Grafting versus Crosslinking of Silk Fibroin-g-PNIPAM via Tyrosine-NIPAM Bridges
Abstract
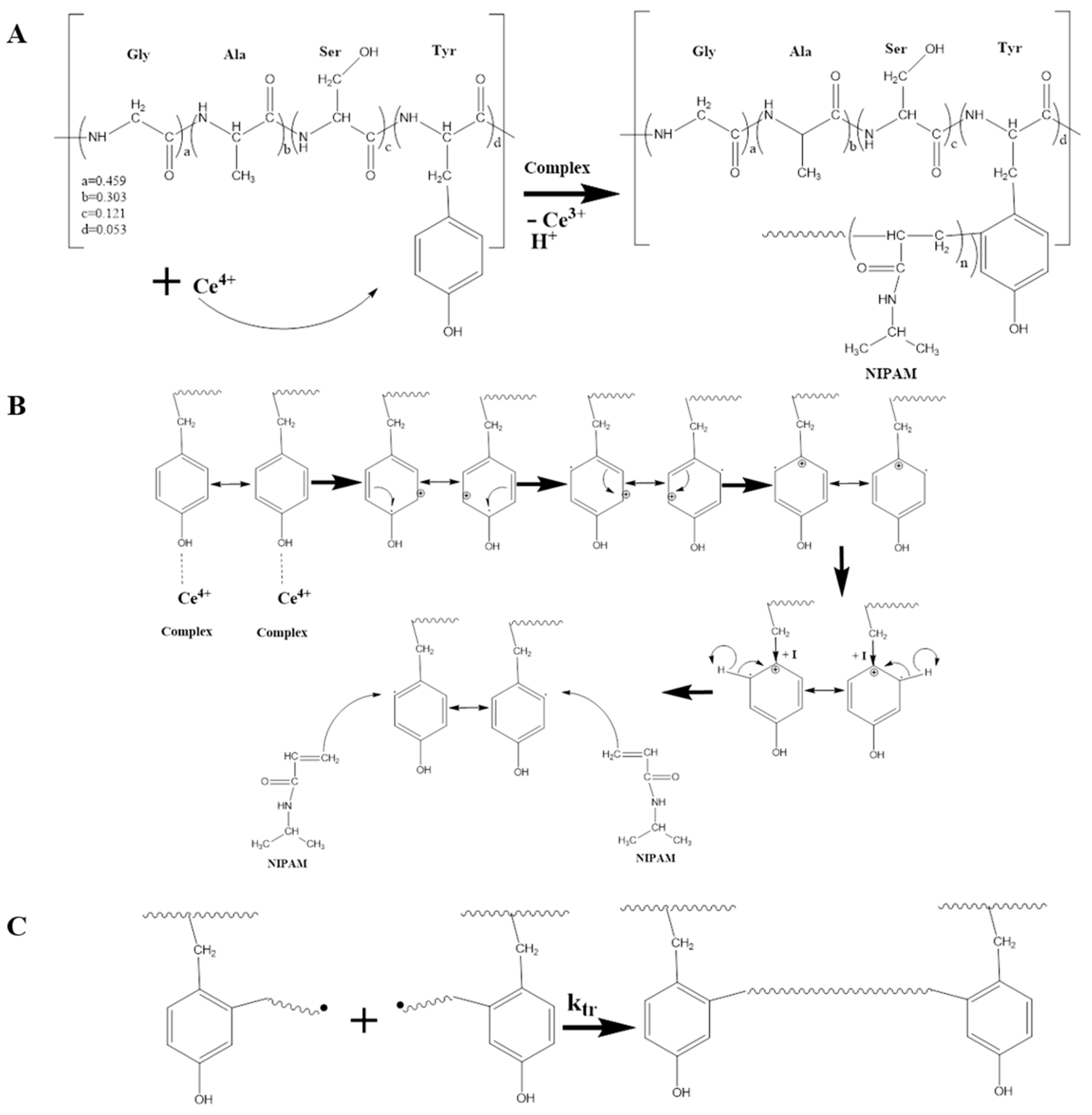
1. Introduction
2. Results and Discussion
2.1. Physico-Chemical Characterization
2.1.1. DLS Analysis
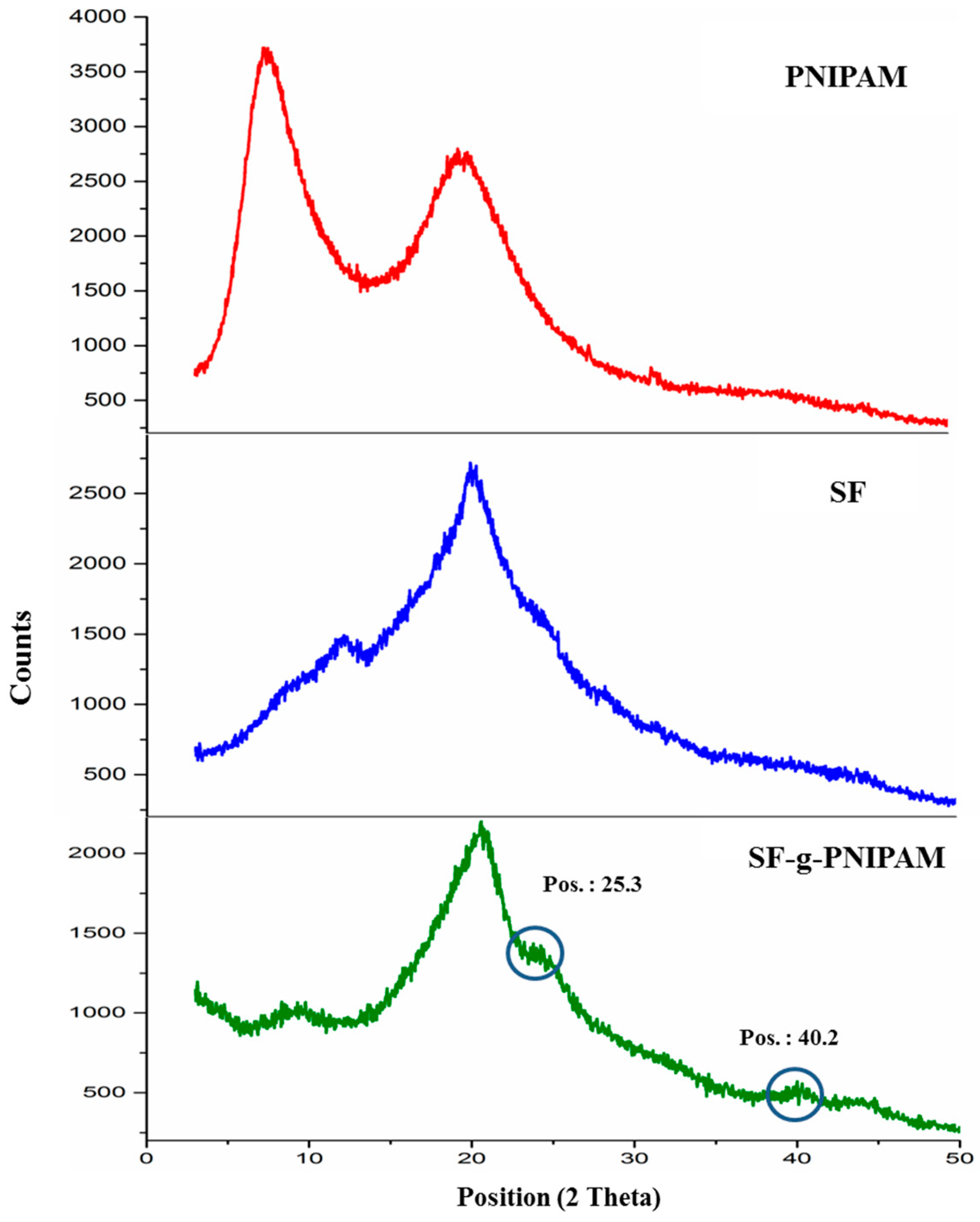
2.1.2. XRD Investigation
2.2. Thermal Behavior of the SF and SF-g-PNIPAM
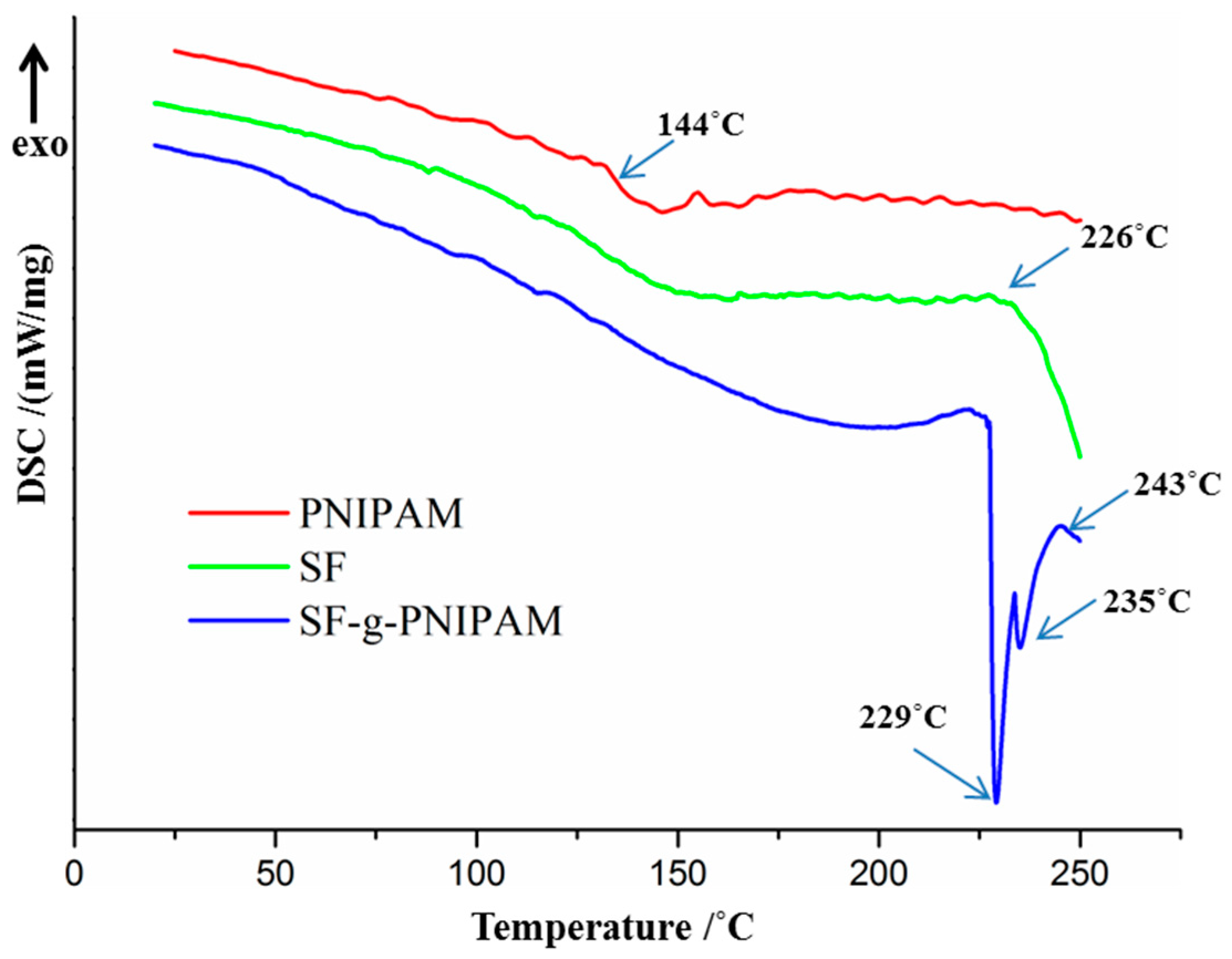
2.2.1. DSC Analysis
2.2.2. TGA Analysis
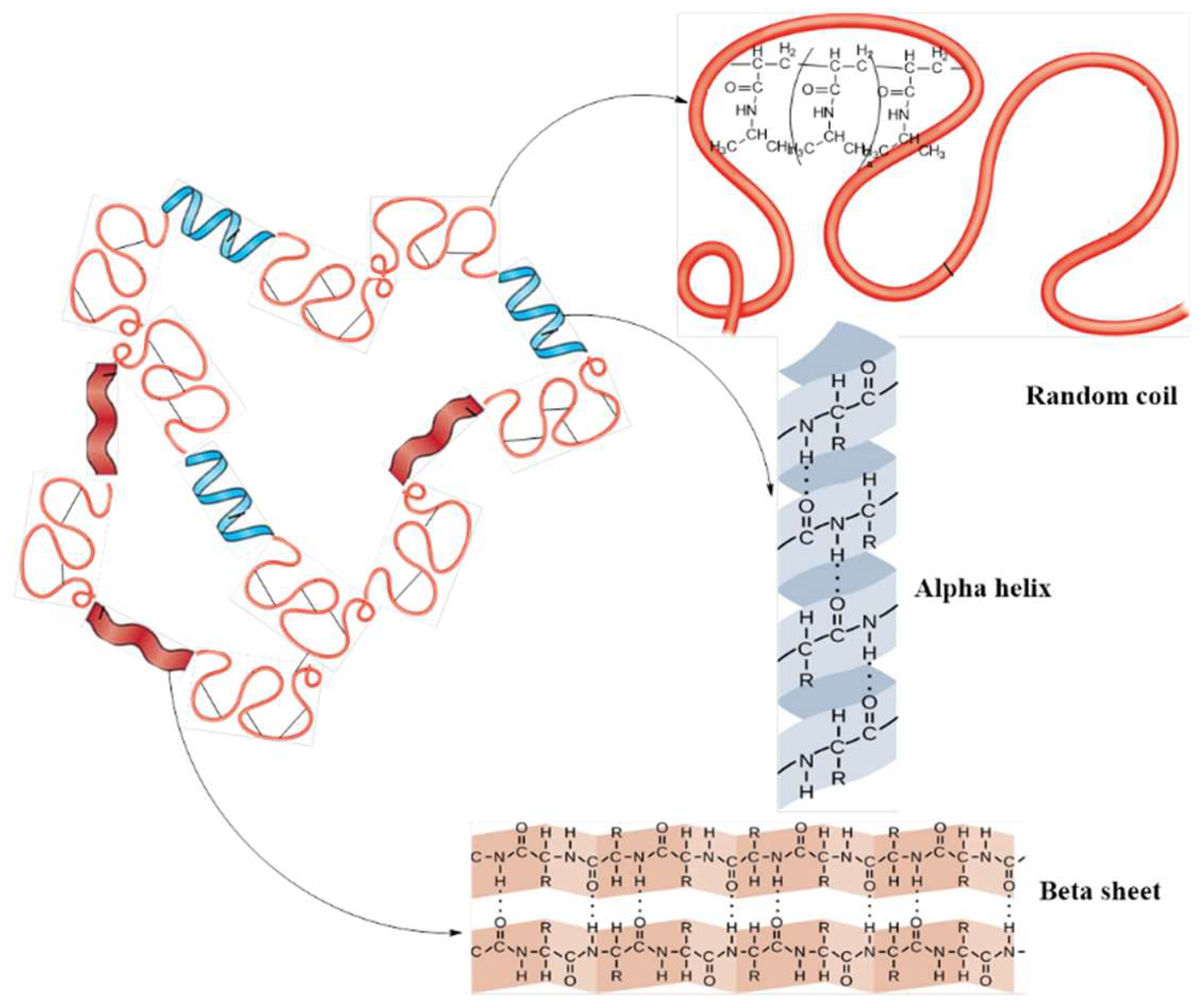
2.3. Conformational Analysis by Circular Dichroism (CD)
2.4. Solubility of Silk Fibroin-Grafted Poly-N-Isopropylacrylamide (SF-g-PNIPAM) and Characterization of the Crosslinked Network
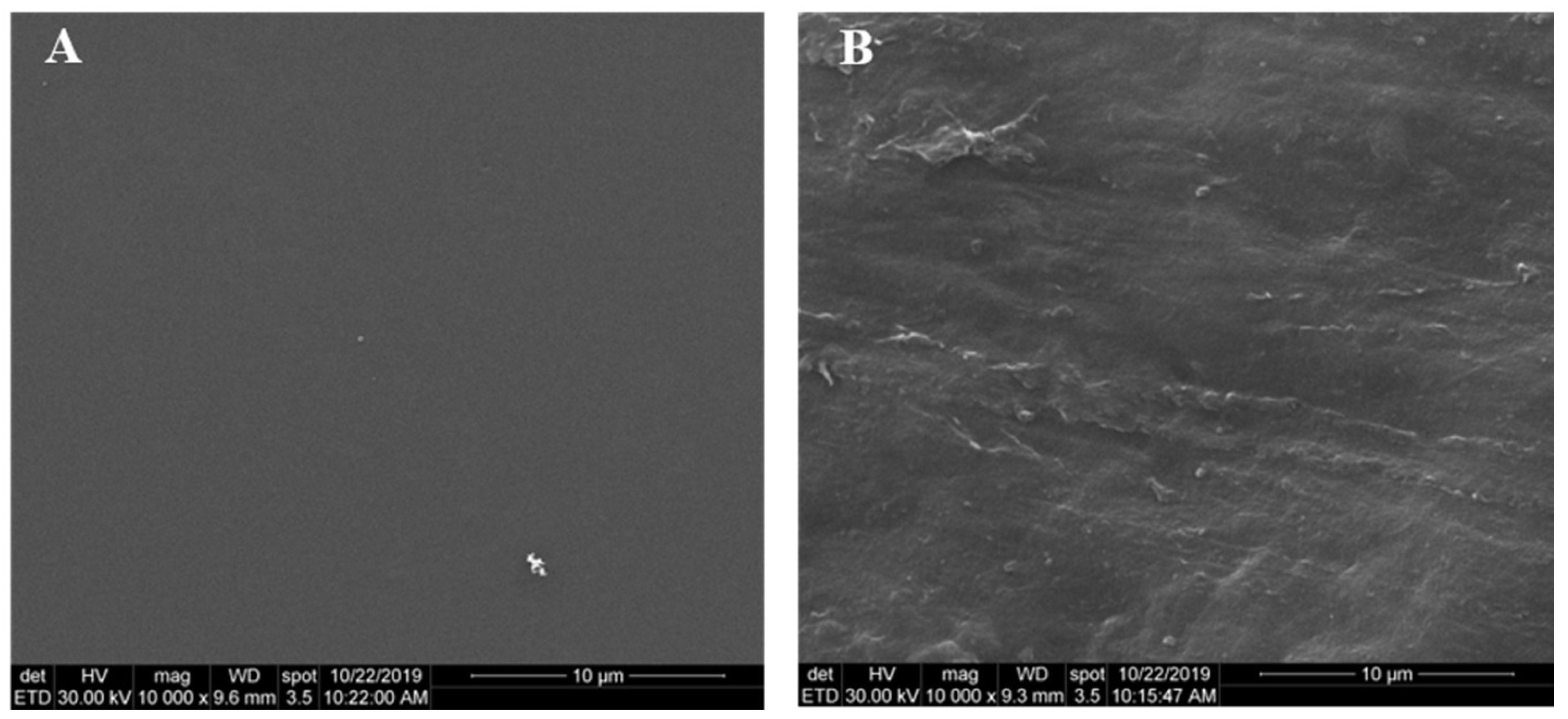
2.5. SEM Analysis
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Obtaining of Silk Fibroin Solution
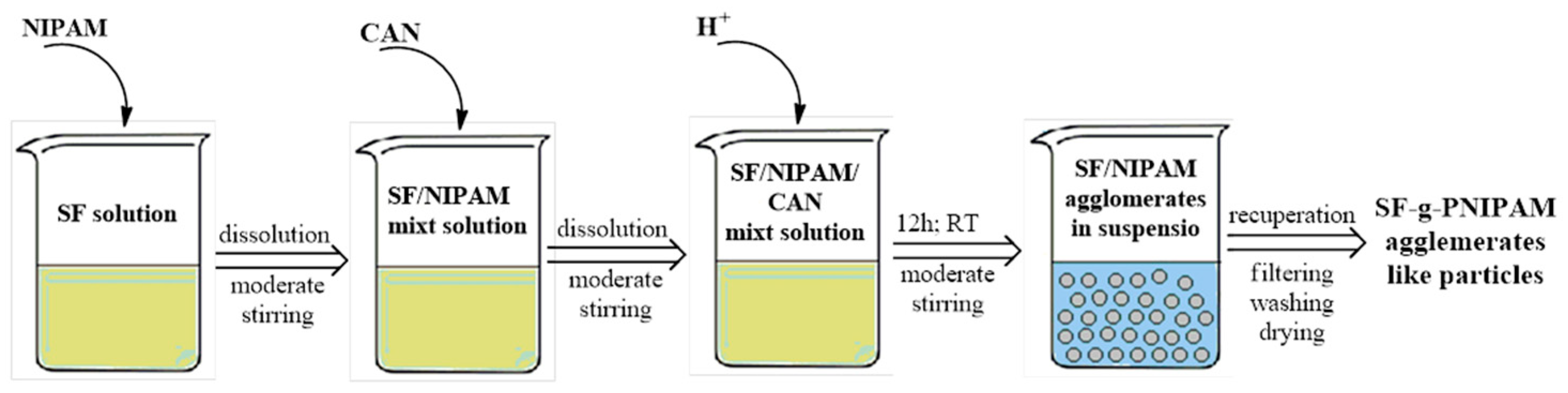
3.2.2. Synthesis of Silk Fibroin-Grafted Poly(N-Isopropylacrylamide) (SF-g-PNIPAM)
3.2.3. Characterization Methods
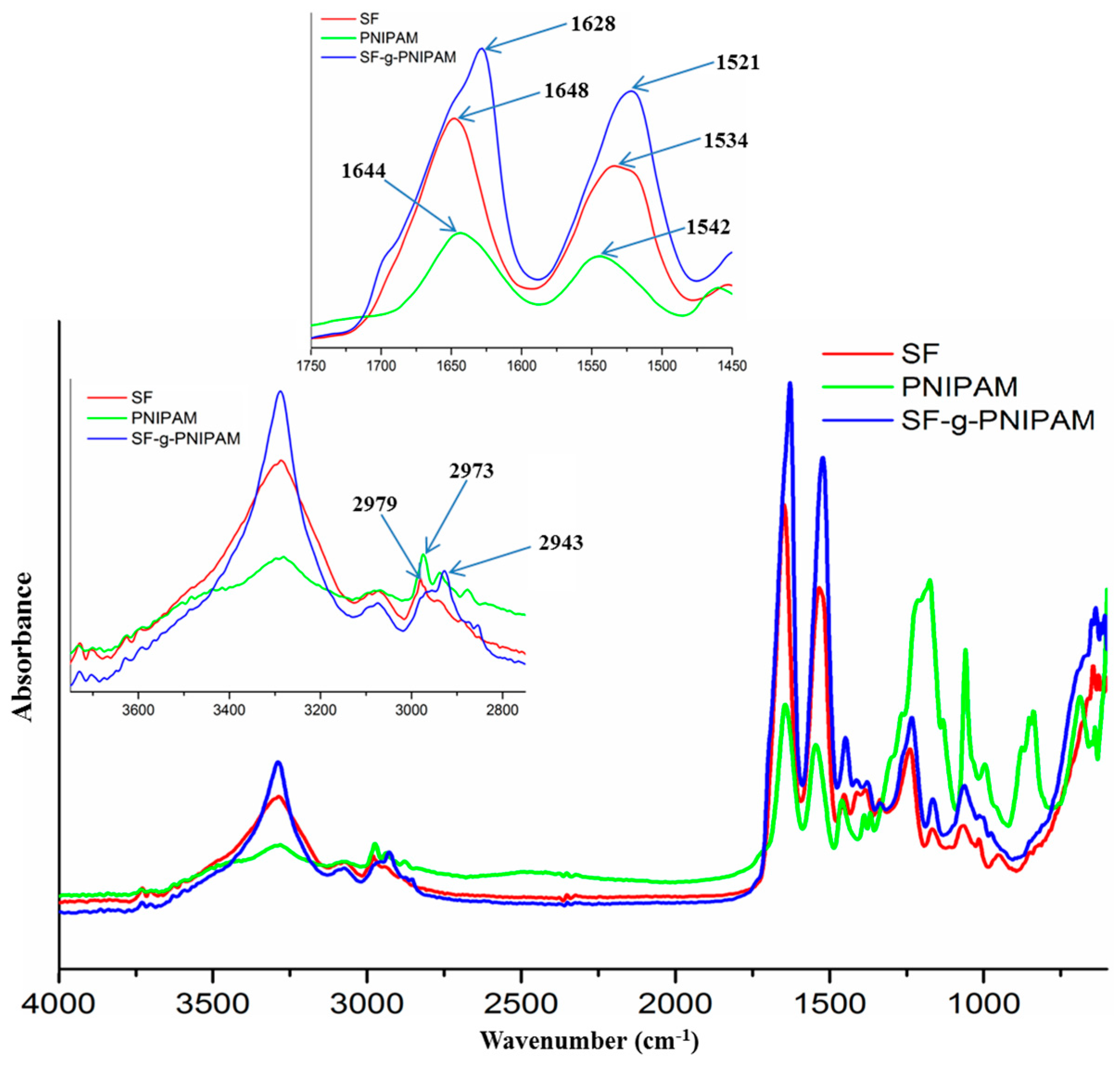
FTIR Analysis
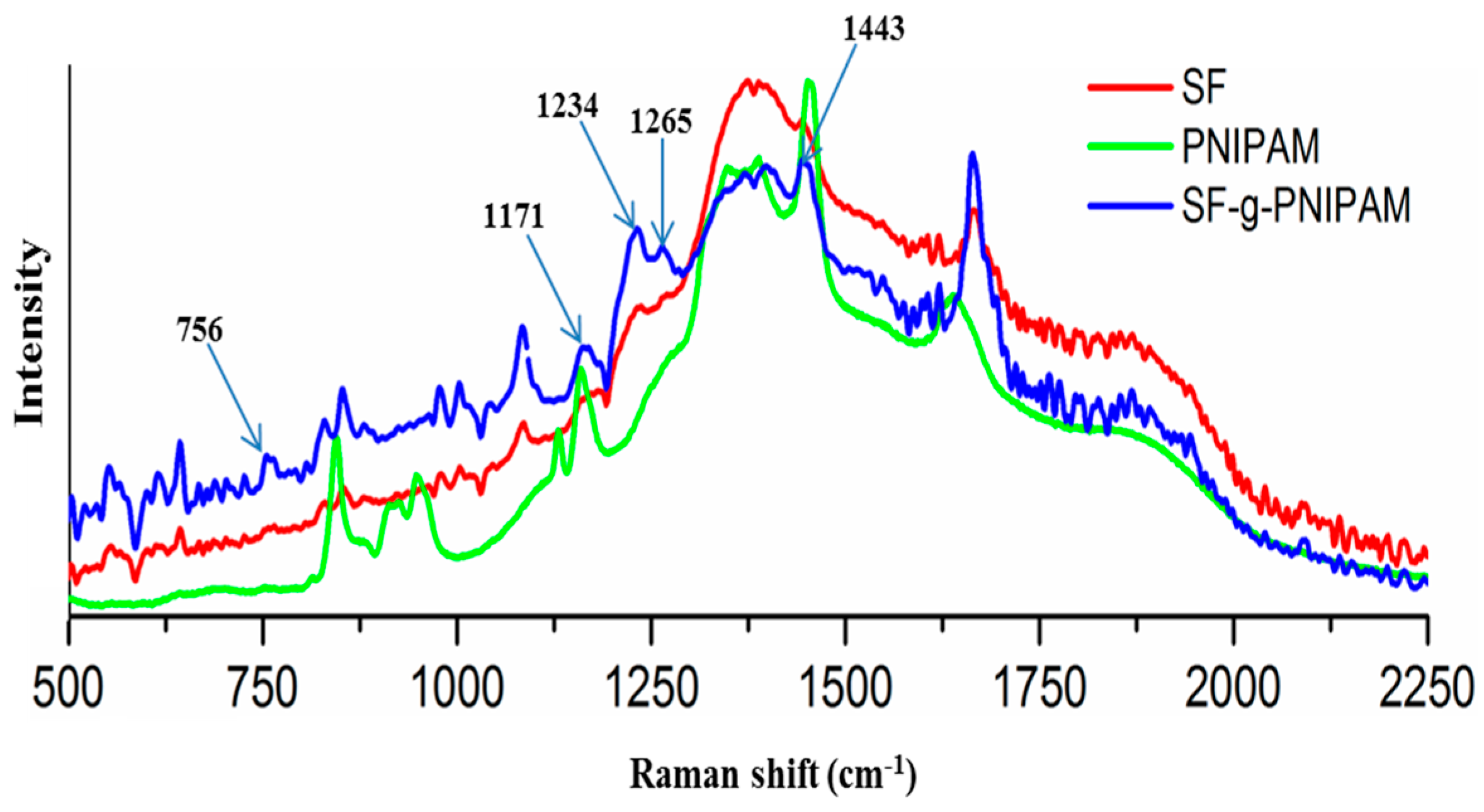
Raman Analysis
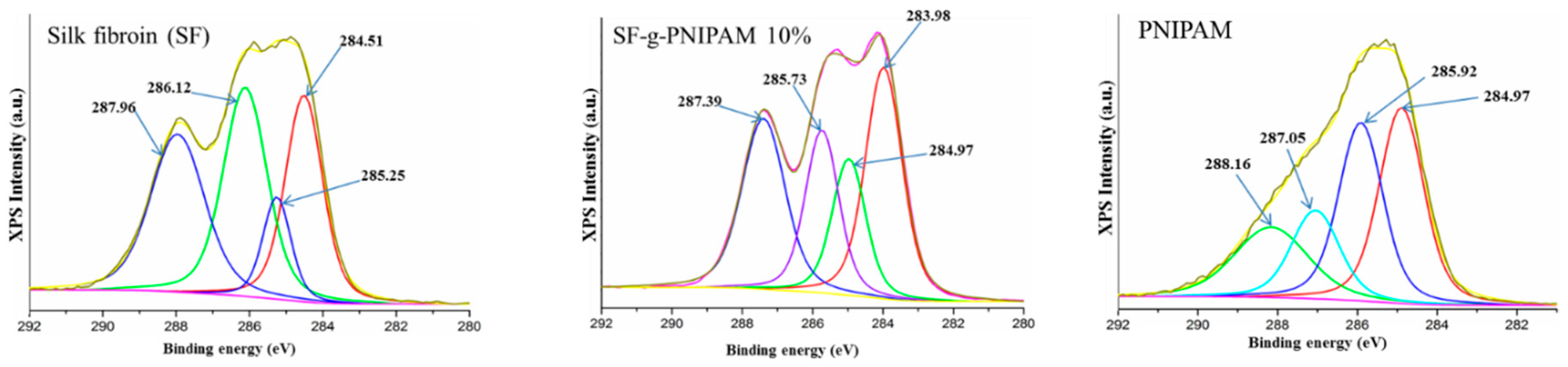
XPS Analysis
Dynamic Light Scattering (DLS)
X-ray Diffraction (XRD) Analysis
Differential Scanning Calorimetry (DSC)
TGA Analysis
Conformational Analysis by Circular Dichroism (CD)
3.2.4. Solubility and Crosslinking Effect of Silk Fibroin-Grafted Poly(N-Isopropylacrylamide) (SF-g-PNIPAM)
3.2.5. SEM Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Behari, K.; Pandey, P.K.; Kumar, R.; Taunk, K. Graft copolymerization of acrylamide onto xanthan gum. Carbohydr. Polym. 2001, 46, 185–189. [Google Scholar] [CrossRef]
- Pandey, P.K.; Srivastava, A.; Tripathy, J.; Behari, K. Graft copolymerization of acrylic acid onto guar gum initiated by vanadium (V)–mercaptosuccinic acid redox pair. Carbohydr. Polym. 2006, 65, 414–420. [Google Scholar] [CrossRef]
- Behari, K.; Banerjee, J.; Srivastava, A.; Mishra, D.K. Studies on graft copolymerization of N-vinyl formamide onto Guar gum initiated by bromate/ascorbic acid redox pair. Indian J. Chem. Technol. 2005, 12, 664–670. [Google Scholar]
