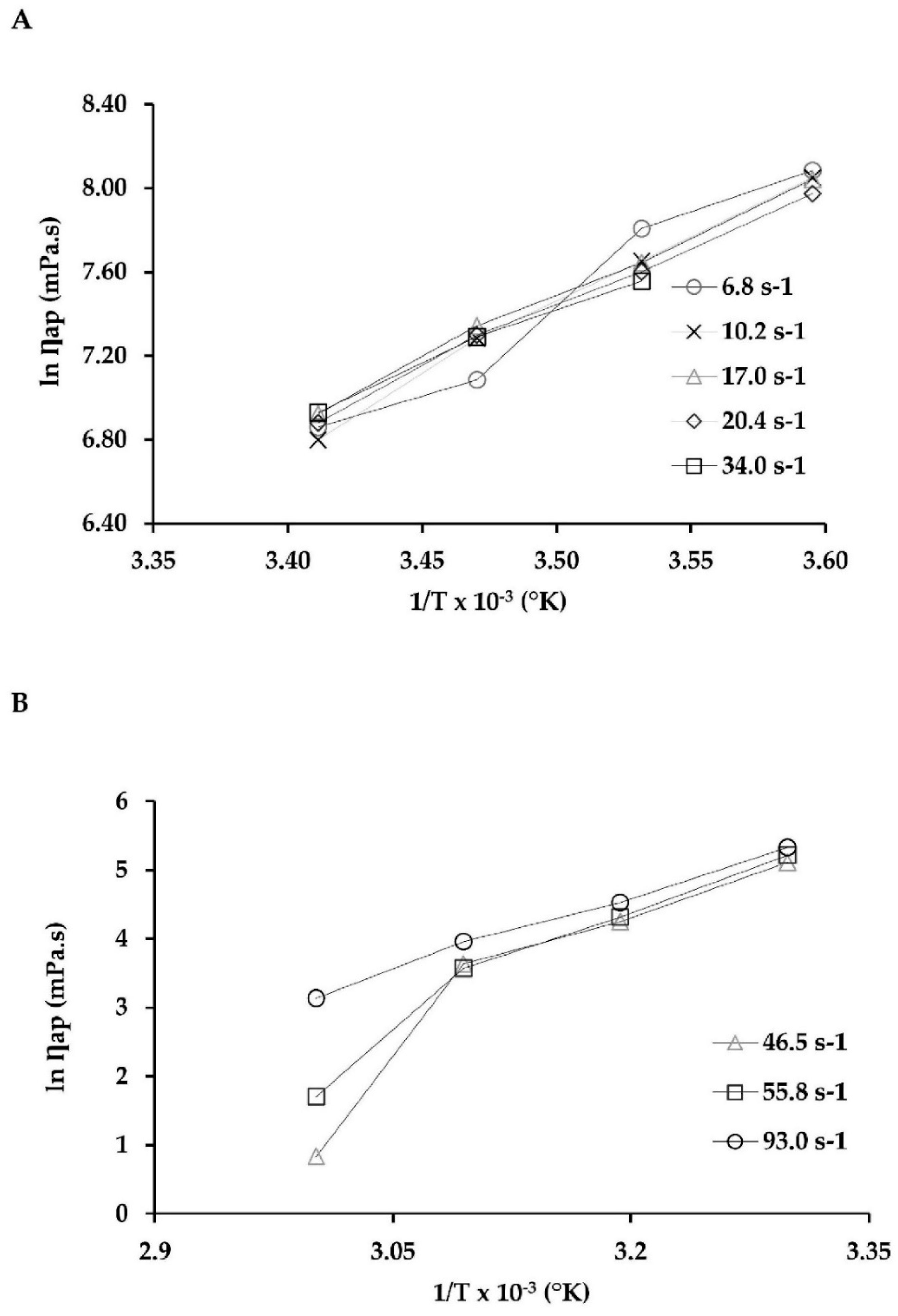
2.4. Viscosity Behavior
3. Materials and Methods
3.1. Materials
3.2. Total Carotenoid Extraction and Quantification
3.3. Sample Preparation and Enzymatic Hydrolysis
3.4. Individual Carotenoid Analysis by High-Performance Liquid Chromatography
3.5. Antioxidant Capacity
3.5.1. Extract of Oleoresin and Radical Scavenging Activity
3.5.2. Ferric Reducing Antioxidant Potential Assay
3.5.3. Oxygen Radical Absorbance Capacity Assay
3.6. Determination of Total Phenol Content
3.7. Fatty Acid Extraction
3.8. Fatty Acid Analysis by Gas Chromatography
3.9. Viscosity Measurements
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Milner, J.A. Functional foods and health promotion. J. Nutr. 1999, 129, 1395S–1397S. [Google Scholar] [CrossRef] [PubMed]
- Michalak, I.; Chojnacka, K. Algae as production systems of bioactive compounds. Eng. Life Sci. 2015, 15, 160–176. [Google Scholar] [CrossRef]
- Amaro, H.M.; Guedes, A.C.; Malcata, F.X. Antimicrobial activities of microalgae: An invited review. In Science Against Microbial Pathogens: Communicating Current Research and Technological Advances; Formatex Research Center: Badajoz, Spain, 2011; Volume 3, pp. 1272–1284. [Google Scholar]
- Gouveia, L.; Coutinho, C.P.; Mendonça, E.; Batista, A.P.; Sousa, I.; Bandarra, N.M.; Raymundo, A. Functional biscuits with PUFA-ω3 fromIsochrysis galbana. J. Sci. Food Agric. 2008, 88, 891–896. [Google Scholar] [CrossRef]
- Rao, A.V.; Rao, L.G. Carotenoids and human health. Pharmacol. Res. 2007, 55, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Plaza, M.; Cifuentes, A.; Ibañez, E. In the search of new functional food ingredients from algae. Trends Food Sci. Technol. 2008, 19, 31–39. [Google Scholar] [CrossRef]
- Varela, J.C.; Pereira, H.; Vila, M.; León, R. Production of carotenoids by microalgae: Achievements and challenges. Photosynth. Res. 2015, 125, 423–436. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wakao, S.; Fischer, B.B.; Niyogi, K.K. Sensing and Responding to Excess Light. Annu. Rev. Plant Biol. 2009, 60, 239–260. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Keum, Y.-S. Carotenoid extraction methods: A review of recent developments. Food Chem. 2018, 240, 90–103. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, E.B.; Filho, N.R.A. Concepts and studies on lipid and pigments of microalgae: A review. Renew. Sustain. Energy Rev. 2016, 58, 832–841. [Google Scholar] [CrossRef]
- Higuera-Ciapara, I.; Felix-Valenzuela, L.; Goycoolea, F.M. Astaxanthin: A Review of its Chemistry and Applications. Crit. Rev. Food Sci. Nutr. 2006, 46, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Reyes, F.A.; Mendiola, J.A.; Ibáñez, E.; Del Valle, J.M. Astaxanthin extraction from Haematococcus pluvialis using CO2-expanded ethanol. J. Supercrit. Fluids 2014, 92, 75–83. [Google Scholar] [CrossRef]
