1. Introduction
Carotenoids are lipophilic compounds with a common C40 backbone of isoprenoid units and are naturally synthesized by photosynthetic organisms and some non-photosynthetic bacteria and fungi [
1,
2]. The high number of conjugated double bonds in carotenoid molecules contributes to their strong antioxidant capacity, and therefore they can potentially protect humans against ageing and diseases that are caused by harmful free radicals. The colourless carotenoids, phytoene ((6
E,10
E,14
E,16
E,18
E,22
E,26
E)-2,6,10,14,19,23,27,31-octamethyldotriaconta-2,6,10,14,16,18,22,26,30-nonaene) and phytofluene ((6
E,10
E,12
E,14
E,16
E,18
E,22
E,26
E)-2,6,10,14,19,23,27,31-octamethyldotriaconta-2,6,10,12,14,16,18,22,26,30-decaene) are of particular interest: phytoene, the progenitor in the carotenoid synthesis pathway, is able to absorb both hazardous UV range B (290–320 nm) and C light (100–290 nm) and phytofluene, to absorb UV range A (315–400 nm). They may protect the skin against erythema, premature skin aging and skin cancer [
3]. Phytoene has also been reported to be anti-inflammatory, hepato-protective and to prevent several other types of cancers [
3,
4]. Ultimately, their colourless features make phytoene and phytofluene valuable as food additives and for cosmetic products [
4,
5]. Phytoene and phytofluene are found in higher plants such as bell pepper, apricot, melon, orange and tomato [
4]. They have also been detected in numerous cyanobacteria and microalgae. However, apart from tomatoes, the amount found in most of the organisms is small [
4,
6].
It is generally considered that carotenoid biosynthesis in photosynthetic plants and algae takes place in the chloroplast with some specific steps in the cytoplasm [
7]. The pathway involves a series of desaturation and isomerization steps to form a diverse range of stereoisomers (
Figure 1). The first committed step in carotenoid biosynthesis is the chain-elongating condensation reaction between two molecules of geranylgeranyl diphosphate (C20) to form
15-cis phytoene (C40). This step is catalysed by the enzyme phytoene synthase (PSY) and is followed by a two-step desaturation reaction catalysed by
15-cis-phytoene desaturase (PDS) with
9,15-di-cis-phytofluene (equation 1), and 9,9’,15-
tri-
cis-ζ-carotene (equation 2) as end products (R = C
53H
80O
2 plastoquinone), and plastoquinone as hydrogen acceptor [
8,
9,
10,
11].
The subsequent steps involve further desaturation and isomerization reactions catalysed by ζ-carotene desaturase (ZDS) and carotenoid isomerase (CrtISO) respectively, to form
all-trans lycopene [
8,
9,
12].
All-trans lycopene is cyclized into either β-carotene or α-carotene catalysed by different groups of lycopene cyclases (LYC). Xanthophyll products, which include zeaxanthin, antheraxanthin, violaxanthin, lutein and their derivatives, are formed via the hydroxylation of β-carotene or α-carotene [
7].
The halotolerant green microalga
Dunaliella salina is one of the richest sources of carotenoids, especially
all-trans- and
9-cis β-carotene. However, it will also accumulate phytoene and phytofluene in the presence of a phytoene desaturase (PDS) inhibitor [
13,
14].
D. salina is easy to culture, as it requires light and a few other nutrients (nitrogen, phosphate and salts) to grow. For these reasons, it is considered a promising species to produce carotenoids [
15].
