Identification of Auxin Metabolites in Brassicaceae by Ultra-Performance Liquid Chromatography Coupled with High-Resolution Mass Spectrometry
Abstract
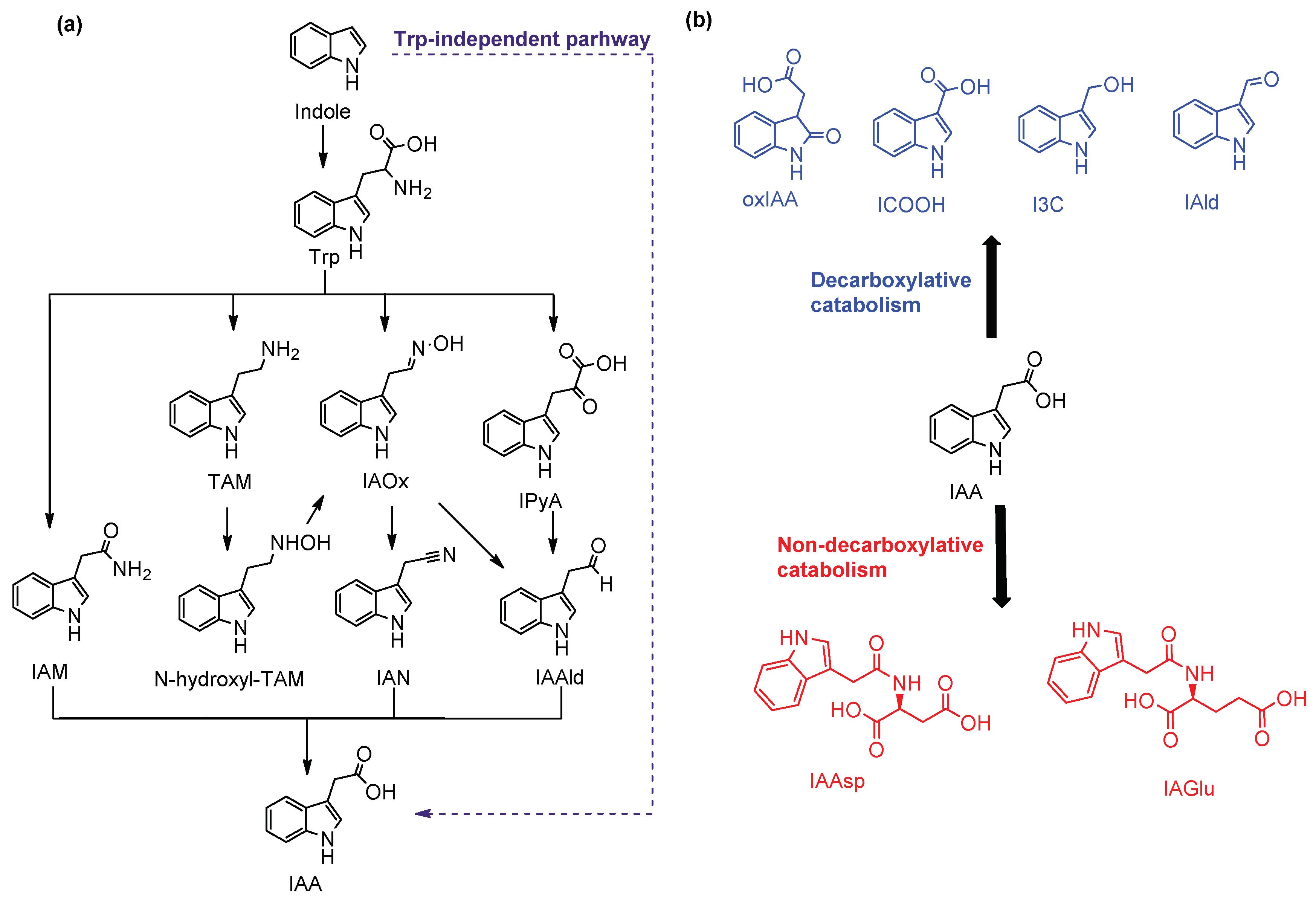
1. Introduction
2. Results and Discussion
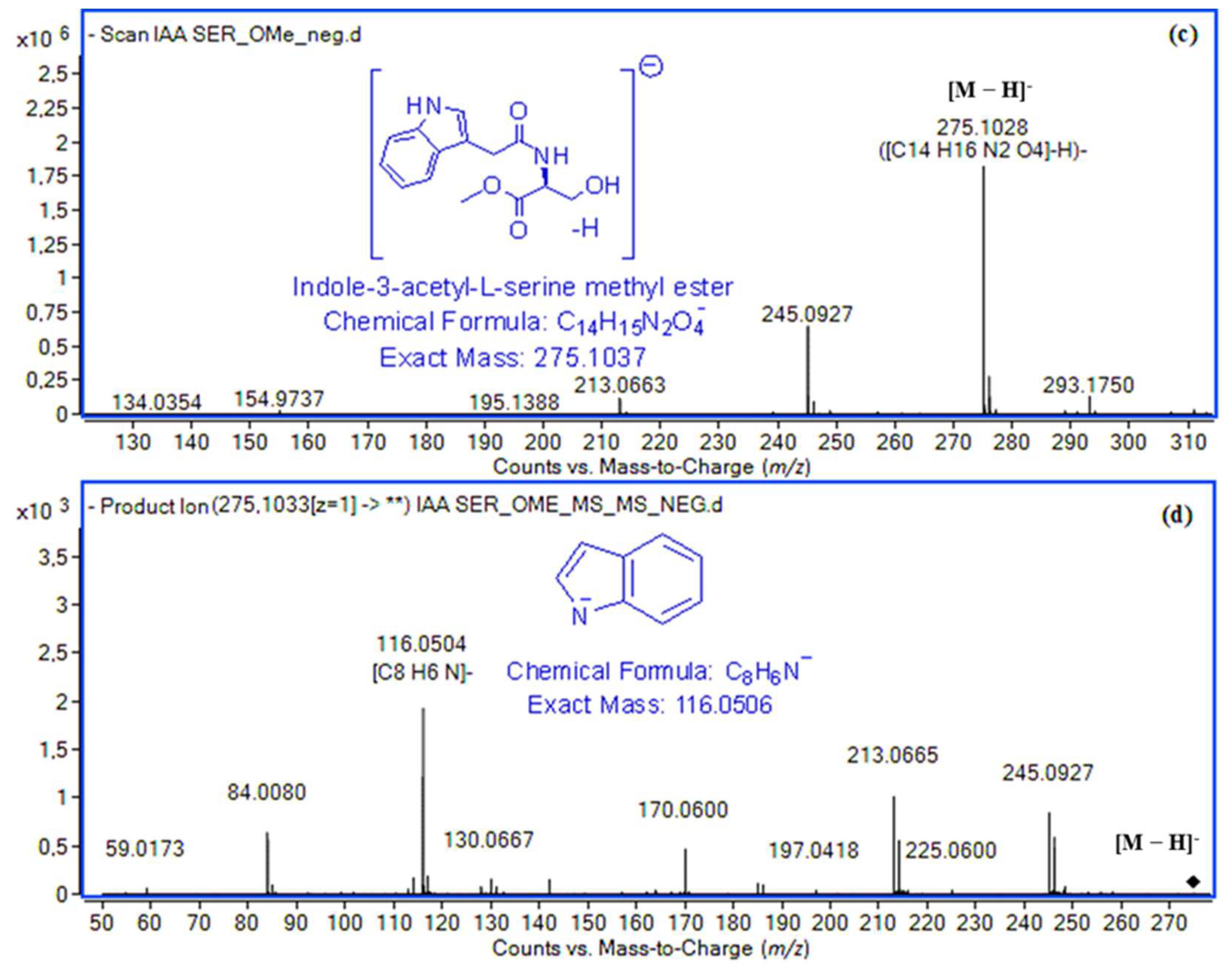
2.1. High-Resolution Mass Spectrometry Study
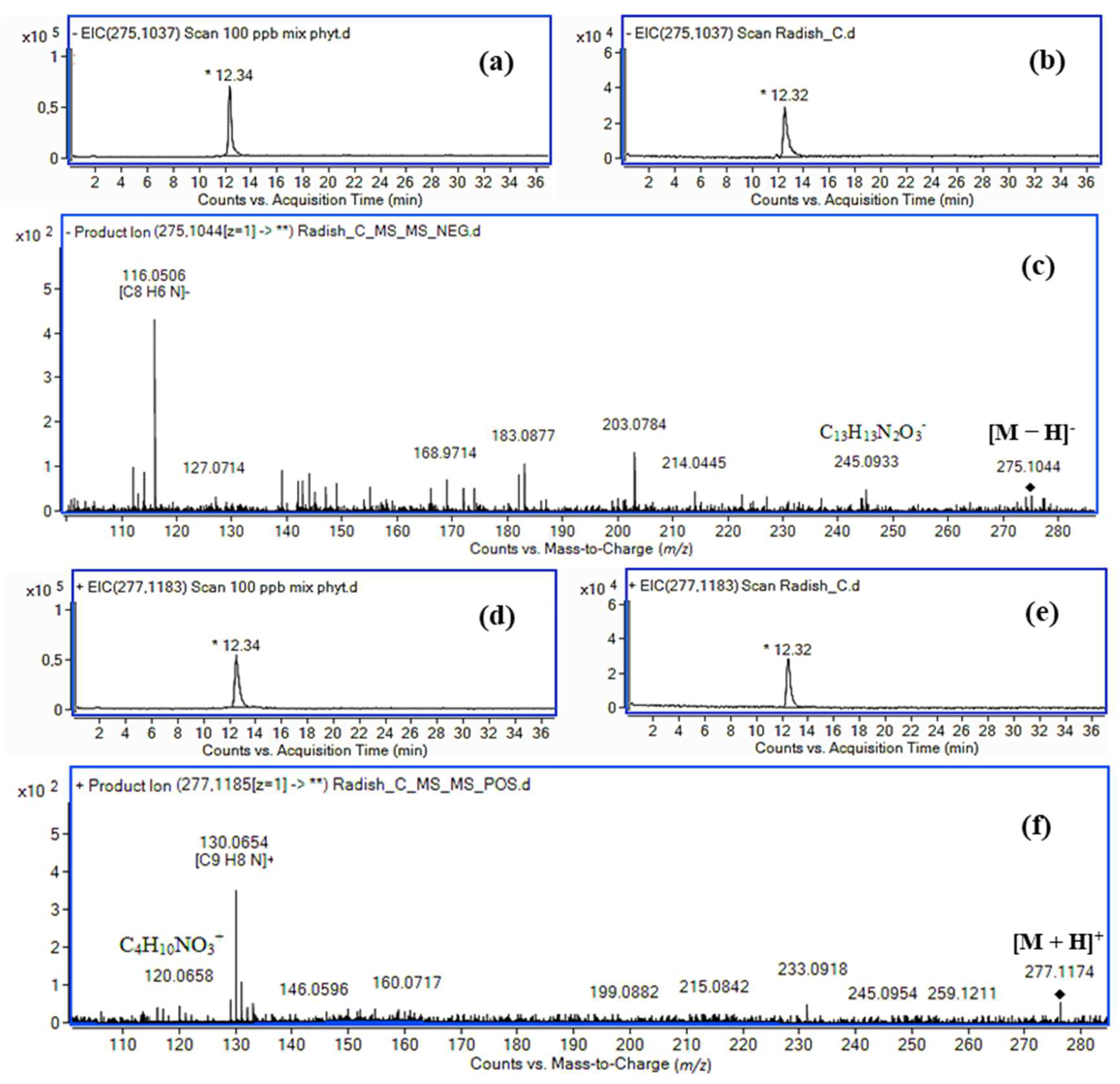
2.2. Analysis of Brassicaceae Vegetables
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Reagents and Materials
3.3. Sampling
3.4. Preparation of Extracts
3.5. Standard Solutions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Darwin, C.; Darwin, F. The Power of Movement in Plants; John Murray: London, UK, 1880. [Google Scholar]
- Teale, W.D.; Paponov, I.A.; Palme, K. Auxin in action: Signalling, transport and the control of plant growth and development. Nat. Rev. Mol. Cell Boil. 2006, 7, 847–859. [Google Scholar] [CrossRef] [PubMed]
- Slovin, J.P.; Bandurski, R.S.; Cohen, J.D. Auxin. In Biochemistry and Molecular Biology of Plant Hormones, 1st ed.; Hooykaas, P.J.J., Hall, M.A., Libbenga, K.R., Eds.; Elsevier Science: Amsterdam, The Netherlands, 1999; Volume 33, pp. 116–140. [Google Scholar]
- Woodward, A.W.; Bartel, B. Auxin: Regulation, Action, and Interaction. Ann. Bot. 2005, 95, 707–735. [Google Scholar] [CrossRef] [PubMed]
- Ljung, K.; Hull, A.K.; Kowalczyk, M.; Marchant, A.; Celenza, J.; Cohen, J.D.; Sandberg, G. Biosynthesis, conjugation, catabolism and homeostasis of indole-3-acetic acid in Arabidopsis thaliana. Auxin Mol. Biol. 2002, 49, 249–272. [Google Scholar]
- Sugawara, S.; Hishiyama, S.; Jikumaru, Y.; Hanada, A.; Nishimura, T.; Koshiba, T.; Zhao, Y.; Kamiya, Y.; Kasahara, H. Biochemical analyses of indole-3-acetaldoxime-dependent auxin biosynthesis in Arabidopsis. Proc. Natl. Acad. Sci. USA 2009, 106, 5430–5435. [Google Scholar] [CrossRef] [PubMed]
- Östin, A. Metabolism of Indole-3-Acetic Acid in Arabidopsis. Plant Physiol. 1998, 118, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.D.; Bandurski, R.S. Chemistry and Physiology of the Bound Auxins. Annu. Rev. Plant Physiol. 1982, 33, 403–430. [Google Scholar] [CrossRef]
- Cohen, J.D.; Gray, W.M. Auxin metabolism and signaling. In Annual Plant Reviews Plant Hormone Signaling, 1st ed.; Hedden, P., Thomas, S.G., Eds.; Blackwell Publishing Ltd.: Oxford, UK, 2006; Volume 24, pp. 37–66. [Google Scholar]
- Cohen, J.D.; Bandurski, R.S. The bound auxins: Protection of indole-3-acetic acid from peroxidase-catalyzed oxidation. Planta 1978, 139, 203–208. [Google Scholar] [CrossRef] [PubMed]
- González-Lamothe, R.; El Oirdi, M.; Brisson, N.; Bouarab, K. The Conjugated Auxin Indole-3-Acetic Acid–Aspartic Acid Promotes Plant Disease Development. Plant Cell 2012, 24, 762–777. [Google Scholar] [CrossRef]
- Oetiker, J.H.; Aeschbacher, G. Temperature-Sensitive Plant Cells with Shunted Indole-3-Acetic Acid Conjugation. Plant Physiol. 1997, 114, 1385–1395. [Google Scholar] [CrossRef]
- Lu, Q.; Zhang, L.; Chen, T.; Lu, M.; Ping, T.; Chen, G. Identification and quantitation of auxins in plants by liquid chromatography/electrospray ionization ion trap mass spectrometry. Rapid Commun. Mass Spectrom. 2008, 22, 2565–2572. [Google Scholar] [CrossRef]
