Synergistic/Antagonistic Potential of Natural Preparations Based on Essential Oils Against Streptococcus mutans from the Oral Cavity
Abstract
1. Introduction
2. Results and Discussion
2.1. GC-MS Composition of EOS
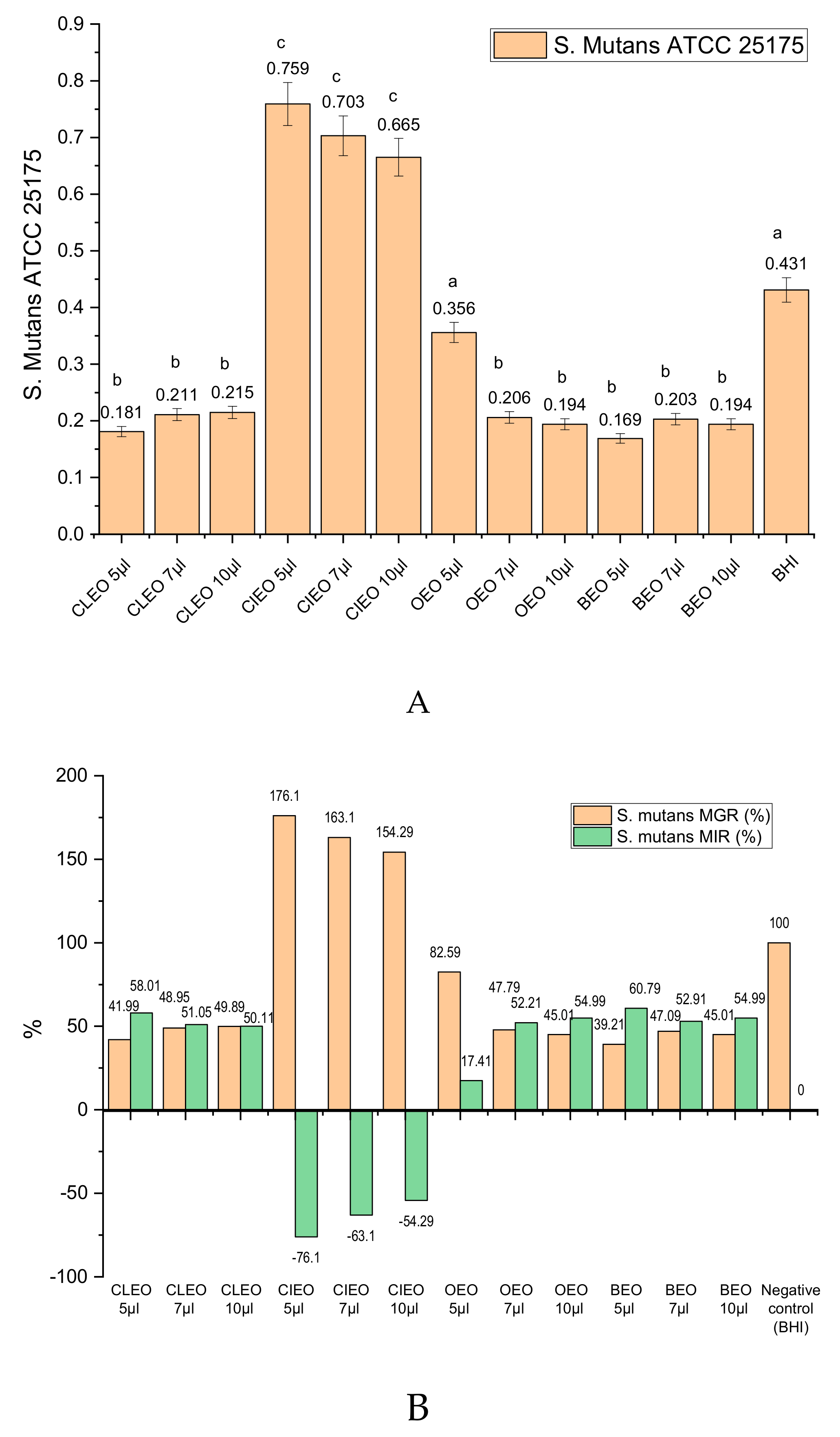
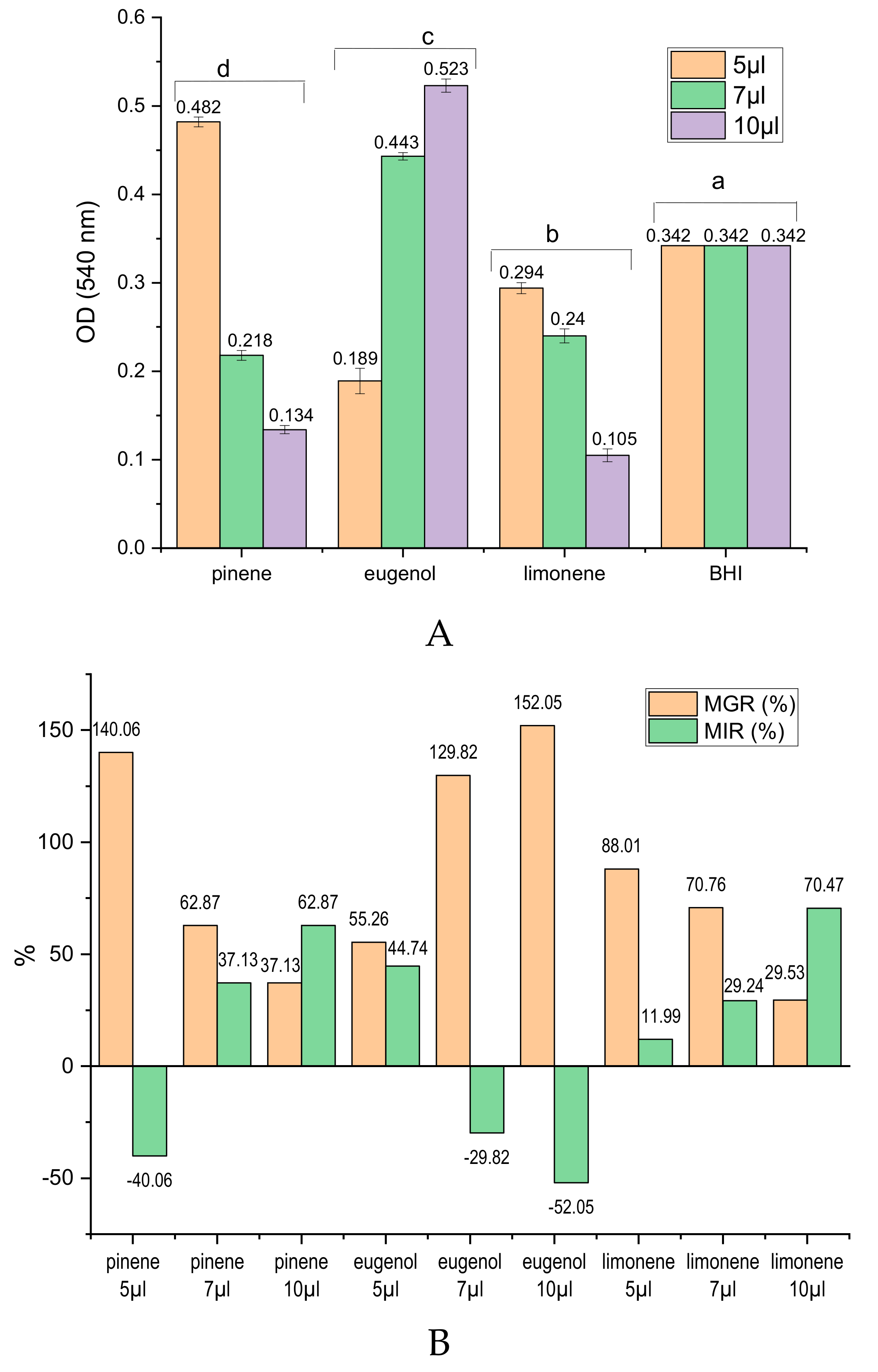
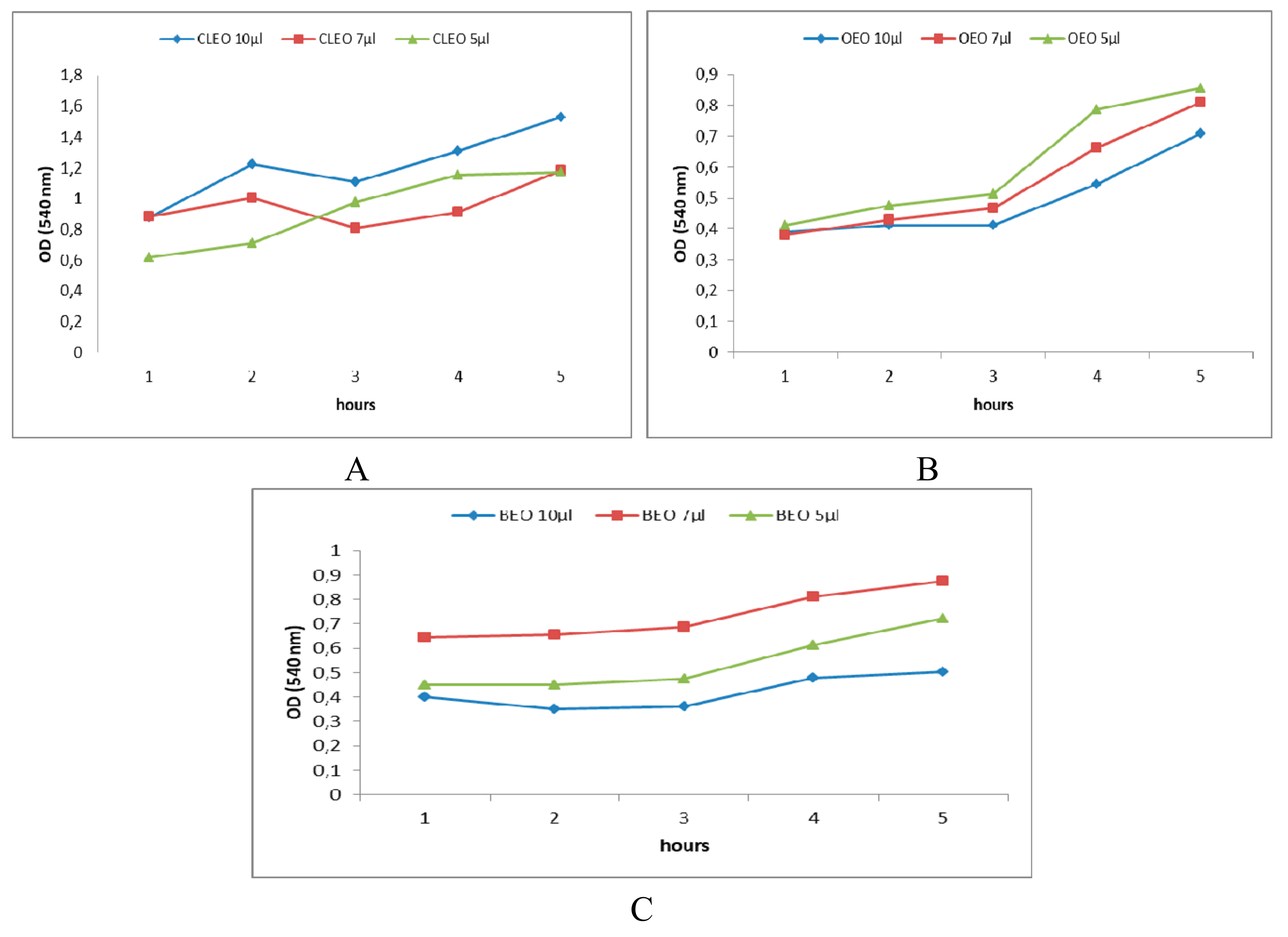