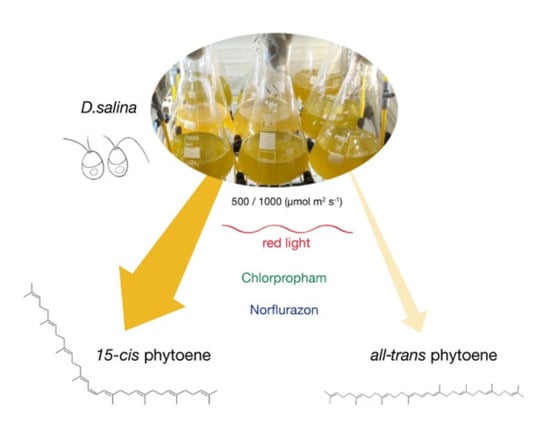
Bleaching herbicides such as norflurazon are known to boost phytoene accumulation in the cells of
D. salina and other higher plants, by inhibiting PDS, which prevents the conversion of phytoene to phytofluene [
14,
16,
17]. However, little information has been provided on the isomer composition of the accumulated phytoene and available data to date are contradictory. Ebenezer [
18] for example showed by using Nuclear Magnetic Resonance (NMR) methods that the main phytoene isomer, which accumulated in norflurazon-treated
D. bardawil cultures
(Dunaliella bardawil is a variant of
Dunaliella salina), was the
15-cis isomer, whilst Werman et al. [
6] and Ben-Amotz et al. [
13] using High Performance Liquid Chromatography (HPLC) techniques, reported that the phytoene-rich
D. bardawil powder used in their study contained the
9-cis phytoene isomer together with
all-trans phytoene. This leads to an unresolved point in regard to the carotenoid biosynthetic pathway of
D. salina: whether the high quantity of
9-cis β-carotene derives directly from
9-cis phytoene, as proposed in [
13], or from the isomerization of
all-trans β-carotene, as proposed by Davidi et al. [
19] and by Xu and Harvey [
14,
20]. Resolving the identity of the phytoene isomers produced by
D. salina could provide new insight into the carotenoid synthesis pathway and clarify this point.
The ratio of absorption peak heights at defined wavelengths, which defines fine structure, is useful for distinguishing carotenoids and their isomers and HPLC coupled with UV-Vis detection appears to be the most common method adopted for their study [
21]. However, most studies have examined phytoene extracted from tomato. In ripening tomato,
all-trans phytoene usually has a higher fine structure than
15-cis phytoene [
22]. NMR analysis is necessary to discriminate at a structural level between different isomers. The values of specific chemical shifts for the
cis- and
trans- isomeric forms of phytoene have been assigned in previous studies [
18,
23,
24].
The main objective of this work was to resolve the phytoene isomers in D. salina and to gain new insight into the carotenoid synthesis pathway of D. salina. Methods of sample preparation from microalgal biomass and NMR methods were developed to characterize the phytoene isomers. The effects of cultivation conditions, light wavelength and light intensity, and herbicide treatment with two different classes of herbicides, chlorpropham and norflurazon, on carotenoid synthesis and phytoene isomer accumulation were also investigated.
3. Discussion
