Effects of Gellan Oligosaccharide and NaCl Stress on Growth, Photosynthetic Pigments, Mineral Composition, Antioxidant Capacity and Antimicrobial Activity in Red Perilla
Abstract
1. Introduction
2. Results
2.1. Growth Parameters
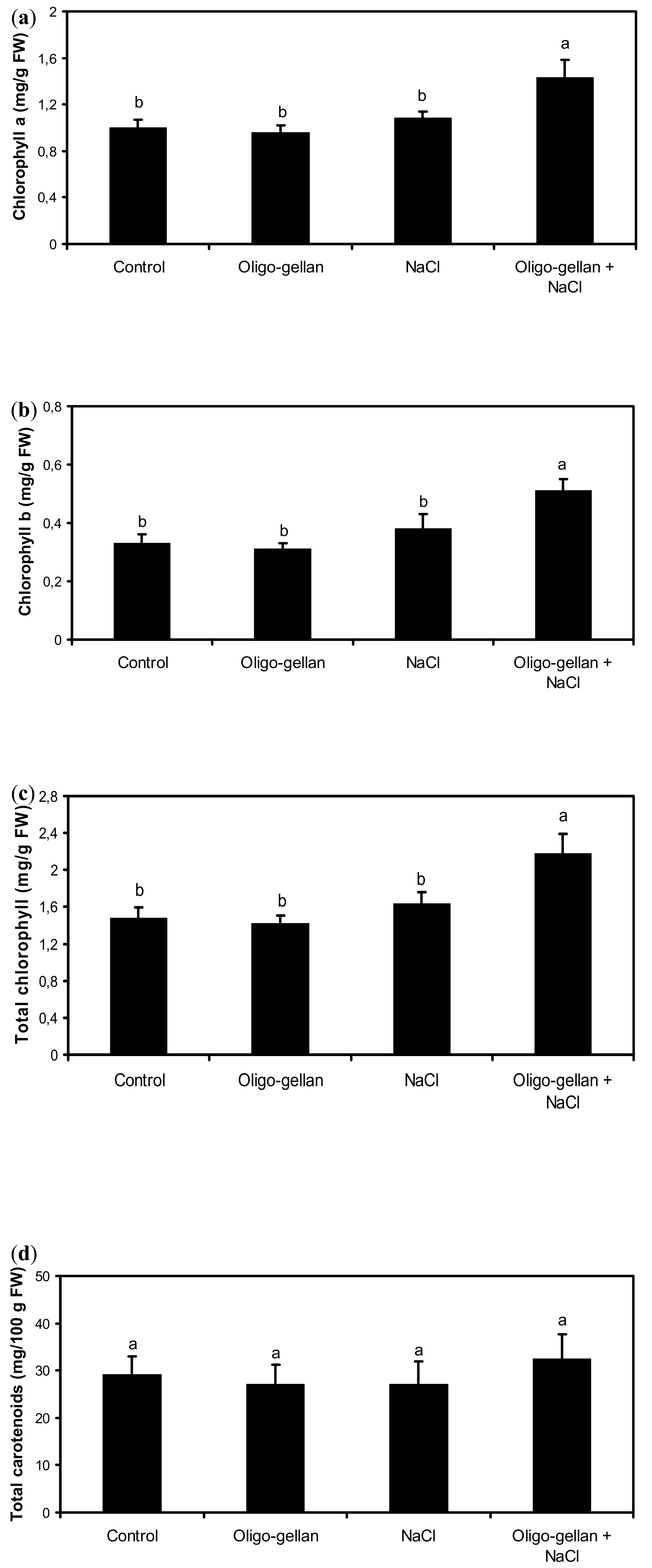
2.2. Photosynthetic Pigments
2.3. Mineral Composition
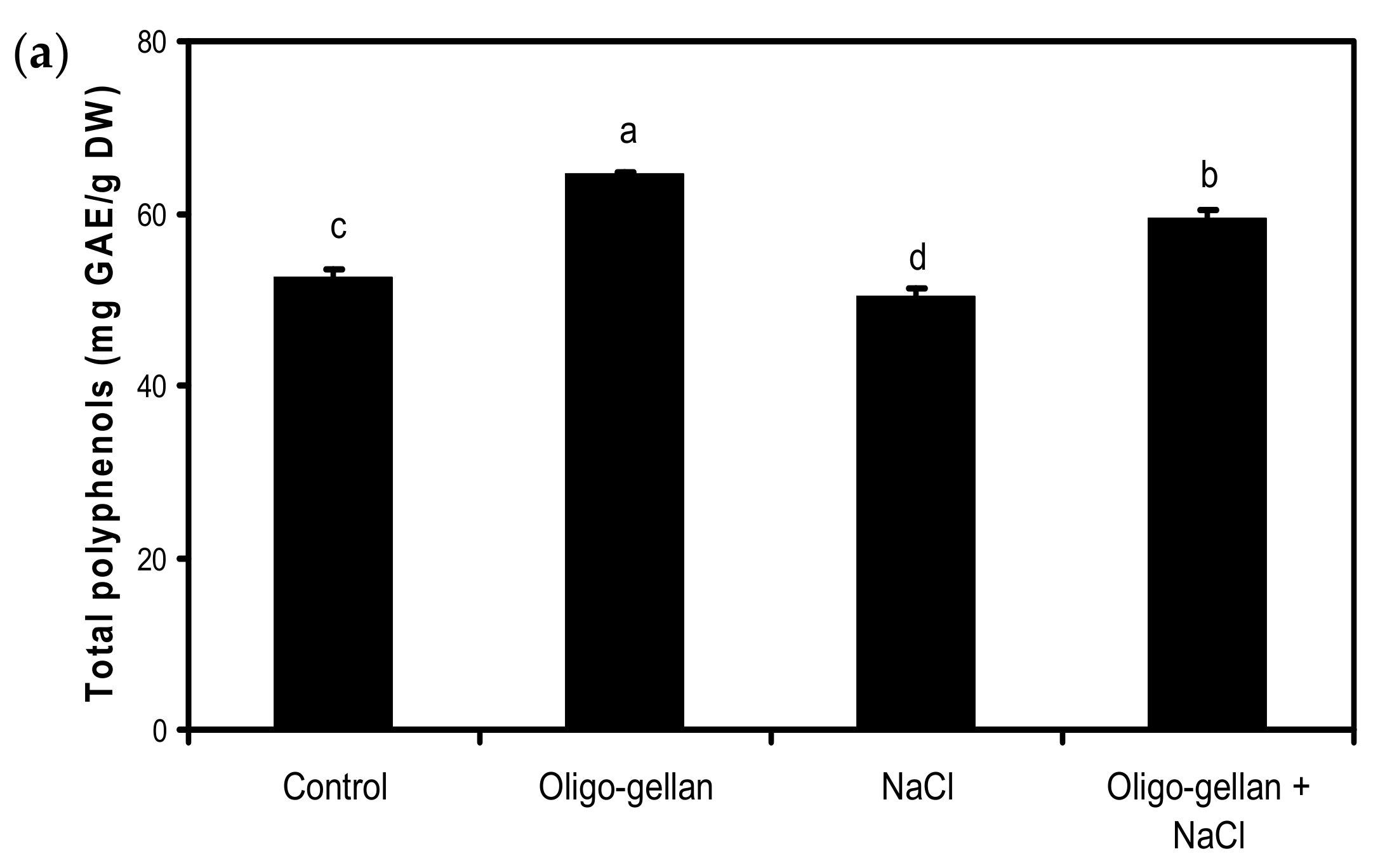
2.4. Total Polyphenols, Total Anthocyanins, and Antioxidant
2.5. Antimicrobial Activity
3. Discussion
4. Materials and Methods
4.1. Characterization of Depolymerized Gellan Gum
4.2. Plant Material and Experimental Conditions
4.3. Treatments
- Non-treated controls;
- Plants drenched with oligo-gellan solution;
- Plants drenched with NaCl solution;
- Plants drenched first with oligo-gellan and then with NaCl.