- Escobar, R.A.; Ortega, A.; Cortés, C.; Pinot, A.; Pereira, E.B.; Martins, F.R.; Boland, J. Solar Energy Resource Assessment in Chile: Satellite Estimation and Ground Station Measurement. Energy Procedia 2014, 57, 1257–1265. [Google Scholar] [CrossRef][Green Version]
- Gross, G.J.; Hazen, S.L.; Lockwood, S.F. Seven day oral supplementation with Cardax TM (disodium disuccinate astaxanthin) provides significant cardioprotection and reduces oxidative stress in rats. Mol. Cell. Biochem. 2006, 283, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Ciccone, M.M.; Cortese, F.; Gesualdo, M.; Carbonara, S.; Zito, A.; Ricci, G.; De Pascalis, F.; Scicchitano, P.; Riccioni, G. Dietary intake of carotenoids and their antioxidant and anti-inflammatory effects in cardiovascular care. Mediat. Inflamm. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Spolaore, P.; Joannis-Cassan, C.; Duran, E.; Isambert, A. Commercial applications of microalgae. J. Biosci. Bioeng. 2006, 101, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, J.; Wang, J.; Liu, T. Attached cultivation of Haematococcus pluvialis for astaxanthin production. Bioresour. Technol. 2014, 158, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Grosso, C.; Valentão, P.; Ferreres, F.; Andrade, P.B. Alternative and Efficient Extraction Methods for Marine-Derived Compounds. Mar. Drugs 2015, 13, 3182–3230. [Google Scholar] [CrossRef] [PubMed]
- Sowbhagya, H.B.; Chitra, V.N. Enzyme-Assisted Extraction of Flavorings and Colorants from Plant Materials. Crit. Rev. Food Sci. Nutr. 2010, 50, 146–161. [Google Scholar] [CrossRef] [PubMed]
- Hachemi, I.; Murzin, D.Y.; Mäki-Arvela, P.; Mäki-Arvela, P. Comparative study of the extraction methods for recovery of carotenoids from algae: Extraction kinetics and effect of different extraction parameters. J. Chem. Technol. Biotechnol. 2014, 89, 1607–1626. [Google Scholar]
- Yen, H.W.; Yang, S.C.; Chen, C.H.; Chang, J.S. Supercritical fluid extraction of valuable compounds from microalgal biomass. Bioresour. Technol. 2015, 184, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Gałuszka, A.; Migaszewski, Z.; Namieśnik, J. The 12 principles of green analytical chemistry and the SIGNIFICANCE mnemonic of green analytical practices. TrAC Trends Anal. Chem. 2013, 50, 78–84. [Google Scholar] [CrossRef]
- Da Silva, R.P.; Rocha-Santos, T.A.; Duarte, A.C. Supercritical fluid extraction of bioactive compounds. TrAC Trends Anal. Chem. 2016, 76, 40–51. [Google Scholar] [CrossRef]
- Martinez, J.L. Supercritical Fluid Extraction of Nutraceuticals and Bioactive Compounds; CRC Press: Boca Raton, FL, USA, 2007; pp. 275–304. [Google Scholar]
- Rao, A.R.; Sarada, R.; Ravishankar, G.A. Stabilization of astaxanthin in edible oils and its use as an antioxidant. J. Sci. Food Agric. 2007, 87, 957–965. [Google Scholar] [CrossRef]
- Pu, J.; Bechtel, P.J.; Sathivel, S. Extraction of shrimp astaxanthin with flaxseed oil: Effects on lipid oxidation and astaxanthin degradation rates. Biosyst. Eng. 2010, 107, 364–371. [Google Scholar] [CrossRef]