- Yadav, M.; Sand, A.; Kumar Mishra, D.; Behari, K. A study toward the physicochemical properties of graft copolymer (partially carboxymethylated guar gum-g-N,N′-dimethylacrylamide): Synthesis and characterization. J. Appl. Polym. Sci. 2010, 117, 974–981. [Google Scholar] [CrossRef]
- Yadav, M.; Sand, A.; Mishra, M.M.; Tripathy, J.; Pandey, V.S.; Behari, K. Synthesis, characterization and applications of graft copolymer (κ-carrageenan-g-vinylsulfonic acid). Int. J. Biol. Macromol. 2012, 50, 826–832. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, G.S.; Guleria, L.; Sharma, R. Synthesis, characterization and metal ion sorption studies of graft copolymers of cellulose with glycidyl methacrylate and some comonomers. Cellulose 2005, 12, 97–110. [Google Scholar] [CrossRef]
- Qudsieh, I.Y.M.; Fakhru’l-Razi, A.; Muyibi, S.A.; Ahmad, M.B.; Rahman, M.Z.A.; Zin Wan Yunus, W.M. Preparation and characterization of poly(methyl methacrylate) grafted sago starch using potassium persulfate as redox initiator. J. Appl. Polym. Sci. 2004, 94, 1891–1897. [Google Scholar] [CrossRef]
- Trimnell, D.; Fanta, G.F.; Salch, J.H. Graft polymerization of methyl acrylate onto granular starch: Comparison of the Fe+2/H2O2 and ceric initiating systems. J. Appl. Polym. Sci. 1996, 60, 285–292. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, L.; Shi, Z.; Li, J. Graft copolymerization of methyl acrylate onto cellulose initiated by potassium ditelluratoargentate(III). Polym. Int. 2004, 53, 1561–1566. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Misra, B.N. Grafting: A versatile means to modify polymers: Techniques, factors and applications. Prog. Polym. Sci. 2004, 29, 767–814. [Google Scholar] [CrossRef]
- Sugino, Y.; Yamamoto, K.; Miwa, Y.; Sakaguchi, M.; Shimada, S. Controlled grafting of poly(styrene-ran-n-butyl methacrylate) to isotactic polypropylene with nitroxidemediated polymerization. e-Polymers 2003, 3. [Google Scholar] [CrossRef]
