Bicyclo [6.3.0] Undecane Sesquiterpenoids: Structures, Biological Activities, and Syntheses
Abstract
1. Introduction
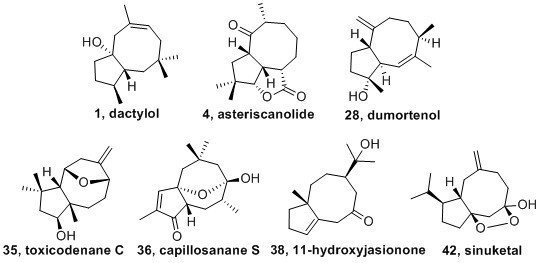
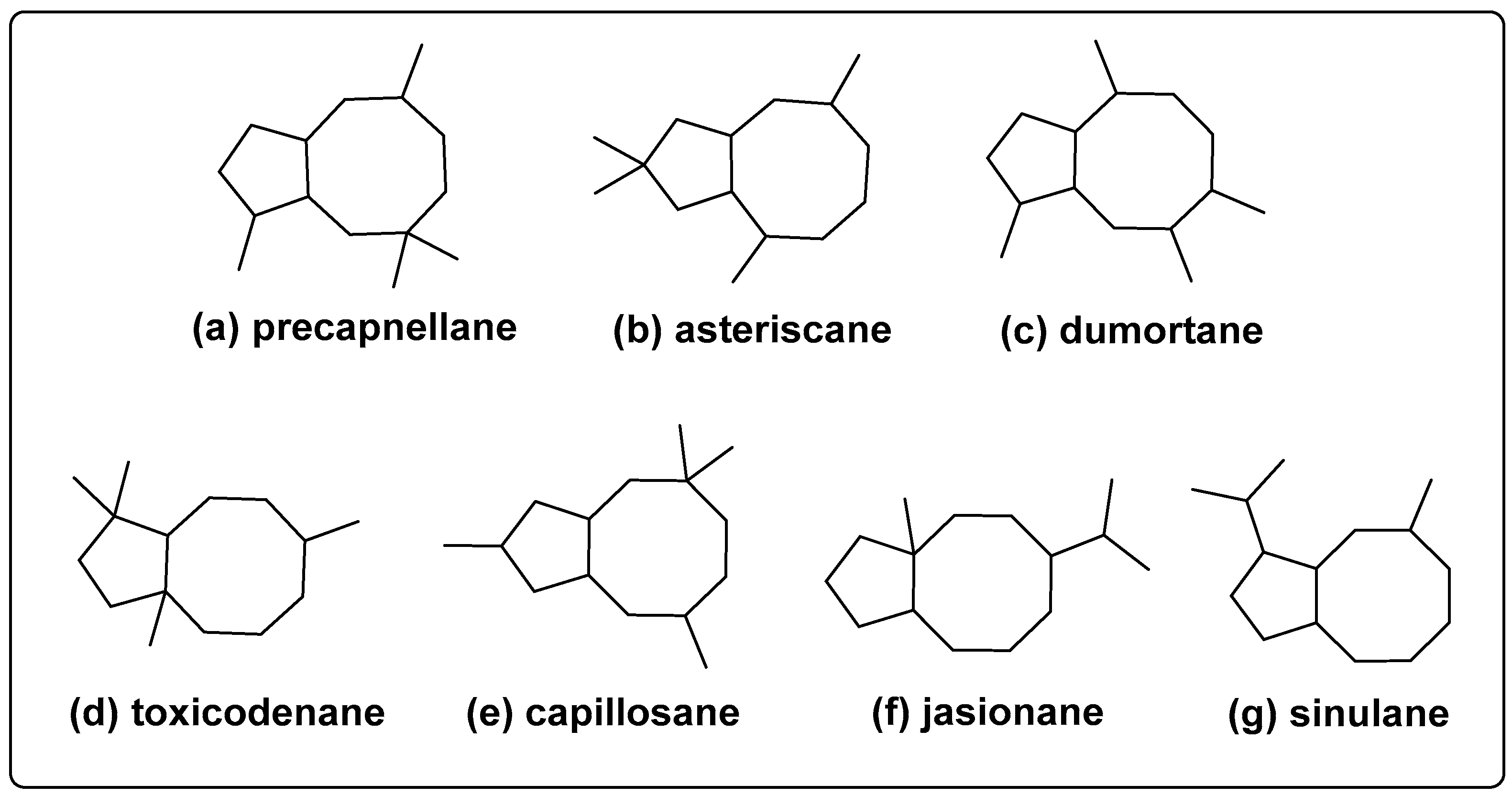
2. Four Methyl Type Bicyclo [6.3.0] Undecane Sesquiterpenoids
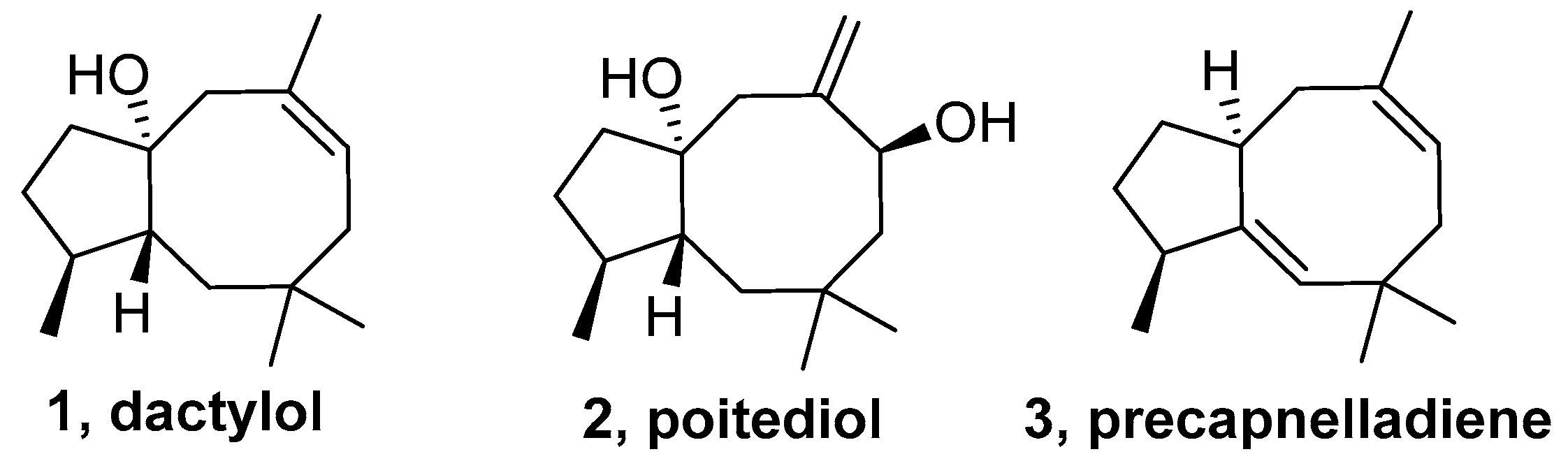
2.1. Precapnellane-Sesquiterpenoid
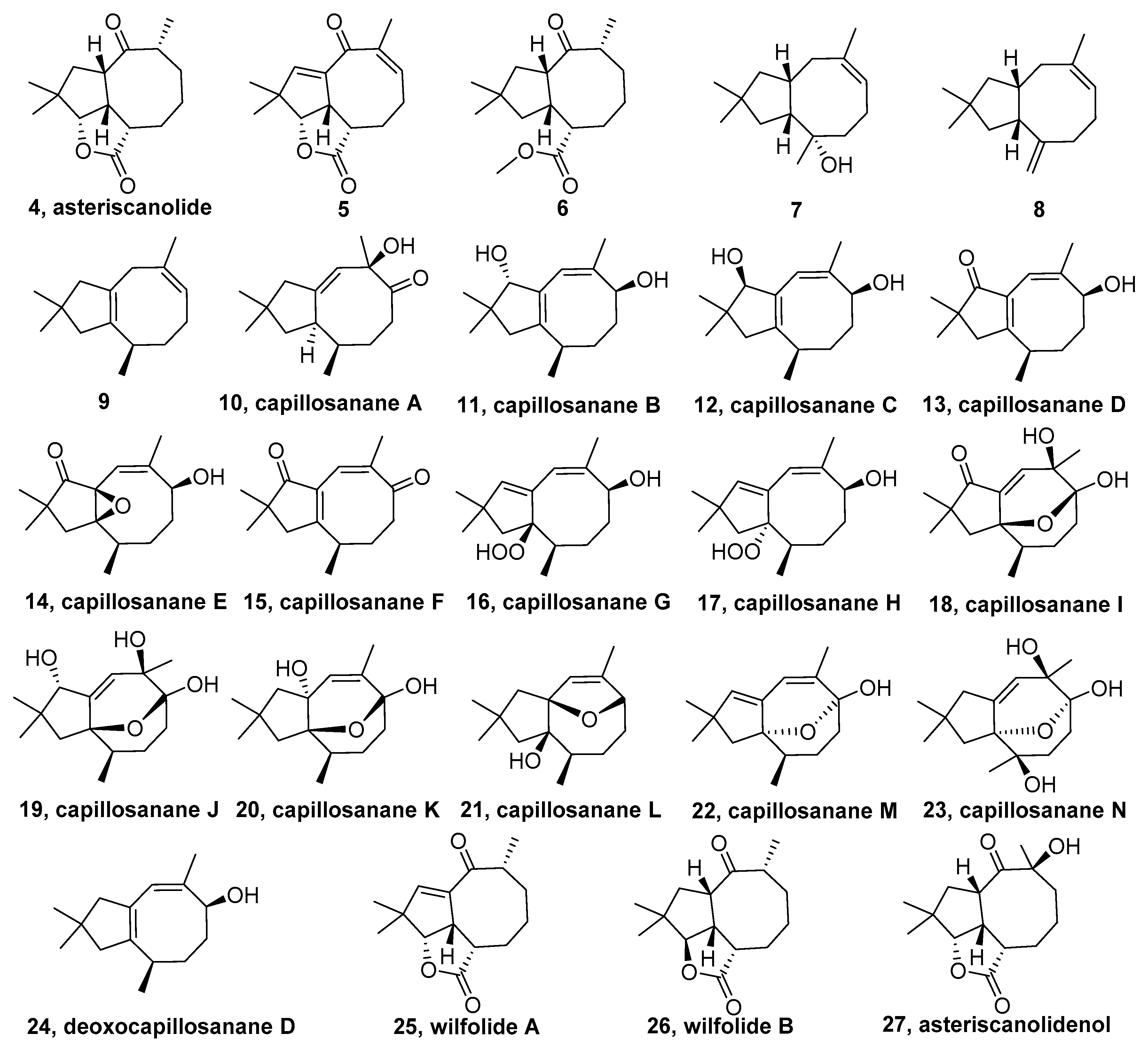
2.2. Asteriscane-Sesquiterpenoid
2.3. Dumortane-Sesquiterpenoid
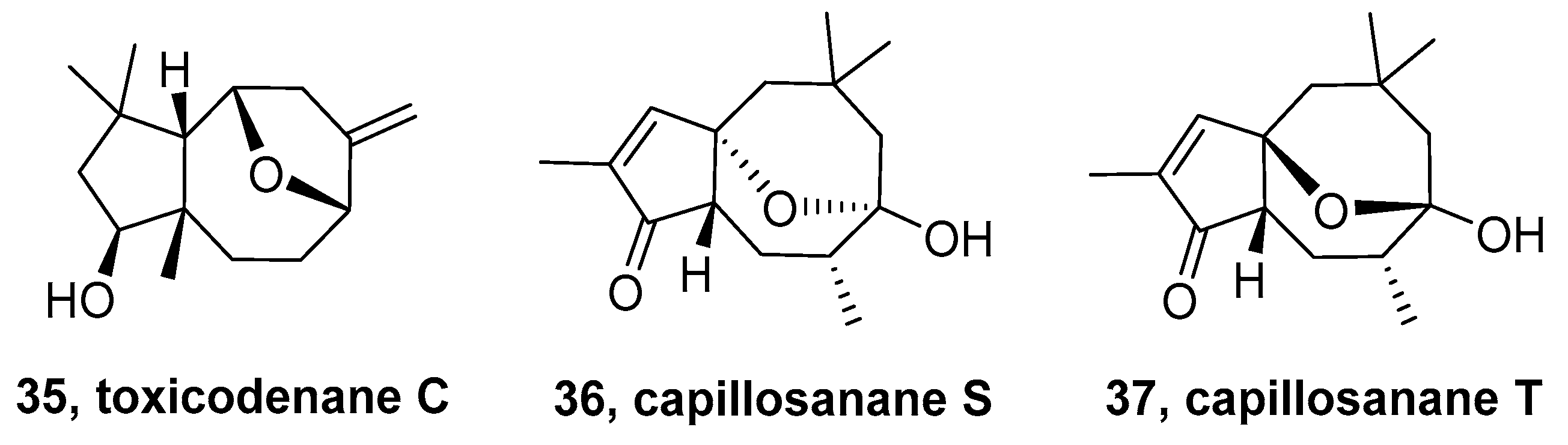
2.4. Toxicodenane-Sesquiterpenoid
2.5. Capillosane-Sesquiterpenoid
3. Isopropyl Type Bicyclo [6.3.0] Undecane Sesquiterpenoids
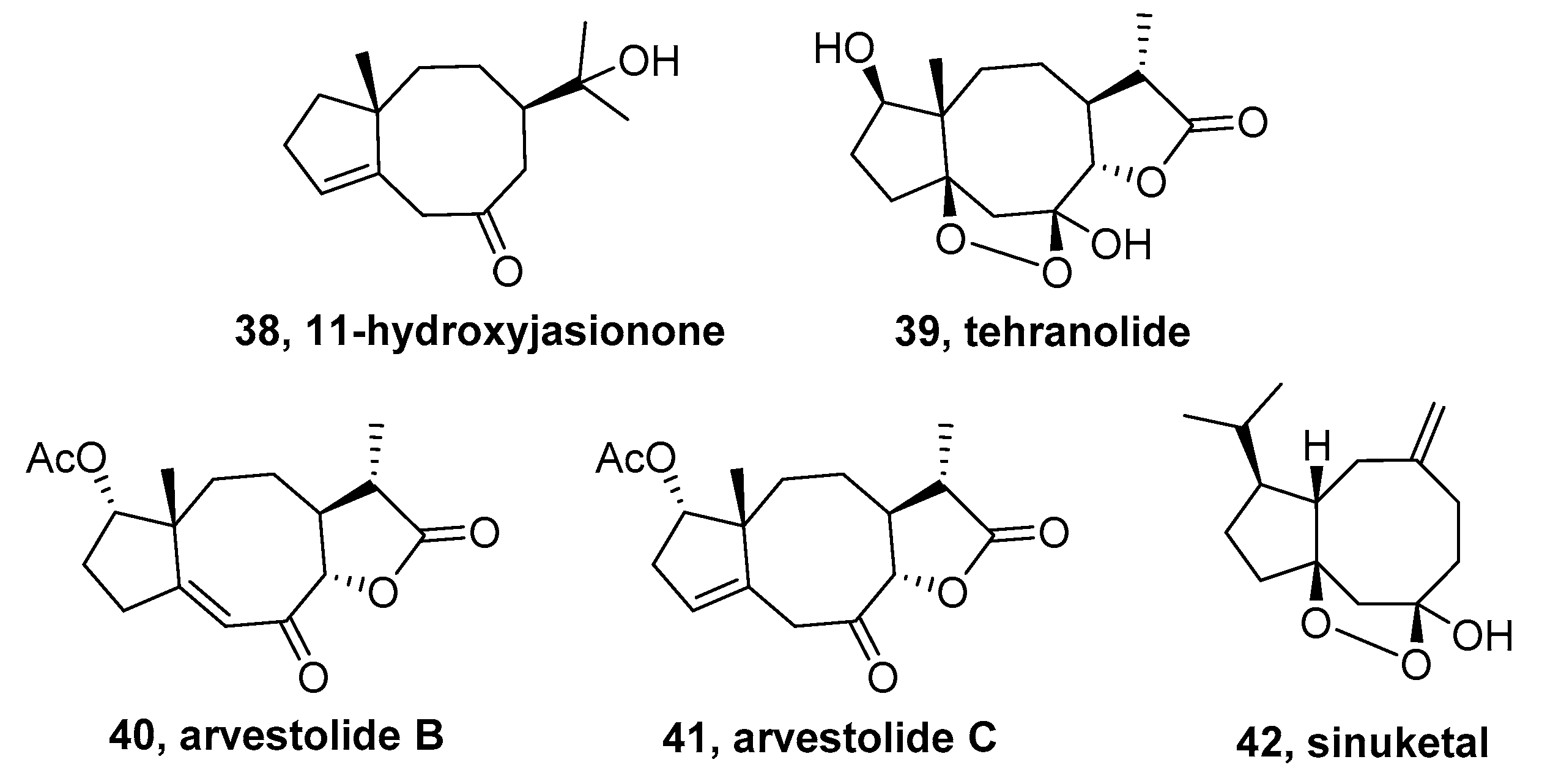
3.1. Jasionane-Sesquiterpenoid
3.2. Sinulane-Sesquiterpenoid
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef]
- Dias, D.A.; Urban, S.; Roessner, U. A historical overview of natural products in drug discovery. Metabolites 2012, 2, 303–336. [Google Scholar] [CrossRef] [PubMed]
- Harvey, A.L. Natural products in drug discovery. Drug Discov. Today 2008, 13, 894–901. [Google Scholar] [CrossRef] [PubMed]
- Mishra, B.B.; Tiwari, V.K. Natural products: An evolving role in future drug discovery. Eur. J. Med. Chem. 2011, 46, 4769–4807. [Google Scholar] [CrossRef] [PubMed]
- Fraga, B.M. Natural sesquiterpenoids. Nat. Prod. Rep. 2013, 30, 1226–1264. [Google Scholar] [CrossRef]
- Fraga, B.M. Natural sesquiterpenoids. Nat. Prod. Rep. 2012, 29, 1334–1366. [Google Scholar] [CrossRef] [PubMed]
- Fraga, B.M. Natural sesquiterpenoids. Nat. Prod. Rep. 2011, 28, 1580–1610. [Google Scholar] [CrossRef] [PubMed]
- Fraga, B.M. Natural sesquiterpenoids. Nat. Prod. Rep. 2010, 27, 1681–1708. [Google Scholar] [CrossRef]
- Schmitz, F.J.; Hollenbeak, K.H.; Vanderah, D.J. Marine natural products: Dactylol, a new sesquiterpene alcohol from a sea hare. Tetrahedron 1978, 34, 2719–2722. [Google Scholar] [CrossRef]
- Gadwood, R.C.; Lett, R.M.; Wissinger, J.E. Total synthesis of (±)-poitediol and (±)-dactylol. J. Am. Chem. Soc. 1986, 108, 6343–6350. [Google Scholar] [CrossRef]
