Chemical Discrimination of Astragalus mongholicus and Astragalus membranaceus Based on Metabolomics Using UHPLC-ESI-Q-TOF-MS/MS Approach
Abstract
1. Introduction
2. Results and Discussion
2.1. Selection of Extraction Methods
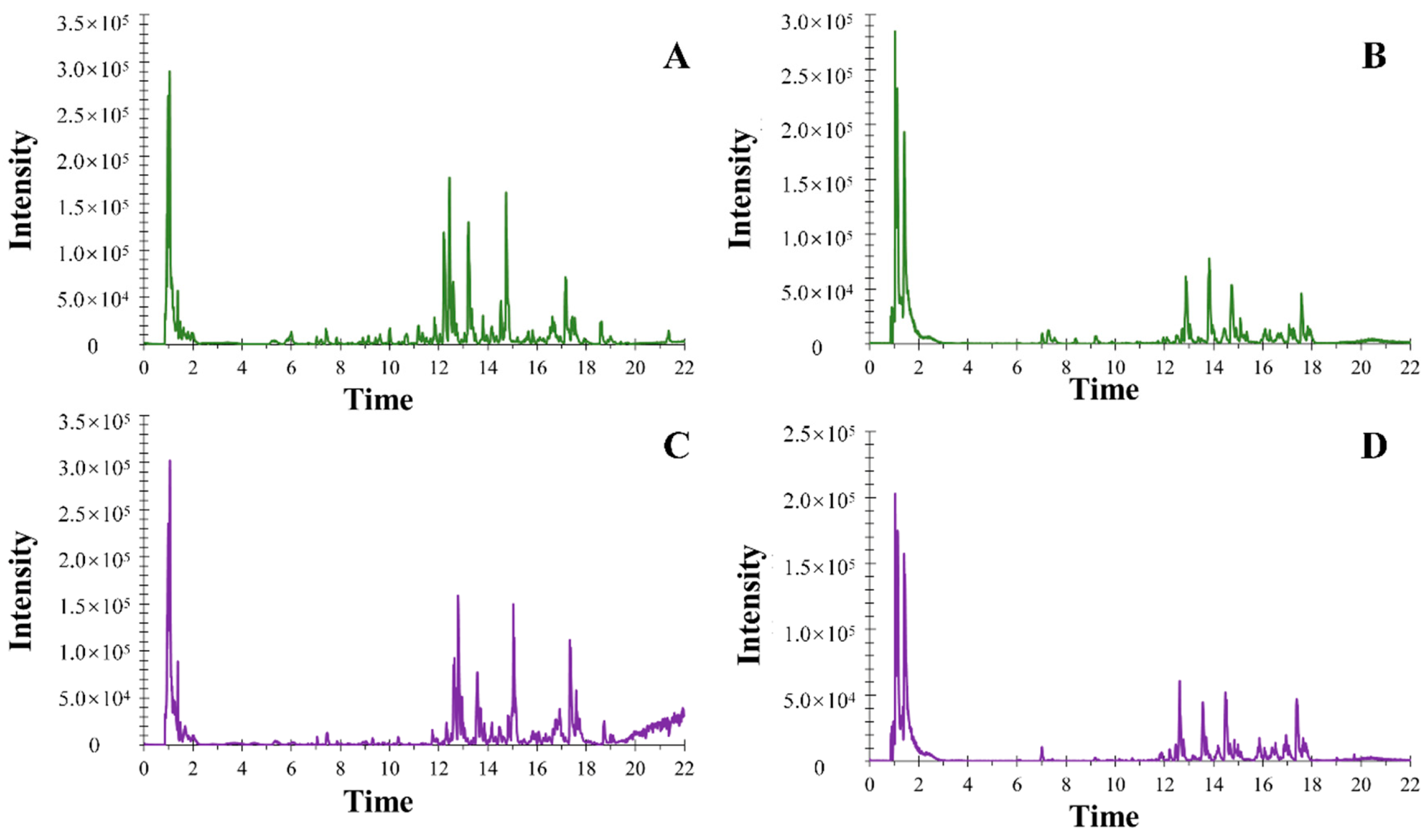
2.2. Optimization of UHPLC-ESI-Q-TOF-MS/MS Conditions
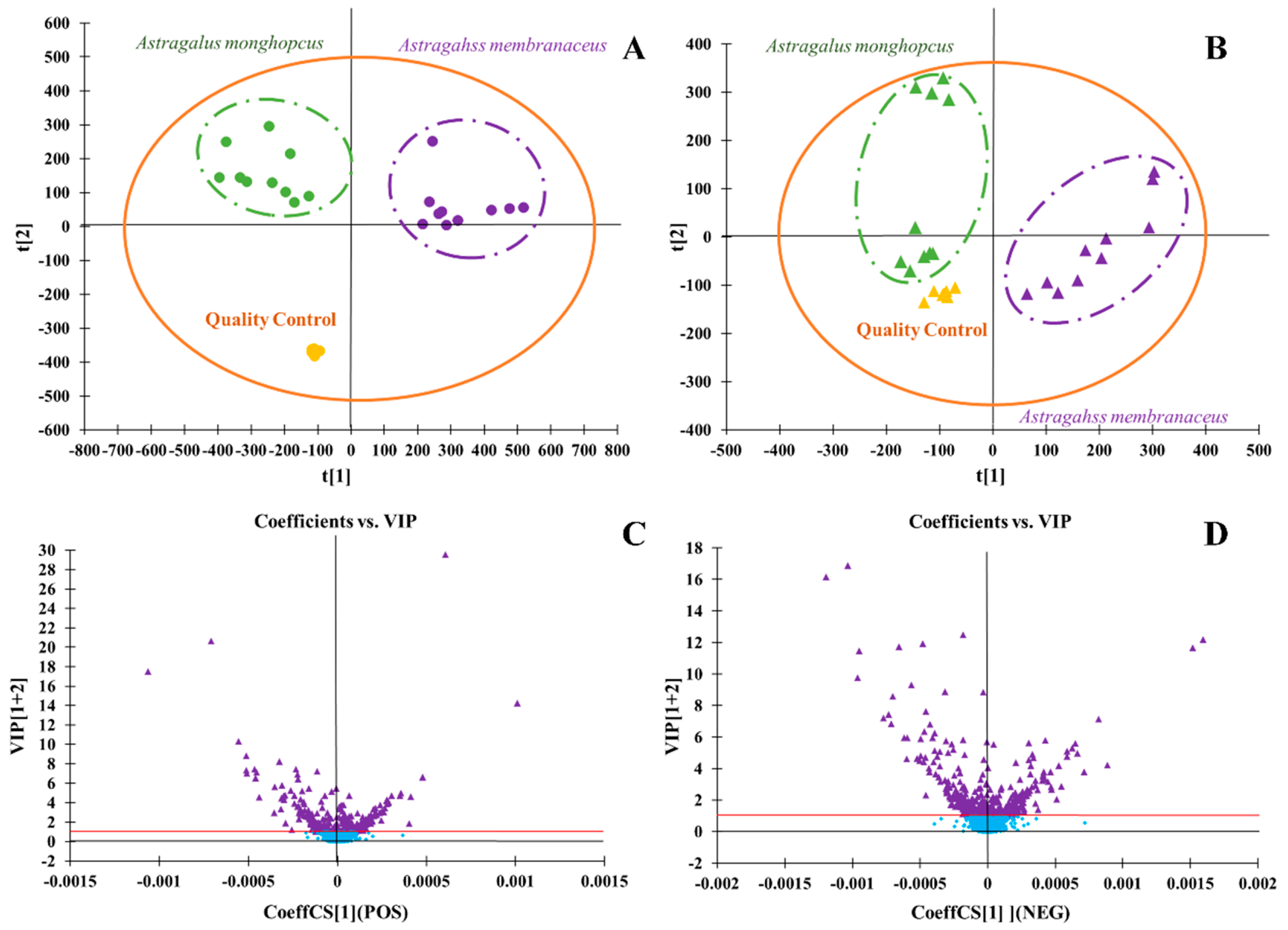
2.3. Characteristic Multivariate Data Analysis of Metabalomics
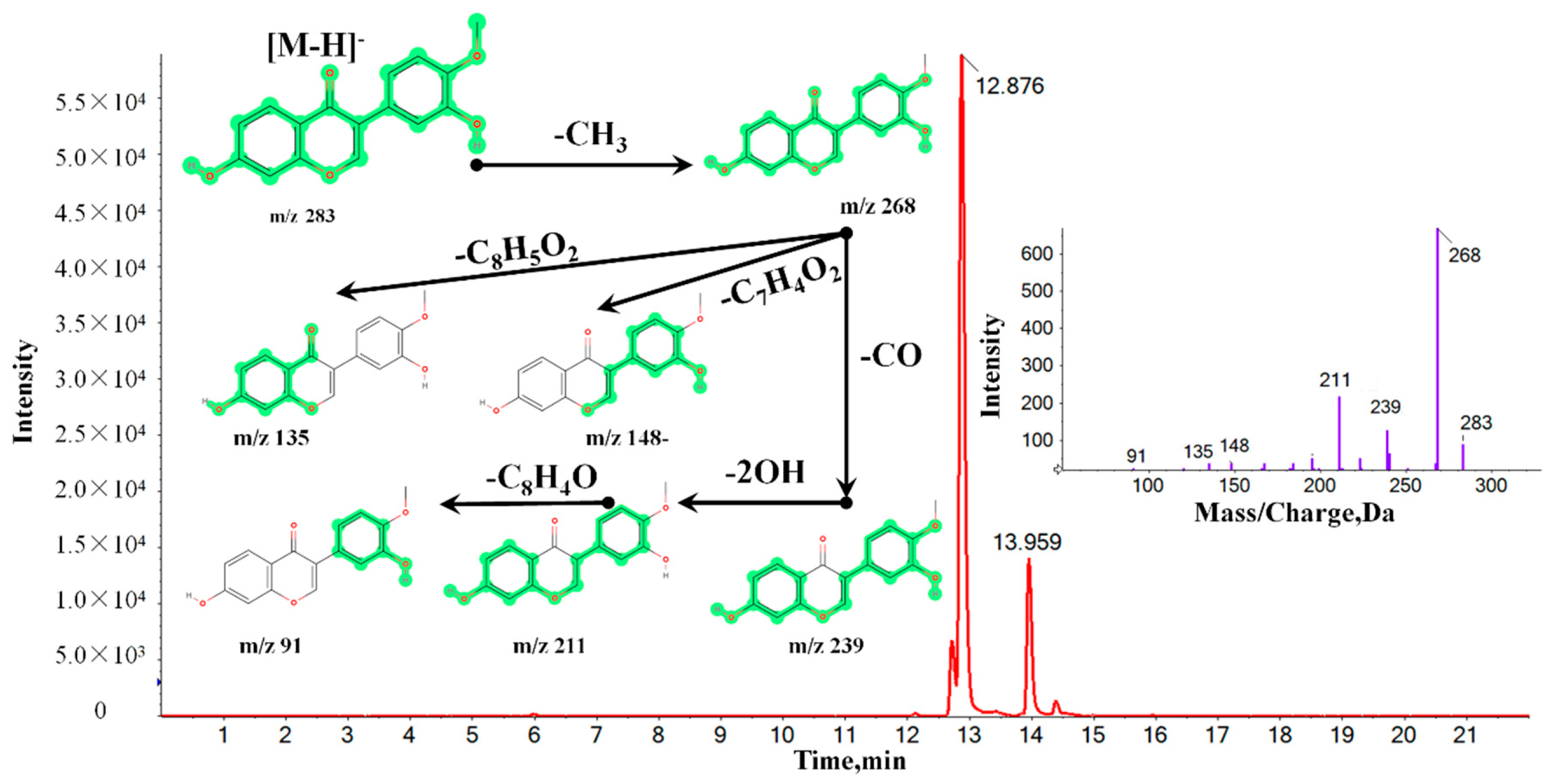
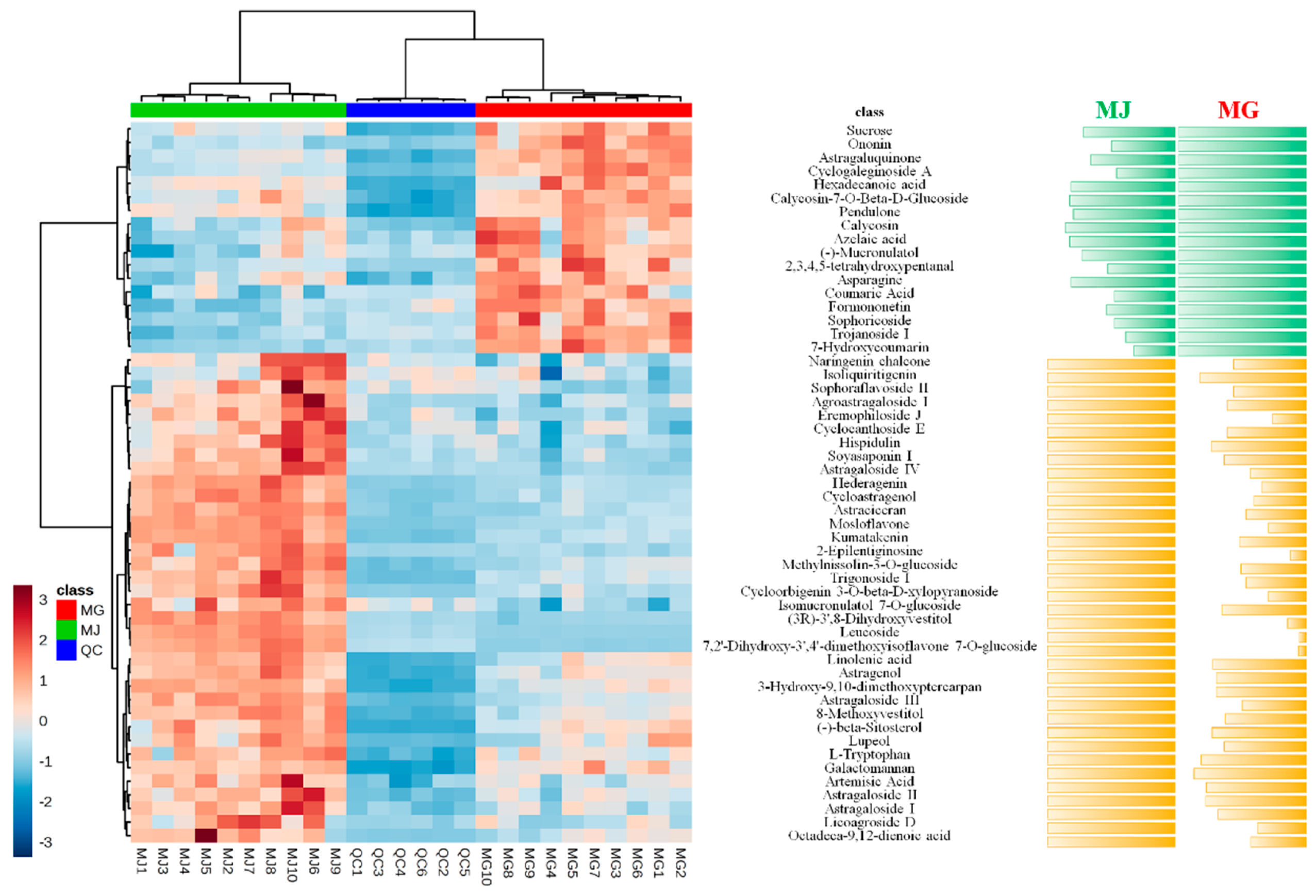
2.4. Identification of Chemical Markers between MG and MJ
2.5. Relative Intensity Comparison of Chemical Markers between MG and MJ
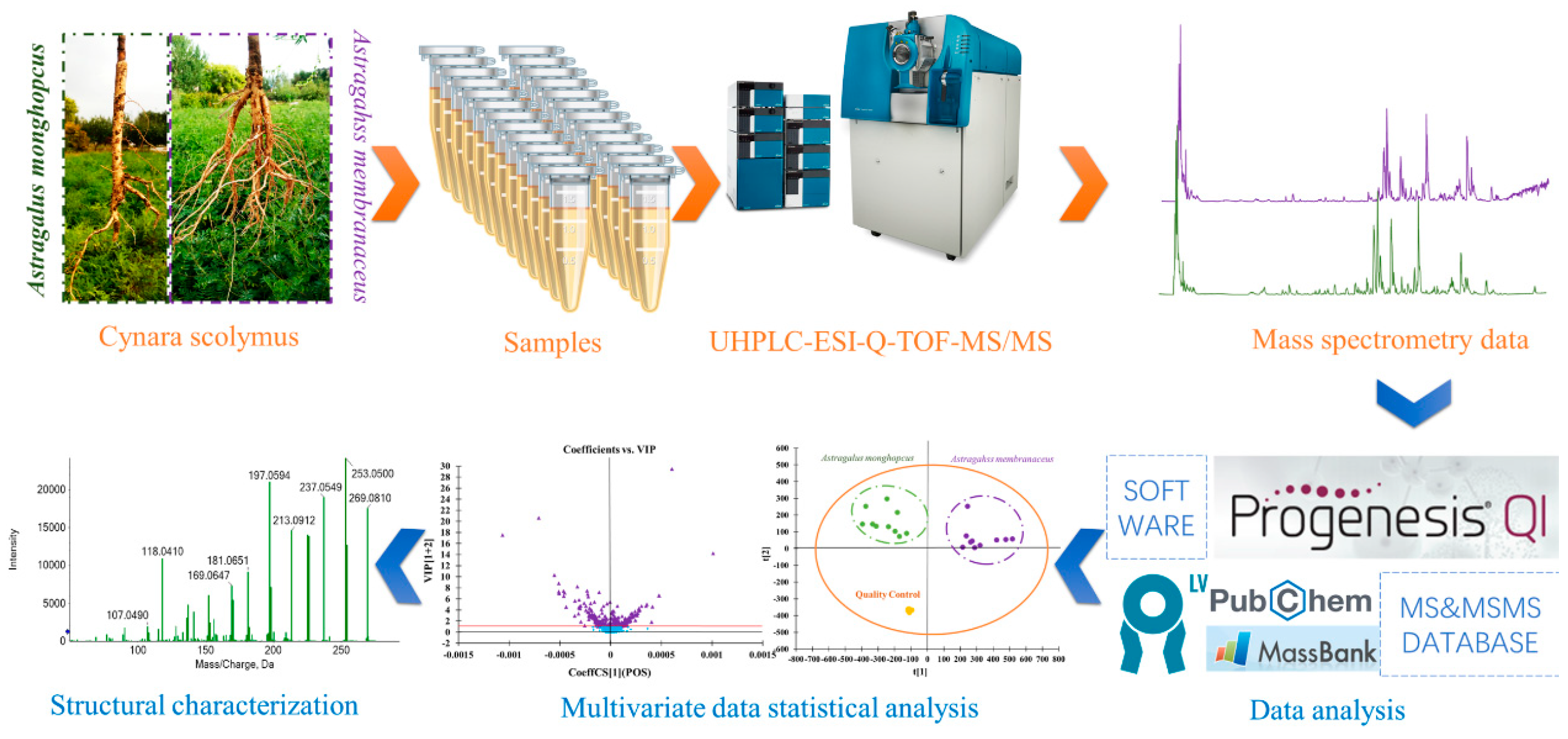
3. Materials and Methods
3.1. Plant Materials, Reagents and Chemicals
3.2. Preparation of MG Extraction and MJ Extraction for UHPLC-ESI-Q-TOF-MS/MS Analysis
3.3. Conditions of Analysis Platform
3.4. Multivariate Data Processing
3.5. Identification of Potential Chemical Markers
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Wu, S. Effect of dietary Astragalus membranaceus polysaccharide on the growth performance and immunity of juvenile broilers. Poult. Sci. 2018, 97, 3489–3493. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Dai, X.; Qi, J.; Ao, Y.; Yang, C.; Li, Y. Astragalus mongholicus (Fisch.) Bge Improves Peripheral Treg Cell Immunity Imbalance in the Children with Viral Myocarditis by Reducing the Levels of miR-146b and miR-155. Front. Pediatr. 2018, 6, 139. [Google Scholar] [CrossRef] [PubMed]
- Li, N.Y.; Yu, H.; Li, X.L.; Wang, Q.Y.; Zhang, X.W.; Ma, R.X.; Zhao, Y.; Xu, H.; Liang, W.; Bai, F.; et al. Astragalus Membranaceus Improving Asymptomatic Left Ventricular Diastolic Dysfunction in Postmenopausal Hypertensive Women with Metabolic Syndrome: A Prospective, Open-Labeled, Randomized Controlled Trial. Chin. Med. J. (Engl.) 2018, 131, 516–526. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Ji, H.; Liu, A. Alcohol-soluble polysaccharide from Astragalus membranaceus: Preparation, characteristics and antitumor activity. Int. J. Biol. Macromol. 2018, 118, 2057–2064. [Google Scholar] [CrossRef]
