Structure Water-Solubility Relationship in α-Helix-Rich Films Cast from Aqueous and 1,1,1,3,3,3-Hexafluoro-2-Propanol Solutions of S. c. ricini Silk Fibroin
Abstract
1. Introduction
2. Results and Discussion
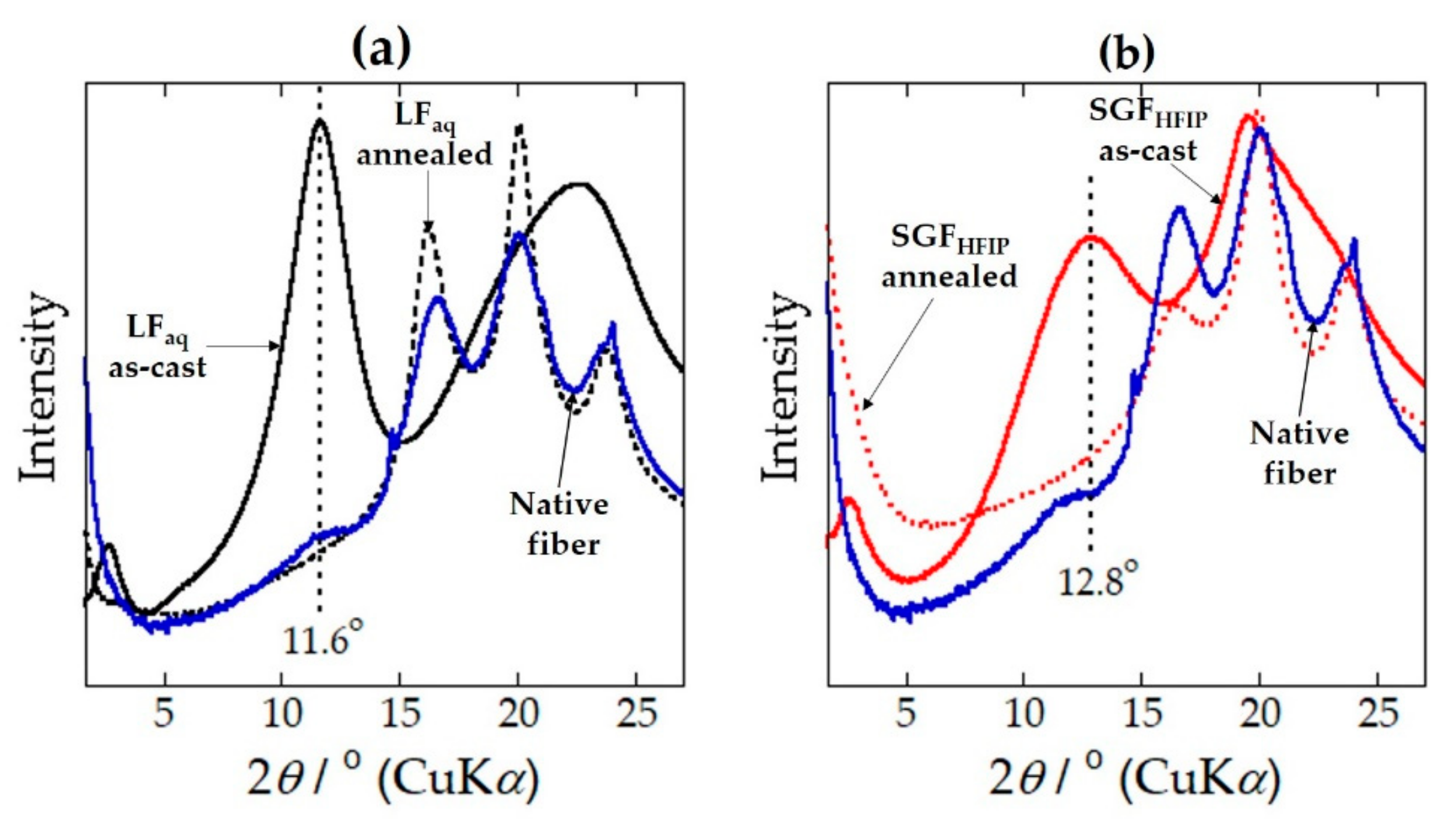
2.1. Structure and Properties of the LFaq and SGFHFIP As-Cast Films
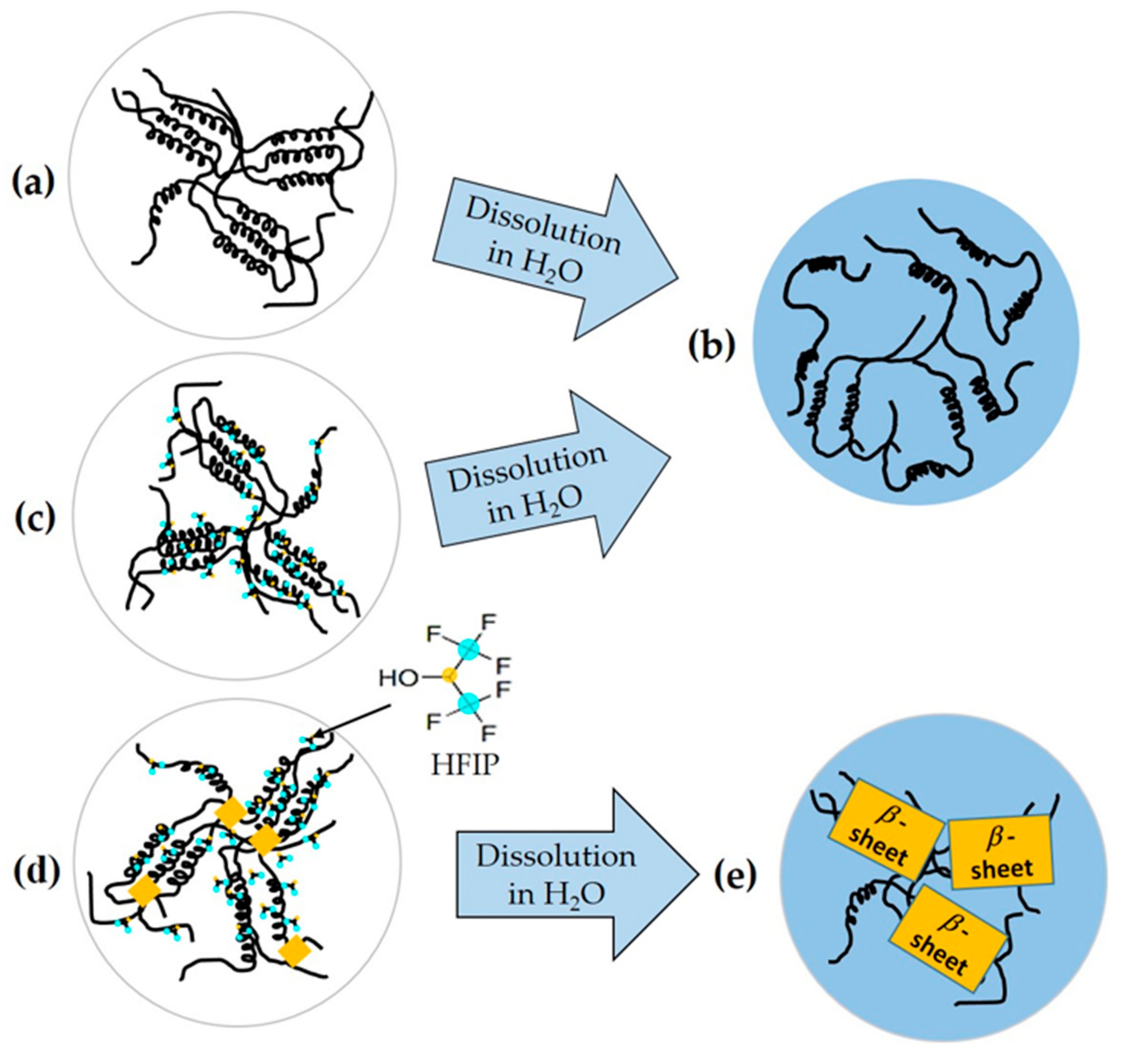
2.2. Ordered α-Helix Structure in the SGFHFIP As-Cast Film
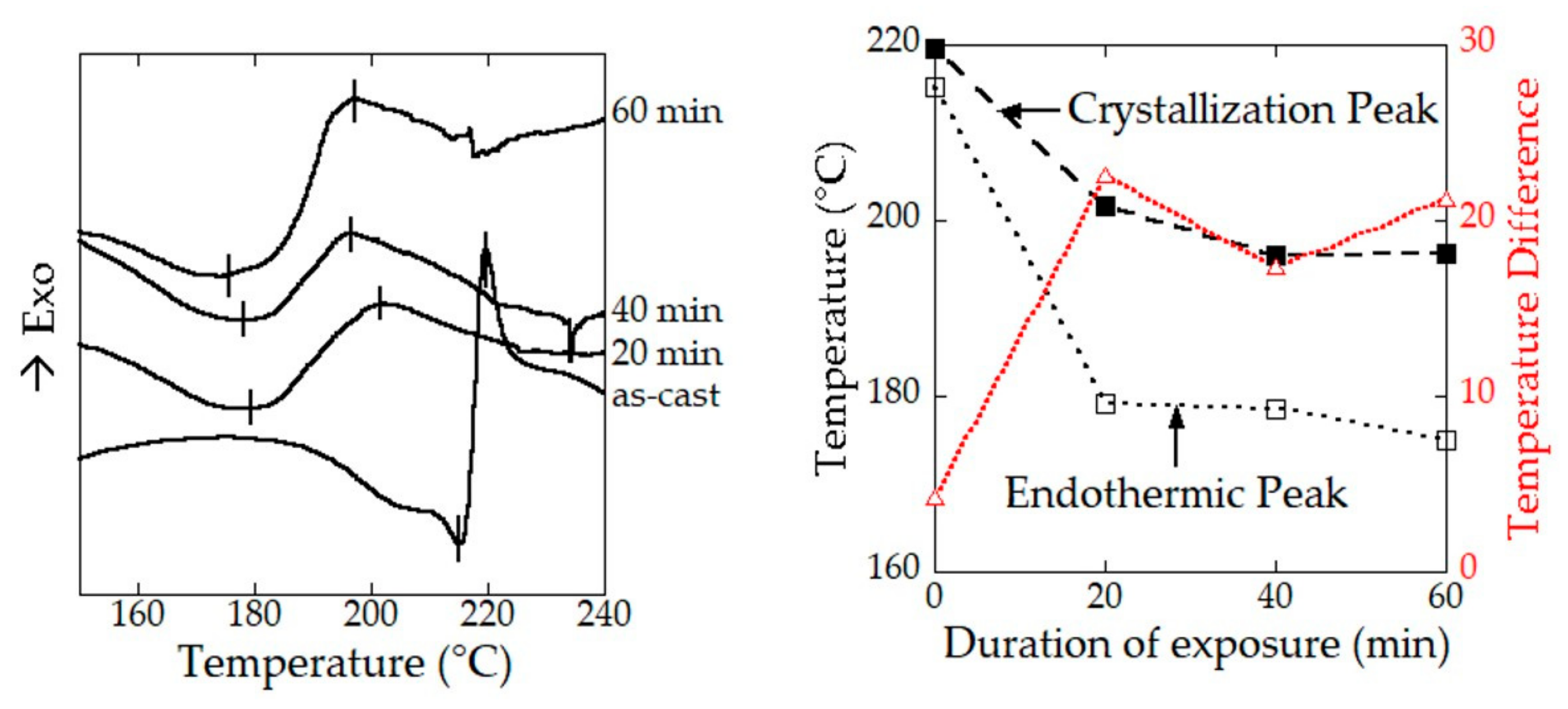
2.3. Structural Origin of the Water-Resistance Property of the SGFHFIP As-Cast Film
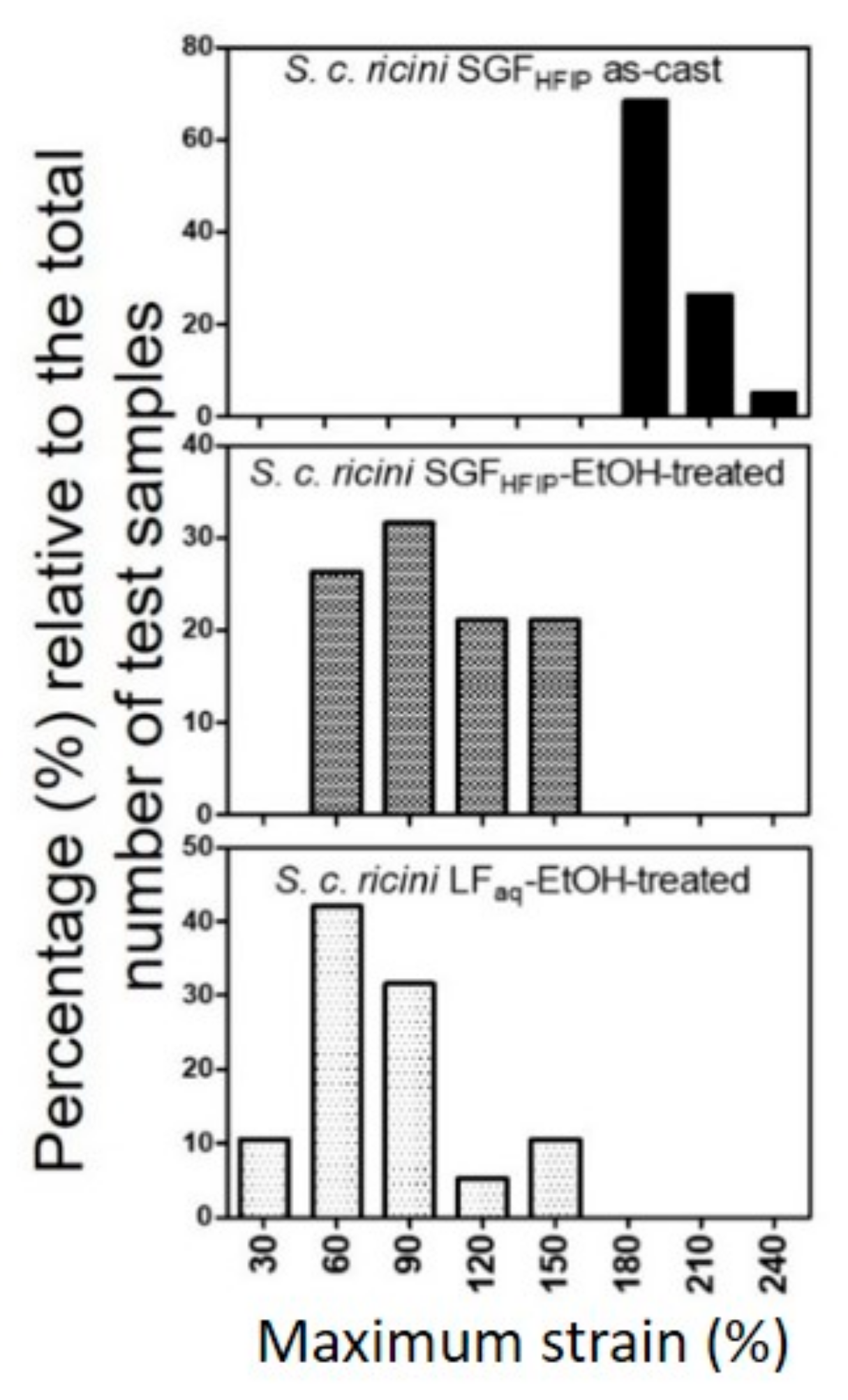
2.4. Superior Wet-Drawability of the SGFHFIP As-Cast Film
2.5. Industrial Prospects of the Current Fabrication Strategy
3. Experimental Section
3.1. Fabrication of Cast Films
3.2. Determination of Molecular Weight (Mw)
3.3. Amino Acid Composition Analysis
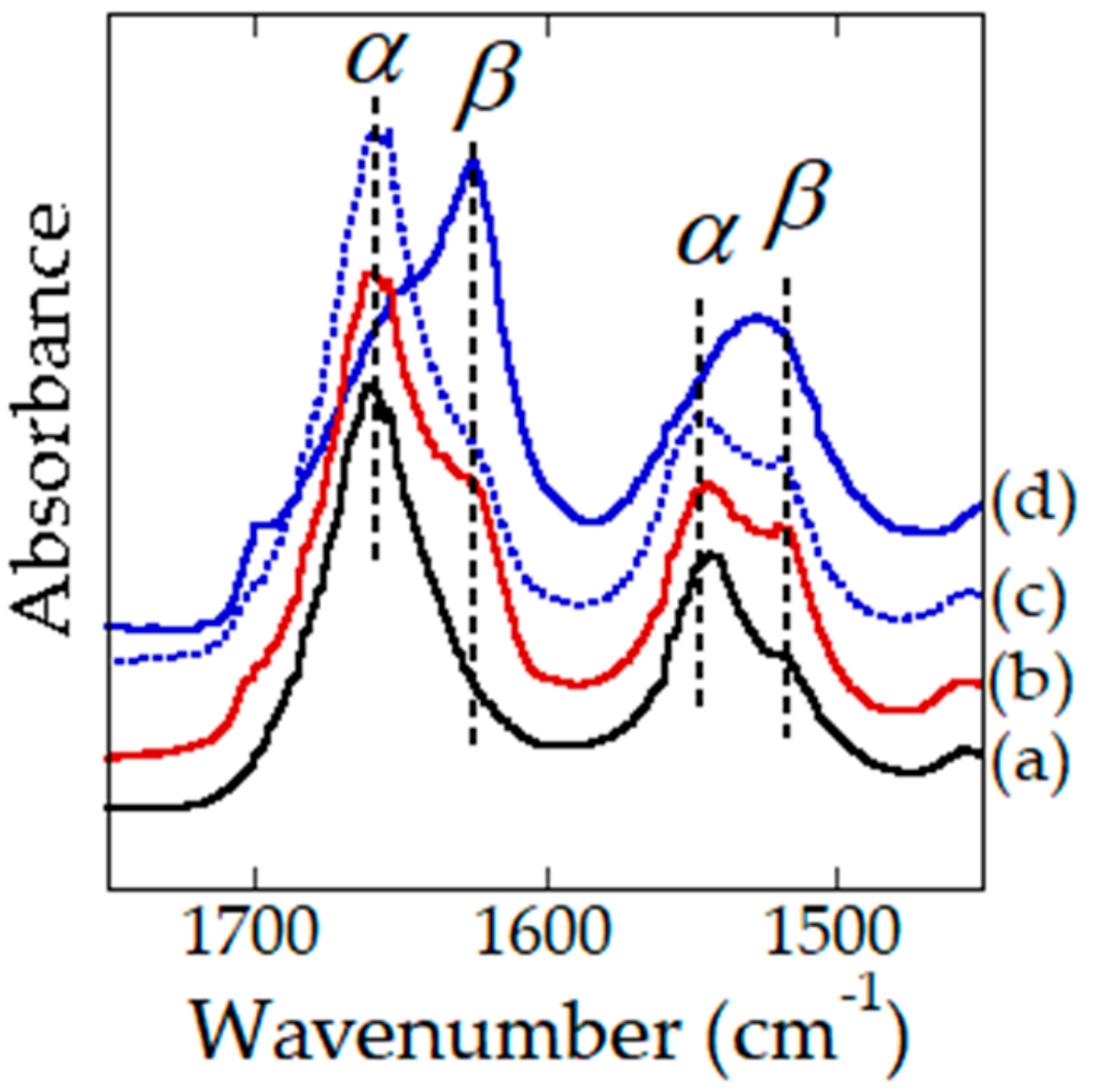
3.4. Fourier Transform Infrared (FTIR) Spectroscopy
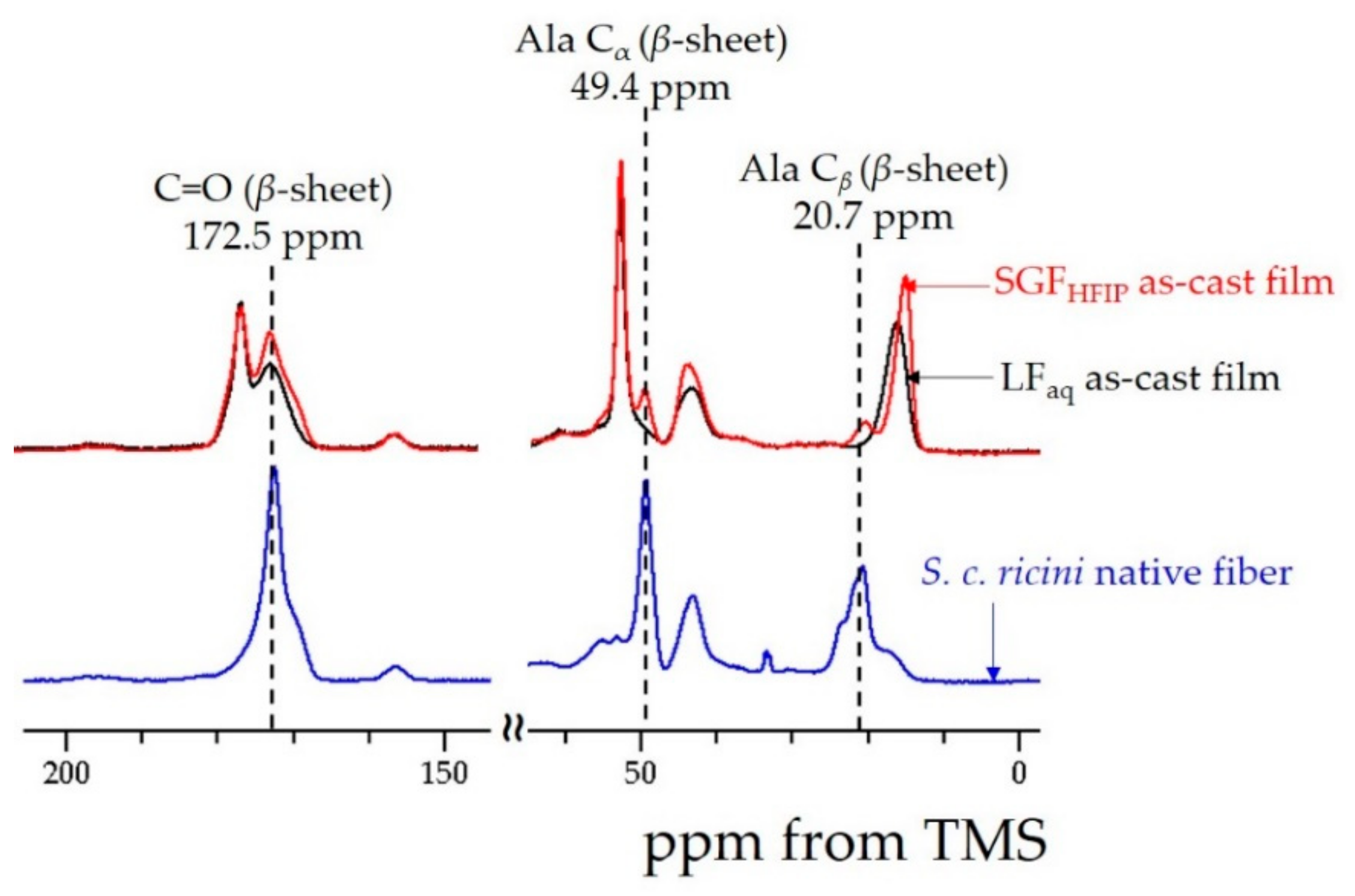
3.5. 13C Cross-Polarization Magic-Angle Spinning Solid-State NMR Spectroscopy
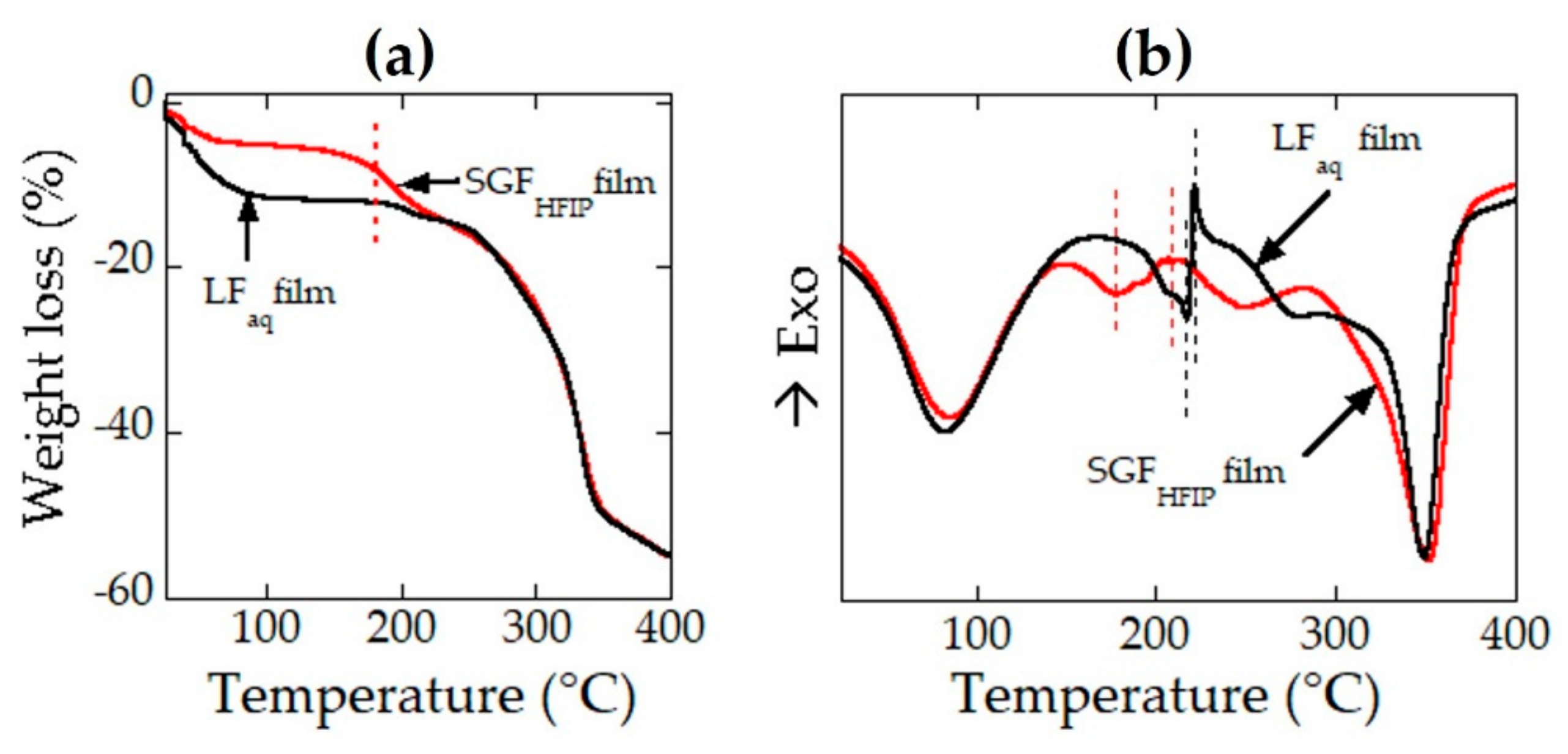
3.6. Thermal Analyses
3.7. Wide-Angle X-Ray Diffraction Analyses
3.8. Wet-Drawing of the Cast films
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sezutsu, H.; Yukuhiro, K. The complete nucleotide sequence of the Eri-silkworm (Samia cynthia ricini) fibroin gene. J. Insect Biotechnol. Sericol. 2014, 83, 59–70. [Google Scholar] [CrossRef]