2.2. In Vitro Antibacterial Activity of EOs and of Main Chemical Compounds against S. mutans
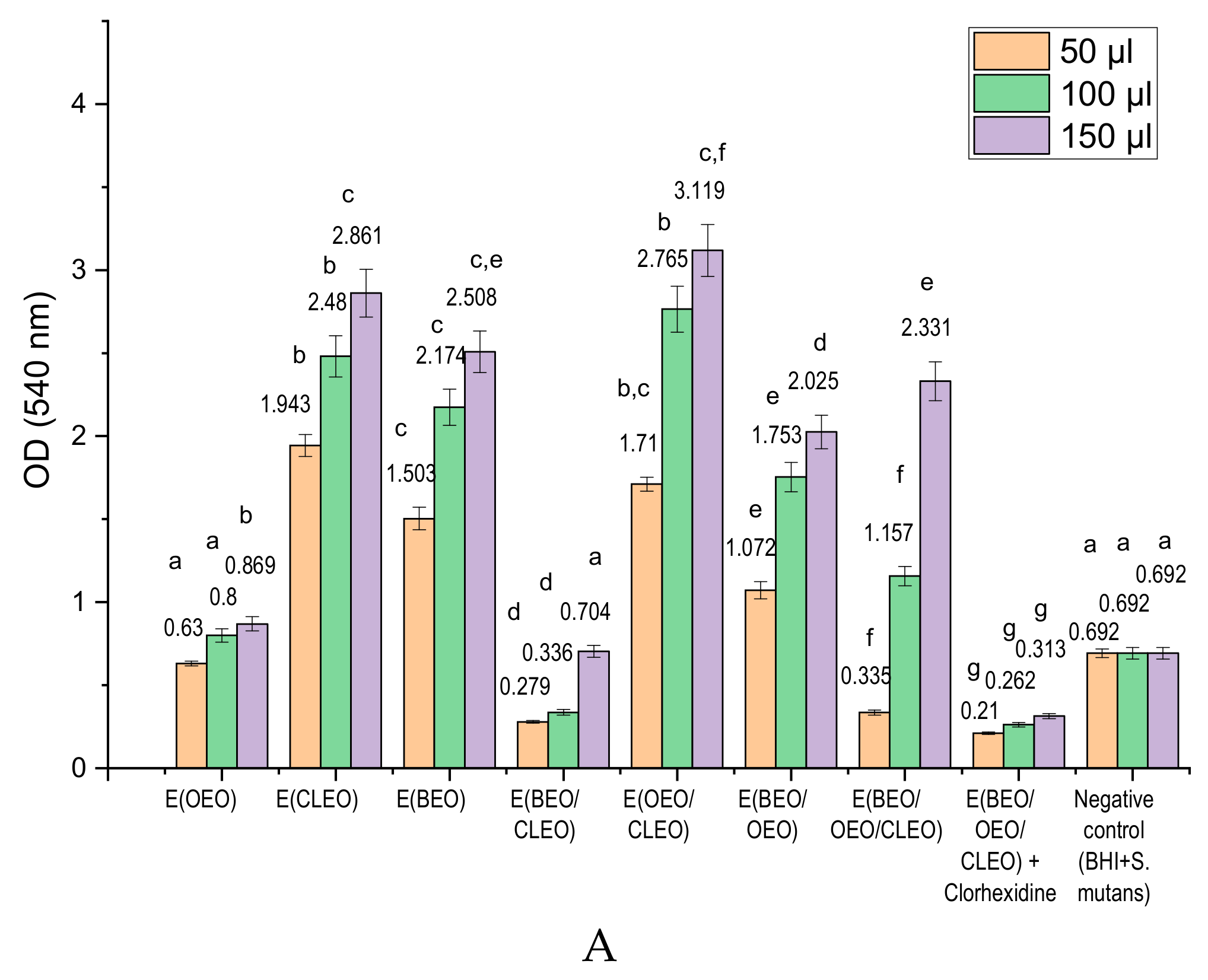
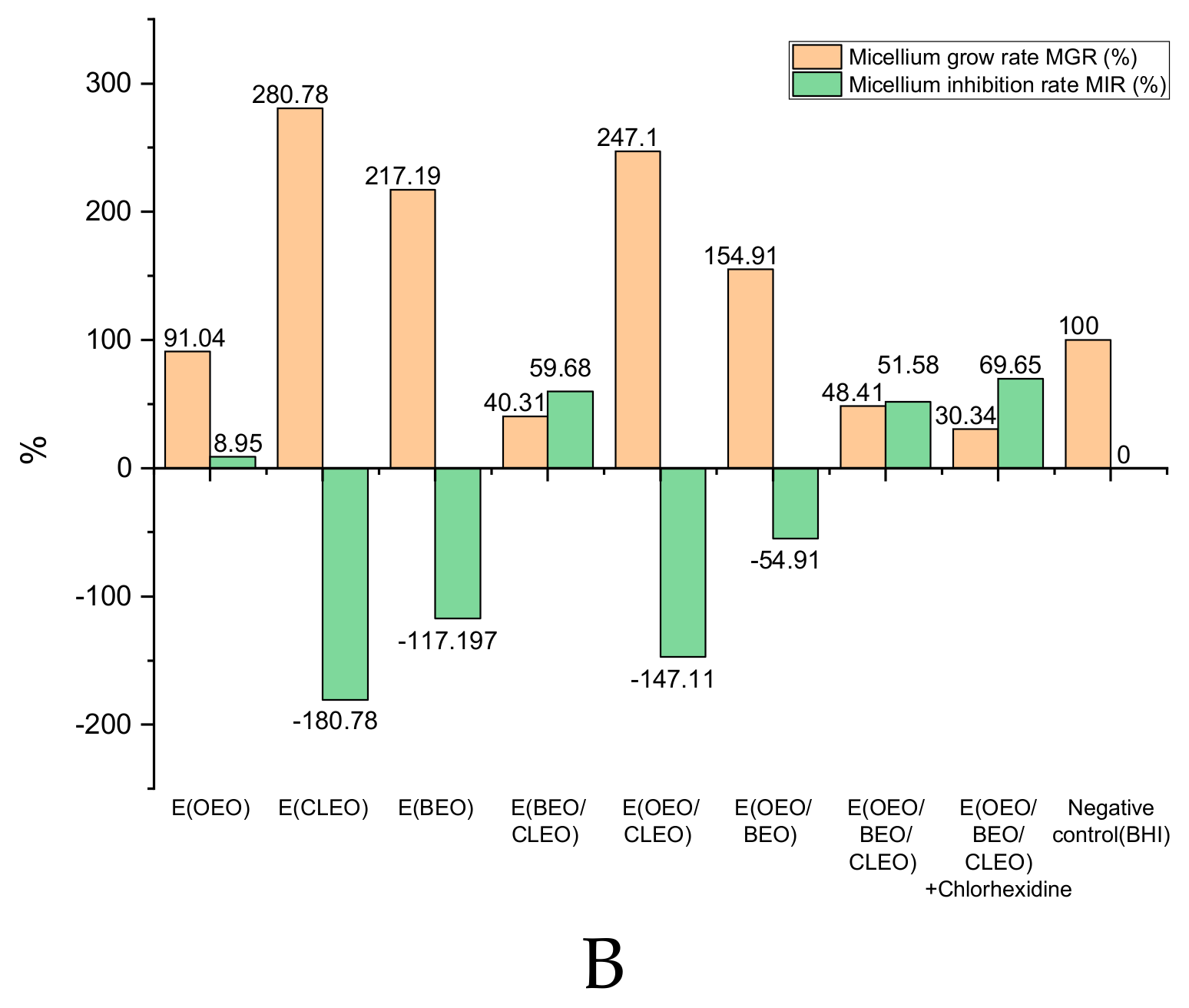
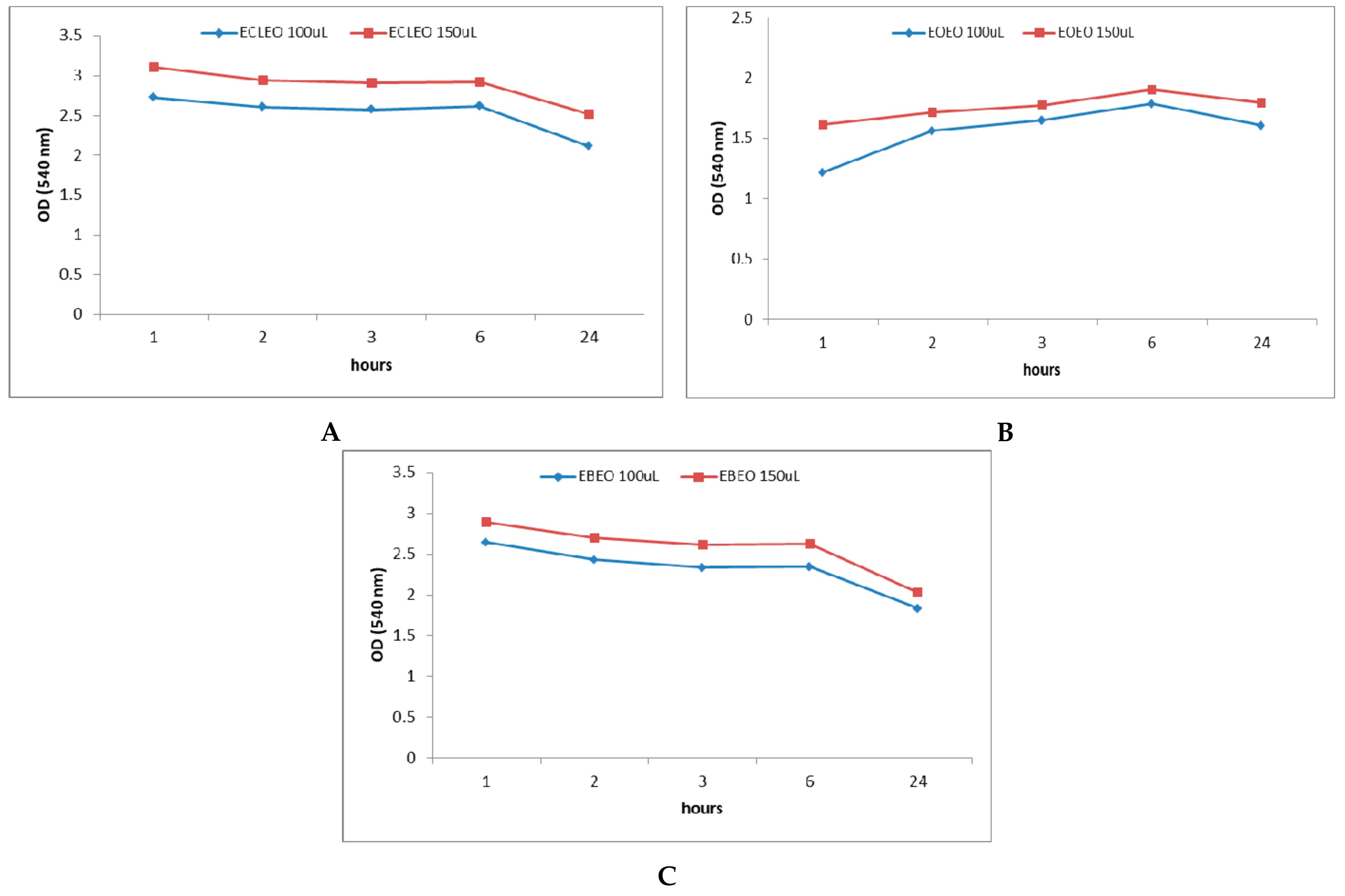
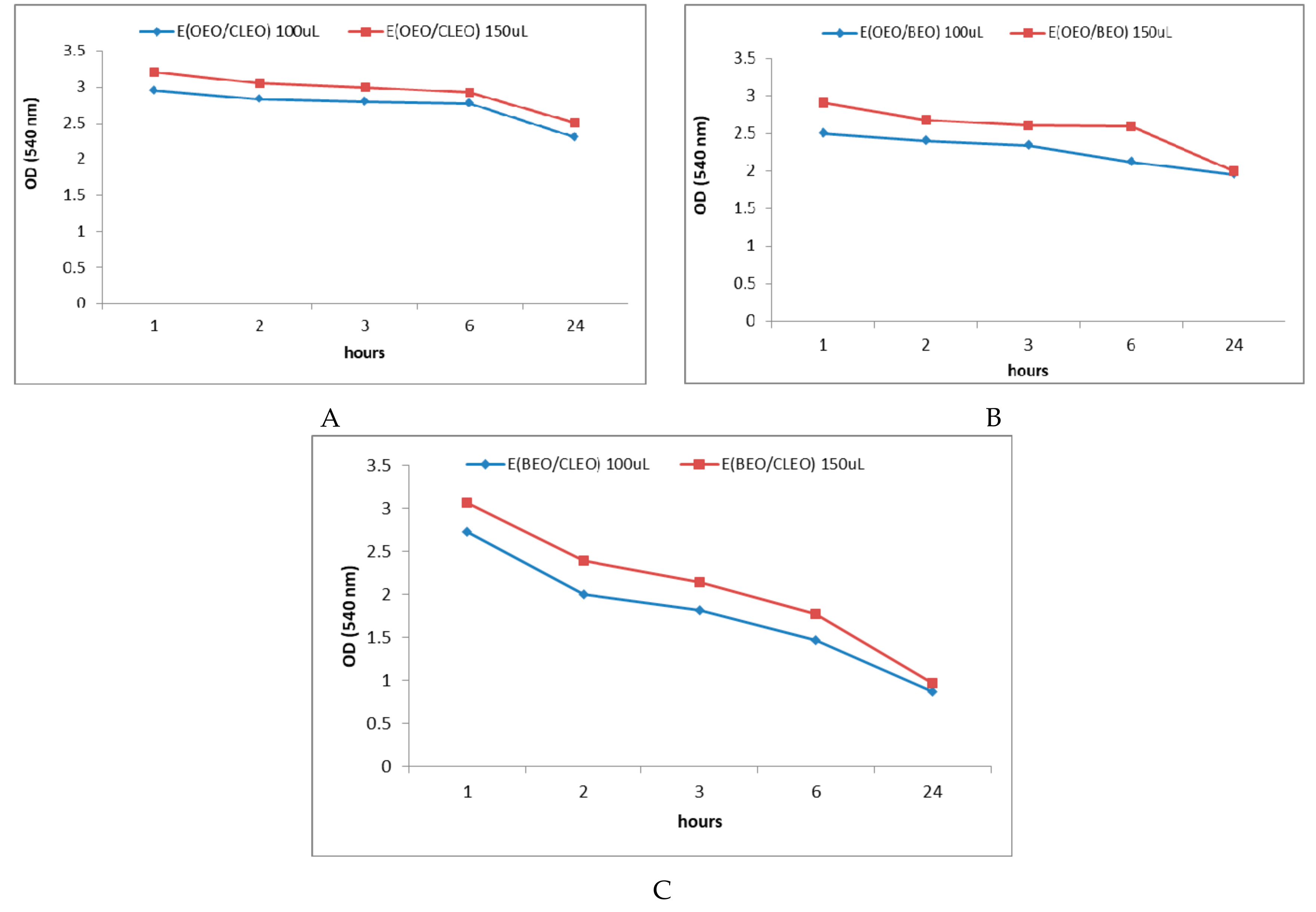
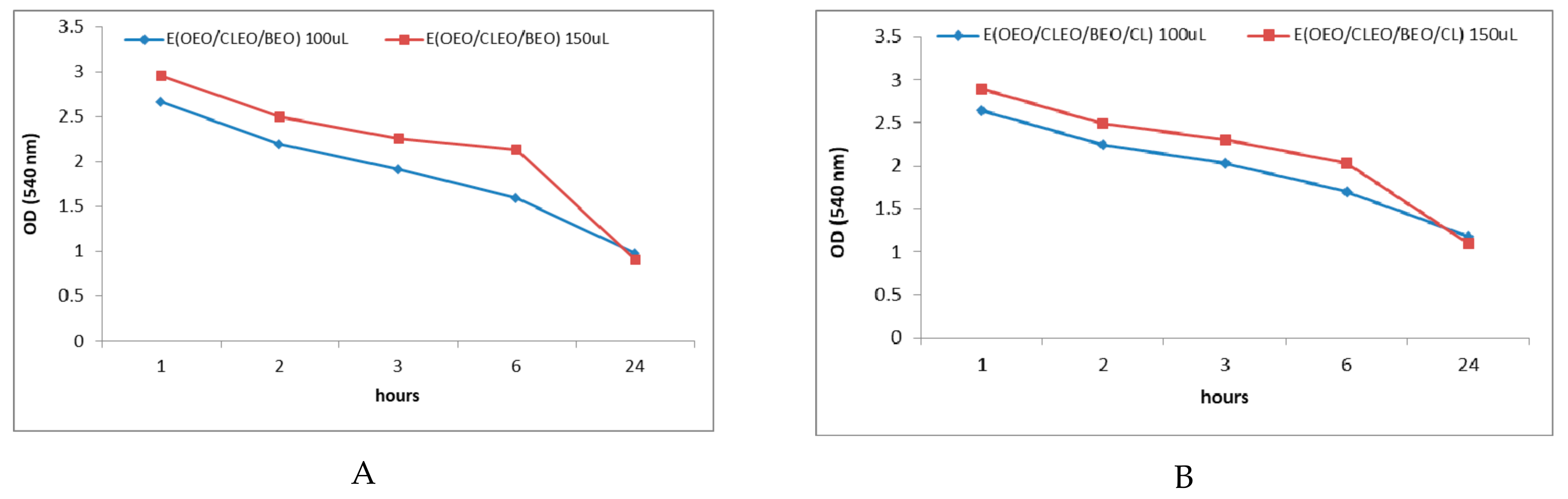
2.3. In Vitro Antibacterial Activity of Natural Emulsions Against S. mutans
