Puccinellia maritima, Spartina maritime, and Spartina patens Halophytic Grasses: Characterization of Polyphenolic and Chlorophyll Profiles and Evaluation of Their Biological Activities
Abstract
1. Introduction
2. Results and Discussion
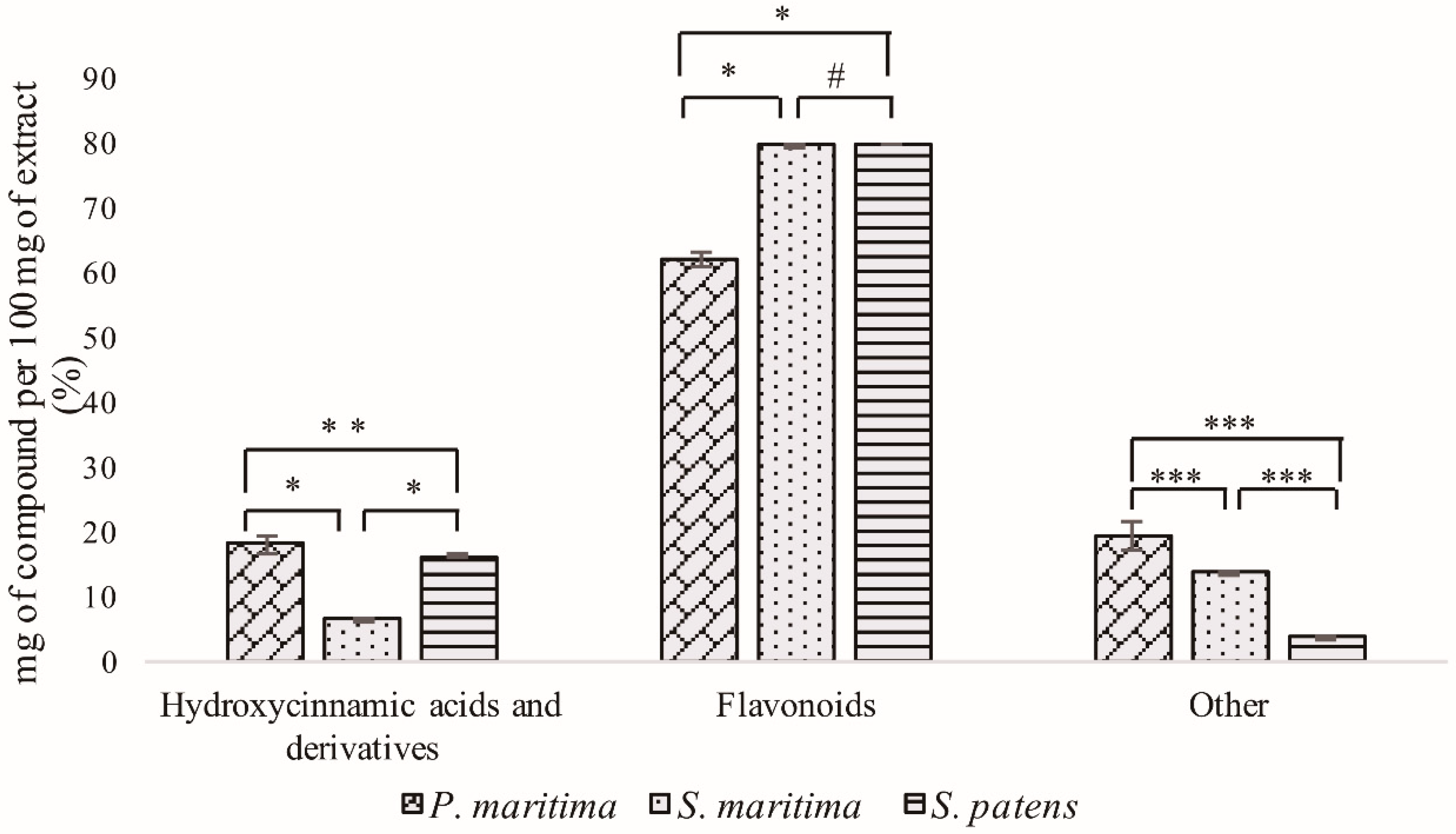
2.1. Polyphenolic Rich Extracts UHPLC-DAD-ESI/MSn Analysis
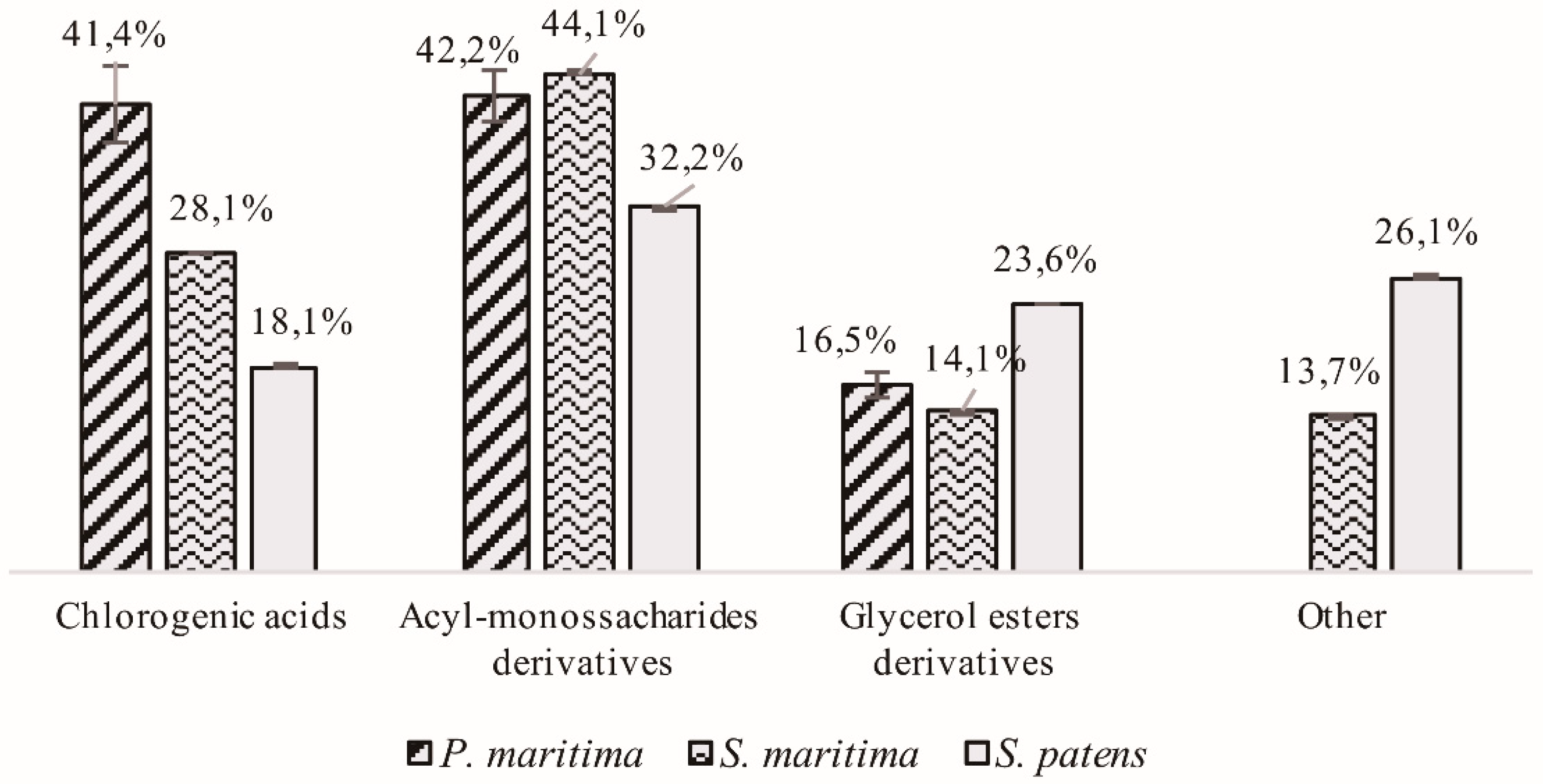
2.1.1. Hydroxycinnamic Acid Derivatives
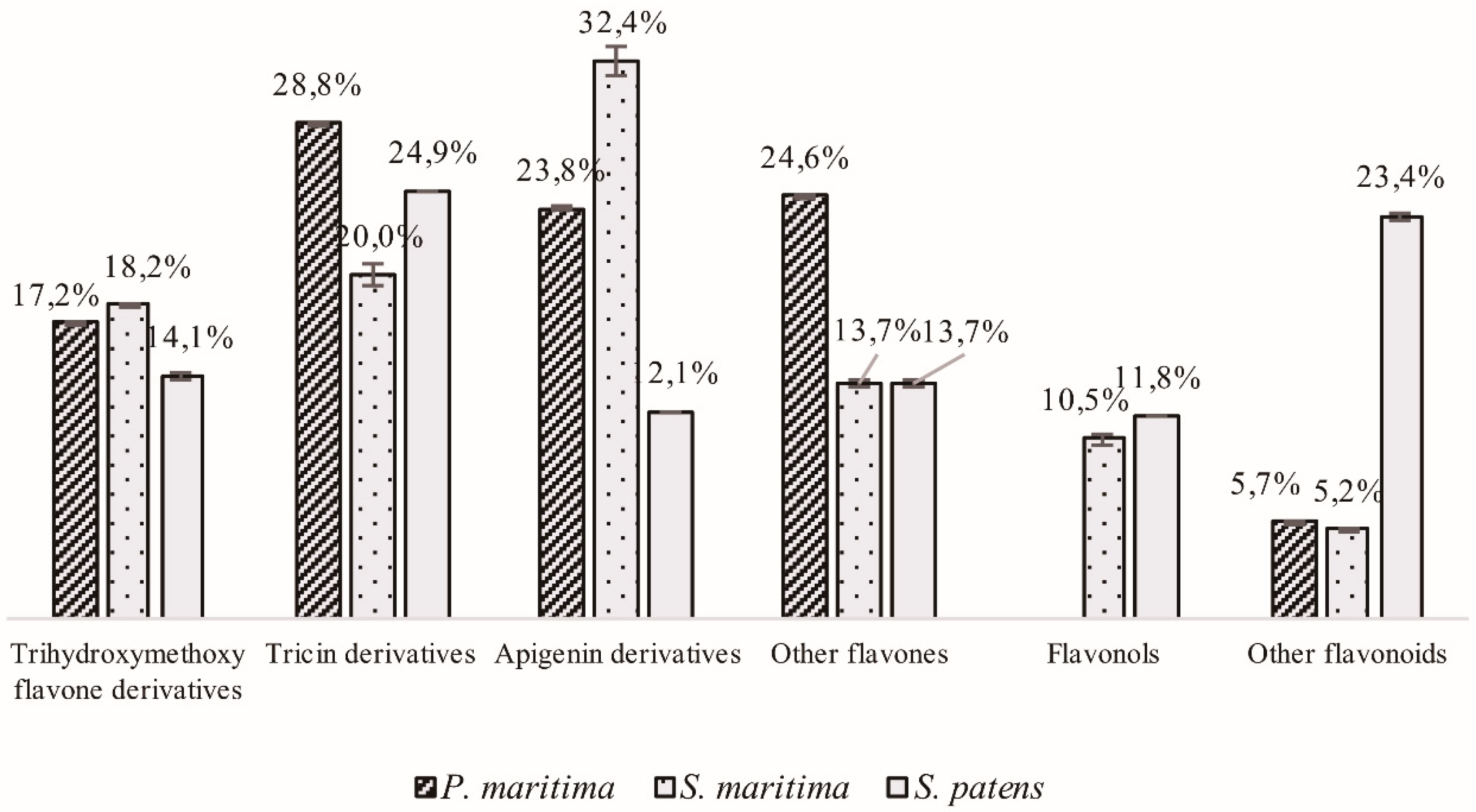
2.1.2. Flavonoid Derivatives
2.1.3. Other Compounds
2.2. Chlorophyll-rich Extracts UHPLC-DAD-ESI/MSn Analysis
2.3. Evaluation of Antioxidant Activity of the Polyphenolic-Rich Extracts
2.4. Inhibition of Acetylcholinesterase by the Polyphenolic Rich Extracts
2.5. Photophysical Properties of the Chlorophyll-rich Extracts
2.5.1. Optical Properties and Photostability
2.5.2. Singlet Oxygen Generation
2.6. Antibacterial and Antifungal Activity Assays
3. Materials and Methods
3.1. Standards and Reagents
3.2. Plant Collection