- Zhao, L.; Tan, S.; Zhang, H.; Liu, P.; Tan, Y.; Li, J.; Jia, D.; Shen, X. Astragalus polysaccharides exerts anti-infective activity by inducing human cathelicidin antimicrobial peptide LL-37 in respiratory epithelial cells. Phytother. Res. 2018, 32, 1521–1529. [Google Scholar] [CrossRef]
- Chang, X.; Lu, K.; Wang, L.; Lv, M.; Fu, W. Astraglaus polysaccharide protects diabetic cardiomyopathy by activating NRG1/ErbB pathway. Biosci. Trends 2018, 12, 149–156. [Google Scholar] [CrossRef]
- Adesso, S.; Russo, R.; Quaroni, A.; Autore, G.; Marzocco, S. Astragalus membranaceus Extract Attenuates Inflammation and Oxidative Stress in Intestinal Epithelial Cells via NF-κB Activation and NrfResponse. Int. J. Mol. Sci. 2018, 19, 800. [Google Scholar] [CrossRef]
- Rahman, M.; Kim, H.; Kim, S.; Kim, M.; Kim, D.; Lee, H. Chondroprotective Effects of a Standardized Extract (KBH-JP-040) from Kalopanax pictus, Hericium erinaceus, and Astragalus membranaceus in Experimentally Induced In Vitro and In Vivo Osteoarthritis Models. Nutrients 2018, 10, 356. [Google Scholar] [CrossRef]
- Li, Y.; Guo, S.; Zhu, Y.; Yan, H.; Qian, D.; Wang, H.; Yu, J.; Duan, J. Flowers of Astragalus membranaceus var. mongholicus as a Novel High Potential By-Product: Phytochemical Characterization and Antioxidant Activity. Molecules 2019, 24, 434. [Google Scholar] [CrossRef]
- Huang, Y.; Tsay, H.; Lu, M.; Lin, C.; Yeh, C.; Liu, H.; Shiao, Y. Astragalus membranaceus-Polysaccharides Ameliorates Obesity, Hepatic Steatosis, Neuroinflammation and Cognition Impairment without Affecting Amyloid Deposition in Metabolically Stressed APPswe/PS1dEMice. Int. J. Mol. Sci. 2017, 18, 2746. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.; Wu, K.; Guo, X.; Tang, Z. A rapid method for sensitive profiling of bioactive triterpene and flavonoid from Astragalus mongholicus and Astragalus membranaceus by ultra-pressure liquid chromatography with tandem mass spectrometry. J. Chromatogr. B 2018, 1085, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Shan, G.; Zhang, F.; Chen, J.; Jia, T. Metabolomics analysis and rapid identification of changes in chemical ingredients in crude and processed Astragali Radix by UPLC-QTOF-MS combined with novel informatics UNIFI platform. Chin. J. Nat. Med. 2018, 16, 714–720. [Google Scholar] [CrossRef]