- Paquette, L.A.; Ham, W.H.; Dime, D.S. A formal total synthesis of dactylol. Tetrahedron Lett. 1985, 26, 4983–4986. [Google Scholar] [CrossRef]
- Paquette, L.A.; Ham, W.H. Total synthesis of the marine sesquiterpenes dactylol and africanol. De novo construction of a cyclooctanoid natural product from cycloheptane precursors. J. Am. Chem. Soc. 1987, 109, 3025–3036. [Google Scholar] [CrossRef]
- Feldman, K.S.; Wu, M.J.; Rotella, D.P. Application of an intramolecular tropone-alkene photocyclization to the total synthesis of (±)-dactylol. J. Am. Chem. Soc. 1989, 111, 6457–6458. [Google Scholar] [CrossRef]
- Feldman, K.S.; Wu, M.J.; Rotella, D.P. Total synthesis of (±)-dactylol and related studies. J. Am. Chem. Soc. 1990, 112, 8490–8496. [Google Scholar] [CrossRef]
- Harmata, M.; Rashatasakhon, P. Intramolecular 4+3 cycloadditions. Aspects of stereocontrol in the synthesis of cyclooctanoids. A synthesis of (+)-dactylol. Org. Lett. 2000, 2, 2913–2915. [Google Scholar] [CrossRef]
- Harmata, M. Exploration of fundamental and synthetic aspects of the intramolecular 4+3 cycloaddition reaction. Acc. Chem. Res. 2001, 34, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Fürstner, A.; Langemann, K. A concise total synthesis of dactylol via ring closing metathesis. J. Org. Chem. 1996, 61, 8746–8749. [Google Scholar] [CrossRef]
- Dowling, M.S.; Vanderwal, C.D. Ring-closing metathesis of allylsilanes as a flexible strategy toward cyclic terpenes. Short syntheses of teucladiol, isoteucladiol, poitediol, and dactylol and an attempted synthesis of caryophyllene. J. Org. Chem. 2010, 75, 6908–6922. [Google Scholar] [CrossRef]
- Molander, G.A.; Eastwood, P.R. Total synthesis of (+)-dactylol via a novel [3+5] annulation approach. J. Org. Chem. 1995, 60, 4559–4565. [Google Scholar] [CrossRef]
- Gadwood, R.C.; Lett, R.M.; Wissinger, J.E. Total synthesis of (±)-poitediol and (±)-4-epipoitediol. J. Am. Chem. Soc. 1984, 106, 3869–3870. [Google Scholar] [CrossRef]