- Jana, S.C.; Maiti, S.; Biswas, S. Graft copolymerization of acrylonitrile onto poly(vinyl alcohol) in presence of air using ceric ammonium nitrate-natural gums. J. Appl. Polym. Sci. 2000, 78, 1586–1590. [Google Scholar] [CrossRef]
- Furuzono, T.; Ishihara, K.; Nakabayashi, N.; Tamada, Y. Chemical modification of silk fibroin with 2-methacryloyloxyethyl phosphorylcholine. II. Graft-polymerization onto fabric through 2-methacryloyloxyethyl isocyanate and interaction between fabric and platelets. Biomaterials 2000, 21, 327–333. [Google Scholar] [CrossRef]
- Zaharia, C.; Buga, M.R.; Stanescu, P.; Vasile, E.; Cincu, C. Silk fibroin films for tissue bioengineering applications. J. Optoelectron. Adv. Mater. 2012, 14, 163–168. [Google Scholar]
- Song, Y.; Wei, D. Preparation and Characterization of Graft Copolymers of Silk Sericin and Methyl Methacrylate. Polym. Polym. Compos. 2006, 14, 169–174. [Google Scholar] [CrossRef]
- Zhang, T.; Li, G.; Guo, L.; Chen, H. Synthesis of thermo-sensitive CS-g-PNIPAM/CMC complex nanoparticles for controlled release of 5-FU. Int. J. Biol. Macromol. 2012, 51, 1109–1115. [Google Scholar] [CrossRef] [PubMed]
- Al-Qalaf, F.; Mekheimer, R.A.; Sadek, K.U. Cerium (IV) ammonium nitrate (CAN) catalyzed one-pot synthesis of 2-arylbenzothiazoles. Molecules 2008, 13, 2908–2914. [Google Scholar] [CrossRef]
- Li, G.; Song, S.; Zhang, T.; Qi, M.; Liu, J. pH-sensitive polyelectrolyte complex micelles assembled from CS-g-PNIPAM and ALG-g-P(NIPAM-co-NVP) for drug delivery. Int. J. Biol. Macromol. 2013, 62, 203–210. [Google Scholar] [CrossRef]
- Ghaeli, I.; de Moraes, M.A.; Beppu, M.M.; Lewandowska, K.; Sionkowska, A.; Ferreira-da-Silva, F.; Ferraz, M.P.; Monteiro, F.J. Phase Behaviour and Miscibility Studies of Collagen/Silk Fibroin Macromolecular System in Dilute Solutions and Solid State. Molecules 2017, 22, 1368. [Google Scholar] [CrossRef]