4.4. Determination of Growth Parameters
4.5. Estimation of Chlorophyll and Carotenoid Content
104 × (20.13 × E646 − 5.03 × E663) ] × [V/1000 × (m × 229)],
4.6. Macronutrient Analysis
4.7. Determination of Total Anthocyanin Content
4.7.1. Anthocyanin Extraction
4.7.2. Anthocyanin Content Determination
4.8. Estimation of Total Polyphenol Content and Antioxidant Activity
4.8.1. Preparation of Plant Extracts
4.8.2. Total Polyphenol Content
4.8.3. Determination of DPPH Radical Scavenging Capacity
4.8.4. Determination of Free-Radical Scavenging Ability Using a Stable ABTS Radical Cation
4.9. Antibacterial Activity
4.9.1. Extract Preparation
4.9.2. Antibacterial Analysis
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Darvill, A.; Augur, C.; Bergmann, C.; Carlson, R.W.; Cheong, J.J.; Eberhard, S.; Hahn, M.G.; Lo, V.M.; Marfa, V.; Meyer, B.; et al. Oligosaccharins- oligosaccharides that regulate growth, development and defence responses in plants. Glycobiology 1992, 2, 181–198. [Google Scholar] [CrossRef] [PubMed]
- Thilip, C.; Mehaboob, V.M.; Varutharaju, K.; Faizal, K.; Raja, P.; Aslam, A.; Shajahan, A. Elicitation of withaferin- A in hairy root culture of Withania somnifera (L.) Dunal using natural polysaccharide. Biologia 2019, 74, 961–968. [Google Scholar] [CrossRef]
- Muley, A.B.; Shingote, P.R.; Patil, A.P.; Dalvi, S.G.; Suprasanna, P. Gamma radiation degradation of chitosan for application in growth promotion and induction of stress tolerance in potato (Solanum tuberosum L.). Carbohydr. Polym. 2019, 210, 289–301. [Google Scholar] [CrossRef] [PubMed]
- El-Mohdy, H.L.A. Radiation-induced degradation of sodium alginate and its plant growth promotion effect. Arab. J. Chem. 2017, 10, 431–438. [Google Scholar] [CrossRef]
- Abad, L.V.; Aurigue, F.B.; Montefalcon, D.R.V.; Manguiat, P.H.; Carandang, F.F.; Mabborang, S.A.; Hizon, M.G.S.; Abella, M.E.S. Effect of radiation-modified kappa-carrageenan as plant growth promoter on peanut (Arachis hypogaea L.). Radiat. Phys. Chem. 2018, 153, 239–244. [Google Scholar] [CrossRef]
- Ahmad, B.; Jaleel, H.; Shabbir, A.; Khan, M.M.A.; Sadiq, Y. Concomitant application of depolymerized chitosan and GA3 modulates photosynthesis, essential oil and menthol production in peppermint (Mentha piperita L.). Sci. Hortic. 2019, 246, 371–379. [Google Scholar] [CrossRef]
- Sadiq, Y.; Khan, M.M.A.; Shabbir, A.; Ahmad, B.; Jaleel, H.; Uddin, M.; Varshney, L. Structural re-arrangement of depolymerized sodium alginate enriches peltate glandular trichomes and essential oil production of spearmint. Int. J. Biol. Macromol. 2017, 105, 1043–1050. [Google Scholar] [CrossRef]
- Aftab, T.; Naeem, M.; Idrees, M.; Khan, M.M.A.; Moinuddin; Varshney, L. Simultaneous use of irradiated sodium alginate and nitrogen and phosphorus fertilizers enhance growth, biomass and artemisinin biosynthesis in Artemisia annua L. J. Appl. Res. Med. Aroma. Plants 2016, 3, 186–194. [Google Scholar]
- Ahmad, B.; Jahan, A.; Sadiq, Y.; Shabbir, A.; Jaleel, H.; Khan, M.M.A. Radiation mediated molecular weight reduction and structural modification in carrageenan potentiates improved photosynthesis and secondary metabolism in peppermint (Mentha piperita L.). Int. J. Biol. Macromol. 2019, 124, 1069–1079. [Google Scholar] [CrossRef]