- Dowling, M.S.; Vanderwal, C.D. Ring-closing metathesis of allylsilanes/electrophilic desilylation to prepare exo-methylidenecycloalkanes. Short syntheses of teucladial and poitediol. J. Am. Chem. Soc. 2009, 131, 15090–15091. [Google Scholar] [CrossRef]
- Mehta, G.; Murty, A.N. Total synthesis of the marine natural product (±)-precapnelladiene. J. Chem. Soc 1984, 16, 1058–1060. [Google Scholar] [CrossRef]
- Mehta, G.; Murty, A.N. A general stereocontrolled approach to the 5-8 fused ring system. Application to the total synthesis of marine natural product (±)-precapnelladiene. J. Org. Chem. 1987, 52, 2875–2881. [Google Scholar] [CrossRef]
- Kinney, W.A.; Coghlan, M.J.; Paquette, L.A. Claisen rearrangement of 6-alkenyl-2-methylenetetrahydropyrans. A new approach to annulated 4-cyclooctenones and a stereospecific synthesis of precapnelladiene. J. Am. Chem. Soc. 1984, 106, 6868–6870. [Google Scholar] [CrossRef]
- Kinney, W.A.; Coghlan, M.J.; Paquette, L.A. General approach to annulated 4-cyclooctenones by aliphatic Claisen rearrangement. Stereospecific total synthesis of (±)-precapnelladiene. J. Am. Chem. Soc. 1985, 107, 7352–7360. [Google Scholar] [CrossRef]
- Petasis, N.A.; Patane, M.A. A Claisen rearrangement strategy for the three-atom ring expansion of cyclic ketones. A total synthesis of (±)-precapnelladiene. Tetrahedron Lett. 1990, 31, 6799–6802. [Google Scholar] [CrossRef]
- MacDougall, J.M.; Turnbull, P.; Verma, S.K.; Moore, H.W. Synthesis of highly substituted bicyclo [3.2.0] heptanones from 3-homoallylcyclobutenones. A total synthesis of (±)-precapnelladiene. J.Org.Chem. 1997, 62, 3792–3793. [Google Scholar] [CrossRef]
- MacDougall, J.M.; Santora, V.J.; Verma, S.K.; Turnbull, P.; Hernandez, C.R.; Moore, H.W. Cyclobutenone-based syntheses of polyquinanes and bicyclo [6.3.0] undecanes by tandem anionic oxy-Cope reactions. Total synthesis of (±)-precapnelladiene. J. Org. Chem. 1998, 63, 6905–6913. [Google Scholar] [CrossRef]