- Asakura, T.; Nakazawa, Y. Structure and structural changes of the silk fibroin from Samia cynthia ricini using nuclear magnetic resonance spectroscopy. Macromol. Biosci. 2004, 4, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.Z.; Confalonieri, F.; Jacquet, M.; Perasso, R.; Li, Z.G.; Janin, J. Silk fibroin: Structural implications of a remarkable amino acid sequence. Proteins 2001, 44, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Yamazaki, T.; Aoki, A.; Shindo, H.; Asakura, T. NMR study of the structures of repeated sequences, GAGXGA (X = S, Y, V), in Bombyx mori liquid silk. Biomacromolecules 2014, 15, 104–112. [Google Scholar] [CrossRef]
- van Beek, J.D.; Beaulieu, L.; Schäfer, H.; Demura, M.; Asakura, T.; Meier, B.H. Solid-state NMR determination of the secondary structure of Samia cynthia ricini silk. Nature 2000, 405, 1077–1079. [Google Scholar] [CrossRef]
- Yang, M.; Yao, J.; Sonoyama, M.; Asakura, T. Spectroscopic characterization of heterogeneous structure of Samia cynthia ricini silk fibroin induced by stretching and molecular dynamics simulation. Macromolecules 2004, 37, 3497–3504. [Google Scholar] [CrossRef]
- Rajkhowa, R.; Wang, L.; Kanwar, J.R.; Wang, X. Molecular weight and secondary structure change in Eri silk during alkali degumming and powdering. J. Appl. Polym. Sci. 2011, 119, 1339–1347. [Google Scholar] [CrossRef]
- Chattopadhyay, D.; Chakraborty, A.; Chatterjee, S.M. Studies on degumming of eri silk cocoons. J. Text. Inst. 2017, 108, 1327–1339. [Google Scholar] [CrossRef]