- Rakpenthai, A.; Khaksar, G.; Burow, M.; Olsen, C.E.; Sirikantaramas, S. Metabolic changes and increased levels of bioactive compounds in white radish (Raphanus sativus L. cv. 01) sprouts elicited by oligochitosan. Agronomy 2019, 9, 467. [Google Scholar] [CrossRef]
- Singh, M.; Khan, M.M.A.; Uddin, M.; Naeem, M.; Qureshi, M.I. Proliferating effect of radiolytically depolymerized carrageenan on physiological attributes, plant water relation parameters, essential oil production and active constituents of Cymbopogon flexuosus Steud. under drought stress. PLoS ONE 2017, 12, e0180129. [Google Scholar] [CrossRef] [PubMed]
- Ben Salah, I.; Aghrouss, S.; Douira, A.; Aissam, S.; El Alaoui-Talibi, Z.; Filali-Maltouf, A.; El Modafar, C. Seaweed polysaccharides as bio-elicitors of natural defenses in olive trees against verticillium wilt of olive. J. Plant Interact. 2018, 13, 248–255. [Google Scholar] [CrossRef]
- Lee, S.Y.; Ahn, J.-Y.; Kim, M.; Sekhon, S.S.; Cho, S.-J.; Kim, Y.-C.; Kim, Y.-H. Phenotypic and proteomic analysis of positively regulated gellan biosynthesis pathway in Sphingomonas elodea. Anim. Cells Syst. 2017, 21, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, V.M.F.; Reis, A.; Domingues, M.R.M.; Lopes-da-Silva, J.A.; Fialho, A.M.; Moreira, L.M.; Sa-Correia, I.; Coimbra, M.A. Structural analysis of gellans produced by Sphingomonas elodea strains by electrospray tandem mass spectrometry. Carbohydr. Polym. 2009, 77, 10–19. [Google Scholar]
- Lelu-Walter, M.A.; Gautier, F.; Eliášová, K.; Sanchez, L.; Teyssier, C.; Lomenech, A.M.; Le Metté, C.; Hargreaves, C.; Trontin, J.F.; Reeves, C. High gellan gum concentration and secondary somatic embryogenesis: Two key factors to improve somatic embryo development in Pseudotsuga menziesii Mirb. Plant Cell Tiss. Org. 2017, 132, 137–155. [Google Scholar] [CrossRef]
- Moncalean, P.; Garcia-Mendiguren, O.; Novak, O.; Strnad, M.; Goicoa, T.; Ugarte, M.D.; Montalban, I.A. Temperature and water availability during maturation affect the cytokinins and auxins profile of radiata pine somatic embryos. Front. Plant Sci. 2018, 9, 1898. [Google Scholar] [CrossRef]
- Salachna, P.; Mizielińska, M.; Soból, M. Exopolysaccharide gellan gum and derived oligo-gellan enhance growth and antimicrobial activity in Eucomis plants. Polymers 2018, 10, 242. [Google Scholar] [CrossRef]
- Daliakopoulos, I.N.; Tsanis, I.K.; Koutroulis, A.; Kourgialas, N.N.; Varouchakis, A.E.; Karatzas, G.; Ritsema, C.J. The threat of soil salinity: A European scale review. Sci. Total Environ. 2016, 573, 727–739. [Google Scholar] [CrossRef]
- Parvaiz, A.; Satyawati, S. Salt stress and phyto-biochemical responses of plants–A review. Plant Soil Environ. 2008, 54, 89. [Google Scholar] [CrossRef]
- Yadav, S.; Irfan, M.; Ahmad, A.; Hayat, S. Causes of salinity and plant manifestations to salt stress: A review. J. Environ. Biol. 2011, 32, 667. [Google Scholar]
- Niu, X.; Bressan, R.A.; Hasegawa, P.M.; Pardo, J.M. Ion homeostasis in NaCl stress environments. Plant Physiol. 1995, 109, 735. [Google Scholar] [CrossRef] [PubMed]