- Kowalczyk, M.; Sandberg, G. Quantitative Analysis of Indole-3-Acetic Acid Metabolites in Arabidopsis. Plant Physiol. 2001, 127, 1845–1853. [Google Scholar] [CrossRef] [PubMed]
- Sohlberg, J.J.; Myrenås, M.; Kuusk, S.; Lagercrantz, U.; Kowalczyk, M.; Sandberg, G.; Sundberg, E. STY1regulates auxin homeostasis and affects apical-basal patterning of the Arabidopsis gynoecium. Plant J. 2006, 47, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, F.; Miyazawa, H.; Wakasa, K.; Miyagawa, H. Quantification of Indole-3-Acetic Acid and Amino Acid Conjugates in Rice by Liquid Chromatography-Electrospray Ionization-Tandem Mass Spectrometry. Biosci. Biotechnol. Biochem. 2005, 69, 778–783. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Hegeman, A.D.; Cohen, J.D. A facile means for the identification of indolic compounds from plant tissues. Plant J. 2014, 79, 1065–1075. [Google Scholar] [CrossRef]
- Pěnčík, A.; Rolčík, J.; Novak, O.; Magnus, V.; Barták, P.; Buchtik, R.; Salopek-Sondi, B.; Strnad, M. Isolation of novel indole-3-acetic acid conjugates by immunoaffinity extraction. Talanta 2009, 80, 651–655. [Google Scholar] [CrossRef] [PubMed]
- Van Meulebroek, L.; Bussche, J.V.; Steppe, K.; Vanhaecke, L. Ultra-high-performance liquid chromatography coupled to high resolution Orbitrap mass spectrometry for metabolomic profiling of the endogenous phytohormonal status of the tomato plant. J. Chromatogr. A 2012, 1260, 67–80. [Google Scholar] [CrossRef]
- Tam, Y.Y. Characterization of Auxin Conjugates in Arabidopsis. Low Steady-State Levels of Indole-3-Acetyl-Aspartate, Indole-3-Acetyl-Glutamate, and Indole-3-Acetyl-Glucose. Plant Physiol. 2000, 123, 589–596. [Google Scholar] [CrossRef]
- Krauss, M.; Singer, H.; Hollender, J. LC–high resolution MS in environmental analysis: From target screening to the identification of unknowns. Anal. Bioanal. Chem. 2010, 397, 943–951. [Google Scholar] [CrossRef]
- Zhao, H.; Jiang, M.; Liang, Q.; Xie, C.; Song, S.; Wang, J.; Bai, G.; Luo, G. Fragmentation pathway studies of several plant hormones using an electrospray ionization-quadrupole/time-of-flight mass spectrometer. Int. J. Mass Spectrom. 2013, 335, 7–15. [Google Scholar] [CrossRef]
- Avula, B.; Sagi, S.; Wang, Y.-H.; Zweigenbaum, J.; Wang, M.; Khan, I.A. Characterization and screening of pyrrolizidine alkaloids and N-oxides from botanicals and dietary supplements using UHPLC-high resolution mass spectrometry. Food Chem. 2015, 178, 136–148. [Google Scholar] [CrossRef]
- Aquino, A.J.; Alves, T.D.C.; Oliveira, R.V.; Ferreira, A.G.; Cass, Q.B. Chemical secondary metabolite profiling of Bauhinia longifolia ethanolic leaves extracts. Ind. Crops Prod. 2019, 132, 59–68. [Google Scholar] [CrossRef]
- Marczak, Ł.; Znajdek-Awiżeń, P.; Bylka, W. The Use of Mass Spectrometric Techniques to Differentiate Isobaric and Isomeric Flavonoid Conjugates from Axyris amaranthoides. Molecules 2016, 21, 1229. [Google Scholar] [CrossRef] [PubMed]
- Sangthong, S.; Weerapreeyakul, N.; Lehtonen, M.; Leppanen, J.; Rautio, J. High-accuracy mass spectrometry for identification of sulphur-containing bioactive constituents and flavonoids in extracts of Raphanus sativus var. caudatus Alef (Thai rat-tailed radish). J. Funct. Foods 2017, 31, 237–247. [Google Scholar] [CrossRef]