- Franco-Zavaleta, M.; Jiménez-Pichardo, R.; Tomasini-Campocosio, A.; Guerrero-Legarreta, I. Astaxanthin Extraction from Shrimp Wastes and its Stability in 2 Model Systems. J. Food Sci. 2010, 75, C394–C399. [Google Scholar] [CrossRef] [PubMed]
- Bustos, R.; Romo, L.; Yáñez, K.; Díaz, G.; Romo, C. Oxidative stability of carotenoid pigments and polyunsaturated fatty acids in microparticulate diets containing krill oil for nutrition of marine fish larvae. J. Food Eng. 2003, 56, 289–293. [Google Scholar] [CrossRef]
- Pu, J.; Bankston, J.D.; Sathivel, S. Production of Microencapsulated Crawfish (Procambarus clarkii) Astaxanthin in Oil by Spray Drying Technology. Dry. Technol. 2011, 29, 1150–1160. [Google Scholar] [CrossRef]
- Bustamante, A.; Masson, L.; Velasco, J.; Del Valle, J.M.; Robert, P. Microencapsulation of H. pluvialis oleoresins with different fatty acid composition: Kinetic stability of astaxanthin and alpha-tocopherol. Food Chem. 2016, 190, 1013–1021. [Google Scholar] [CrossRef] [PubMed]
- Boussiba, S. Carotenogenesis in the green alga Haematococcus pluvialis: Cellular physiology and stress response. Physiol. Plant. 2000, 108, 111–117. [Google Scholar] [CrossRef]
- Montero, P.; Calvo, M.; Gómez-Guillén, M.; Gómez-Estaca, J. Microcapsules containing astaxanthin from shrimp waste as potential food coloring and functional ingredient: Characterization, stability, and bioaccessibility. LWT 2016, 70, 229–236. [Google Scholar] [CrossRef]
- Guerin, M.; Huntley, M.E.; Olaizola, M. Haematococcus astaxanthin: Applications for human health and nutrition. Trends Biotechnol. 2003, 21, 210–216. [Google Scholar] [CrossRef]
- Baker, R.; Günther, C. The role of carotenoids in consumer choice and the likely benefits from their inclusion into products for human consumption. Trends Food Sci. Technol. 2004, 15, 484–488. [Google Scholar] [CrossRef]
- Landrum, J.T.; Bone, R.A. Lutein, Zeaxanthin, and the Macular Pigment. Arch. Biochem. Biophys. 2001, 385, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Gong, M.; Bassi, A. Carotenoids from microalgae: A review of recent developments. Biotechnol. Adv. 2016, 34, 1396–1412. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Huang, Y.; Zhang, R.; Wang, S.; Liu, Y. Four different methods comparison for extraction of astaxanthin from green alga haematococcus pluvialis. Sci. World J. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Christaki, E.; Bonos, E.; Giannenas, I.; Florou-Paneri, P. Functional properties of carotenoids originating from algae. J. Sci. Food Agric. 2013, 93, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Plaza, M.; Herrero, M.; Cifuentes, A.; Ibanez, E. Innovative Natural Functional Ingredients from Microalgae. J. Agric. Food Chem. 2009, 57, 7159–7170. [Google Scholar] [CrossRef] [PubMed]