3.3. Extracts Preparation
3.4. UHPLC-DAD-ESI/MSn Analysis
3.5. Identification and Quantification of the Secondary Metabolites
3.6. Evaluation of Antioxidant Activity of the Polyphenolic Rich Extracts
3.6.1. DPPH Radical Scavenging Assay
3.6.2. ABTS radical Scavenging Assay
3.6.3. Ferric Reducing Antioxidant Power (FRAP) Assay
3.6.4. Inhibition of Acetylcholinesterase by the Polyphenolic Extracts
3.7. Photophysical Properties of the Chlorophyll-Rich Extracts
3.7.1. Photostability
3.7.2. Singlet Oxygen Generation
3.8. Antibacterial and Antifungal Activity Assays
3.8.1. Microbial Strains and Culture Conditions
3.8.2. Dilution Agar Assay
3.9. Statistics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jachak, S.; Saklani, A. Challenges and opportunities in drug discovery from plant. Curr. Sci. 2007, 92, 1251–1257. [Google Scholar]
- Dias, D.A.; Urban, S.; Roessner, U. A historical overview of natural products in drug discovery. Metabolites 2012, 2, 303–336. [Google Scholar] [CrossRef] [PubMed]
- Petrovska, B.B. Historical review of medicinal plants’ usage. Pharmacog. Rev. 2012, 6, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Rahmouni, N.; Pinto, D.C.G.A.; Beghidja, N.; Benayache, S.; Silva, A.M.S. Scabiosa stellata L. phenolic content clarifies its antioxidant activity. Molecules 2018, 23, 1285. [Google Scholar] [CrossRef] [PubMed]
- Diogo, P.; Mota, M.; Fernandes, C.; Sequeira, D.; Palma, P.; Caramelo, F.; Neves, M.G.P.M.S.; Faustino, M.A.F.; Gonçalves, T.; Santos, J.M. Is the chlorophyll derivative Zn(II)e6Me a good photosensitizer to be used in root canal disinfection? Photodiagn. Photodyn. Ther. 2018, 22, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Solymosi, K.; Mysliwa-Kurdziel, B. Chlorophylls and their derivatives used in food industry and medicine. Mini-Rev. Med. Chem. 2017, 17, 1194–1222. [Google Scholar] [CrossRef]