2.4. Antibacterial Activity of EOs and Natural Emulsions Against S. mutans in Saliva Enhanced Medium
3. Materials and Methods
3.1. The GC-MS Characterisation of EOs
3.2. Microbial Strain and Culture Preparation
3.3. Antimicrobial Assay; Optical Density Loss (OD)
3.4. Preparation of Emulsion Based on Essential Oils
- Emulsion E(OEO): Prepared with 500 µL OEO, 6 mg lecithin, 10 mL water;
- Emulsion E(CLEO): Prepared with 500 µL CLEO, 6 mg lecithin, 10 mL water;
- Emulsion E(BEO): Prepared with 500 µL BEO, 6 mg lecithin, 10 mL water;
- Emulsion E(CLEO/BEO): Prepared with 500 µL CLEO+500 µL BEO, 6 mg lecithin, 9.5 mL water;
- Emulsion E(OEO/BEO): Prepared with 500 µL OEO+500 µL BEO, 6 mg lecithin, 9.5 mL water;
- Emulsion E(BEO/CLEO): Prepared with 500 µL BEO+500 µL CLEO, 6 mg lecithin, 9.5 mL water;
- Emulsion E(OEO/CLEO/BEO): Prepared with 500 µL OEO+500 µL CLEO+500 µL BEO, 6 mg lecithin, 9 mL water;
- Emulsion E(OEO/CLEO/BEO) Clorhexidine: Prepared with 500 µL OEO+500 µL CLEO+500 µL BEO, 6 mg lecithin, 1 mL clorhexidine 0.2%, 8 mL water.