- Takenaka, Y.; Ito, H.; Iguchi, K. Enantioselective formal synthesis of (+)-precapnelladiene by chiral copper-catalyzed asymmetric [2+2]-cycloaddition reaction. Tetrahedron 2007, 63, 510–513. [Google Scholar] [CrossRef]
- Wender, P.A.; Ihle, N.C.; Correia, C.R.D. Nickel-catalyzed intramolecular [4+4] cycloadditions. 4. enantioselective total synthesis of (+)-asteriscanolide. J. Am. Chem. Soc. 1988, 110, 5904–5906. [Google Scholar] [CrossRef]
- Paquette, L.A.; Tae, J.; Arrington, M.P.; Sadoun, A.H. Enantioselective double Michael addition/cyclization with an oxygen-centered nucleophile as the first step in a concise synthesis of natural (+)-asteriscanolide. J. Am. Chem. Soc. 2000, 122, 2742–2748. [Google Scholar] [CrossRef]
- Krafft, M.E.; Cheung, Y.Y.; Juliano-Capucao, C.A. Synthesis of the first “inside-outside” eight-membered ring via ring-closing metathesis: A total synthesis of (+/-)-asteriscanolide. Synthesis 2000, 7, 1020–1026. [Google Scholar] [CrossRef]
- Krafft, M.E.; Cheung, Y.Y.; Abboud, K.A. Total synthesis of (±)-asteriscanolide. J. Org. Chem. 2001, 66, 7443–7448. [Google Scholar] [CrossRef] [PubMed]
- Limanto, J.; Snapper, M.L. Sequential intramolecular cyclobutadiene cycloaddition, ring-opening metathesis, and Cope rearrangement: Total syntheses of (+)- and (−)-asteriscanolide. J. Am. Chem. Soc. 2000, 122, 8071–8072. [Google Scholar] [CrossRef]
- Liang, Y.; Jiang, X.; Yu, Z.X. Enantioselective total synthesis of (+)-asteriscanolide via Rh(I)-catalyzed [(5+2)+1] reaction. Chem. Commun. 2011, 47, 6659–6661. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yu, Z.X. Rhodium-catalyzed [5+2+1] cycloaddition of the enevinylcyclopropanes and CO: Reaction design, development, application in natural product synthesis, and inspiration for developing new reactions for synthesis of eight-membered carbocycles. Acc. Chem. Res. 2015, 48, 2288–2296. [Google Scholar] [CrossRef] [PubMed]