- Deinlein, U.; Stephan, A.B.; Horie, T.; Luo, W.; Xu, G.; Schroeder, J.I. Plant salt-tolerance mechanisms. Trends Plant Sci. 2014, 19, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Ma, X.; Wan, P.; Liu, L. Plant salt-tolerance mechanism: A review. Biochem. Biophys. Res. Commun. 2018, 495, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Kabar, K. Alleviation of salinity stress by plant growth regulators on seed germination. J. Plant Physiol. 1987, 128, 179–183. [Google Scholar] [CrossRef]
- Karapouloutidou, S.; Gasparatos, D. Effects of biostimulant and organic amendment on soil properties and nutrient status of Lactuca sativa in a calcareous saline-sodic soil. Agriculture 2019, 9, 164. [Google Scholar] [CrossRef]
- Saporta, R.; Bou, C.; Frías, V.; Mulet, J.M. A Method for a fast evaluation of the biostimulant potential of different natural extracts for promoting growth or tolerance against abiotic stress. Agronomy 2019, 9, 143. [Google Scholar] [CrossRef]
- Van Oosten, M.J.; Pepe, O.; De Pascale, S.; Silletti, S.; Maggio, A. The role of biostimulants and bioeffectors as alleviators of abiotic stress in crop plants. Chem. Biol. Technol. Agric. 2017, 4, 5. [Google Scholar] [CrossRef]
- Bulgari, R.; Franzoni, G.; Ferrante, A. Biostimulants application in horticultural crops under abiotic stress conditions. Agronomy 2019, 9, 306. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Lin, X.; Yan, G.; Liu, L.; Zheng, H.; Zhao, B.; Tang, J.; Guo, Y.D. Alginate derived oligosaccharides promote water stress tolerance in cucumber (Cucumis sativus L.). Plant Physiol. Biochem. 2018, 130, 80–88. [Google Scholar] [CrossRef]
- Zou, P.; Lu, X.; Jing, C.; Yuan, Y.; Lu, Y.; Zhang, C.; Li, Y. Low-molecular-weightt polysaccharides from Pyropia yezoensis enhance tolerance of wheat seedlings (Triticum aestivum L.) to salt stress. Front. Plant Sci. 2018, 9, 427. [Google Scholar] [CrossRef]
- González-Pérez, L.; Páez-Watson, T.; Álvarez-Suarez, J.M.; Obando-Rojas, M.C.; Bonifaz-Arcos, E.; Viteri, G.; Cabrera, J.C. Application of exogenous xyloglucan oligosaccharides affects molecular responses to salt stress in Arabidopsis thaliana seedlings. J. Soil Sci. Plant Nutr. 2018, 18, 1187–1205. [Google Scholar] [CrossRef]
- Ahmed, H.M. Ethnomedicinal, phytochemical and pharmacological investigations of Perilla frutescens (L.) Britt. Molecules 2019, 24, 102. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Qiu, J.F.; Ma, L.J.; Hu, Y.J.; Li, P.; Wan, J.B. Phytochemical and phytopharmacological review of Perilla frutescens L. (Labiatae), a traditional edible-medicinal herb in China. Food Chem. Toxicol. 2017, 108, 375–391. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Park, Y.M.; Hong, J. Effect of light intensity on the growth of Perilla frutescens var. acuta. J. Korea Soc. Environ. Restor. Technol. 2004, 7, 73–77. [Google Scholar]
- Yamazaki, M.; Ueda, H. Antiinflammatory and Antiallergic Activity of Perilla Extract. In Perilla: The Genus Perilla; Yu, H.-C., Kosuna, K., Haga, M., Eds.; Harwood Academic Publishers: Amsterdam, The Netherlands, 1997; pp. 47–54. [Google Scholar]
- Yu, H.-C. Introduction. In Perilla: The Genus Perilla; Yu, H.-C., Kosuna, K., Haga, M., Eds.; Harwood Academic Publishers: Amsterdam, The Netherlands, 1997; pp. 1–8. [Google Scholar]
- Ogawa, E.; Hikosaka, S.; Goto, E. Effects of nutrient solution temperature on the concentration of major bioactive compounds in red perilla. J. Agric. Meteorol. 2018, 74, 71–78. [Google Scholar] [CrossRef]