- Silva, S.S.; Oliveira, N.M.; Oliveira, M.B.; da Costa, D.P.S.; Naskar, D.; Mano, J.F.; Kundu, S.C.; Reis, R.L. Fabrication and characterization of Eri silk fibers-based sponges for biomedical application. Acta Biomater. 2016, 32, 178–189. [Google Scholar] [CrossRef]
- Dutta, S.; Talukdar, B.; Bharali, R.; Rajkhowa, R.; Devi, D. Fabrication and characterization of biomaterial film from gland silk of Muga and Eri silkworms. Biopolymers 2013, 99, 326–333. [Google Scholar] [CrossRef]
- Perotto, G.; Zhang, Y.; Naskar, D.; Patel, N.; Kaplan, D.L.; Kundu, S.C.; Omenetto, F.G. The optical properties of regenerated silk fibroin films obtained from different sources. Appl. Phys. Lett. 2017, 111, 103702. [Google Scholar] [CrossRef]
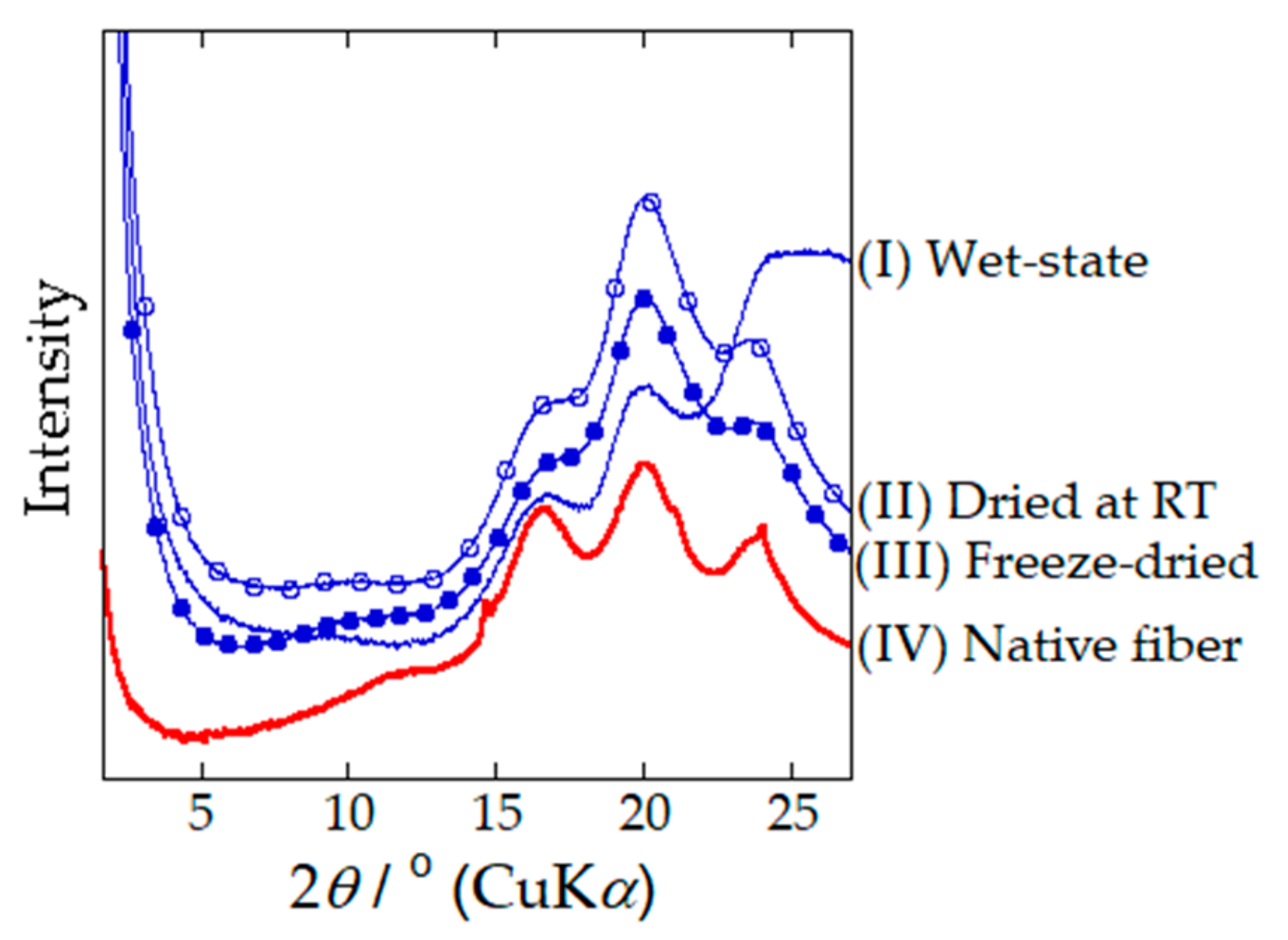
- Moseti, K.O.; Yoshioka, T.; Kameda, T.; Nakazawa, Y. Aggregation state of residual α-helices and their influence on physical properties of S. c. ricini native fiber. Molecules 2019, 24, 3471. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, T.; Hata, T.; Kojima, K.; Nakazawa, Y.; Kameda, T. Fabrication scheme for obtaining transparent, flexible, and water-insoluble silk films from apparently dissolved silk-gland fibroin of Bombyx mori silkworm. ACS Biomater. Sci. Eng. 2017, 3, 3207–3214. [Google Scholar] [CrossRef]