- Choi, K.Y.; Chung, H.; Min, K.H.; Yoon, H.Y.; Kim, K.; Park, J.H.; Kwon, I.C.; Jeong, S.Y. Self-assembled hyaluronic acid nanoparticles for active tumor targeting. Biomaterials 2010, 31, 106–114. [Google Scholar] [CrossRef]
- Numata, K.; Kaplan, D.L. Silk-based delivery systems of bioactive molecules. Adv. Drug Deliv. Rev. 2010, 62, 1497–1508. [Google Scholar] [CrossRef] [PubMed]
- Murphy, A.R.; Romero, I.S. 8—Biochemical and biophysical properties of native Bombyx mori silk for tissue engineering applications. In Silk Biomaterials for Tissue Engineering and Regenerative Medicine; Kundu, S.C., Ed.; Woodhead Publishing: Cambridge, UK, 2014; pp. 219–238. [Google Scholar] [CrossRef]
- Kundu, B.; Rajkhowa, R.; Kundu, S.C.; Wang, X. Silk fibroin biomaterials for tissue regenerations. Adv. Drug Deliv. Rev. 2013, 65, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Mottaghitalab, F.; Farokhi, M.; Shokrgozar, M.A.; Atyabi, F.; Hosseinkhani, H. Silk fibroin nanoparticle as a novel drug delivery system. J. Control. Release 2015, 206, 161–176. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Lin, Y.; Wu, P. Structure Analysis of Poly(N-isopropylacrylamide) Using Near-Infrared Spectroscopy and Generalized Two-Dimensional Correlation Infrared Spectroscopy. Appl. Spectrosc. 2007, 61, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Beattie, D.A.; Addai-Mensah, J.; Beaussart, A.; Franks, G.V.; Yeap, K.-Y. In situ particle film ATR FTIR spectroscopy of poly (N-isopropyl acrylamide) (PNIPAM) adsorption onto talc. Phys. Chem. Chem. Phys. 2014, 16, 25143–25151. [Google Scholar] [CrossRef]
- Sun, T.; Qing, G. Biomimetic Smart Interface Materials for Biological Applications. Adv. Mater. 2011, 23, H57–H77. [Google Scholar] [CrossRef]
- Hu, X.; Kaplan, D.; Cebe, P. Determining Beta-Sheet Crystallinity in Fibrous Proteins by Thermal Analysis and Infrared Spectroscopy. Macromolecules 2006, 39, 6161–6170. [Google Scholar] [CrossRef]
- Wongkrongsak, S.; Tangthong, T.; Pasanphan, W. Electron beam induced water-soluble silk fibroin nanoparticles as a natural antioxidant and reducing agent for a green synthesis of gold nanocolloid. Radiat. Phys. Chem. 2016, 118, 27–34. [Google Scholar] [CrossRef]