- Xu, H.; Niu, H.; He, B.; Cui, C.; Li, Q.; Bi, K. Comprehensive Qualitative Ingredient Profiling of Chinese Herbal Formula Wu-Zhu-Yu Decoction via a Mass Defect and Fragment Filtering Approach Using High Resolution Mass Spectrometry. Molecules 2016, 21, 664. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Kuang, G.; Chen, X.; Zeng, R. Identification of Chemical Composition of Leaves and Flowers from Paeonia rockii by UHPLC-Q-Exactive Orbitrap HRMS. Molecules 2016, 21, 947. [Google Scholar] [CrossRef]
- Piccolella, S.; Bianco, A.; Crescente, G.; Santillo, A.; Chieffi Baccari, G.; Pacifico, S. Recovering Cucurbita pepo cv. ‘Lungo Fiorentino’ wastes: UHPLC-HRMS/MS metabolic profile, the basis for establishing their nutra- and cosmeceutical valorization. Molecules 2016, 24, 1479. [Google Scholar] [CrossRef]
- Wang, H.; Li, Y.; Huang, Y.; Zhao, C.; Cheung, H.-Y. Chemical Profiling of Lobelia chinensis with High-Performance Liquid Chromatography/Quadrupole Time-of-Flight Mass Spectrometry (HPLC/Q-ToF MS) Reveals Absence of Lobeline in the Herb. Molecules 2018, 23, 3258. [Google Scholar] [CrossRef]
- Khan, H.; Ali, J. UHPLC/Q-ToF-MS Technique: Introduction and Applications. Lett. Org. Chem. 2015, 12, 371–378. [Google Scholar] [CrossRef]
- Kokotou, M.G.; Revelou, P.-K.; Pappas, C.; Constantinou-Kokotou, V. High resolution mass spectrometry studies of sulforaphane and indole-3-carbinol in broccoli. Food Chem. 2017, 237, 566–573. [Google Scholar] [CrossRef]
- Khmel’Nitskii, R.A. Mass spectrometry of indole compounds (review). Chem. Heterocycl. Compd. 1974, 10, 253–267. [Google Scholar] [CrossRef]
- Prinsen, E.; Van Dongen, W.; Esmans, E.L.; Van Onckelen, H.A. HPLC Linked Electrospray Tandem Mass Spectrometry: A Rapid and Reliable Method to Analyse Indole-3-Acetic Acid Metabolism in Bacteria. J. Mass Spectrom. 1997, 32, 12–22. [Google Scholar] [CrossRef]
- Ferrer, I.; Thurman, E.M. Measuring the Mass of an Electron by LC/ToF-MS: A Study of “Twin Ions”. Anal. Chem. 2005, 77, 3394–3400. [Google Scholar] [CrossRef] [PubMed]
- Revelou, P.-K.; Constantinou-Kokotou, V. Preparation of synthetic auxin-amino acid conjugates. Synth. Commun. 2019, 49, 1708–1712. [Google Scholar] [CrossRef]
- Revelou, P.-K.; Kokotou, M.G.; Constantinou-Kokotou, V. Determination of indole-type phytonutrients in cruciferous vegetables. Nat. Prod. Res. 2018, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Revelou, P.; Kokotou, M.; Pappas, C.; Constantinou-Kokotou, V. Direct determination of total isothiocyanate content in broccoli using attenuated total reflectance infrared Fourier transform spectroscopy. J. Food Compos. Anal. 2017, 61, 47–51. [Google Scholar] [CrossRef]
- Mikhaleva, A.I.; Ivanov, A.V.; Skital’tseva, E.V.; Ushakov, I.A.; Vasil’tsov, A.M.; Trofimov, B.A. An efficient route to 1-vinylpyrrole-2-carbaldehydes. Synthesis 2009, 4, 587–590. [Google Scholar]
- Somei, M.; Yamada, F.; Hashizume, T. Simple One Step Syntheses of Indole-3-acetonitriles from Indole-3-carboxaldehydes. Heterocycles 1998, 47, 509. [Google Scholar] [CrossRef]
- Reid, A.E.; Kim, S.W.; Seiner, B.; Fowler, F.W.; Hooker, J.; Ferrieri, R.; Babst, B.; Fowler, J.S. Radiosynthesis of C-11 labeled auxin (3-indolyl[1-11C]acetic acid) and its derivatives from gramine. J. Label. Compd. Radiopharm. 2011, 54, 433–437. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |


| Compounds | Rt 1 (min) | Positive Ion Mode | Negative Ion Mode | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ion Species | Elemental Composition | Theoretical Mass (m/z) | Observed Mass (m/z) (Mass Error, ∆ ppm) | Peak Intensity | Score (Iso. abund.) 2 | Ion Species | Elemental Composition | Theoretical Mass (m/z) | Observed Mass (m/z) (Mass Error, ∆ (ppm) | Peak Intensity | Score (Iso. abund.) 2 | ||
| Indole-3-acetic acid (IAA) | 13.12 | [M + H]+ | C10H10NO2+ | 176.0706 | 176.0704 (1.1) | 1.8 × 104 | 94.9 | [M − H]− | C10H8NO2− | 174.0561 | 174.0555 (3.4) | 4.0 × 103 | 90.63 |
| [Μ + Νa]+ | C10H9NNaO2+ | 198.0525 | 198.0528 (1.5) | 0.6 × 104 | 90.79 | 4 | C9H8N− | 130.0662 | 130.0656 (4.6) | 2.2 × 103 | 91.21 | ||
| 2 | C9H8N+ | 130.0651 | 130.0651 (0) | 6.7 × 103 | 94.76 | ||||||||
| 4-Chloroindole-3-acetic acid (4-Cl-IAA) | 13.93 | [M + H]+ | C10H9ClNO2+ | 210.0316 | 210.0317 (0.5) | 1.4 × 105 | 99.71 | C10H7ClNO2− | 208.0171 | 208.0167 (1.9) | 8.2 × 105 | 99.25 | |
| [M + H]+ | C10H9ClNO2+ | 212.0287 | 212.0292 (2.4) | 0.5 × 105 | C10H7ClNO2− | 210.0142 | 210.0136 (2.9) | 2.9 × 105 | |||||
| [Μ + Νa]+ | C10H8ClNNaO2+ | 232.0136 | 232.0137 (0.4) | 2.1 × 105 | 99.51 | C9H7ClN− | 164.0273 | 164.0271 (1.2) | 4.9 × 103 | 90.86 | |||
| [Μ + Νa]+ | C10H8ClNNaO2+ | 234.0106 | 234.0110 (1.7) | 7.1 × 104 | C9H7ClN− | 166.0244 | 166.0246 (1.2) | 2.3 × 103 | |||||
| C9H7ClN+ | 164.0252 | 164.0252 (0) | 0.9 × 104 | 90.06 | |||||||||
| C9H7ClN+ | 166.0223 | 166.0231 (4.8) | 0.3 × 104 | ||||||||||
| Indole-3-aldehyde (IAld) | 12.82 | [M + H]+ | C9H8NO+ | 146.06 | 146.0601 (0.7) | 8.3 × 105 | 99.2 | [M − H]− | C9H6NO− | 144.0455 | 146.0451 (0.7) | 3.1 × 106 | 98.68 |
| [Μ + Νa]+ | C9H7NNaO+ | 168.042 | 168.0420 (0) | 1.5 × 105 | 99.88 | 5 | C8H6N− | 116.0506 | 116.0503 (2.6) | 7.2 × 102 | 90.67 | ||
| [M + H − CO]+ | C8H8N+ | 118.0651 | 118.0653 (1.7) | 8.1 × 103 | 99.92 | ||||||||
| Indole-3-acetonitrile (IAN) | 13.38 | [M + H]+ | C10H9N2+ | 157.076 | 157.0765 (3.2) | 6.0 × 104 | 98.84 | C10H7N2− | 155.0615 | 155.0615 (0) | 4.0 × 105 | 99.53 | |
| [Μ + Νa]+ | C10H8N2Na+ | 179.0579 | 179.0583 (2.2) | 7.8 × 104 | 99.46 | C9H6N− | 128.0506 | 128.0508 (2.3) | 4.0 × 102 | 91.46 | |||
| 2 | C9H8N+ | 130.0651 | 130.0655 (3.1) | 2.6 × 102 | 98.01 | ||||||||
| Indole-3-acetamide (IAM) | 12.81 | [M + H]+ | C10H11N2O+ | 175.0866 | 175.0866 (0) | 1.0 × 105 | 90.6 | [M − H]− | C10H9N2O− | 173.072 | 173.0716 (2.3) | 4.2 × 104 | 90.2 |
| [Μ + Νa]+ | C10H10N2NaO+ | 197.0685 | 197.0683 (1.0) | 1.9 × 106 | 98.9 | 4 | C9H8N− | 130.0662 | 130.0663 (0.8) | 3.5 × 103 | 99.94 | ||
| 2 | C9H8N+ | 130.0651 | 130.0650 (0.8) | 3.1 × 104 | 99.8 | ||||||||
| Indole-3-acetyl-l-alanine (IAAla) | 12.64 | [M + H]+ | C13H15N2O3+ | 247.1077 | 247.1077 (0) | 7.9 × 105 | 99.91 | [M − H]− | C13H13N2O3− | 245.0932 | 245.0928 (1.6) | 2.3 × 105 | 99.95 |
| [Μ + Νa]+ | C13H14N2NaO3+ | 269.0896 | 269.0896 (0) | 8.5 × 105 | 99.65 | [H–Ala–OH − H]− | C3H6NO2− | 88.0404 | 88.0405 (1.1) | 6.4 × 103 | 98.32 | ||
| 2 | C9H8N+ | 130.0651 | 130.0649 (1.5) | 6.9 × 103 | 98.91 | ||||||||