- Minoura, N.; Aiba, S.; Higuchi, M.; Gotoh, Y.; Tsukada, M.; Imai, Y. Attachment and growth of fibroblast cells on silk fibroin. Biochem. Biophys. Res. Commun. 1995, 208, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Lock, R.L. Process for Making Silk Fibroin Fibers. US Patent 5,252,285, 12 October 1993. [Google Scholar]
- Wang, Q.; Chen, Q.; Yang, Y.; Shao, Z. Effect of various dissolution systems on the molecular weight of regenerated silk fibroin. Biomacromolecules 2013, 14, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, T.; Tashiro, K.; Ohta, N. Molecular orientation enhancement of silk by the hot-stretching-induced transition from α-helix-HFIP complex to β-sheet. Biomacromolecules 2016, 17, 1437–1448. [Google Scholar] [CrossRef] [PubMed]
- Drummy, L.F.; Phillips, D.M.; Stone, M.O.; Farmer, B.L.; Naik, R.R. Thermally induced α-helix to β-sheet transition in regenerated silk fibers and films. Biomacromolecules 2005, 6, 3328–3333. [Google Scholar] [CrossRef]
- Zarkoob, S.; Eby, R.K.; Reneker, D.H.; Hudson, S.D.; Ertley, D.; Adams, W.W. Structure and morphology of electrospun silk nanofibers. Polymer 2004, 45, 3973–3977. [Google Scholar] [CrossRef]
- Spek, E.J.; Olson, C.A.; Shi, Z.; Kallenbach, N.R. Alanine is an intrinsic α-helix stabilizing amino acid. J. Am. Chem. Soc. 1999, 121, 5571–5572. [Google Scholar] [CrossRef]
- Nakazawa, Y.; Asakura, T. Structure determination of a peptide model of the repeated helical domain in Samia cynthia ricini silk fibroin before spinning by a combination of advanced solid-state NMR methods. J. Am. Chem. Soc. 2003, 125, 7230–7237. [Google Scholar] [CrossRef]