Phytoene demand has increased recently due to its potential health benefits. In higher plants and fruits, such as tomato, phytoene is found predominantly in the 15-cis form. However, little work has been done to characterize the phytoene isomers in microalgae, such as D. salina, which is a rich source of natural carotenoids, especially β-carotene with a high ratio of the valuable 9-cis β-carotene. This work characterized phytoene isomers in D. salina and compared the production of phytoene in D. salina cultures treated with the two different classes of herbicides norflurazon and chlorpropham. After both treatments, the concentration of 15-cis phytoene increased. Moreover, the colourless phytofluene isomers over-accumulated in chlorpropham, but not norflurazon-treated D. salina cultures.
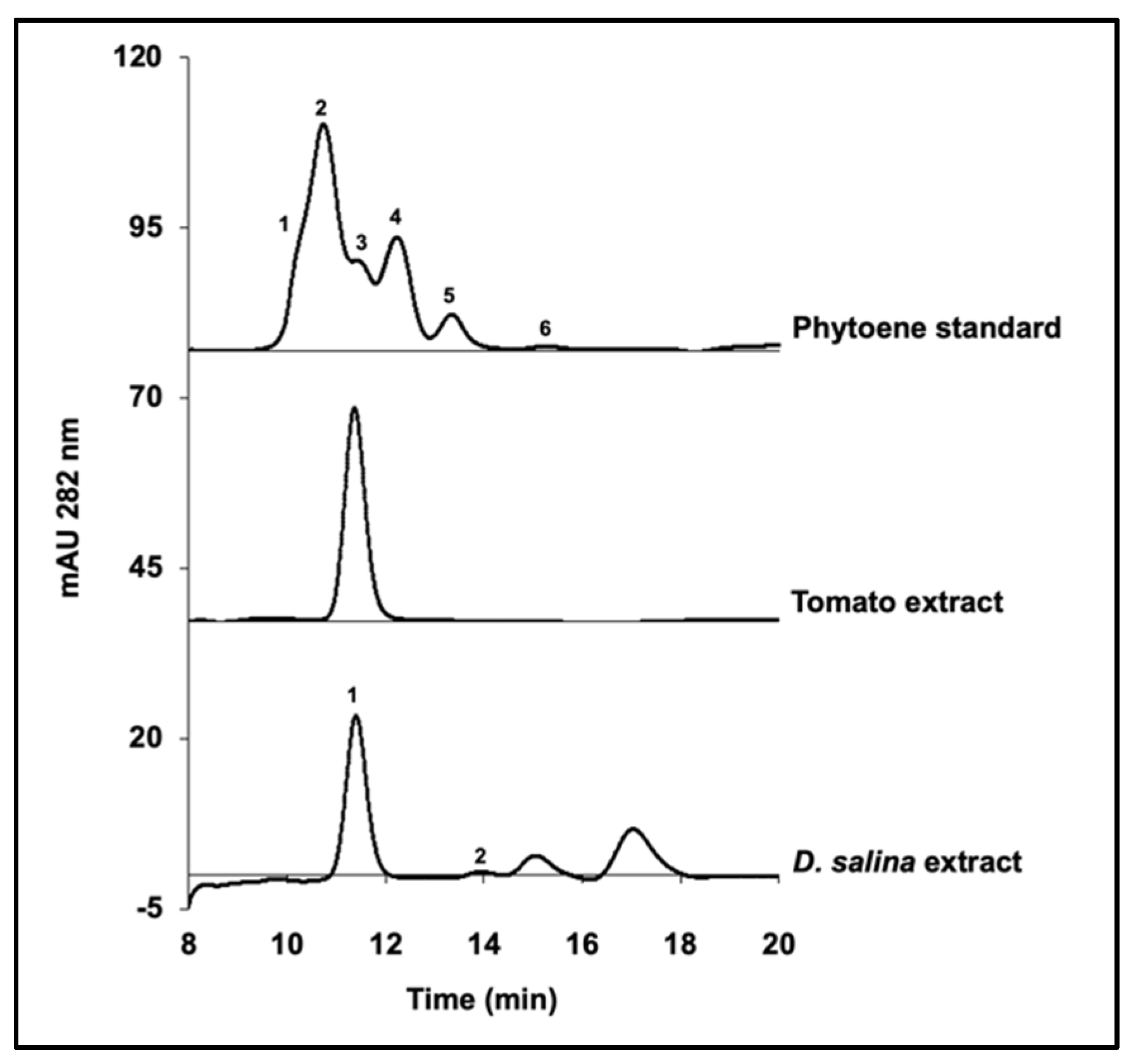
Results of HPLC analysis, UPC
2 analysis and NMR analysis each showed that
15-cis phytoene was the main phytoene isomer that accumulated in
D. salina (>98% of total phytoene). Furthermore, the
15-cis phytoene increased with the treatment of both the herbicides norflurazon and chlorpropham and with increasing light intensity. Identification of
15-cis phytoene with only trace amounts of
all-trans phytoene is in concord with findings for ripe tomato and higher plants [
8,
22] and with results obtained by Ebenezer et al. for
D. bardawil [
18], but not with the results obtained by Ben-Amotz et al. [
13] and Werman et al. [
6], who showed that both
9-cis phytoene and
all-trans phytoene accumulated in
D. bardawil treated with herbicides.
15-cis phytofluene has been considered by many to be the predominant phytofluene isomer (for a review see [
4]). However, Koschmieder et al. [
26] compared synthetic
15-cis phytofluene and
all-trans phytofluene with phytofluene isomers from tomato and norflurazon-treated
Dunaliella bardawil, and on the basis of RT obtained after HPLC analysis as well as absorption maxima at 331nm, 348 nm and 366 nm, concluded that phytofluene in tomato extract was predominately
9,15-di-cis phytofluene, which also exists in
Dunaliella bardawil in a small proportion. They also determined that the main phytofluene isomer in norflurazon treated
D. bardawil was
9-cis phytofluene but did not present the isomeric configurations in untreated
D. bardawil cultures. In contrast, our results showed that the main phytofluene isomer in both untreated and norflurazon in chlorpropham treated
D. salina was the same as in tomato extracts, which is
9,15-di-cis phytofluene. The
9-cis phytofluene shown by Koschmieder et al. [
26] is likely not a phytofluene peak because norflurazon inhibits PDS function and blocks the desaturation of phytoene to subsequent phytofluene products.