| [H–Ala–OH + H]+ | C3H8NO2+ | 90.055 | 90.0550 (0) | 1.8 × 103 | 99.36 | ||||||||
| Indole-3-acetyl-l-valine (IAVal) | 13.86 | [M + H]+ | C15H19N2O3+ | 275.139 | 275.1391 (0.4) | 1.3 × 106 | 99.75 | [M − H]− | C15H17N2O3− | 273.1245 | 273.1240 (1.8) | 2.2 × 106 | 99.6 |
| [Μ + Νa]+ | C15H18N2NaO3+ | 297.1209 | 297.1208 (0.3) | 2.0 × 106 | 99.47 | [H–Val–OH − H]− | C5H10NO2− | 116.0717 | 116.0717 (0) | 6.0 × 103 | 90.33 | ||
| 2 | C9H8N+ | 130.0651 | 130.0651 (0) | 1.2 × 104 | 98.71 | ||||||||
| [H–Val–OH + H]+ | C5H12NO2+ | 118.0863 | 118.0861 (1.7) | 3.5 × 103 | 90.83 | ||||||||
| Indole-3-acetyl-l-glycine (IAGly) | 11.76 | [M + H]+ | C12H13N2O3+ | 233.0921 | 233.0919 (0.9) | 2.5 × 105 | 99.83 | [M − H]− | C12H11N2O3− | 231.0775 | 231.0770 (2.2) | 1.3 × 106 | 99.75 |
| [Μ + Νa]+ | C12H12N2NaO3+ | 255.074 | 255.0742 (0.4) | 5.3 × 105 | 99.64 | [H–Gly–OH − H]− | C2H4NO2− | 74.0248 | 74.0248 (0) | 5.0 × 103 | 90.54 | ||
| 2 | C9H8N+ | 130.0651 | 130.0649 (1.5) | 5.9 × 103 | 93.33 | ||||||||
| [H–Gly–OH + H]+ | C2H6NO2+ | 76.0393 | 76.0393 (0) | 4.1 × 102 | 90.49 | ||||||||
| Indole-3-acetyl-l-methionine (IAMet) | 13.72 | [M + H]+ | C15H19N2O3S+ | 307.1111 | 307.1113 (0.7) | 8.0 × 105 | 98.87 | [M − H]− | C15H17N2O3S− | 305.0965 | 305.0960 (1.6) | 2.0 × 106 | 98.73 |
| [Μ + Νa]+ | C15H18N2NaO3S+ | 329.093 | 329.0926 (1.2) | 2.0 × 106 | 98.55 | [H–Met–OH − H]− | C5H10NO2S− | 148.0438 | 148.0438 (0) | 5.2 × 103 | 96.69 | ||
| [H–Met–OH + H]+ | C5H12NO2S+ | 150.0583 | 150.0583 (0) | 9.0 × 102 | 89.51 | ||||||||
| 2 | C9H8N+ | 130.0651 | 130.0650 (0.8) | 3.8 × 105 | 88.7 | ||||||||
| Indole-3-acetyl-l-tryptophan (IATrp) | 14.15 | [M + H]+ | C21H20N3O3+ | 362.1499 | 362.1499 (0) | 8.2 × 105 | 99.45 | [M − H]− | C21H18N3O3− | 360.1354 | 360.1351 (0.8) | 8.0 × 105 | 98.46 |
| [Μ + Νa]+ | C21H19N3NaO3+ | 384.1318 | 384.1319 (0.3) | 8.2 × 105 | 99.8 | [H–Trp–OH − H]− | C11H11N2O2− | 203.0826 | 203.0825 (0.5) | 5.8 × 103 | 90.69 | ||
| [H–Trp–OH + H]+ | C11H13N2O2+ | 205.0972 | 205.0972 (0) | 0.9 × 103 | 94.82 | 5 | C8H6N− | 116.0506 | 116.0502 (3.4) | 8.1 × 102 | 89.74 | ||
| 2 | C9H8N+ | 130.0651 | 130.0649 (1.5) | 4.1 × 103 | 95.63 | ||||||||
| Indole-3-acetyl-l-tyrosine (IATyr) | 13.53 | [M + H]+ | C19H19N2O4+ | 339.1339 | 339.1341 (0.6) | 2.8 × 105 | 99.61 | [M − H]− | C19H17N2O4− | 337.1194 | 337.1191 (0.9) | 1.1 × 106 | 99.73 |
| [Μ + Νa]+ | C19H18N2NaO4+ | 361.1159 | 361.1156 (0.8) | 8.5 × 105 | 99.49 | [H–Tyr–OH − H]− | C9H10NO3+ | 180.0666 | 180.0663 (1.7) | 4.9 × 103 | 90.58 | ||
| [H–Tyr–OH + H]+ | C9H12NO3+ | 182.0812 | 182.0816 (2.2) | 2.5 × 102 | 90.55 | ||||||||
| 2 | C9H8N+ | 130.0651 | 130.0649 (1.5) | 1.1 × 103 | 97.7 | ||||||||
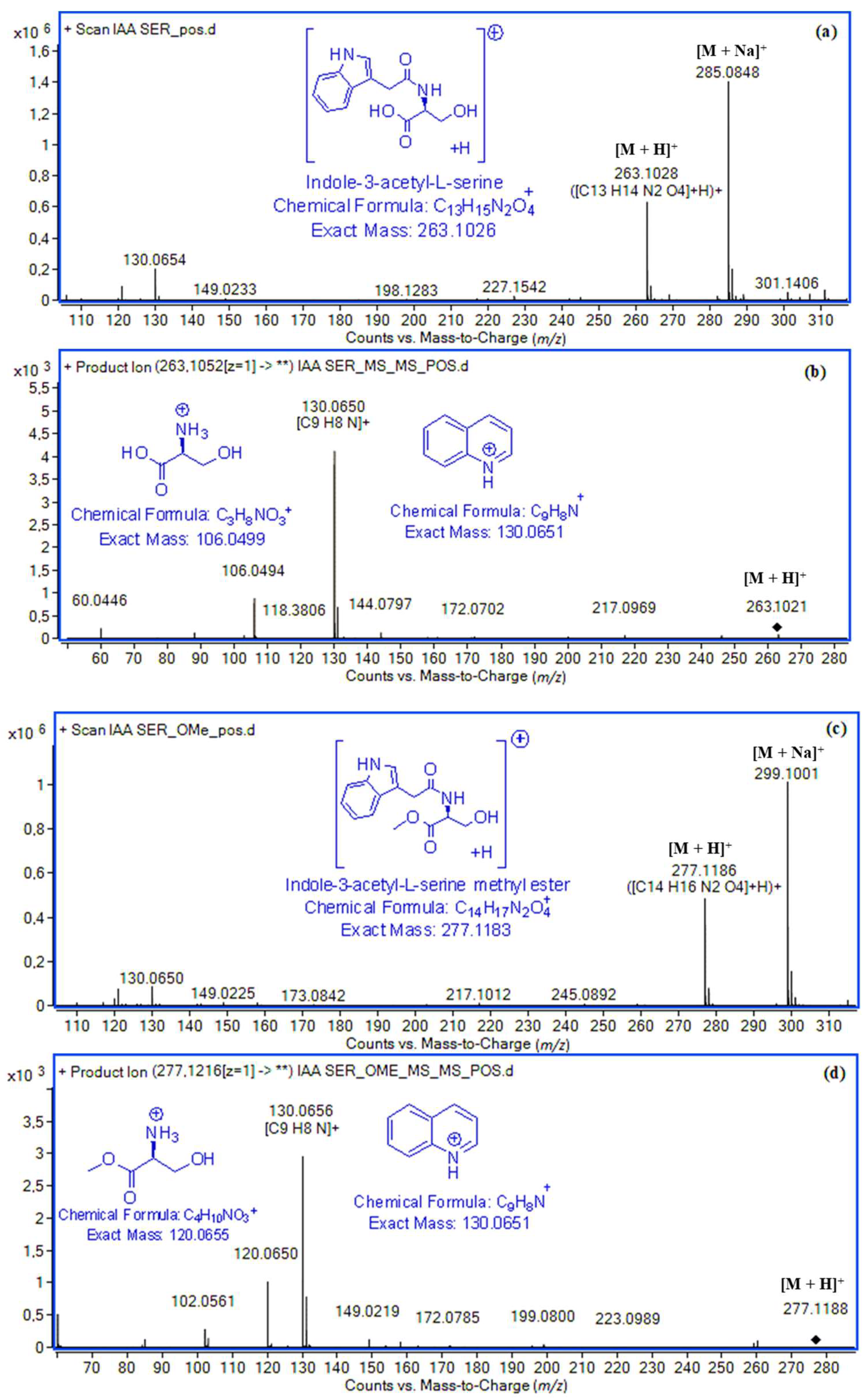
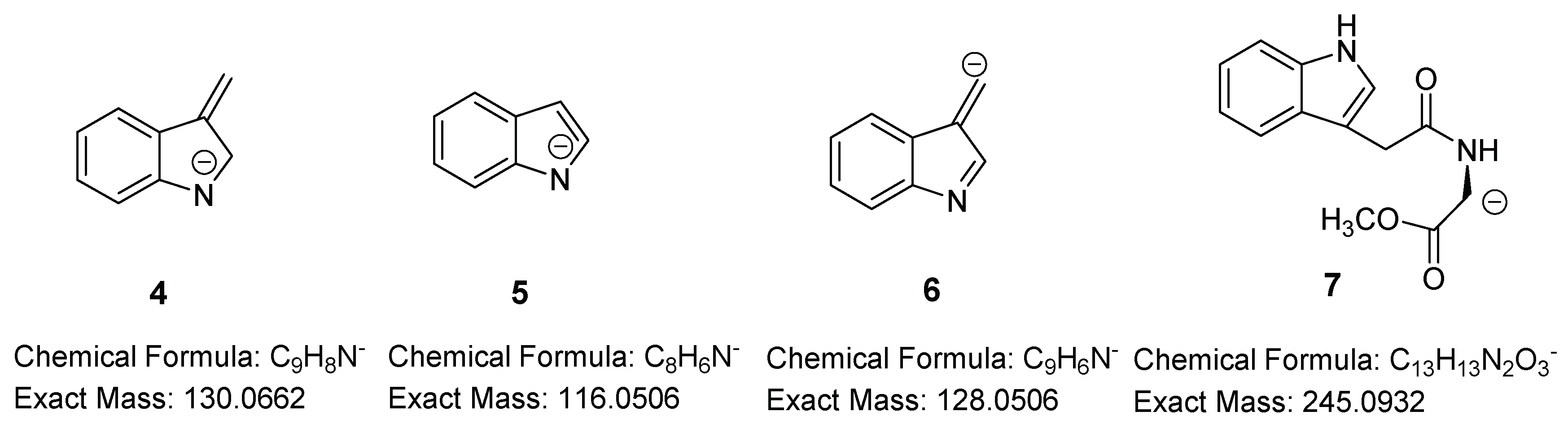
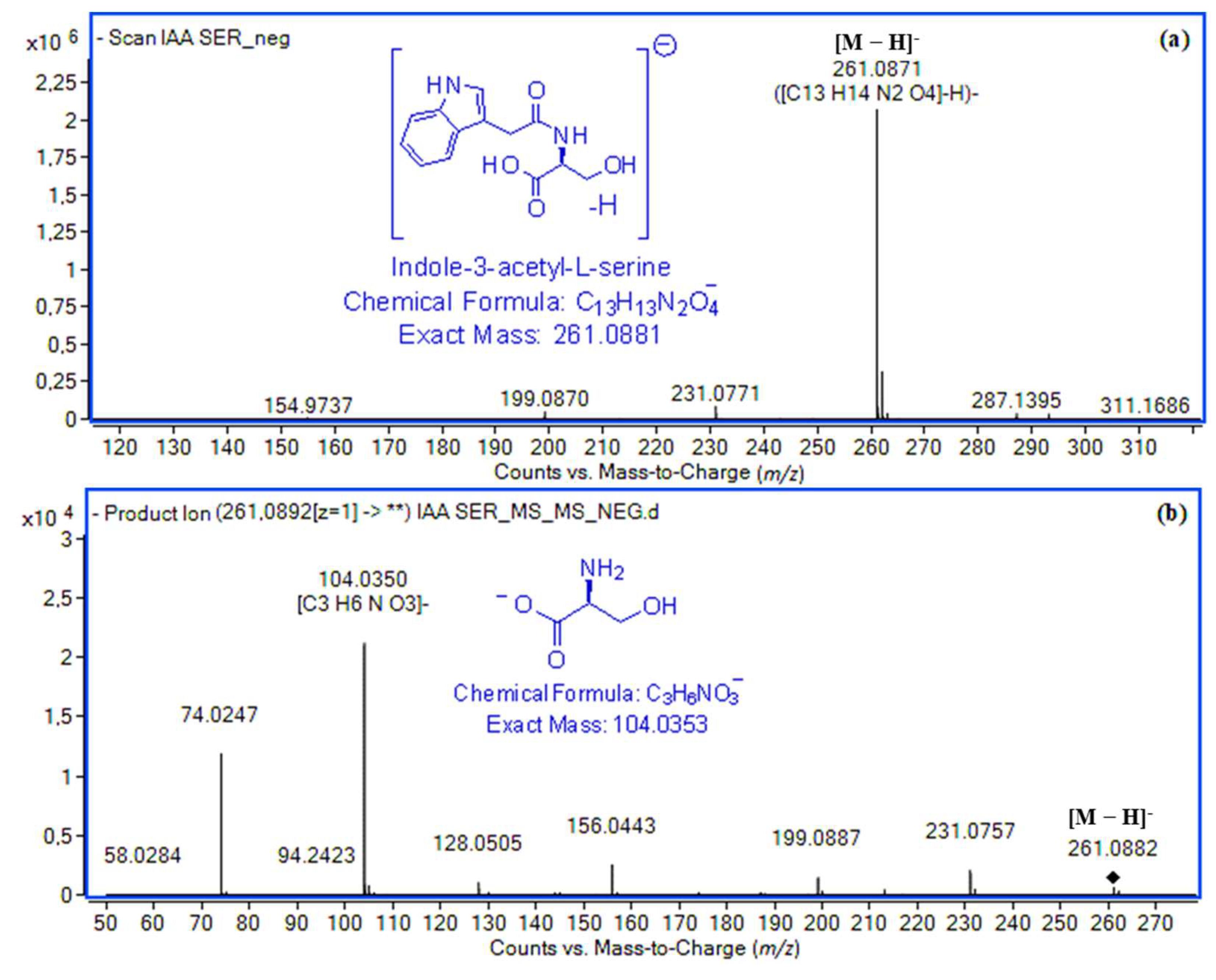
| Indole-3-acetyl-l-serine (IASer) | 11.29 | [M + H]+ | C13H15N2O4+ | 263.1026 | 263.1028 (0.8) | 7.0 × 105 | 99.85 | [M − H]− | C13H13N2O4− | 261.0881 | 261.0871 (3.8) | 2.1 × 106 | 99.69 |
| [Μ + Νa]+ | C13H14N2NaO4+ | 285.0846 | 285.0848 (0.7) | 1.5 × 106 | 99.64 | [H–Ser–OH − H]− | C3H6NO3+ | 104.0353 | 104.0350 (2.9) | 2.2 × 104 | 99.26 | ||
| 2 | C9H8N+ | 130.0651 | 130.0650 (0.8) | 4.1 × 103 | 98.81 | ||||||||