- Kluge, J.A.; Kahn, B.T.; Brown, J.E.; Omenetto, F.G.; Kaplan, D.L. Optimizing molecular weight of lyophilized silk as a shelf-stable source material. ACS Biomater. Sci. Eng. 2016, 4, 595–605. [Google Scholar] [CrossRef]
- Inoue, S.I.; Tsuda, H.; Tanaka, T.; Kobayashi, M.; Magoshi, Y.; Magoshi, J. Nanostructure of natural fibrous protein: In vitro nanofabric formation of Samia cynthia ricini wild silk fibroin by self-assembling. Nano Lett. 2003, 3, 1329–1332. [Google Scholar] [CrossRef]
- Kameda, T.; Kojima, K.; Miyazawa, M.; Fujiwara, S. Film formation and structural characterization of silk of the hornet Vespa simillima xanthoptera Cameron. Z. Naturforsch. 2005, 60, 906–914. [Google Scholar] [CrossRef] [PubMed]
- Magoshi, J.; Magoshi, Y.; Nakamura, S. Chapter 25—Mechanism of fiber formation of silkworm. In Silk Polymers; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1993; Volume 544, pp. 292–310. [Google Scholar] [CrossRef]
- Otikovs, M.; Andersson, M.; Jia, Q.; Nordling, K.; Meng, Q.; Andreas, L.B.; Pintacuda, J.; Johansson, J.; Rising, A.; Jaudzems, K. Degree of biomimicry of artificial spider silk spinning assessed by NMR spectroscopy. Angew. Chemie 2017, 56, 12571–12575. [Google Scholar] [CrossRef] [PubMed]
- Levy, Y.; Jortner, J.; Becker, O.M. Solvent effects on the energy landscapes and folding kinetics of polyalanine. PNAS 2001, 98, 2188–2193. [Google Scholar] [CrossRef]