- Trost, B.M.; Parquette, J.R. A TMM cycloaddition strategy to the bicyclo [6.3.0] undecyl ring system. A total synthesis of 11-hydroxyjasionone. J. Org. Chem. 1994, 59, 7568–7569. [Google Scholar] [CrossRef]
- Fenical, W.; Schulte, G.R.; Finer, J.; Clardy, J. Poitediol, a new nonisoprenoid sesquiterpene diol from the marine alga Laurencia poitei. J. Org. Chem. 1978, 43, 3628–3630. [Google Scholar] [CrossRef]
- Ayanoglu, E.; Gebreyesus, T.; Beechan, C.M.; Djerassi, C. Terpenoids—LXXVI: Precapnelladiene, a possible biosynthetic precursor of the capnellane skeleton. Tetrahedron 1979, 35, 1035–1039. [Google Scholar] [CrossRef]
- San Feliciano, A.; Barrero, A.F.; Medarde, M.; del Corral, J.M.; Aramburu, A. Asteriscanolide. A sesquiterpene lactone with a new natural skeleton. Tetrahedron Lett. 1985, 26, 2369–2372. [Google Scholar] [CrossRef]
- Eldahmy, S.; Jakupovic, J.; Bohlmann, F.; Sagn, T.M. New humulene derivatives from Asteriscus graveolens. Tetrahedron Lett. 1985, 41, 309–316. [Google Scholar] [CrossRef]
- Catalán, C.A.N.; De Lampasona, M.E.P.; Cerda-Garcia-Rojas, C.M.; Joseph-Nathan, P. Trace constituents of Lippia integrifolia. J. Nat. Prod. 1995, 58, 1713–1717. [Google Scholar] [CrossRef]
- Fricke, C.; Hardt, I.H.; König, W.A.; Joulain, D.; Zygadlo, J.A.; Guzmàn, C.A. Sesquiterpenes from Lippia integrifolia essential oil. J. Nat. Prod. 1999, 62, 694–696. [Google Scholar] [CrossRef]
- Mao, S.C.; Gavagnin, M.; Mollo, E.; Guo, Y.W. A new rare asteriscane sesquiterpene and other related derivatives from the Hainan aeolid nudibranch Phyllodesmium magnum. Biochem. Syst. Ecol. 2011, 39, 408–411. [Google Scholar] [CrossRef]
- Chen, D.W.; Chen, W.; Liu, D.; van Ofwegen, L.; Proksch, P.; Lin, W.H. Asteriscane-type sesquiterpenoids from the soft coral Sinularia capillosa. J. Nat. Prod. 2013, 76, 1753–1763. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.P.; Cheng, S.M.; Fu, W.T.; Zhao, M.; Li, X.B.; Cai, Y.P.; Dong, J.Y.; Huang, K.X.; Gustafson, K.R.; Yan, P.C. Structurally diverse metabolites from the soft coral Sinularia verruca collected in the South China Sea. J. Nat. Prod. 2016, 79, 1124–1131. [Google Scholar] [CrossRef] [PubMed]