- Sharma, S.; Bano, S.; Ghosh, A.S.; Mandal, M.; Kim, H.-W.; Dey, T.; Kundu, S.C. Silk fibroin nanoparticles support in vitro sustained antibiotic release and osteogenesis on titanium surface. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 1193–1204. [Google Scholar] [CrossRef]
- Fischer, W.B.; Eysel, H.H. Polarized Raman spectra and intensities of aromatic amino acids phenylalanine, tyrosine and tryptophan. Spectrochim. Acta Part A Mol. Spectrosc. 1992, 48, 725–732. [Google Scholar] [CrossRef]
- Zhu, G.; Zhu, X.; Fan, Q.; Wan, X. Raman spectra of amino acids and their aqueous solutions. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 78, 1187–1195. [Google Scholar] [CrossRef] [PubMed]
- Hwu, J.R.; King, K.-Y. Versatile reagent ceric ammonium nitrate in modern chemical synthesis. Curr. Sci. 2001, 81, 1043–1053. [Google Scholar]
- Shao, J.; Liu, J.; Zheng, J.; Carr, C.M. X-ray photoelectron spectroscopic study of silk fibroin surface. Polym. Int. 2002, 51, 1479–1483. [Google Scholar] [CrossRef]
- Kobayashi, J.; Arisaka, Y.; Yui, N.; Akiyama, Y.; Yamato, M.; Okano, T. Effect of Temperature Changes on Serum Protein Adsorption on Thermoresponsive Cell-Culture Surfaces Monitored by A Quartz Crystal Microbalance with Dissipation. Int. J. Mol. Sci. 2018, 19, 1516. [Google Scholar] [CrossRef] [PubMed]
- Tu, H.; Heitzman, C.E.; Braun, P.V. Patterned Poly(N-isopropylacrylamide) Brushes on Silica Surfaces by Microcontact Printing Followed by Surface-Initiated Polymerization. Langmuir 2004, 20, 8313–8320. [Google Scholar] [CrossRef] [PubMed]
- Titantah, J.T.; Lamoen, D. Carbon and nitrogen 1s energy levels in amorphous carbon nitride systems: XPS interpretation using first-principles. Diam. Relat. Mater. 2007, 16, 581–588. [Google Scholar] [CrossRef]
- Kamalha, E.; Zheng, Y.; Zeng, Y. Analysis of the secondary crystalline structure of regenerated Bombyx mori fibroin. Res. Rev. Biosci. 2013, 7, 76–83. [Google Scholar]