- Nobre, B.; Marcelo, F.; Passos, R.; Beirão, L.; Palavra, A.; Gouveia, L.; Mendes, R. Supercritical carbon dioxide extraction of astaxanthin and other carotenoids from the microalga Haematococcus pluvialis. Eur. Food Res. Technol. 2006, 223, 787–790. [Google Scholar] [CrossRef]
- Beutner, S.; Bloedorn, B.; Frixel, S.; Blanco, I.H.; Hoffmann, T.; Martin, H.-D.; Mayer, B.; Noack, P.; Ruck, C.; Schmidt, M.; et al. Quantitative assessment of antioxidant properties of natural colorants and phytochemicals: Carotenoids, flavonoids, phenols and indigoids. The role of β-carotene in antioxidant functions. J. Sci. Food Agric. 2001, 81, 559–568. [Google Scholar] [CrossRef]
- Krinsky, N.I. Antioxidant functions of carotenoids. Free Radic. Biol. Med. 1989, 7, 617–635. [Google Scholar] [CrossRef]
- Molino, A.; Rimauro, J.; Casella, P.; Cerbone, A.; LaRocca, V.; Chianese, S.; Karatza, D.; Mehariya, S.; Ferraro, A.; Hristoforou, E.; et al. Extraction of astaxanthin from microalga Haematococcus pluvialis in red phase by using generally recognized as safe solvents and accelerated extraction. J. Biotechnol. 2018, 283, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Machu, L.; Misurcova, L.; Ambrozova, J.V.; Orsavová, J.; Mlcek, J.; Sochor, J.; Jurikova, T. Phenolic content and antioxidant capacity in algal food products. Molecules 2015, 20, 1118–1133. [Google Scholar] [CrossRef] [PubMed]
- Batista, A.P.; Niccolai, A.; Fradinho, P.; Fragoso, S.; Bursic, I.; Rodolfi, L.; Biondi, N.; Tredici, M.R.; Sousa, I.; Raymundo, A. Microalgae biomass as an alternative ingredient in cookies: Sensory, physical and chemical properties, antioxidant activity and in vitro digestibility. Algal Res. 2017, 26, 161–171. [Google Scholar] [CrossRef]
- Gilbert-López, B.; Mendiola, J.A.; Broek, L.A.V.D.; Houweling-Tan, B.; Sijtsma, L.; Cifuentes, A.; Herrero, M.; Ibáñez, E. Green compressed fluid technologies for downstream processing of Scenedesmus obliquus in a biorefinery approach. Algal Res. 2017, 24, 111–121. [Google Scholar] [CrossRef]
- Gilbert-López, B.; Barranco, A.; Herrero, M.; Cifuentes, A.; Ibáñez, E. Development of new green processes for the recovery of bioactives from Phaeodactylum tricornutum. Food Res. Int. 2017, 99, 1056–1065. [Google Scholar] [CrossRef] [PubMed]
- Goiris, K.; Muylaert, K.; Fraeye, I.; Foubert, I.; De Brabanter, J.; De Cooman, L. Antioxidant potential of microalgae in relation to their phenolic and carotenoid content. J. Appl. Phycol. 2012, 24, 1477–1486. [Google Scholar] [CrossRef]
- Alam, M.N.; Bristi, N.J.; Rafiquzzaman, M. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm. J. 2013, 21, 143–152. [Google Scholar] [CrossRef] [PubMed]
- López-Alarcón, C.; Denicola, A. Evaluating the antioxidant capacity of natural products: A review on chemical and cellular-based assays. Anal. Chim. Acta 2013, 763, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Régnier, P.; Bastias, J.; Rodriguez-Ruiz, V.; Caballero-Casero, N.; Caballo, C.; Sicilia, D.; Fuentes, A.; Maire, M.; Crepin, M.; Letourneur, D. Astaxanthin from haematococcus pluvialis prevents oxidative stress on human endothelial cells without toxicity. Mar. Drugs 2015, 13, 2857–2874. [Google Scholar] [CrossRef] [PubMed]