- Fujita, T.; Nakayama, M. Chemical Studies on the Constituents of Perilla frutescens. In Perilla: The Genus Perilla; Yu, H.-C., Kosuna, K., Haga, M., Eds.; Harwood Academic Publishers: Amsterdam, The Netherlands, 1997; pp. 109–128. [Google Scholar]
- Peiretti, P.G. Fatty acid content and chemiacal composition of vegetative parts of perilla (Perilla frutescens L.) after different growth lengths. Res. J. Med. Plant 2011, 5, 72–78. [Google Scholar]
- Asif, M. Phytochemical study of polyphenols in Perilla frutescens as an antioxidant. Avicenna J. Phytomed. 2012, 2, 169–178. [Google Scholar]
- Grbic, N.; Pinker, I.; Böhme, M. The Nutritional Treasure of Leafy Vegetables – Perilla frutescens. In Proceedings of the Conference on International Research on Food Security, Natural Resource Management and Rural Development, and University of Natural Resources and Life Sciences (BOKU), Vienna, Austria, 18–21 September 2016. [Google Scholar]
- Martinetti, L.; Ferrante, A.; Bassoli, A.; Borgonovo, G.; Tosca, A.; Spoleto, P. Characterization of some qualitative traits in different perilla cultivars. Acta Hortic. 2012, 939, 301–308. [Google Scholar] [CrossRef]
- Rouphael, Y.; Kyriacou, M.C.; Carillo, P.; Pizzolongo, F.; Romano, R.; Sifola, M.I. Chemical eustress elicits tailored responses and enhances the functional quality of novel food Perilla frutescens. Molecules 2019, 24, 185. [Google Scholar] [CrossRef]
- Fujiwara, Y.; Kono, M.; Ito, A.; Ito, M. Anthocyanins in perilla plants and dried leaves. Phytochemistry 2018, 147, 158–166. [Google Scholar] [CrossRef]
- Meng, L.; Lozano, Y.; Bombarda, I.; Gaydou, E.M.; Li, B. Polyphenol extraction from eight Perilla frutescens cultivars. C. R. Chim. 2009, 12, 602–611. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, H.; Qiao, S.; Zhang, X.; Liu, P.; Liu, X. Effect of salinity on seed germination, seedling growth, and physiological characteristics of Perilla frutescens. Plant Biosyst. 2012, 146, 245–251. [Google Scholar] [CrossRef]
- Povero, G.; Mejia, J.F.; Di Tommaso, D.; Piaggesi, A.; Warrior, P. A systematic approach to discover and characterize natural plant biostimulants. Front. Plant Sci. 2016, 7, 435. [Google Scholar] [CrossRef] [PubMed]
- Parađiković, N.; Teklić, T.; Zeljković, S.; Lisjak, M.; Špoljarević, M. Biostimulants research in some horticultural plant species—A review. Food Energy Secur. 2019, 8, e00162. [Google Scholar] [CrossRef]
- Kurepin, L.V.; Zaman, M.; Pharis, R.P. Phytohormonal basis for the plant growth promoting action of naturally occurring biostimulators. J. Sci. Food Agric. 2014, 94, 1715–1722. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G. Synergistic biostimulatory action: Designing the next generation of plant biostimulants for sustainable agriculture. Front. Plant Sci. 2018, 9, 1655. [Google Scholar] [CrossRef]
- Yakhin, O.I.; Lubyanov, A.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in plant science: A global perspective. Front. Plant Sci. 2017, 7, 2049. [Google Scholar] [CrossRef]
- Salachna, P.; Grzeszczuk, M.; Meller, E.; Soból, M. Oligo-alginate with low molecular mass improves growth and physiological activity of Eucomis autumnalis under salinity stress. Molecules 2018, 23, 812. [Google Scholar] [CrossRef]