- Li, R.; Yin, M.; Yang, M.; Chu, S.; Han, X.; Wang, M.; Peng, H. Developmental anatomy of anomalous structure and classification of commercial specifications and grades of theAstragalus membranaceus var.mongholicus. Microsc. Res. Tech. 2018, 81, 1165–1172. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Kong, L.; Zhang, A.; Han, Y.; Liu, Z.; Sun, H.; Liu, L.; Wang, X. High-Throughput Metabolomics for Discovering Potential Metabolite Biomarkers and Metabolic Mechanism from the APPswe/PS1dETransgenic Model of Alzheimer’s Disease. J. Proteome Res. 2017, 16, 3219–3228. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Zhang, A.; Dong, H.; Yan, G.; Sun, H.; Wu, X.; Han, Y.; Wang, X. Toxicity and Detoxification Effects of Herbal Caowu via Ultra Performance Liquid Chromatography/Mass Spectrometry Metabolomics Analyzed using Pattern Recognition Method. Pharmacogn. Mag. 2017, 13, 683–692. [Google Scholar]
- Ma, Y.; Zhou, W.; Chen, P.; Urriola, P.E.; Shurson, G.C.; Ruan, R.; Chen, C. Metabolomic Evaluation of Scenedesmus sp. as a Feed Ingredient Revealed Dose-Dependent Effects on Redox Balance, Intermediary and Microbial Metabolism in a Mouse Model. Nutrients 2019, 11, 1971. [Google Scholar] [CrossRef]
- Ghisoni, S.; Lucini, L.; Rocchetti, G.; Chiodelli, G.; Farinelli, D.; Tombesi, S.; Trevisan, M. Untargeted metabolomics with multivariate analysis to discriminate hazelnut (Corylus avellana L.) cultivars and their geographical origin. J. Sci. Food Agric. 2019. [Google Scholar] [CrossRef]
- Ragusa, A.; Centonze, C.; Grasso, M.E.; Latronico, M.F.; Mastrangelo, P.F.; Sparascio, F.; Fanizzi, F.P.; Maffia, M. A Comparative Study of Phenols in Apulian Italian Wines. Foods 2017, 6, 24. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, A.; Wang, L.; Yan, G.; Zhao, H.; Sun, H.; Zou, S.; Han, J.; Ma, C.W.; Kong, L.; et al. High-throughput chinmedomics-based prediction of effective components and targets from herbal medicine AS1350. Sci. Rep.-UK 2016, 6, 38487. [Google Scholar] [CrossRef]