The fact that only
15-cis phytoene accumulated in
D. salina when treated with phytoene desaturase inhibitor, while no accumulation of
all-trans phytoene was found, is consistent with
15-cis phytoene as the most likely precursor of phytofluene.
All-trans phytoene present in
D. salina is likely to be formed by the isomerisation of the predominant
15-cis phytoene and this reaction step along with the conversion of
15-cis phytoene to phytofluene might be fast enough to occur prior to the interference of the chlorpropham herbicide action. As a second hypothesis,
all-trans phytoene may also be formed directly from the precursor of geranylgeranyl pyrophosphate as suggested by Gregonis and Rilling [
27]. An early study on a green microalga
Scenedesmus obliquus also supports the idea that
15-cis phytoene is converted via
15-cis phytofluene and
15-cis ζ-carotene into
all-trans-gz-carotene and
trans-bicyclic carotenoids such as α-carotene and β-carotene [
28]. Our results show that
15-cis phytoene is the main stereoisomer produced by
D. salina and that it is over-accumulated with red light and with the use of herbicides. Our results also support the recent finding that
9-cis β-carotene is formed by isomerisation of
all-trans β-carotene with the function of a
9-cis/
all-trans β-carotene isomerase [
19,
20], rather than synthesised from the precursor of
9-cis phytoene.
4. Materials and Methods
4.1. Algal Cultivation
D. salina strain CCAP 19/41 (PLY DF15) was obtained from the Marine Biological Association, Plymouth, UK (MBA). Algae were cultured as described in previous work [
14]. Inoculum was prepared by adding 10 mL of the culture stock of
D. salina to 250 mL of fresh media until the culture reached the exponential phase. Algae were cultured in 500 mL Modified Johnsons Medium containing 1.5 M NaCl and 10 mM NaHCO
3 in an illuminated incubator (Varicon Aqua, Worcester, UK) under white light of 500 µmol·m
−2·s
−1 at 25 °C. To study the effect of light wavelength, algae were cultivated in Algem photobioreactor under red or blue light of 500 µmol·m
−2·s
−1 at 25 °C. To study the effect of herbicides, cultures were treated with either 5 µM of norflurazon or 20 µM of chlorpropham for 48 h before carotenoid extraction. All the experiments were performed in triplicate.
4.2. Standards and Solvents
Phytoene standard (mixture of isomers (E/Z), 95% purity) was purchased from Sigma–Aldrich (Merck KGaA, Darmstadt, Germany). Methanol (MeOH) and Methyl tert Butyl Ether (MtBE), both HPLC grade, were purchased from Fischer Scientific UK Ltd. (Loughborough, Leicestershire, UK).
4.3. Carotenoids Extraction
The carotenoids were extracted from wet alga biomass as follows: the samples were centrifuged at 3000× g for 5 min at 5 °C; 10 mL MeOH-MtBE (80:20) were added to the pellets and sonicated and vortexed for 20 s; extracts were clarified at the centrifuge then filtered (0.20 µm filter) into amber HPLC vials before HPLC analysis.
15-cis phytoene was also extracted from ripe tomatoes (purchased from Waitrose and Partners, UK) to be used as control material because it favourably accumulates the compound at high concentration.