| [H–Ser–OH + H]+ | C3H8NO3+ | 106.0499 | 106.0494 (4.7) | 9.5 × 102 | 90.41 | ||||||||
| Indole-3-acetyl-l-phenylalanine (IAPhe) | 14.36 | [M + H]+ | C19H19N2O3+ | 323.139 | 323.1392 (0.6) | 6.1 × 105 | 98.84 | [M − H]− | C19H17N2O3− | 321.1245 | 321.1241 (1.2) | 1.7 × 106 | 99.46 |
| [Μ + Νa]+ | C19H18N2NaO3+ | 345.1209 | 345.1210 (0.3) | 7.0 × 105 | 99.42 | [H–Phe–OH − H]− | C9H10NO2− | 164.0717 | 164.0715 (1.2) | 7.0 × 103 | 96.73 | ||
| [H–Phe–OH + H]+ | C9H12NO2+ | 166.0863 | 166.0863 (0) | 1.5 × 103 | 95.1 | ||||||||
| 2 | C9H8N+ | 130.0651 | 130.0649 (1.5) | 5.5 × 103 | 97.64 | ||||||||
| Indole-3-acetyl-l-aspartic acid (IAAsp) | 11.73 | [M + H]+ | C14H15N2O5+ | 291.0975 | 291.0979 (1.4) | 6.0 × 105 | 93.49 | [M − H]− | C14H13N2O5− | 289.083 | 289.0821 (3.1) | 2.3 × 106 | 99.81 |
| [Μ + Νa]+ | C14H14N2NaO5+ | 313.0795 | 313.0795 (0) | 1.1 × 106 | 99.72 | [H–Asp–OH − H]− | C4H6NO4− | 132.0302 | 132.0300 (1.5) | 2.2 × 103 | 90.38 | ||
| [H–Asp–OH + H]+ | C4H8NO4+ | 134.0448 | 134.0447 (0.7) | 1.0 × 104 | 90.8 | [H–Ala–OH − H]− | C3H6NO2− | 88.0404 | 88.0404 (0) | 1.7 × 103 | 99.04 | ||
| 2 | C9H8N+ | 130.0651 | 130.0652 (0.8) | 9.3 × 104 | 91.9 | ||||||||
| Indole-3-acetyl-l-glutamic acid (IAGlu) | 11.99 | [M + H]+ | C15H17N2O5+ | 305.1132 | 305.1139 (2.3) | 5.0 × 105 | 99.83 | [M − H]− | C15H15N2O5− | 303.0986 | 303.0980 (2.0) | 2.5 × 106 | 99.99 |
| [Μ + Νa]+ | C15H16N2NaO5+ | 327.0951 | 327.0952 (0.3) | 6.9 × 105 | 99.91 | [H–Glu–OH − H]− | C5H8NO4− | 146.0459 | 146.0459 (0) | 1.8 × 103 | 90.63 | ||
| 2 | C9H8N+ | 130.0651 | 130.0651 (0) | 9.9 × 103 | 90.2 | ||||||||
| [H–Clu–OH + H]+ | C5H10NO4+ | 148.0604 | 148.0604 (0) | 1.2 × 103 | 90.66 | ||||||||
| Indole-3-acetyl-glycine methyl ester (IAGly-Me) | 12.64 | [M + H]+ | C13H15N2O3+ | 247.1077 | 247.1084 (2.8) | 3.0 × 105 | 99.92 | [M − H]− | C13H13N2O3− | 245.0932 | 245.0923 (3.7) | 3.1 × 106 | 99.62 |
| [Μ + Νa]+ | C13H14N2NaO3+ | 269.0896 | 269.0899 (1.1) | 7.5 × 105 | 99.59 | 5 | C8H6N− | 116.0506 | 116.0505 (0.9) | 2.7 × 103 | 99.04 | ||
| 2 | C9H8N+ | 130.0651 | 130.0651 (0) | 7.8 × 103 | 99.57 | ||||||||
| [H–Gly–OMe + H]+ | C3H8NO2+ | 90.055 | 90.0553 (3.3) | 7.0 × 103 | 99.3 | ||||||||
| Indole-3-acetyl-l-alanine methyl ester (IAAla-Me) | 13.22 | [M + H]+ | C14H17N2O3+ | 261.1234 | 261.1235 (0.4) | 8.2 × 105 | 98.6 | [M − H]− | C14H15N2O3− | 259.1088 | 259.1082 (2.3) | 3.2 × 106 | 99.85 |
| [Μ + Νa]+ | C14H16N2NaO3+ | 283.1053 | 283.1053 (0) | 1.7 × 106 | 99.96 | 5 | C8H6N− | 116.0506 | 116.0506 (0) | 2.5 × 103 | 90.82 | ||
| 2 | C9H8N+ | 130.0651 | 130.0648 (2.3) | 7.2 × 103 | 99.21 | ||||||||
| [H–Ala–OMe + H]+ | C4H10NO2+ | 104.0706 | 104.0703 (2.9) | 1.8 × 103 | 99.34 | ||||||||
| Indole-3-acetyl-l-valine methyl ester (IAVal-Me) | 14.24 | [M + H]+ | C16H21N2O3+ | 289.1547 | 289.1550 (1.0) | 1.5 × 106 | 99.85 | [M − H]− | C16H19N2O3− | 287.1401 | 287.1396 (1.7) | 4.82106 | 99.97 |
| [Μ + Νa]+ | C16H20N2NaO3+ | 311.1366 | 311.1368 (0.6) | 1.6 × 106 | 99.95 | 5 | C8H6N− | 116.0506 | 116.0504 (1.7) | 3.1 × 103 | 99.88 | ||
| [H–Val–OMe + H]+ | C6H14NO2+ | 132.1019 | 132.1017 (1.5) | 3.3 × 103 | 94.29 | ||||||||
| 2 | C9H8N+ | 130.0651 | 130.0651 (0) | 9.2 × 103 | 98.28 | ||||||||