- Zhang, A.; Liu, Q.; Zhao, H.; Zhou, X.; Sun, H.; Nan, Y.; Zou, S.; Ma, C.W.; Wang, X. Phenotypic characterization of nanshi oral liquid alters metabolic signatures during disease prevention. Sci. Rep.-UK 2016, 6, 19333. [Google Scholar] [CrossRef]
- Zhang, W.N.; Li, A.P.; Qi, Y.S.; Qin, X.M.; Li, Z.Y. Metabolomics coupled with system pharmacology reveal the protective effect of total flavonoids of Astragali Radix against adriamycin-induced rat nephropathy model. J. Pharm. Biomed. Anal. 2018, 158, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Fan, Z.; Xiao, Y. Comprehensive relative quantitative metabolomics analysis of lycopodium alkaloids in different tissues of Huperzia serrata. Synth. Syst. Biotechnol. 2018, 3, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.; Zhang, A.H.; Zhao, Q.Q.; Yan, G.L.; Sun, H.; Wang, X.J. Discovery of quality-marker ingredients of Panax quinquefolius driven by high-throughput chinmedomics approach. Phytomedicine 2019, 152928. [Google Scholar] [CrossRef] [PubMed]
- Sawada, Y.; Sato, M.; Okamoto, M.; Masuda, J.; Yamaki, S.; Tamari, M.; Tanokashira, Y.; Kishimoto, S.; Ohmiya, A.; Abe, T.; et al. Metabolome-based discrimination of chrysanthemum cultivars for the efficient generation of flower color variations in mutation breeding. Metabolomics 2019, 15, 118. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Yang, L.; Li, M.X.; Fang, H.; Zhang, A.H.; Song, Q.; Liu, X.Y.; Su, J.; Yu, M.D.; Makino, T.; et al. UPLC-G2Si-HDMS untargeted metabolomics for identification of metabolic targets of Yin-Chen-Hao-Tang used as a therapeutic agent of dampness-heat jaundice syndrome. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2018, 1081, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.H.; Yu, J.B.; Sun, H.; Kong, L.; Wang, X.Q.; Zhang, Q.Y.; Wang, X.J. Identifying quality-markers from Shengmai San protects against transgenic mouse model of Alzheimer’s disease using chinmedomics approach. Phytomedicine 2018, 45, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.J.; Zhang, A.H.; Kong, L.; Yu, J.B.; Gao, H.L.; Liu, Z.D.; Sun, H. Rapid discovery of quality-markers from Kaixin San using chinmedomics analysis approach. Phytomedicine 2019, 54, 371–381. [Google Scholar] [CrossRef]