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Friedman, J.Y. The Role of S. mutans in the Formation of Dental Caries: An Ecological Perspective. Sci. J. Lander Coll. Arts Sci. 2011, 5, 5. [Google Scholar]
- Forssten, S.D.; Björklund, M.; Ouwehand, A.C. Streptococcus mutans, caries and simulation models. Nutrients 2010, 2, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; Hanna, M.N.; Svensäter, G.; Ellen, R.P.; Cvitkovitch, D.G. Cell Density Modulates Acid Adaptation in Streptococcus mutans: Implications for Survival in Biofilms. J. Bacteriol. 2001, 183, 6875–6884. [Google Scholar] [CrossRef] [PubMed]
- Tehrani, M.H.; Asghari, G.; Hajiahmadi, M. Comparing Streptococcus mutans and Lactobacillus colony count changes following green tea mouth rinse or sodium fluoride mouth rinse use in children (Randomized double-blind controlled clinical trial). Dent. Res. J. 2011, 8 (Suppl. 1), 58–63. [Google Scholar]
- Murray, P.; Rosenthal, K.; Pfaller, M. Medical Microbiology, 8th ed.; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Freires, I.; Denny, C.; Benso, B. Antibacterial Activity of Essential Oils and Their Isolated Constituents against Cariogenic Bacteria: A Systematic Review. Molecules 2015, 20, 7329–7358. [Google Scholar] [CrossRef]
- Duarte, A.; Fernandes, J.; Bernardes, J. Citrus as a Component of the Mediterranean Diet. J. Spat. Organ. Dyn. 2016, 4, 289–304. [Google Scholar]
- Song, J.K.; Bae, J.M. Citrus fruit intake and breast cancer risk: A quantitative systematic review. J. Breast Cancer 2016, 16, 72–76. [Google Scholar] [CrossRef]
- Al-Kalaldeh, J.Z.; Abu-Dahab, R.; Afifi, F.U. Volatile oil composition and antiproliferative activity of Laurus nobilis, Origanum syriacum, Origanum vulgare, and Salvia triloba against human breast adenocarcinoma cells. Nutr. Res. 2010, 30, 271–278. [Google Scholar] [CrossRef]
- Roussos, P.A. Orange (Citrus sinensis (L.) Osbeck). In Nutritional Composition of Fruit Cultivars; Elsevier Science: Amsterdam, The Netherlands, 2016; pp. 469–496. [Google Scholar] [CrossRef]
- Curk, F.; Ollitrault, F.; Garcia-Lor, A. Phylogenetic origin of limes and lemons revealed by cytoplasmic and nuclear markers. Ann. Bot. 2016, 117, 565–583. [Google Scholar] [CrossRef]
- Sawamura, M.; Onishi, Y.; Ikemoto, J.; Tu, N.T.M.; Phi, N.T.L. Characteristic odour components of bergamot (Citrus bergamia Risso) essential oil. Flavour Fragr. J. 2006, 21, 609–615. [Google Scholar] [CrossRef]
- Danthu, P.; Penot, E.; Ranoarisoa, K.M.; Rakotondravelo, J.C.; Michel, I.; Tiollier, M.; Michels, T.; Normand, F.; Razafimamonjison, G.; Fawbush, F.; et al. The clove tree of Madagascar: A success story with an unpredictable future. Bois For. Trop. 2014, 320, 83–96. [Google Scholar] [CrossRef]
- Cortés-Rojas, D.F.; Fernandes de Souza, C.R.; Oliveira, W.P. Clove (Syzygium aromaticum): A precious spice. Asian Pac. J. Trop. Biomed. 2014, 4, 90–96. [Google Scholar] [CrossRef]
- Chaudhari, L.K.; Jawale, B.A.; Sharma, S.; Sharma, H.; Kumar, C.D.; Kulkarni, P.A. Antimicrobial activity of commercially available essential oils against Streptococcus mutans. J. Contemp. Dent. Pract. 2012, 13, 71–74. [Google Scholar] [PubMed]
- Nabavi, S.F.; Di Lorenzo, A.; Izadi, M.; Sobarzo-Sánchez, E.; Daglia, M.; Nabavi, S.M. Antibacterial Effects of Cinnamon: From Farm to Food, Cosmetic and Pharmaceutical Industries. Nutrients 2015, 7, 7729–7748. [Google Scholar] [CrossRef]
- Wiwattanarattanabut, K.; Choonharuangdej, S.; Srithavaj, T. In Vitro Anti-Cariogenic Plaque Effects of Essential Oils Extracted from Culinary Herbs. J. Clin. Diagn. Res. 2017, 11, 30–35. [Google Scholar] [CrossRef]
- Mohagheghniapour, A.; Mohammad, J.S.; Mohammad, T.G. Variations in chemical compositions of essential oil from sour orange (Citrus aurantium L.) blossoms by different isolation methods. Sustain. Chem. Pharm. 2018, 10, 118–124. [Google Scholar] [CrossRef]
- Verzera, A.; Trozzi, A.; Dugo, G.; Di Bella, G.; Cotroneo, A. Biological lemon and sweet orange essential oil composition. Flavour Fragr. J. 2004, 19, 544–548. [Google Scholar] [CrossRef]
- Moufida, S.; Marzouk, B. Biochemical characterization of blood orange, sweet orange, lemon, bergamot and bitter orange. Phytochemistry 2003, 62, 1283–1289. [Google Scholar] [CrossRef]
- Azar, A.P.; Nekoei, M.; Larijani, K.; Bahraminasab, S. Chemical composition of the essential oils of Citrus sinensis cv. valencia and a quantitative structureretention relationship study for the prediction of retention indices by multiple linear regression. J. Serb. Chem. Soc. 2011, 76, 1627–1637. [Google Scholar] [CrossRef]