- Hossain, A.; Brennan, M.A.; Mason, S.L.; Guo, X.; Zeng, X.A.; Brennan, C.S. The effect of astaxanthin-rich microalgae “haematococcus pluvialis” and wholemeal flours incorporation in improving the physical and functional properties of cookies. Foods 2017, 6, 57. [Google Scholar] [CrossRef] [PubMed]
- Giorgis, M.; Garella, D.; Cena, C.; Boffa, L.; Cravotto, G.; Marini, E. An evaluation of the antioxidant properties of arthrospira maxima extracts obtained using non-conventional techniques. Eur. Food Res. Technol. 2017, 243, 227–237. [Google Scholar] [CrossRef]
- Chatzikonstantinou, M.; Kalliampakou, A.; Gatzogia, M.; Flemetakis, E.; Katharios, P.; Labrou, N.E. Comparative analyses and evaluation of the cosmeceutical potential of selected chlorella strains. J. Appl. Phycol. 2017, 29, 179–188. [Google Scholar] [CrossRef]
- Assefa, A.D.; Keum, Y.S. Effect of extraction solvent and various drying methods on polyphenol content and antioxidant activities of yuzu (citrus junos sieb ex tanaka). J. Food Meas. Charact. 2017, 11, 576–585. [Google Scholar] [CrossRef]
- Yu, J.-H.; Wang, Y.; Sun, J.; Bian, F.; Chen, G.; Zhang, Y.; Bi, Y.-P.; Wu, Y.-J. Antioxidant activity of alcohol aqueous extracts of Crypthecodinium cohnii and Schizochytrium sp. J. Zhejiang Univ. Sci. B 2017, 18, 797–806. [Google Scholar] [CrossRef]
- Naguib, Y.M.A. Antioxidant activities of astaxanthin and related carotenoids. J. Agric. Food Chem. 2000, 48, 1150–1154. [Google Scholar] [CrossRef] [PubMed]
- Zuluaga, M.; Barzegari, A.; Letourneur, D.; Gueguen, V.; Pavon-Djavid, G. Oxidative stress regulation on endothelial cells by hydrophilic astaxanthin complex: Chemical, biological, and molecular antioxidant activity evaluation. Oxidative Med. Cell. Longev. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Safafar, H.; Van Wagenen, J.; Møller, P.; Jacobsen, C. Carotenoids, phenolic compounds and tocopherols contribute to the antioxidative properties of some microalgae species grown on industrial wastewater. Mar. Drugs 2015, 13, 7339–7356. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Zhai, Y.; Xu, D.; Ye, N.; Zhang, X.; Wang, Y.; Zhang, W.; Yu, J. Correlation between lipid and carotenoid synthesis and photosynthetic capacity in haematococcus pluvialis grown under high light and nitrogen deprivation stress. Grasas Y Aceites 2015, 66, 077. [Google Scholar]
- Bilbao, P.G.S.; Damiani, C.; Salvador, G.A.; Leonardi, P. Haematococcus pluvialis as a source of fatty acids and phytosterols: Potential nutritional and biological implications. J. Appl. Phycol. 2016, 28, 3283–3294. [Google Scholar] [CrossRef]
- Armenta, R.E.; Valentine, M.C. Single-cell oils as a source of omega-3 fatty acids: An overview of recent advances. J. Am. Oil Chem. Soc. 2013, 90, 167–182. [Google Scholar] [CrossRef]