- Ahmed, K.B.M.; Khan, M.M.A.; Siddiqui, H.; Jahan, A. Chitosan and its oligosaccharides, a promising option for sustainable crop production-a review. Carbohydr. Polym. 2019, 17, 115331. [Google Scholar]
- Zhou, Y.; Tang, N.; Huang, L.; Zhao, Y.; Tang, X.; Wang, K. Effects of salt stress on plant growth, antioxidant capacity, glandular trichome density, and volatile exudates of Schizonepeta tenuifolia Briq. Int. J. Mol. Sci. 2018, 19, 252. [Google Scholar] [CrossRef]
- Poljsak, B.; Šuput, D.; Milisav, I. Achieving the balance between ROS and antioxidants: When to use the synthetic antioxidants. Oxid. Med. Cell Longev. 2013, 956792. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef]
- Salachna, P.; Grzeszczuk, M.; Soból, M. Effects of chitooligosaccharide coating combined with selected ionic polymers on the stimulation of Ornithogalum saundersiae growth. Molecules 2017, 22, 1903. [Google Scholar] [CrossRef] [PubMed]
- Shukla, P.S.; Borza, T.; Critchley, A.T.; Prithiviraj, B. Carrageenans from red seaweeds as promoters of growth and elicitors of defense response in plants. Front. Mar. Sci. 2016, 3, 81. [Google Scholar] [CrossRef]
- González, A.; Castro, J.; Vera, J.; Moenne, A. Seaweed oligosaccharides stimulate plant growth by enhancing carbon and nitrogen assimilation, basal metabolism, and cell division. J. Plant Growth Regul. 2013, 32, 443–448. [Google Scholar] [CrossRef]
- Zou, P.; Li, K.; Liu, S.; Xing, R.; Qin, Y.; Yu, H.; Li, P. Effect of chitooligosaccharides with different degrees of acetylation on wheat seedlings under salt stress. Carbohydr. Polym. 2015, 126, 62–69. [Google Scholar] [CrossRef]
- Kang, R.; Helms, R.; Stout, M.J.; Jaber, H.; Chen, Z.; Nakatsu, T. Antimicrobial activity of the volatile constituents of Perilla frutescens and its synergistic effects with polygodial. J. Agric. Food Chew. 1992, 40, 2328–2330. [Google Scholar] [CrossRef]
- Yamamoto, H.; Ogawa, T. Antimicrobial activity of perilla seed polyphenols against oral pathogenic bacteria. Biosci. Biotechnol. Biochem. 2002, 66, 921–924. [Google Scholar] [CrossRef]
- Lee, C.W.; Choi, H.M.; Kim, S.Y.; Lee, J.R.; Kim, H.J.; Jo, C.; Jung, S. Influence of Perilla frutescens var. acuta water extract on the shelf life and physicochemical qualities of cooked beef patties. Korean J. Food Sci. Anim. Resour. 2015, 35, 389–397. [Google Scholar]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Ostrowska, A.; Gawliński, S.; Szczubiałka, Z. Methods for Analyzing and Assessing the Properties of Soil and Plants; Instytut Ochrony Środowiska: Warsaw, Poland, 1991; pp. 1–333. (In Polish) [Google Scholar]
- Anuar, N.; Adnan, A.F.M.; Saat, N.; Aziz, N.; Taha, R.M. Optimization of extraction parameters by using response surface methodology, purification, and identification of anthocyanin pigments in Melastoma malabathricum fruit. Sci. World J. 2013, 2, 810547. [Google Scholar]
- Lee, J.; Durst, R.W.; Wrolstad, R.E. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural color-ants, and wines by the pH differential method: Collaborative study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [PubMed]
- Wojdyło, A.; Oszmiański, J.; Czemerys, R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007, 105, 940–949. [Google Scholar] [CrossRef]