- Ksouri, R.; Ksouri, W.M.; Jallali, I.; Debez, A.; Magné, C.; Hiroko, I.; Abdelly, C. Medicinal halophytes: Potent source of health promoting biomolecules with medicinal, nutraceutical and food applications. Crit. Rev. Biotechnol. 2012, 32, 289–326. [Google Scholar] [CrossRef]
- Barreira, L.; Resek, E.; Rodrigues, M.J.; Rocha, M.I.; Pereira, H.; Bandarra, N.; Silva, M.M.; Varela, J.; Custódio, L. Halophytes: Gourmet food with nutritional health benefits? J. Food Comp. Anal. 2017, 59, 35–42. [Google Scholar] [CrossRef]
- Ventura, Y.; Eshel, A.; Pasternak, D.; Sagi, M. The development of halophyte-based agriculture: Past and present. Ann. Bot. 2015, 115, 529–540. [Google Scholar] [CrossRef]
- Lu, X.; Chen, Q.; Cui, X.; Abozeid, A.; Liu, Y.; Liu, J.; Tang, Z. Comparative metabolomics of two saline-alkali tolerant plants Suaeda glauca and Puccinellia tenuiflora based on GC-MS platform. Nat. Prod. Res. 2019. [Google Scholar] [CrossRef]
- Halophytes (eHALOPH -Halophytes Database). Available online: https://www.sussex.ac.uk/affiliates/halophytes/ (accessed on 2 October 2019).