- Jia, L.; Lv, D.; Zhang, S.; Wang, Z.; Zhou, B. Astragaloside IV Inhibits the Progression of Non-Small Cell Lung Cancer Through the Akt/GSK-3beta/beta-Catenin Pathway. Oncol. Res. 2019, 27, 503–508. [Google Scholar] [CrossRef]
- Lin, J.; Fang, L.; Li, H.; Li, Z.; Lyu, L.; Wang, H.; Xiao, J. Astragaloside IV alleviates doxorubicin induced cardiomyopathy by inhibiting NADPH oxidase derived oxidative stress. Eur. J. Pharmacol. 2019, 859, 172490. [Google Scholar] [CrossRef]
- Qian, W.; Cai, X.; Qian, Q.; Zhuang, Q.; Yang, W.; Zhang, X.; Zhao, L. Astragaloside IV protects endothelial progenitor cells from the damage of ox-LDL via the LOX-1/NLRP3 inflammasome pathway. Drug Des. Dev. Ther. 2019, 13, 2579–2589. [Google Scholar] [CrossRef]
- Zhang, L.; Deng, S. Effects of astragaloside IV on inflammation and immunity in rats with experimental periodontitis. Braz. Oral Res. 2019, 33, 32. [Google Scholar] [CrossRef] [PubMed]
- Nie, Q.; Zhu, L.; Zhang, L.; Leng, B.; Wang, H. Astragaloside IV protects against hyperglycemia-induced vascular endothelial dysfunction by inhibiting oxidative stress and Calpain-1 activation. Life Sci. 2019, 232, 116662. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.; Zhou, P.; Liu, L.; Jiang, W.; Xie, H.; Zhang, L.; Xie, D. Protective Effect of Astragaloside IV on Hepatic Injury Induced by Iron Overload. BioMed Res. Int. 2019, 2019, 3103946. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Zhou, Y.P.; Chang, X.C.; Wang, F.; Li, G.W. Astragaloside regulates Nrf2/Bach1/HO-1 signaling pathway and inhibits H9c2 cardiomyocyte injury induced by hypoxia-reoxygenation. Zhongguo Zhong Yao Za Zhi 2019, 44, 2331–2337. [Google Scholar] [PubMed]
- Nie, P.; Meng, F.; Zhang, J.; Wei, X.; Shen, C. Astragaloside IV Exerts a Myocardial Protective Effect against Cardiac Hypertrophy in Rats, Partially via Activating the Nrf2/HO-Signaling Pathway. Oxid. Med. Cell. Longev. 2019, 2019, 4625912. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |
| No. | Rt | m/z | CAS&Pubchem ID | Identification | Adducts | Formula | Error (ppm) | p-Value |
|---|---|---|---|---|---|---|---|---|
| 1 | 1.01 | 132.0541 | 3130-87-8 | Asparagine | M − H2O − H, M − H | C4H8N2O3 | 4.74 | 3.7 × 10−4 |