- Li, J.L.; Fu, Y.; Zhang, H.Y.; Zhao, W.M. Two new humulanolides from the roots of Cynanchum wilfordii. Tetrahedron Lett. 2015, 56, 6503–6505. [Google Scholar] [CrossRef]
- Triana, J.; Eiroa, J.L.; Morales, M.; Perez, F.J.; Brouard, I.; Quintana, J.; Ruiz-Estévez, M.; Estévez, F.; León, F. Sesquiterpenoids isolated from two species of the Asteriscus alliance. J. Nat. Prod. 2016, 79, 1292–1297. [Google Scholar] [CrossRef]
- Toyota, M.; Bardón, A.; Kamiya, N.; Takaoka, S.; Asakawa, Y. Dumortenols, novel skeletal sesquiterpenoids from the Argentinian liverwort Dumortiera hirsuta. Chem. Pharm. Bull. 1997, 45, 2119–2121. [Google Scholar] [CrossRef]
- Bardón, A.; Kamiya, N.; Toyota, M.; Asakawa, Y. A 7-nordumortenone and other dumortane derivatives from the Argentine liverwort Dumortiera hirsuta. Phytochemistry 1999, 51, 281–287. [Google Scholar] [CrossRef]
- Cheng, S.Y.; Huang, K.J.; Wang, S.K.; Wen, Z.H.; Chen, P.W.; Duh, C.Y. Antiviral and anti-inflammatory metabolites from the soft coral Sinularia capillosa. J. Nat. Prod. 2010, 73, 771–775. [Google Scholar] [CrossRef]
- Chen, D.W.; Cheng, W.; Liu, D.; van Ofwegen, L.; Proksch, P.; Lin, W.H. Capillosananes S–Z, new sesquiterpenoids from the soft coral Sinularia capillosa. Tetrahedron Lett. 2014, 55, 3077–3082. [Google Scholar] [CrossRef]
- Lu, C.H.; Liu, S.S.; Wang, J.Y.; Wang, M.Z.; Shen, Y.M. Characterization of eight new secondary metabolites from the mutant strain G-444 of Tubercularia sp. TF5. Helv. Chim. Acta. 2014, 97, 334–344. [Google Scholar] [CrossRef]
- He, J.B.; Luo, J.; Zhang, L.; Yan, Y.M.; Cheng, Y.X. Sesquiterpenoids with new carbon skeletons from the resin of Toxicodendron vernicifluum as new types of extracellular matrix inhibitors. Org. Lett. 2013, 15, 3602–3605. [Google Scholar] [CrossRef] [PubMed]