| Indole-3-acetyl-l-tryptophan methyl ester (IATrp-Me) | 14.43 | [M + H]+ | C22H22N3O3+ | 376.1656 | 376.1660 (1.1) | 4.4 × 105 | 99.67 | [M − H]− | C22H20N3O3− | 374.151 | 374.1502 (2.1) | 1.4 × 106 | 99.95 |
| [Μ + Νa]+ | C22H21N3NaO3+ | 398.1475 | 398.1477 (0.5) | 6.2 × 105 | 99.45 | 7 | C13H13N2O3− | 245.0932 | 245.0934 (0.8) | 4.6 × 103 | 97.87 | ||
| [H–Trp–OMe + H]+ | C12H15N2O2+ | 219.1128 | 219.1129 (0.5) | 5.1 × 102 | 90.51 | 5 | C8H6N− | 116.0505 | 116.0502 (2.6) | 9.0 × 102 | 96.53 | ||
| 2 | C9H8N+ | 130.0651 | 130.0651 (0) | 5.5 × 103 | 96.39 | ||||||||
| Indole-3-acetyl-l-tyrosine methyl ester (IATyr-Me) | 13.86 | [M + H]+ | C20H21N2O4+ | 353.1496 | 353.1497 (0.3) | 1.1 × 106 | 99.93 | [M − H]− | C20H19N2O4− | 351.135 | 351.1344 (1.7) | 1.3 × 106 | 99.65 |
| [Μ + Νa]+ | C20H20N2NaO4+ | 375.1315 | 375.1321 (1.6) | 6.5 × 105 | 99.48 | 7 | C13H13N2O3− | 245.0932 | 245.0933 (0.4) | 4.9 × 103 | 93.52 | ||
| [H–Tyr–-OMe + H]+ | C10H14NO3+ | 196.0968 | 196.0970 (1.0) | 1.4 × 103 | 97.44 | 5 | C8H6N− | 116.0505 | 116.0503 (1.7) | 9.8 × 102 | 95.36 | ||
| 2 | C9H8N+ | 130.0651 | 130.0650 (0.8) | 4.5 × 103 | 99.62 | ||||||||
| Indole-3-acetyl-l-serine methyl ester (IASer-Me) | 12.34 | [M + H]+ | C14H17N2O4+ | 277.1183 | 275.1186 (1.1) | 5.6 × 105 | 99.76 | [M − H]− | C14H15N2O4− | 275.1037 | 275.1028 (3.3) | 2.0 × 106 | 99.72 |
| [Μ + Νa]+ | C14H16N2NaO4+ | 299.1002 | 299.1001 (0.3) | 1.1 × 106 | 90.66 | 7 | C13H13N2O3− | 245.0932 | 245.0927 (1.2) | 8.0 × 105 | 90.7 | ||
| 2 | C9H8N+ | 130.0651 | 130.0656 (3.8) | 3.0 × 103 | 94.54 | 5 | C8H6N− | 116.0506 | 116.0504 (1.7) | 2.0 × 103 | 94.39 | ||
| [H–Ser–OMe + H]+ | C4H10NO3+ | 120.0655 | 120.0650 (4.2) | 1.0 × 103 | 99.18 | ||||||||
| Indole-3-acetyl-l-phenylalanine methyl ester (IAPhe-Me) | 14.56 | [M + H]+ | C20H21N2O3+ | 337.1547 | 337.1547 (0) | 7.9 × 105 | 98.99 | [M − H]− | C20H19N2O3− | 335.1401 | 335.1395 (1.8) | 2.7 × 106 | 99.99 |
| [Μ + Νa]+ | C20H20N2NaO3+ | 359.1366 | 359.1366 (0) | 1.2 × 106 | 99.08 | 5 | C8H6N+ | 116.0506 | 116.0503 (2.6) | 1.5 × 103 | 92.53 | ||
| [H–Phe–OMe + H]+ | C10H14NO2+ | 180.1019 | 180.1019 (0) | 2.4 × 103 | 90.6 | ||||||||
| 2 | C9H8N+ | 130.0651 | 130.0655 (3.1) | 6.1 × 103 | 97.34 | ||||||||
| Indole-3-acetyl-l-methionine methyl ester (IAMet-Me) | 14.04 | [M + H]+ | C16H21N2O3S+ | 321.1276 | 321.1273 (1.9) | 6.0 × 105 | 98.68 | [M − H]− | C16H19N2O3S− | 319.1122 | 319.1117 (1.6) | 3.2 × 106 | 98.55 |
| [Μ + Νa]+ | C16H20N2NaO3S+ | 343.1087 | 343.1088 (0.3) | 8.1 × 105 | 98.42 | 5 | C8H6N− | 116.0506 | 116.0506 (0) | 3.3 × 103 | 99.8 | ||
| [H–Met–OMe + H]+ | C6H14NO2S+ | 164.074 | 164.0739 (0.6) | 1.5 × 103 | 98.04 | ||||||||
| 2 | C9H8N+ | 130.0651 | 130.0651 (0) | 6.0 × 103 | 99.42 | ||||||||
| Indole-3-acetyl-l-aspartic acid dimethyl ester (IAAsp-Me2) | 13.31 | [M + H]+ | C16H19N2O5+ | 319.1288 | 319.1288 (0) | 1.9 × 106 | 99.81 | [M − H]− | C16H17N2O5− | 317.1143 | 317.1140 (0.9) | 2.8 × 106 | 99.81 |
| [Μ + Νa]+ | C16H18N2NaO5+ | 341.1108 | 341.1106 (0.6) | 4.5 × 106 | 100 | [IAM − H]− | C10H9N2O− | 173.072 | 173.0723 (1.7) | 3.6 × 103 | 99.09 | ||
| [H–Asp-(OMe)2 + H]+ | C6H12NO4+ | 162.0761 | 162.0766 (3.1) | 2.2 × 103 | 90.56 | 5 | C8H6N− | 116.0506 | 116.0507 (0.9) | 5.3 × 102 | 90.9 | ||
| 2 | C9H8N+ | 130.0651 | 130.0652 (0.8) | 5.2 × 103 | 98.94 | ||||||||