- Spiess, K.; Ene, R.; Keenan, C.D.; Senker, J.; Kremer, F.; Scheibel, T. Impact of initial solvent on thermal stability and mechanical properties of recombinant spider silk films. J. Mater. Chem. 2011, 21, 13594–13604. [Google Scholar] [CrossRef]
- Rousseau, M.-E.; Beaulieu, L.; Lefèvre, T.; Paradis, J.; Asakura, T.; Pèzolet, M. Characterization by Raman microspectroscopy of the strain-induced conformational transition in fibroin fibers from the silkworm Samia cynthia ricini. Biomacromolecules 2006, 7, 2512–2521. [Google Scholar] [CrossRef]
- Tucker, C.L.; Jones, J.A.; Bringhurst, H.N.; Copeland, C.G.; Addison, J.B.; Weber, W.S.; Mou, Q.; Yarger, J.L.; Lewis, R.V. Mechanical and physical properties of recombinant spider silk films using organic and aqueous solvents. Biomacromolecules 2014, 15, 3158–3170. [Google Scholar] [CrossRef]
- Yoshioka, T.; Kameda, T.; Tashiro, K.; Schaper, A.K. Transformation of coiled α-helices into cross-β-sheets superstructure. Biomacromolecules 2017, 18, 3892–3903. [Google Scholar] [CrossRef]
- Kameda, T.; Kojima, K.; Togawa, E.; Sezutsu, H.; Zhang, Q.; Teramoto, H.; Tamada, Y. Drawing-induced changes in morphology and mechanical properties of hornet silk gel films. Biomacromolecules 2010, 11, 1009–1018. [Google Scholar] [CrossRef]
- Fedic, R.; Zurovec, M.; Sehnal, F. The silk of Lepidoptera. J. Insect Biotechnol. Sericol. 2002, 71, 1–15. [Google Scholar] [CrossRef]