- Asakura, T.; Kuzuhara, A.; Tabeta, R.; Saito, H. Conformational characterization of Bombyx mori silk fibroin in the solid state by high-frequency carbon-13 cross polarization-magic angle spinning NMR, x-ray diffraction, and infrared spectroscopy. Macromolecules 1985, 18, 1841–1845. [Google Scholar] [CrossRef]
- Motta, A.; Fambri, L.; Migliaresi, C. Regenerated silk fibroin films: Thermal and dynamic mechanical analysis. Macromol. Chem. Phys. 2002, 203, 1658–1665. [Google Scholar] [CrossRef]
- Jaramillo-Quiceno, N.; Álvarez-López, C.; Restrepo-Osorio, A. Structural and thermal properties of silk fibroin films obtained from cocoon and waste silk fibers as raw materials. Procedia Eng. 2017, 200, 384–388. [Google Scholar] [CrossRef]
- Nielsen, L.E. Cross-Linking–Effect on Physical Properties of Polymers. J. Macromol. Sci. Part C 1969, 3, 69–103. [Google Scholar] [CrossRef]
- Krumova, M.; López, D.; Benavente, R.; Mijangos, C.; Pereña, J.M. Effect of crosslinking on the mechanical and thermal properties of poly(vinyl alcohol). Polymer 2000, 41, 9265–9272. [Google Scholar] [CrossRef]
- Somo, S.I.; Langert, K.; Yang, C.-Y.; Vaicik, M.K.; Ibarra, V.; Appel, A.A.; Akar, B.; Cheng, M.-H.; Brey, E.M. Synthesis and evaluation of dual crosslinked alginate microbeads. Acta Biomater. 2018, 65, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Shinde, V.S.; Girme, M.R.; Pawar, V.U. Thermoresponsive polystyrene-b-poly(N-isopropylacrylamide) copolymers by atom transfer radical polymerization. Indian J. Chem. 2011, 50A, 781–787. [Google Scholar]
- Dhumure, A.B.; Patil, A.B.; Kulkarni, A.S.; Voevodina, I.; Scandola, M.; Shinde, V.S. Thermoresponsive copolymers with pendant d-galactosyl 1,2,3-triazole groups: Synthesis, characterization and thermal behavior. New J. Chem. 2015, 39, 8179–8187. [Google Scholar] [CrossRef]