- Faustino, M.V.; Pinto, D.C.G.A.; Gonçalves, M.J.; Salgueiro, L.; Silveira, P.; Silva, A.M.S. Calendula L. species polyphenolic profile and in vitro antifungal activity. J. Func. Foods 2018, 45, 254–267. [Google Scholar] [CrossRef]
- Seca, A.M.L.; Gouveia, V.L.M.; Barreto, M.C.; Silva, A.M.S.; Pinto, D.C.G.A. Comparative study by GC-MS and chemometrics on the chemical and nutritional profile of Fucus spiralis L. juvenile and mature life-cycle phases. J. App. Phycol. 2018, 30, 2539–2548. [Google Scholar] [CrossRef]
- Clifford, M.N.; Johnston, K.L.; Knight, S.; Kuhnert, N. Hierarchical scheme for LC-MSn identification of chlorogenic acids. J. Agric. Food Chem. 2003, 51, 2900–2911. [Google Scholar] [CrossRef] [PubMed]
- Clifford, M.N.; Knight, S.; Kuhnert, N. Discriminating between the six isomers of dicaffeoylquinic acid by LC-MS(n). J. Agric. Food Chem. 2005, 53, 3821–3832. [Google Scholar] [CrossRef] [PubMed]
- Willems, J.L.; Khamis, M.M.; Saeid, W.M.; Purves, R.W.; Katselis, G.; Low, N.H.; El-Aneed, A. Analysis of a series of chlorogenic acid isomers using differential ion mobility and tandem mass spectrometry. Anal. Chim. Acta 2016, 933, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Kuhnert, N.; Jaiswal, R.; Matei, M.F.; Sovdat, T.; Deshpande, S. How to distinguish between feruloyl quinic acids and isoferuloyl quinic acids by liquid chromatography/tandem mass spectrometry: Distinguishing between feruloyl quinic and isoferuloyl quinic acids. Rap. Commun. Mass Spectrom. 2010, 24, 1575–1582. [Google Scholar] [CrossRef] [PubMed]
- Barros, L.; Dueñas, M.; Pinela, J.; Carvalho, A.M.; Buelga, C.S.; Ferreira, I.C.F.R. Characterization and quantification of phenolic compounds in four tomato (Lycopersicon esculentum L.) farmers’ varieties in Northeastern Portugal homegardens. Plant Foods Hum. Nutr. 2012, 67, 229–234. [Google Scholar] [CrossRef]
- Mocan, A.; Vlase, L.; Leti, A.; Arsene, I.; Vodnar, D.; Bischin, C.; Silaghi-Dumitrescu, R.; Cri, G. HPLC/MS analysis of caffeic and chlorogenic acids from three romanian Veronica species and their antioxidant and antimicrobial properties. Farmacia 2015, 63, 890–896. [Google Scholar]
- Falcão, S.I.; Vale, N.; Gomes, P.; Domingues, M.R.M.; Freire, C.; Cardoso, S.M.; Vilas-Boas, M. Phenolic profiling of Portuguese propolis by LC-MS spectrometry: Uncommon propolis rich in flavonoid glycosides. Phytochem. Anal. 2013, 24, 309–318. [Google Scholar] [CrossRef]
- Ma, C.; Xiao, S.; Li, Z.; Wang, W.; Du, L. Characterization of active phenolic components in the ethanolic extract of Ananas comosus L. leaves using high-performance liquid chromatography with diode array detection and tandem mass spectrometry. J. Chromatogr. A 2007, 1165, 39–44. [Google Scholar] [CrossRef]
- Adetunji, A.I.; Duodu, K.G.; Taylor, J.R.N. Inactivation of tannins in milled sorghum grain through steeping in dilute NaOH solution. Food Chem. 2015, 175, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Johnson, S.K.; Bornman, J.F.; Bennett, S.J.; Clarke, M.W.; Singh, V.; Fang, Z. Growth temperature and genotype both play important roles in sorghum grain phenolic composition. Sci. Rep. 2016, 6, 21835. [Google Scholar] [CrossRef] [PubMed]