| Indole-3-acetyl-l-glutamic acid dimethyl ester (IAGlu-Me2) | 13.5 | [M + H]+ | C17H21N2O5+ | 333.1445 | 333.1441 (1.2) | 1.1 × 106 | 99.8 | [M − H]− | C17H19N2O5− | 331.1299 | 331.1290 (2.7) | 2.4 × 106 | 99.89 |
| [Μ + Νa]+ | C17H20N2NaO5+ | 355.1264 | 355.1259 (1.4) | 1.9 × 106 | 99.97 | 7 | C13H13N2O3− | 245.0932 | 245.0932 (0) | 1.9 × 103 | 90.37 | ||
| [H–Glu–(OMe)2 + H]+ | C7H14NO4+ | 176.0917 | 176.0920 (1.7) | 1.8 × 103 | 96.24 | 4 | C9H6N− | 130.0661 | 130.0662 (0.8) | 4.7 × 102 | 90.47 | ||
| 2 | C9H8N+ | 130.0651 | 10.0651 (0) | 6.9 × 103 | 98.32 | 5 | C8H6N− | 116.0506 | 116.0503 (2.6) | 1.5 × 103 | 90.35 | ||
| Compounds | B. oleracea var. capitata | B. oleracea var. rubra | B. rapa subsp. rapifera | B. oleracea var. botrytis cv. Zarka | B. oleracea var. italica cv. Calabrese | B. oleracea var. italica cv. Viοlleto | R. raphanistrum subsp. sativus | E. sativa |
|---|---|---|---|---|---|---|---|---|
| IAA | + | + | + | + | + | + | + | + |
| 4-Cl-IAA | − | + | − | − | − | − | + | − |
| IAld | + | + | + | + | + | + | + | + |
| IAN | + | + | + | + | + | + | + | − |
| IAM | − | − | − | − | − | + | + | − |
| IAAla | + | + | + | + | + | + | + | + |
| IAVal | + | − | + | + | + | + | + | − |
| IAGly | + | − | − | − | − | − | + | + |
| IAMet | + | + | − | − | − | − | − | − |
| IATrp | + | + | + | + | + | + | + | − |
| IATyr | − | − | − | − | − | − | + | − |
| IASer | − | − | − | − | − | + | + | − |
| IAPhe | + | + | + | − | + | + | + | − |
| IAAsp | − | − | + | − | − | − | − | − |
| IAGlu | − | + | − | + | − | + | − | |
| IAGly-Me | + | + | + | + | + | − | + | − |
| IAAla-Me | − | − | + | − | + | − | + | − |
| IAVal-Me | + | + | + | − | + | + | + | + |
| IATrp-Me | − | − | − | − | − | − | − | − |
| IATyr-Me | + | − | + | − | − | + | + | + |
| IASer-Me | + | + | + | − | + | + | + | + |
| IAPhe-Me | + | − | − | − | − | − | − | − |
| IAMet-Me | − | − | − | − | − | − | − | − |
| IAAsp-(Me)2 | + | − | − | − | − | − | − | − |
| IAAGlu-(Me)2 | + | − | − | − | − | − | − | − |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Revelou, P.-K.; Kokotou, M.G.; Constantinou-Kokotou, V. Identification of Auxin Metabolites in Brassicaceae by Ultra-Performance Liquid Chromatography Coupled with High-Resolution Mass Spectrometry. Molecules 2019, 24, 2615. https://doi.org/10.3390/molecules24142615
Revelou P-K, Kokotou MG, Constantinou-Kokotou V. Identification of Auxin Metabolites in Brassicaceae by Ultra-Performance Liquid Chromatography Coupled with High-Resolution Mass Spectrometry. Molecules. 2019; 24(14):2615. https://doi.org/10.3390/molecules24142615
Chicago/Turabian StyleRevelou, Panagiota-Kyriaki, Maroula G. Kokotou, and Violetta Constantinou-Kokotou. 2019. "Identification of Auxin Metabolites in Brassicaceae by Ultra-Performance Liquid Chromatography Coupled with High-Resolution Mass Spectrometry" Molecules 24, no. 14: 2615. https://doi.org/10.3390/molecules24142615
APA StyleRevelou, P.-K., Kokotou, M. G., & Constantinou-Kokotou, V. (2019). Identification of Auxin Metabolites in Brassicaceae by Ultra-Performance Liquid Chromatography Coupled with High-Resolution Mass Spectrometry. Molecules, 24(14), 2615. https://doi.org/10.3390/molecules24142615