- Lewicki, J.P.; Pielichowski, K.; De La Croix, P.T.; Janowski, B.; Todd, D.; Liggat, J.J. Thermal degradation studies of polyurethane/POSS nanohybrid elastomers. Polym. Degrad. Stab. 2010, 95, 1099–1105. [Google Scholar] [CrossRef]
- Sharon, M.K.; Nicholas, C.P. The Use of Circular Dichroism in the Investigation of Protein Structure and Function. Curr. Protein Pept. Sci. 2000, 1, 349–384. [Google Scholar] [CrossRef]
- Yagci, C.; Yildiz, U. Redox polymerization of methyl methacrylate with allyl alcohol 1,2-butoxylate-block-ethoxylate initiated by Ce(IV)/HNO3 redox system. Eur. Polym. J. 2005, 41, 177–184. [Google Scholar] [CrossRef]
- Nair, V.; Deepthi, A. Cerium(IV) Ammonium NitrateA Versatile Single-Electron Oxidant. Chem. Rev. 2007, 107, 1862–1891. [Google Scholar] [CrossRef]
- Takahashi, T.; Hori, Y.; Sato, I. Coordinated radical polymerization and redox polymerization of acrylamide by ceric ammonium nitrate. J. Polym. Sci. Part A 1 Polym. Chem. 1968, 6, 2091–2102. [Google Scholar] [CrossRef]
- Halperin, A.; Kröger, M.; Winnik, F.M. Poly(N-isopropylacrylamid)-Phasendiagramme: 50 Jahre Forschung. Angew. Chem. 2015, 127, 15558–15586. [Google Scholar] [CrossRef]
- Virtanen, O.L.J. Insight into Precipitation Polymerization of N-Isopropylacrylamide: Reaction Mechanism, Particle Formation and Particle Structure. Ph.D. Thesis, RWTH Aachen University, Aachen, Germany, 2016. [Google Scholar]
- Murphy, A.R.; Kaplan, D.L. Biomedical applications of chemically-modified silk fibroin. J. Mater. Chem. 2009, 19, 6443–6450. [Google Scholar] [CrossRef] [PubMed]
- Flory, P.J. Fundamental Principles of Condensation Polymerization. Chem. Rev. 1946, 39, 137–197. [Google Scholar] [CrossRef] [PubMed]
- Asakura, T.; Suita, K.; Kameda, T.; Afonin, S.; Ulrich, A.S. Structural role of tyrosine in Bombyx mori silk fibroin, studied by solid-state NMR and molecular mechanics on a model peptide prepared as silk I and II. Magn. Reson. Chem. 2004, 42, 258–266. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds silk fibroin and SF-g-PNIPAM are available from the authors. |



| Sample | Swelling Degree (%) |