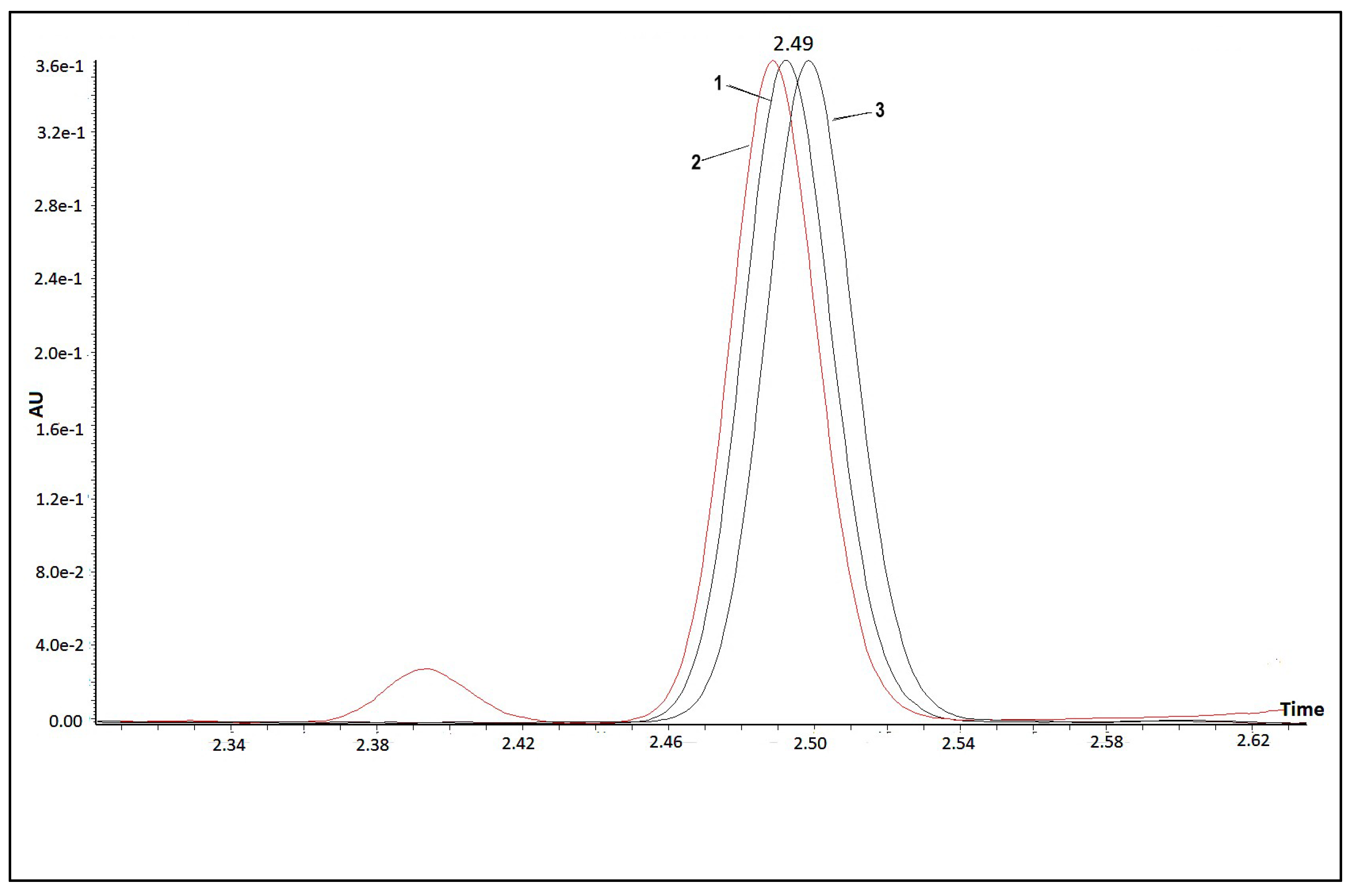
4.4. HPLC Analysis
High-Performance Liquid Chromatography equipped with Diode-Array Detection (HPLC-DAD; Agilent Technologies 1200 series, Agilent, Santa Clara, CA, United States), on-line degasser, a quaternary pump system, a YMC30 250 × 4.9 mm I.D S-5µ column (YMC, Europe GmbH) was used to resolve the phytoene and the phytofluene from the carotenoid extracts. The column temperature was set at 15 °C, the gradient solvent system was MeOH (A): MtBE (B) running at 80% A for the first 10 min at a flow rate of 1 mL/min, then increased to 100% A for the next 10 min at a flow rate of 0.5 mL/min before going back to the initial conditions at 20 min. Total run time was 45 min. The absorbance at four wavelengths (276, 282, 293 and 350) was monitored. All data were acquired and analysed with Chemstation for LC System software. Phytoene extracted from ripe tomatoes was stereo-mutated similarly to Melendez-Martinez et al. [
29]. The sample extracted was heated at 75 °C for a total of 240 min and it was then injected in the HPLC system for analysis. Methods parameters were as follow: the column was set at 15 °C, the isocratic solvent system was MeOH (A): MtBE (B) running at 88% A at a flow rate of 0.5 mL/min.