| 2 | 1.05 | 195.0513 | 70849-23-9 | 2,3,4,5-tetrahydroxypentanal | M + FA − H | C5H10O5 | 2.15 | 2.0 × 10−5 |
| 3 | 1.10 | 342.1158 | 57-50-1 | Sucrose | M − H, M + FA − H | C12H22O11 | −1.09 | 1.7 × 10−4 |
| 4 | 7.43 | 203.0829 | 73-22-3 | L-Tryptophan | M − H | C11H12N2O2 | 1.58 | 6.1 × 10−3 |
| 5 | 10.23 | 161.0249 | 93-35-6 | 7-Hydroxycoumarin | M − H | C9H6O3 | 3.09 | 3.4 × 10−10 |
| 6 | 11.37 | 209.0460 | 25429-38-3 | Coumaric Acid | M + FA − H | C9H8O3 | 3.00 | 7.7 × 10−5 |
| 7 | 11.72 | 431.0987 | 152-95-4 | Sophoricoside | M−H | C21H20O10 | 0.77 | 1.5 × 10−6 |
| 8 | 12.88 | 283.0608 | 20575-57-9 | Calycosin | M − H | C16H12O5 | −1.52 | 2.5 × 10−3 |
| 9 | 12.91 | 447.1312 | 464196-55-2 | Licoagroside D | M − H | C22H24O10 | 3.46 | 4.2 × 10−3 |
| 10 | 13.02 | 187.0979 | 66923-62-4 | Azelaic acid | M − H | C9H16O4 | 1.61 | 2.9 × 10−3 |
| 11 | 13.40 | 299.0560 | 1447-88-7 | Hispidulin | M − H | C16H12O6 | −0.47 | 1.8 × 10−5 |
| 12 | 13.76 | 255.0663 | 961-29-5 | Isoliquiritigenin | M − H | C15H12O4 | 0.07 | 2.3 × 10−2 |
| 13 | 13.76 | 463.1618 | 94367-43-8 | Isomucronulatol 7-O-glucoside | M − H | C23H28O10 | 1.71 | 3.3 × 10−6 |
| 14 | 13.82 | 267.0658 | 485-72-3 | Formononetin | M − H | C16H12O4 | −1.66 | 9.1 × 10−09 |
| 15 | 14.09 | 971.4902 | 147540-80-5 | Sophoraflavoside II | M − H | C48H76O20 | 4.61 | 1.2 × 10−3 |
| 16 | 14.42 | 271.0614 | 25515-46-2 | Naringenin chalcone | M − H | C15H12O5 | 0.63 | 9.0 × 10−4 |
| 17 | 14.42 | 831.4781 | 170969-74-1 | Cyclocanthoside E | M + FA − H | C41H70O14 | 4.21 | 4.1 × 10−4 |
| 18 | 14.85 | 827.4408 | 1037218-20-4 | Eremophiloside J | M + FA − H | C41H66O14 | −1.67 | 1.5 × 10−6 |
| 19 | 14.89 | 784.4650 | 84687-43-4 | Astragaloside IV | M − H, M + FA − H | C41H68O14 | 4.28 | 2.6 × 10−8 |
| 20 | 15.17 | 942.5210 | 51330-27-9 | Soyasaponin I | M − H, M + FA − H | C48H78O18 | 2.30 | 1.3 × 10−5 |
| 21 | 15.25 | 871.4705 | 84676-89-1 | Astragaloside II | M + FA − H | C43H70O15 | 0.96 | 6.0 × 10−4 |
| 22 | 15.35 | 301.1075 | 20878-97-1 | (−)-Mucronulatol | M − H | C17H18O5 | −2.23 | 4.2 × 10−4 |
| 23 | 15.51 | 915.4903 | 156769-94-7 | Agroastragaloside I | M + FA − H | C45H74O16 | −4.90 | 1.9 × 10−4 |