- Freddi, G.; Monti, P.; Nagura, M.; Gotoh, Y.; Tsukada, M. Structure and molecular conformation of Tussah silk fibroin films: Effect of heat treatment. J. Polym. Sci. Polym. Phys. 1997, 35, 841–847. [Google Scholar] [CrossRef]
- Guo, C.; Zhang, J.; Jordan, J.S.; Wang, X.; Henning, R.W.; Yarger, J.L. Structural comparison of various silkworm silks: An insight into the structure–property relationship. Biomacromolecules 2018, 19, 906–917. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, T.; Kameda, T. X-ray scattering analyses quantitatively revealed periodic hierarchical structure of polyalanine β-sheet and non-polyalanine amorphous domains in Antheraea assamensis (Muga) silk. J. Silk Sci. Tech. Jpn. 2019, 27, 95–101. [Google Scholar] [CrossRef]
Sample Availability: Samples of S. c. ricini LFaq and SGFHFIP as-cast films, and native fiber are available from the authors upon reasonable request. |



| Amino Acid | LFaq | SGFHFIP (a) | SGFHFIP (b) |
|---|---|---|---|
| Gly | 33.8 | 34.5 | 34.9 |
| Ala | 44.2 | 40.0 | 41.3 |
| Pro | 0.4 | 0.5 | 0.5 |
| Val | 0.5 | 0.5 | 0.6 |
| Leu | 0.4 | 0.5 | 0.5 |
| Ile | 0.4 | 0.4 | 0.4 |
| Met * | * | * | * |
| Ser | 6.1 | 7.2 | 5.6 |
| Thr | 0.5 | 0.8 | 0.7 |
| Cys * | * | * | * |
| Asx ** | 3.7 | 4.0 | 4.0 |
| Glx ** | 0.9 | 1.0 | 1.1 |
| Arg | 1.8 | 2.1 | 2.1 |
| Lys | 0.4 | 0.5 | 0.5 |
| His | 1.6 | 1.8 | 1.9 |
| Tyr | 5.3 | 6.3 | 5.9 |
| Phe | 0.3 | 0.2 | 0.2 |
| Trp # | - | - | - |
| Gly/Ala | 0.8 | 0.9 | 0.9 |
| Acidic | 4.6 | 5.0 | 5.1 |
| Basic | 3.8 | 4.3 | 4.5 |
| Sample | Strain at Break (%) | ||