- Kumaran, A.; Karunakaran, R.J. In vitro antioxidant activities of methanol extracts of five Phyllanthus species from India. LWT-Food Sci. Technol. 2007, 40, 344–352. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Chew, K.K.; Khoo, M.Z.; Ng, S.Y.; Thoo, Y.Y.; Wan Aida, W.M.; Ho, C.W. Effect of ethanol concentration, extraction time and extraction temperature on the recovery of phenolic compounds and antioxidant capacity of Orthosiphon stamineus extracts. Int. Food Res. J. 2011, 18, 1427–1435. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |


| Treatment | Plant Height (cm) | Number of Shoots per Plant | Fresh Weight of Above-Ground Part (g) | Fresh Weight of Leaves per Plant (g) | Fresh Weight of Shoots per Plant (g) |
|---|---|---|---|---|---|
| Control | 84.8 ± 4.54 b | 37.5 ± 1.75 a | 305.8 ± 4.60 b | 176.4 ± 3.45 b | 105.5 ± 3.72 b |
| Oligo-gellan | 93.5 ± 1.25 a | 40.1 ± 1.83 a | 328.0 ± 6.68 a | 192.8 ± 9.47 a | 117.1 ± 4.65 a |
| NaCl | 75.3 ± 0.65 c | 33.8 ± 0.99 b | 239.6 ± 6.46 d | 141.0 ± 4.11 c | 75.6 ± 3.97 c |
| Oligo-gellan + NaCl | 85.2 ± 2.00 b | 39.4 ± 0.95 a | 284.2 ± 8.12 c | 191.7 ± 2.59 a | 98.5 ± 4.86 b |
| Treatment | N (% DW) | P (% DW) | K (% DW) | Mg (% DW) | Na (% DW) | K/Na |
|---|---|---|---|---|---|---|
| Control | 2.20 ± 0.13 b | 0.37 ± 0.02 a | 2.63 ± 0.12 b | 0.25 ± 0.02 b | 0.17 ± 0.02 c | 15.7 ± 2.53 a |
| Oligo-gellan | 2.58 ± 0.04 a | 0.37 ± 0.02 a | 3.04 ± 0.13 a | 0.28 ± 0.01 a | 0.23 ± 0.03 c | 13.3 ± 0.74 a |
| NaCl | 1.67 ± 0.12 c | 0.29 ± 0.01 b | 2.14 ± 0.13 c | 0.22 ± 0.01 b | 1.45 ± 0.11 a | 1.48 ± 0.17 b |
| Oligo-gellan + NaCl | 1.94 ± 0.08 b | 0.35 ± 0.01 a | 2.84 ± 0.07 b | 0.30 ± 0.02 a | 1.03 ± 0.08 b | 2.78 ± 0.25 b |
| Treatment | Concentration of Extract (%) | Concentration of Bacterial Cells | |
|---|---|---|---|
| S. aureus (103 CFU/mL) | E. coli (103 CFU/mL) | ||
| Control | 50 25 | 0 166.00 ± 23.30 | 0 42.50 ± 17.70 |
| Oligo-gellan | 50 25 | 0 0 | 0 27.50 ± 4.95 |
| NaCl | 50 25 | 0 0 | 0 12.00 ± 2.83 |
| Oligo-gellan + NaCl | 50 25 | 0 5.20 ± 1.70 | 0 9.00 ± 5.66 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salachna, P.; Grzeszczuk, M.; Meller, E.; Mizielińska, M. Effects of Gellan Oligosaccharide and NaCl Stress on Growth, Photosynthetic Pigments, Mineral Composition, Antioxidant Capacity and Antimicrobial Activity in Red Perilla. Molecules 2019, 24, 3925. https://doi.org/10.3390/molecules24213925
Salachna P, Grzeszczuk M, Meller E, Mizielińska M. Effects of Gellan Oligosaccharide and NaCl Stress on Growth, Photosynthetic Pigments, Mineral Composition, Antioxidant Capacity and Antimicrobial Activity in Red Perilla. Molecules. 2019; 24(21):3925. https://doi.org/10.3390/molecules24213925
Chicago/Turabian StyleSalachna, Piotr, Monika Grzeszczuk, Edward Meller, and Małgorzata Mizielińska. 2019. "Effects of Gellan Oligosaccharide and NaCl Stress on Growth, Photosynthetic Pigments, Mineral Composition, Antioxidant Capacity and Antimicrobial Activity in Red Perilla" Molecules 24, no. 21: 3925. https://doi.org/10.3390/molecules24213925
APA StyleSalachna, P., Grzeszczuk, M., Meller, E., & Mizielińska, M. (2019). Effects of Gellan Oligosaccharide and NaCl Stress on Growth, Photosynthetic Pigments, Mineral Composition, Antioxidant Capacity and Antimicrobial Activity in Red Perilla. Molecules, 24(21), 3925. https://doi.org/10.3390/molecules24213925