| 24 | 15.60 | 909.4863 | 386273-42-3 | Trojanoside I | M − H | C47H74O17 | 1.04 | 3.0 × 10−9 |
| 25 | 16.10 | 868.4847 | 84680-75-1 | Astragaloside I | M + Cl, M + FA − H | C45H72O16 | 3.08 | 1.8 × 10−4 |
| 26 | 17.59 | 296.2344 | 80286-58-4 | Artemisic Acid | M − H2O − H, M − H, M + Cl | C18H32O3 | −2.37 | 6.9 × 10−4 |
| 27 | 1.08 | 543.1320 | 439336 | Galactomannan | M + K | C18H32O16 | −0.30 | 4.6 × 10−2 |
| 28 | 1.39 | 158.1173 | 108866-42-8 | 2-Epilentiginosine | M + H | C8H15NO2 | −1.69 | 1.6 × 10−7 |
| 29 | 12.41 | 446.1217 | 20633-67-4 | Calycosin-7-O-Beta-d-Glucoside | M + H, M + Na | C22H22O10 | 0.97 | 1.6 × 10−3 |
| 30 | 12.61 | 477.1388 | 113235-89-5 | 7,2′-Dihydroxy-3′,4′-dimethoxyisoflavone 7-O-glucoside | M + H | C23H24O11 | −0.78 | 5.3 × 10−15 |
| 31 | 13.47 | 563.1392 | 27661-51-4 | Leucoside | M + H − H2O | C26H28O15 | −0.57 | 8.2 × 10−17 |
| 32 | 14.07 | 430.1267 | 486-62-4 | Ononin | M + H, M + Na | C22H22O9 | 0.73 | 4.3 × 10−7 |
| 33 | 14.12 | 287.0911 | 122587-87-5 | (3R)-3′,8-Dihydroxyvestitol | M + H − H2O | C16H16O6 | −0.98 | 6.9 × 10−13 |
| 34 | 14.27 | 316.0946 | 158991-20-9 | Astragaluquinone | M + H, M + Na | C17H16O6 | −0.26 | 3.9 × 10−8 |
| 35 | 14.41 | 462.1519 | 94367-42-7 | Methylnissolin-3-O-glucoside | M + H, M + NH4, M + Na | C23H26O10 | −1.59 | 9.2 × 10−7 |
| 36 | 14.44 | 300.0988 | 72026-91-6 | Astraciceran | M + H − H2O, M + | C17H16O5 | −3.15 | 1.7 × 10−11 |
| 37 | 15.00 | 314.0791 | 3301-49-3 | Kumatakenin | M + H, M + Na, M + K | C17H14O6 | 0.18 | 1.9 × 10−12 |
| 38 | 15.22 | 472.3549 | 465-99-6 | Hederagenin | M + H − H2O, M + H | C30H48O4 | −0.67 | 2.2 × 10−11 |
| 39 | 15.24 | 302.1150 | 158784-72-6 | 8-Methoxyvestitol | M + H, M + Na | C17H18O5 | −1.51 | 1.3 × 10−6 |
| 40 | 15.49 | 316.0946 | 69359-09-7 | Pendulone | M + H−H2O, M H, M + Na | C17H16O6 | −0.24 | 8.9 × 10−4 |
| 41 | 15.86 | 490.3650 | 78574-94-4 | Cycloastragenol | M + H − H2O, M + Na | C30H50O5 | −1.68 | 4.5 × 10−10 |
| 42 | 15.88 | 807.4503 | 84687-42-3 | Astragaloside III | M + Na | C41H68O14 | 0.18 | 1.9 × 10−9 |
| 43 | 16.20 | 298.0842 | 740-33-0 | Mosloflavone | M + H, M + Na | C17H14O5 | 0.34 | 4.2 × 10−14 |