- Tao, N.G.; Liu, Y.J.; Zhang, M.L. Chemical composition and antimicrobial activities of essential oil from the peel of bingtang sweet orange (Citrus sinensis Osbeck). Int. J. Food Sci. Technol. 2009, 44, 1281–1285. [Google Scholar] [CrossRef]
- Njoroge, S.M.; Phi, N.T.L.; Sawamura, M. Chemical composition of peel essential oils of sweet oranges (Citrus sinensis) from Uganda and Rwanda. J. Essent. Oil Bear. Plants 2009, 12, 26–33. [Google Scholar] [CrossRef]
- Kejlova, K.; Jirova, D.; Bendova, H. Phototoxicity of bergamot oil assessed by in vitro techniques in combination with human patch tests. Toxicol. Vitr. 2007, 21, 1298–1303. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.K.; Goswami, P.; Verma, R.S.; Padalia, R.C.; Chauhan, A.; Singh, V.R.; Darokar, M.P. Chemical composition and antimicrobial activity of bergamot-mint (Mentha citrata Ehrh.) essential oils isolated from the herbage and aqueous distillate using different methods. Ind. Crop. Prod. 2016, 91, 152–160. [Google Scholar] [CrossRef]
- Ramesh, G.; Seth, R.K.; Ramamurthy Sujatha, D.S. Evaluation of antibacterial efficacy of lemon grass oil eucalyptus oil and lemon juice, as a hand sanitizer. Int. Ayurvedic Med. J. 2011, 4, 1193–1203. [Google Scholar]
- Fabio, A.; Cermelli, C.; Fabio, G. Screening of the antibacterial effects of a variety of essential oils on microorganisms responsible for respiratory infections. Phytother. Res. 2007, 21, 374–377. [Google Scholar] [CrossRef] [PubMed]
- Mandalari, G.; Bennett, R.N.; Bisignano, G.; Trombetta, D.; Saija, A.; Faulds, C.B.; Gasson, M.J.; Narbad, A. Antimicrobial activity of flavonoids extracted from bergamot (Citrus bergamia Risso) peel, a byproduct of the essential oil industry. J. Appl. Microbiol. 2007, 103, 2056–2064. [Google Scholar] [CrossRef] [PubMed]
- Vaishali, M.; Geetha, R.V. Antibacterial activity of Orange peel oil on Streptococcus mutans and Enterococcus—An In-vitro study. Res. J. Pharm. Technol. 2018, 11, 513–514. [Google Scholar] [CrossRef]
- Radünz, M.; da Trindade, M.L.M.; Camargo, T.M.; Radünz, A.L.; Borges, C.D.; Gandra, E.A.; Helbig, E. Antimicrobial and antioxidant activity of unencapsulated and encapsulated clove (Syzygium aromaticum, L.) essential oil. Food Chem. 2019, 276, 180–186. [Google Scholar]
- Kheawfu, K.; Pikulkaew, S.; Rades, T.; Müllertz, A.; Okonogi, S. Development and characterization of clove oil nanoemulsions and self-microemulsifying drug delivery systems. J. Drug Deliv. Sci. Technol. 2018, 46, 330–338. [Google Scholar] [CrossRef]
- Sharma, M.; Mann, B.; Sharma, R.; Bajaj, R.; Athira, S.; Sarkar, P.; Pothuraju, R. Sodium caseinate stabilized clove oil nanoemulsion: Physicochemical properties. J. Food Eng. 2017, 212, 38–46. [Google Scholar] [CrossRef]
- Hongfang, G.; Hui, Y.; Chuang, W. Controllable preparation and mechanism of nano-silver mediated by the microemulsion system of the clove oil. Results Phys. 2017, 7, 3130–3136. [Google Scholar]
- Heredia-Guerrero, J.A.; Ceseracciu, L.; Guzman-Puyol, S.; Paul, U.C.; Alfaro-Pulido, A.; Grande, C.; Vezzulli, L.; Bandiera, T.; Bertorelli, R.; Russo, D.; et al. Antimicrobial, antioxidant, and waterproof RTV silicone-ethyl cellulose composites containing clove essential oil. Carbohydr. Polym. 2018, 192, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Ravi, S.B.; Nirupad, S.; Chippagiri, P.; Pandurangappa, R. Antibacterial Effects of Natural Herbal Extracts on Streptococcus mutans: Can They Be Potential Additives in Dentifrices? Int. J. Dent. 2017, 2017, 4921614. [Google Scholar]
- Matsui, R.; Cvitkovitch, D. Acid tolerance mechanisms utilized by Streptococcus mutans. Future Microbiol. 2010, 5, 403–417. [Google Scholar] [CrossRef] [PubMed]
- Sumalan, R.M.; Alexa, E.; Popescu, I.; Negrea, M.; Radulov, I.; Obistioiu, D.; Cocan, I. Exploring Ecological Alternatives for Crop Protection Using Coriandrum sativum Essential Oil. Molecules 2019, 24, 2040. [Google Scholar] [CrossRef] [PubMed]
- Alexa, E.; Danciu, C.; Radulov, I.; Obistioiu, D.; Sumalan, R.M.; Morar ADehelean, C.A. Phytochemical screening and Biological activity of Menthe × piperita L. and Lavandula angustifolia Mill. Extracts. Anal. Cell. Pathol. 2018, 2678924. [Google Scholar]
- Mattlock, B.C.; Beringer, R.W.; Ash, D.; Allen, M.W.; Page, A.F. Analyzing differences in bacterial optical densitry measurements between spectrophotometers. Thermofish. Sci. 2011. Available online: https://www.semanticscholar.org/paper/Analyzing-Differences-in-Bacterial-Optical-Density-Matlock-Beringer/44de6ef94cb363c9203151d04cb63d0a9500664c (accessed on 5 August 2019).