4.5. UPC2 Analysis
Supercritical, or near-critical, CO2 was used as the primary eluent in the mobile phase with a MeOH cosolvent gradient to resolve the phytoene from the total-tomato and D. salina extracts using a seven-minute gradient. Supercritical fluid chromatography is very powerful when it comes to identifying compounds based on their retention time, as it allows shorter run times than HPLC and therefore a decrease in shifts in the retention time among runs. The UPC2 (Waters Limited, Herts, UK) was equipped with a binary solvent delivery pump, auto-sampler, column oven, photodiode array detector and back-pressure regulator. Mobile phase A was CO2 and mobile phase B was MeOH with 0.1% formic acid (v/v). The gradient was held at 5% B for 0.5 min, then increased to 50% B at 4.9 min, held at 50% for 0.1 min, ramped to 5% B in 0.1 min, and equilibrated for 1.9 min to give a total run time of 7 min. The binary solvent flow rate was 1.0 mL/min, the isocratic make-up solvent flow was 0.3 mL/min, and the UPC2 was operated with a back-pressure of 2000 psi. The column oven was maintained at a temperature of 50 °C and an Acquity UPC2 HSS C18 SB (1.8 µm, 3.0 mm × 100 mm) column was used. A sample injection volume of 3.0 µL was used with all samples. The UPC2 was fitted with an Acquity PDA detector (190–800 nm) and operated in the wavelength range 210–600 nm with a 1.2 nm resolution. In addition, the absorbance at four wavelengths (276 nm, 282 nm, 293 nm and 450 nm) was monitored with a resolution of 1.2 nm.
4.6. Mass Spectrometry Analysis
All mass spectrometry experiments were collected on a Waters Synapt G2 Q-ToF mass spectrometer (Manchester, UK). The instrument was operated in positive ion mode electrospray with a capillary voltage of 2.0 kV and sampling cone voltage was 30 V. Nitrogen at a flow rate of 650 L/h was used as the desolvation gas with a constant desolvation temperature of 350 °C, a cone gas flow rate of 50 L/h, and a source temperature of 130 °C. Data were acquired over the m/z range 50–800. An integral LockSpray unit infusing Leucine-Enkephalin peptide into the electrospray sample stream was used to collect reference scans. Scans were performed every 10 s with the reference calibrant introduced at a flow rate of 10 µL/min using the fluidics system of the instrument. Single point lock-mass correction was used for the protonated pseudo-molecular ion at m/z 556.2771 (+ve). All data were acquired and analysed with Waters MassLynx v4.1 software (Manchester, UK).
4.7. NMR Sample Preparation
Fresh tomato samples and harvested D. salina cultures were freeze-dried and a total of 20 g of dried tomato powder and dried algae samples was used for the carotenoid extraction. The procedure described above was modified as follow: MtBE was chosen as solvent for extraction due to its lower polarity index (2.4), hence its higher selectivity for only carotenoids (and lipids) in comparison to the MeOH and ethanol solvents; moreover, its high volatility value allowed shorter evaporation times and minimisation of sample losses. The extracts were injected in the HPLC system to confirm the presence of phytoene. The MtBE extracts were evaporated for one and a half hour, flushed with nitrogen and suspended with deuterated chloroform prior to analysis.
4.8. NMR Conditions
The NMR experiments were carried out with a JEOL (Tokyo, Japan) 500 MHz. All the spectra were recorded at ambient temperature. Proton and carbon spectra for carotenoid analysis were referenced to the TMS signal (δ = 0.00 ppm). 1D Proton parameters: 1H 45° flip angle of 6.32 µs, spectral width 9.38 kHz, 65K data points, 64 scans and relaxation delay 2s. 1D Carbon parameters: 13C 30° flip angle of 3.56 µs, spectral width of 39.31 kHz, 65k data points, 1024 scans and relaxation delay 2s. 2D 1H-13C pulse field gradient heteronuclear single quantum correlation spectroscopy (HSQC): pulse of 12.64 µs for 1H, pulse of 10.7 µs for 13C, decoupling, J_constant of 135 Hz, spectral width of 6.26 and 31.45 kHz for the proton and carbon dimensions, respectively; relaxation delay 2s, 2048 total scan and 1024 and 256 data point in f2 and f1, respectively. Data were acquired and analysed with MNova software (12.0.3, Mestrelab Research S.L., Santiago de Compostela, Spain).
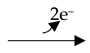 C40H62 + 2 RH2
C40H62 + 2 RH2
 C40H60 + 2 RH2
C40H60 + 2 RH2