- Judé, S.; Roger, S.; Martel, E.; Besson, P.; Richard, S.; Bougnoux, P.; Champeroux, P.; Le Guennec, J.-Y. Dietary long-chain omega-3 fatty acids of marine origin: A comparison of their protective effects on coronary heart disease and breast cancers. Prog. Biophys. Mol. Biol. 2006, 90, 299–325. [Google Scholar] [CrossRef] [PubMed]
- Rose, D.P.; Connolly, J.M. Omega-3 fatty acids as cancer chemopreventive agents. Pharmacol. Ther. 1999, 83, 217–244. [Google Scholar] [CrossRef]
- Coupland, J.N.; McClements, D.J. Physical properties of liquid edible oils. J. Am. Oil Chem. Soc. 1997, 74, 1559–1564. [Google Scholar] [CrossRef]
- Chakraborty, M.; Bart, H.-J. Emulsion liquid membranes: Role of internal droplet size distribution on toluene/n-heptane separation. Colloids Surf. A 2006, 272, 15–21. [Google Scholar] [CrossRef]
- Zhou, G.; Kresta, S.M. Correlation of mean drop size and minimum drop size with the turbulence energy dissipation and the flow in an agitated tank. Chem. Eng. Sci. 1998, 53, 2063–2079. [Google Scholar] [CrossRef]
- Domian, E.; Brynda-Kopytowska, A.; Oleksza, K. Rheological properties and physical stability of o/w emulsions stabilized by osa starch with trehalose. Food Hydrocoll. 2015, 44, 49–58. [Google Scholar] [CrossRef]
- Sahin, S.; Sumnu, S.G. Rheological properties of foods. Phys. Prop. Foods 2006, 39–105. [Google Scholar]
- Lin, L.; Shen, M.; Liu, S.; Tang, W.; Wang, Z.; Xie, M.; Xie, J. An acidic heteropolysaccharide from mesona chinensis: Rheological properties, gelling behavior and texture characteristics. Int. J. Biol. Macromol. 2018, 107, 1591–1598. [Google Scholar] [CrossRef] [PubMed]
- Roenningsen, H.P.; Bjoerndal, B.; Baltzer Hansen, A.; Batsberg Pedersen, W. Wax precipitation from north sea crude oils: 1. Crystallization and dissolution temperatures, and newtonian and non-newtonian flow properties. Energy Fuels 1991, 5, 895–908. [Google Scholar] [CrossRef]
- Pal, R.; Yan, Y.; Masliyah, J.; Schramm, L. Emulsions: Fundamentals and applications in the petroleum industry. Adv. Chem. Ser. 1992, 231, 295–312. [Google Scholar]
- Belyamani, I.; Otaigbe, J.U.; Nelson, C.D.; Strom, B.; Roberds, J. Rheological properties of southern pine oleoresins. Appl. Rheol. 2015, 25, 49–60. [Google Scholar]
- Haminiuk, C.; Sierakowski, M.; Izidoro, D.; Masson, M. Rheological characterization of blackberry pulp. Braz. J. Food Technol. 2006, 9, 291–296. [Google Scholar]
- Ahmed, J.; Ramaswamy, H.; Sashidhar, K. Rheological characteristics of tamarind (Tamarindus indica L.) juice concentrates. LWT-Food Sci. Technol. 2007, 40, 225–231. [Google Scholar] [CrossRef]
- Dak, M.; Verma, R.; Jaaffrey, S. Effect of temperature and concentration on rheological properties of “kesar” mango juice. J. Food Eng. 2007, 80, 1011–1015. [Google Scholar] [CrossRef]
- Britton, G. Carotenoids, Vol. 1b, Spectroscopy, Edited By g. Britton, s. Liaaen-Jensen & H. Pfander, Ch. 2; Birkhauser Verlag: Berlin, Germany, 1995. [Google Scholar]