- Salli, K.M.; Gürsoy, U.K.; Söderling, E.M. Effects of Xylitol and Sucrose Mint Products on Streptococcus mutans Colonization in a Dental Simulator Model. Curr. Microbiol. 2017, 74, 1153–1159. [Google Scholar] [CrossRef]
Sample Availability: Samples of the natural preparations are available from the authors. |
| Nr. | Compounds | Type | Retention Time | LRI | % of Total | |||
|---|---|---|---|---|---|---|---|---|
| CLEO | CIEO | BEO | OEO | |||||
| 1. | α-pinene | MH | 5.58 | 1013 | - | - | 32.629 | - |
| 2. | camphene | MH | 6.62 | 1057 | - | - | 0.420 | - |
| 3. | β-pinene | MH | 7.67 | 1092 | - | - | 1.617 | - |
| 4. | thujene | MO | 7.99 | 1416 | - | - | 0.164 | - |
| 5. | β-myrcene | MH | 9.09 | 1164 | - | - | 0.939 | 1.520 |
| 6. | D-limonene | MH | 10.15 | 1189 | - | - | 49.381 | 97.930 |
| 7. | eucalyptol | MO | 10.41 | 1198 | - | - | 0.242 | - |
| 8. | trans-o-cymene | MH | 11.34 | 1238 | - | - | 1.162 | - |
| 9. | cis-o-cymene | MH | 11.43 | 1241 | - | - | 3.819 | - |
| 10. | 4-carene | MH | 12.47 | 1277 | - | - | 6.345 | |
| 11. | butanoic acid hexyl ester β-linalool | - MO | 15.550 19.41 | 1532 | - - | - - | 0.092 3.020 | - 0.550 |
| 12. | α -caryophyllene | SH | 21.08 | 1598 | 5.558 | 2.052 | - | - |
| 13. | β-farnesene | SH | 22.68 | 1664 | - | - | 0.050 | - |
| 14. | α-terpineol | MH | 23.44 | 1695 | - | - | 0.120 | - |
| 15. | cinnamaldehide | MO | 27.45 | 1996 | - | 0.386 | - | - |
| 16. | cinamil-alchool | MO | 30.45 | 2063 | - | 88.452 | - | - |
| 17. | cinnamyl-acetate | MO | 32.54 | 2163 | - | 6.133 | - | - |
| 18. | p-eugenol | MO | 32.83 | 2192 | 80.106 | 2.977 | - | - |
| 19. | eugenol acetate | MO | 30.627 | 2277 | 13.544 | - | - | - |
| 20. | chavicol | MO | 35.94 | 2318 | 0.792 | - | - | - |
| Total of major compounds | 100.00 | 100.0 | 100.00 | 100.00 | ||||
| Monoterpene hydrocarbonates (MH) | 96.524 | 99.45 | ||||||
| Monoterpene oxygenate (MO) | 94.44 | 97.948 | 3.426 | 0.55 | ||||
| Sesquiterpene hydrocarbonates (SH) | 5.56 | 2.052 | 0.050 | - | ||||
| Sesquiterpene oxygenate (SO) | - | - | - | - | ||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alexa, V.T.; Galuscan, A.; Popescu, I.; Tirziu, E.; Obistioiu, D.; Floare, A.D.; Perdiou, A.; Jumanca, D. Synergistic/Antagonistic Potential of Natural Preparations Based on Essential Oils Against Streptococcus mutans from the Oral Cavity. Molecules 2019, 24, 4043. https://doi.org/10.3390/molecules24224043
Alexa VT, Galuscan A, Popescu I, Tirziu E, Obistioiu D, Floare AD, Perdiou A, Jumanca D. Synergistic/Antagonistic Potential of Natural Preparations Based on Essential Oils Against Streptococcus mutans from the Oral Cavity. Molecules. 2019; 24(22):4043. https://doi.org/10.3390/molecules24224043
Chicago/Turabian StyleAlexa, Vlad Tiberiu, Atena Galuscan, Iuliana Popescu, Emil Tirziu, Diana Obistioiu, Alin Daniel Floare, Antonis Perdiou, and Daniela Jumanca. 2019. "Synergistic/Antagonistic Potential of Natural Preparations Based on Essential Oils Against Streptococcus mutans from the Oral Cavity" Molecules 24, no. 22: 4043. https://doi.org/10.3390/molecules24224043
APA StyleAlexa, V. T., Galuscan, A., Popescu, I., Tirziu, E., Obistioiu, D., Floare, A. D., Perdiou, A., & Jumanca, D. (2019). Synergistic/Antagonistic Potential of Natural Preparations Based on Essential Oils Against Streptococcus mutans from the Oral Cavity. Molecules, 24(22), 4043. https://doi.org/10.3390/molecules24224043