| 44 | 16.22 | 274.2743 | 57-10-3 | hexadecanoic acid | M + NH4 | C16H32O2 | 1.05 | 2.4 × 10−2 |
| 45 | 16.22 | 301.1067 | 73340-41-7 | 3-Hydroxy-9,10-dimethoxyptercarpan | M + H | C17H16O5 | −1.09 | 1.2 × 10−6 |
| 46 | 16.41 | 645.3974 | 101683491 | Trigonoside I | M + Na | C35H58O9 | 0.12 | 2.2 × 10−7 |
| 47 | 16.44 | 620.3922 | 13943265 | Cycloorbigenin 3-O-beta-d-xylopyranoside | M + H − H2O, M + Na | C35H56O9 | −0.37 | 8.5 × 10−7 |
| 48 | 17.03 | 473.3617 | 86541-79-9 | Astragenol | M + H − H2O | C30H50O5 | −1.66 | 4.0 × 10−5 |
| 49 | 17.07 | 687.4079 | 133550435 | Cyclogaleginoside A | M + Na | C37H60O10 | 0.11 | 4.0 × 10−8 |
| 50 | 18.42 | 279.2315 | 463-40-1 | Linolenic acid | M + H | C18H30O2 | −1.36 | 7.0 × 10−5 |
| 51 | 19.93 | 263.2367 | 693-77-6 | Octadeca-9,12-dienoic acid | M + H − H2O | C18H32O2 | −0.73 | 5.9 × 10−3 |
| 52 | 21.68 | 409.3823 | 545-47-1 | Lupeol | M + H − H2O | C30H50O | −1.45 | 2.1 × 10−2 |
| 53 | 21.93 | 397.3826 | 83-46-5 | (−)-beta-Sitosterol | M + H − H2O | C29H50O | −0.76 | 1.8 × 10−2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Liu, L.; Ma, Y.; Guo, L.; Sun, Y.; Liu, Q.; Liu, J. Chemical Discrimination of Astragalus mongholicus and Astragalus membranaceus Based on Metabolomics Using UHPLC-ESI-Q-TOF-MS/MS Approach. Molecules 2019, 24, 4064. https://doi.org/10.3390/molecules24224064
Wang Y, Liu L, Ma Y, Guo L, Sun Y, Liu Q, Liu J. Chemical Discrimination of Astragalus mongholicus and Astragalus membranaceus Based on Metabolomics Using UHPLC-ESI-Q-TOF-MS/MS Approach. Molecules. 2019; 24(22):4064. https://doi.org/10.3390/molecules24224064
Chicago/Turabian StyleWang, Yumei, Lei Liu, Yukun Ma, Lina Guo, Yu Sun, Qi Liu, and Jicheng Liu. 2019. "Chemical Discrimination of Astragalus mongholicus and Astragalus membranaceus Based on Metabolomics Using UHPLC-ESI-Q-TOF-MS/MS Approach" Molecules 24, no. 22: 4064. https://doi.org/10.3390/molecules24224064
APA StyleWang, Y., Liu, L., Ma, Y., Guo, L., Sun, Y., Liu, Q., & Liu, J. (2019). Chemical Discrimination of Astragalus mongholicus and Astragalus membranaceus Based on Metabolomics Using UHPLC-ESI-Q-TOF-MS/MS Approach. Molecules, 24(22), 4064. https://doi.org/10.3390/molecules24224064