- Dewick, P.M. Medicinal Natural Products: A Biosynthetic Approach, 3rd ed.; John Wiley and Sons: West Sussex, UK, 2002; pp. 14–16, 210–217. [Google Scholar] [CrossRef]
- Qin, G.F.; Tang, X.L.; Sun, Y.T.; Luo, X.C.; Zhang, J.; van Ofwegen, L.; Sung, P.J.; Li, P.L.; Li, G.Q. Terpenoids from the soft coral Sinularia sp. collected in Yongxing Island. Mar. Drugs 2018, 16, 127. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.A.; Jakupovic, J.; Eid, F.; Ali, A.A. 11-Hydroxyjasionone, a new sesquiterpene type from Jasonia montana. Phytochemistry 1988, 27, 3875–3877. [Google Scholar] [CrossRef]
- Rustaiyan, A.; Sigari, H.; Jakupovic, J.; Grenz, M. A sesquiterpene lactone from Artemisia diffusa. Phytochemistry 1989, 28, 2723–2725. [Google Scholar] [CrossRef]
- Noori, S.; Taghikhani, M.; Hassan, Z.M.; Allameh, A.; Mostafaei, A. Tehranolide could shift the immune response towards Th1 and modulate the intra-tumor infiltrated T regulatory cells. Iran. J. Immunol. 2009, 6, 216–224. [Google Scholar]
- Noori, S.; Taghikhani, M.; Hassan, Z.M.; Allameha, A.; Mostafaei, A. Tehranolide molecule modulates the immune response, reduce regulatory T cell and inhibits tumor growth in vivo. Mol. Immunol. 2010, 47, 1579–1584. [Google Scholar] [CrossRef]
- Noori, S.; Hassan, Z.M. Tehranolide inhibits proliferation of MCF-7 human breast cancer cells by inducing G0/G1 arrest and apoptosis. Free Radic. Bio. Med. 2012, 52, 1987–1999. [Google Scholar] [CrossRef]
- Noori, S.; Hassan, Z.M. Tehranolide inhibits cell proliferation via calmodulin inhibition, PDE, and PKA activation. Tumour Biol. 2014, 35, 257–264. [Google Scholar] [CrossRef][Green Version]
- Taherkhani, M. Mutagenic, anti-mutagenic and cytotoxic activities of artediffusin (tehranolide), in vitro, extracted from Artemisia diffusa. Iran. J. Toxicol. 2015, 9, 1316–1321. [Google Scholar]
- Rustaiyan, A.; Nahrevanian, H.; Kazemi, M. Isolation of artediffusin (tehranolide) as a new antimalarial agent. Asian J. Chem. 2011, 23, 4810–4814. [Google Scholar]