|---|---|
| SF-g-PNIPAM (5% NIPAM/2.5% ACN) | 360 |
| SF-g-PNIPAM (10% NIPAM/5% ACN) | 290 |
| SF-g-PNIPAM (15% NIPAM/7.5% ACN) | 200 |
| SF-g-PNIPAM (20% NIPAM/10% ACN) | 187 |
| SF-g-PNIPAM (25% NIPAM/12.5% ACN) | 181 |
| SF-g-PNIPAM (30% NIPAM/15% ACN) | 187 |
| No. | Composition with Various Amount of NIPAM | Composition with Various Amount of NIPAM and Cerium Ammonium Nitrate (ACN) |
|---|---|---|
| 1 | SF-g-PNIPAM (5% NIPAM/5% ACN) | SF-g-PNIPAM (5% NIPAM/2.5% ACN) |
| 2 | SF-g-PNIPAM (10% NIPAM/5% ACN) | SF-g-PNIPAM (10% NIPAM/5% ACN) |
| 3 | SF-g-PNIPAM (15% NIPAM/5% ACN) | SF-g-PNIPAM (15% NIPAM/7.5% ACN) |
| 4 | SF-g-PNIPAM (20% NIPAM/5% ACN) | SF-g-PNIPAM (20% NIPAM/10% ACN) |
| 5 | SF-g-PNIPAM (25% NIPAM/5%ACN) | SF-g-PNIPAM (25% NIPAM/12.5%ACN) |
| 6 | SF-g-PNIPAM (30% NIPAM/5%ACN) | SF-g-PNIPAM (30% NIPAM/15%ACN) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radu, I.-C.; Biru, I.-E.; Damian, C.-M.; Ion, A.-C.; Iovu, H.; Tanasa, E.; Zaharia, C.; Galateanu, B. Grafting versus Crosslinking of Silk Fibroin-g-PNIPAM via Tyrosine-NIPAM Bridges. Molecules 2019, 24, 4096. https://doi.org/10.3390/molecules24224096
Radu I-C, Biru I-E, Damian C-M, Ion A-C, Iovu H, Tanasa E, Zaharia C, Galateanu B. Grafting versus Crosslinking of Silk Fibroin-g-PNIPAM via Tyrosine-NIPAM Bridges. Molecules. 2019; 24(22):4096. https://doi.org/10.3390/molecules24224096
Chicago/Turabian StyleRadu, Ionut-Cristian, Iuliana-Elena Biru, Celina-Maria Damian, Andreea-Cristina Ion, Horia Iovu, Eugenia Tanasa, Catalin Zaharia, and Bianca Galateanu. 2019. "Grafting versus Crosslinking of Silk Fibroin-g-PNIPAM via Tyrosine-NIPAM Bridges" Molecules 24, no. 22: 4096. https://doi.org/10.3390/molecules24224096
APA StyleRadu, I.-C., Biru, I.-E., Damian, C.-M., Ion, A.-C., Iovu, H., Tanasa, E., Zaharia, C., & Galateanu, B. (2019). Grafting versus Crosslinking of Silk Fibroin-g-PNIPAM via Tyrosine-NIPAM Bridges. Molecules, 24(22), 4096. https://doi.org/10.3390/molecules24224096