- Mezquita, P.C.; Huerta, B.E.B.; Ramírez, J.C.P.; Hinojosa, C.P.O. Milks pigmentation with astaxanthin and determination of colour stability during short period cold storage. J. Food Sci. Technol. 2015, 52, 1634–1641. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mezquita, P.C.; Barragán-Huerta, B.E.; Ramírez, J.P.; Hinojosa, C.O. Stability of astaxanthin in yogurt used to simulate apricot color, under refrigeration. Food Sci. Technol. 2014, 34, 559–565. [Google Scholar] [CrossRef]
- Lorenz, R. Hplc and spectrophotometric analysis of carotenoids from Haematococcus algae powder. BioAstin/Nat. TM Tech. Bull. 2001, 15, 1–10. [Google Scholar]
- Leon, R.; Martín, M.; Vigara, J.; Vilchez, C.; Vega, J.M. Microalgae mediated photoproduction of β-carotene in aqueous–organic two phase systems. Biomol. Eng. 2003, 20, 177–182. [Google Scholar] [CrossRef]
- Mathew, M.; Subramanian, S. In vitro screening for anti-cholinesterase and antioxidant activity of methanolic extracts of ayurvedic medicinal plants used for cognitive disorders. PLoS ONE 2014, 9, e86804. [Google Scholar] [CrossRef] [PubMed]
- Gasca, C.A.; Cabezas, F.A.; Torras, L.; Bastida, J.; Codina, C. Chemical composition and antioxidant activity of the ethanol extract and purified fractions of cadillo (pavonia sepioides). Free Radic. Antioxid. 2013, 3, S55–S61. [Google Scholar] [CrossRef]
- Bekir, J.; Mars, M.; Souchard, J.P.; Bouajila, J. Assessment of antioxidant, anti-inflammatory, anti-cholinesterase and cytotoxic activities of pomegranate (punica granatum) leaves. Food Chem. Toxicol. 2013, 55, 470–475. [Google Scholar] [CrossRef] [PubMed]
- Akter, K.; Barnes, E.C.; Loa-Kum-Cheung, W.L.; Yin, P.; Kichu, M.; Brophy, J.J.; Barrow, R.A.; Imchen, I.; Vemulpad, S.R.; Jamie, J.F. Antimicrobial and antioxidant activity and chemical characterisation of erythrina stricta roxb.(fabaceae). J. Ethnopharmacol. 2016, 185, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Beecher, G.R.; Holden, J.M.; Haytowitz, D.B.; Gebhardt, S.E.; Prior, R.L. Lipophilic and hydrophilic antioxidant capacities of common foods in the united states. J. Agric. Food Chem. 2004, 52, 4026–4037. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using folin-ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef] [PubMed]
- Lamers, P.P.; van de Laak, C.C.; Kaasenbrood, P.S.; Lorier, J.; Janssen, M.; De Vos, R.C.; Bino, R.J.; Wijffels, R.H. Carotenoid and fatty acid metabolism in light-stressed dunaliella salina. Biotechnol. Bioeng. 2010, 106, 638–648. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds H. pluvialis oleoresin are available from the authors. |
| Carotenoid | Specific Name | Content in Oleoresin (mg/g) | Abundance (%wt) |
|---|---|---|---|
| Free astaxanthin | 3,3′-dihydroxy-β, β-carotene-4,4′-dione | 6.82 ± 0.19 | 5.94 |
| Canthaxanthin | β, β-carotene-4,4′-dione | 1.68 ± 0.19 | 1.46 |