- Rustaiyan, A.; Nahrevanian, H.; Zamani, Z.; Taherkhani, M.; Iravani, A. An investigation on anti-malarial effects of tehranolide isolated from Artemisia diffusa against human malaria parasite, Plasmodium falciparum in vitro. Res. J. Parasitol. 2015, 10, 73–78. [Google Scholar] [CrossRef]
- Rustaiyan, A.; Nahrevanian, H.; Kazemi, M. A new antimalarial agent; effect of extracts of Artemisia diffusa against Plasmodium berghei. Pharm. Mag. 2009, 4, 1–7. [Google Scholar] [CrossRef]
- Taherkhani, M.; Rustaiyan, A.; Nahrevanian, H. Effect of extracts of Artemisia diffusa against Plasmodium berghei. Asian J. Chem. 2012, 24, 1591–1595. [Google Scholar]
- Tian, S.H.; Chai, X.Y.; Zan, K.; Zeng, K.W.; Guo, X.Y.; Jiang, Y.; Tu, P.F. Arvestolides A–C, new rare sesquiterpenes from the aerial parts of Artemisia vestita. Tetrahedron Lett. 2013, 54, 5035–5038. [Google Scholar] [CrossRef]
Sample Availability: Not available. |


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, G.-F.; Liang, H.-B.; Liu, W.-X.; Zhu, F.; Li, P.-L.; Li, G.-Q.; Yao, J.-C. Bicyclo [6.3.0] Undecane Sesquiterpenoids: Structures, Biological Activities, and Syntheses. Molecules 2019, 24, 3912. https://doi.org/10.3390/molecules24213912
Qin G-F, Liang H-B, Liu W-X, Zhu F, Li P-L, Li G-Q, Yao J-C. Bicyclo [6.3.0] Undecane Sesquiterpenoids: Structures, Biological Activities, and Syntheses. Molecules. 2019; 24(21):3912. https://doi.org/10.3390/molecules24213912
Chicago/Turabian StyleQin, Guo-Fei, Hong-Bao Liang, Wen-Xiu Liu, Feng Zhu, Ping-Lin Li, Guo-Qiang Li, and Jing-Chun Yao. 2019. "Bicyclo [6.3.0] Undecane Sesquiterpenoids: Structures, Biological Activities, and Syntheses" Molecules 24, no. 21: 3912. https://doi.org/10.3390/molecules24213912
APA StyleQin, G.-F., Liang, H.-B., Liu, W.-X., Zhu, F., Li, P.-L., Li, G.-Q., & Yao, J.-C. (2019). Bicyclo [6.3.0] Undecane Sesquiterpenoids: Structures, Biological Activities, and Syntheses. Molecules, 24(21), 3912. https://doi.org/10.3390/molecules24213912