|---|---|---|---|
| Minimum | Maximum | Average | |
| SGFHFIP as-cast film | 180 | 230 | 202 ± 14 |
| 80%-EtOH-treated SGFHFIP cast film | 40 | 160 | 100 ± 33 |
| 80%-EtOH-treated LFaq cast film | 60 | 150 | 79 ± 29 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moseti, K.O.; Yoshioka, T.; Kameda, T.; Nakazawa, Y. Structure Water-Solubility Relationship in α-Helix-Rich Films Cast from Aqueous and 1,1,1,3,3,3-Hexafluoro-2-Propanol Solutions of S. c. ricini Silk Fibroin. Molecules 2019, 24, 3945. https://doi.org/10.3390/molecules24213945
Moseti KO, Yoshioka T, Kameda T, Nakazawa Y. Structure Water-Solubility Relationship in α-Helix-Rich Films Cast from Aqueous and 1,1,1,3,3,3-Hexafluoro-2-Propanol Solutions of S. c. ricini Silk Fibroin. Molecules. 2019; 24(21):3945. https://doi.org/10.3390/molecules24213945
Chicago/Turabian StyleMoseti, Kelvin O., Taiyo Yoshioka, Tsunenori Kameda, and Yasumoto Nakazawa. 2019. "Structure Water-Solubility Relationship in α-Helix-Rich Films Cast from Aqueous and 1,1,1,3,3,3-Hexafluoro-2-Propanol Solutions of S. c. ricini Silk Fibroin" Molecules 24, no. 21: 3945. https://doi.org/10.3390/molecules24213945
APA StyleMoseti, K. O., Yoshioka, T., Kameda, T., & Nakazawa, Y. (2019). Structure Water-Solubility Relationship in α-Helix-Rich Films Cast from Aqueous and 1,1,1,3,3,3-Hexafluoro-2-Propanol Solutions of S. c. ricini Silk Fibroin. Molecules, 24(21), 3945. https://doi.org/10.3390/molecules24213945