| β-carotene | β, β-carotene | 2.35 ± 0.29 | 2.05 |
| Lutein | β, ε-carotene-3,3′-diol | 1.12 ± 0.23 | 0.97 |
| Total astaxanthin | Free-astaxanthin and astaxanthin esters * | 96.22 ± 1.21 | 83.76 |
| Others | - | 6.68 ± 0.30 | 5.81 |
| Total carotenoids | 114.9 ± 0.89 | 100.0 |
| Analytical Determination | Unit | Value | ±SD | |
|---|---|---|---|---|
| Antioxidant capacity | FRAP | mg TE/g OE | 313.76 | 5.92 |
| ORAC | µmol TE/100 g OE | 5.22 | 0.16 | |
| Total phenols | - | mg GAE/g OE | 74.08 | 3.29 |
| Temperature | η (Pa s) | K (mPa sn) | n | R2 | χ2 | SSE | RMSE | |
|---|---|---|---|---|---|---|---|---|
| Zones | (°C) | |||||||
| Low | 5 | 3.10 ± 0.12 | 3800.45 | 0.92 | 0.998 | 3.41 × 10−03 | 4.55 × 10−03 | 0.067 |
| 10 | 2.11 ± 0.18 | 3085.98 | 0.85 | 0.995 | 5.33 × 10−03 | 6.66 × 10−03 | 0.082 | |
| 15 | 1.43 ± 0.12 | 1043.79 | 1.11 | 0.989 | 8.08 × 10−03 | 1.01 × 10−02 | 0.101 | |
| 20 | 0.97 ± 0.05 | 824.25 | 1.06 | 0.997 | 1.01 × 10−03 | 1.27 × 10−03 | 0.036 | |
| High | 30 | 0.15 ± 0.05 | 12.12 | 1.64 | 0.986 | 2.58 × 10−04 | 3.23 × 10−04 | 0.018 |
| 40 | 0.06 ± 0.03 | 0.47 | 2.23 | 0.965 | 2.78 × 10−04 | 3.47 × 10−04 | 0.019 | |
| 50 | 0.04 ± 0.01 | 4.53 | 1.54 | 0.977 | 4.04 × 10−04 | 6.06 × 10−04 | 0.025 | |
| 60 | 0.01 ± 0.01 | 1.36 × 10−05 | 4.17 | 0.990 | 1.37 × 10−06 | 2.06 × 10−06 | 0.001 | |
| Shear Rate (s−1) | R2 | ||
|---|---|---|---|
| 6.8 | 4.82 × 10−08 | 57.70 | 0.946 |
| 10.2 | 2.04 × 10−07 | 54.24 | 0.957 |
| 17.0 | 2.65 × 10−06 | 48.26 | 0.947 |
| 20.4 | 4.22 × 10−06 | 47.03 | 0.974 |
| 34.0 | 1.74 × 10−05 | 43.65 | 0.984 |
| Shear Rate (s−1) | R2 | ||
|---|---|---|---|
| 18.6 | - | - | - |
| 27.9 | - | - | - |
| 46.5 | 2.61 × 10−17 | 110.08 | 0.863 |
| 55.8 | 2.37 × 10−14 | 92.71 | 0.934 |
| 93.0 | 1.18 × 10−8 | 59.47 | 0.992 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-Domínguez, M.C.; Espinosa, C.; Paredes, A.; Palma, J.; Jaime, C.; Vílchez, C.; Cerezal, P. Determining the Potential of Haematococcus pluvialis Oleoresin as a Rich Source of Antioxidants. Molecules 2019, 24, 4073. https://doi.org/10.3390/molecules24224073
Ruiz-Domínguez MC, Espinosa C, Paredes A, Palma J, Jaime C, Vílchez C, Cerezal P. Determining the Potential of Haematococcus pluvialis Oleoresin as a Rich Source of Antioxidants. Molecules. 2019; 24(22):4073. https://doi.org/10.3390/molecules24224073
Chicago/Turabian StyleRuiz-Domínguez, Mari Carmen, Carolina Espinosa, Adrián Paredes, Jenifer Palma, Carolina Jaime, Carlos Vílchez, and Pedro Cerezal. 2019. "Determining the Potential of Haematococcus pluvialis Oleoresin as a Rich Source of Antioxidants" Molecules 24, no. 22: 4073. https://doi.org/10.3390/molecules24224073
APA StyleRuiz-Domínguez, M. C., Espinosa, C., Paredes, A., Palma, J., Jaime, C., Vílchez, C., & Cerezal, P. (2019). Determining the Potential of Haematococcus pluvialis Oleoresin as a Rich Source of Antioxidants. Molecules, 24(22), 4073. https://doi.org/10.3390/molecules24224073