- Flores, G.; Dastmalchi, K.; Wu, S.B.; Whalen, K.; Dabo, A.J.; Reynertson, K.A.; Foronjy, J.A.; Kennelly, E.J. Phenolic-rich extract from the Costa Rican guava (Psidium friedrichsthalianum) pulp with antioxidant and anti-inflammatory activity. Food Chem. 2013, 141, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Nakabayashi, R.; Okazaki, Y.; Mori, T.; Takamatsu, S.; Kitanaka, S.; Kikuchi, J.; Saito, K. Toward better annotation in plant metabolomics: Isolation and structure elucidation of 36 specialized metabolites from Oryza sativa (rice) by using MS/MS and NMR analyses. Metabolomics 2014, 10, 543–555. [Google Scholar] [CrossRef]
- Kang, J.; Price, W.E.; Ashton, J.; Tapsell, L.C.; Johnson, S. Identification and characterization of phenolic compounds in hydromethanolic extracts of sorghum wholegrains by LC-ESI-MSn. Food Chem. 2016, 211, 215–226. [Google Scholar] [CrossRef]
- Stintzing, F.C.; Kammerer, D.; Schieber, A.; Adama, H.; Nacoulma, O.G.; Carle, R. Betacyanins and phenolic compounds from Amaranthus spinosus L. and Boerhavia erecta L. Z. Nat. C 2004, 59, 1–8. [Google Scholar] [CrossRef]
- Kumar, B.R. Application of HPLC and ESI-MS techniques in the analysis of phenolic acids and flavonoids from green leafy vegetables (GLVs). J. Pharm. Anal. 2017, 7, 349–364. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, Q.; Wang, X.; Yang, J.; Wang, Q. Qualitative analysis and simultaneous quantification of phenolic compounds in the aerial parts of Salvia miltiorrhiza by HPLC-DAD and ESI/MS(n). Phytochem. Anal. 2011, 22, 247–257. [Google Scholar] [CrossRef]
- Ruan, M.; Li, Y.; Li, X.; Luo, J.; Kong, L. Qualitative and quantitative analysis of the major constituents in Chinese medicinal preparation Guan-Xin-Ning injection by HPLC-DAD-ESI-MS(n). J. Pharm. Biomed. Anal. 2012, 59, 184–189. [Google Scholar] [CrossRef]
- Barros, L.; Dueñas, M.; Dias, M.I.; Sousa, M.J.; Santos-Buelga, C.; Ferreira, I.C.F.R. Phenolic profiles of cultivated, in vitro cultured and commercial samples of Melissa officinalis L. infusions. Food Chem. 2013, 136, 1–8. [Google Scholar] [CrossRef]
- Barros, L.; Dueñas, M.; Ferreira, I.C.F.R.; Carvalho, A.M.; Santos-Buelga, C. Use of HPLC–DAD–ESI/MS to profile phenolic compounds in edible wild greens from Portugal. Food Chem. 2011, 127, 169–173. [Google Scholar] [CrossRef]
- Said, R.B.; Ahmad, A.I.; Mahalel, U.A.; Al-Ayed, A.S.; Kowalczyk, M.; Moldoch, J.; Oleszek, W.; Stochmal, A. Tentative characterization of polyphenolic compounds in the male flowers of Phoenix dactylifera by liquid chromatography coupled with mass spectrometry and DFT. Int. J. Molec. Sci. 2017, 18, 512. [Google Scholar] [CrossRef] [PubMed]
- Owen, B.C.; Haupert, L.J.; Jarrell, T.M.; Marcum, C.L.; Parsell, T.H.; Abu-Omar, M.M.; Bozell, J.J.; Black, S.K.; Kenttämaa, H.I. High-performance liquid chromatography/high-resolution multiple stage tandem mass spectrometry using negative-ion-mode hydroxide-doped electrospray ionization for the characterization of lignin degradation products. Anal. Chem. 2012, 84, 6000–6007. [Google Scholar] [CrossRef] [PubMed]
- Lam, P.Y.; Zhu, F.Y.; Chan, W.L.; Liu, H.; Lo, C. Cytochrome P450 93G1 is a flavone synthase II that channels flavanones to the biosynthesis of tricin O-linked conjugates in rice. Plant Physiol. 2014, 165, 1315–1327. [Google Scholar] [CrossRef] [PubMed]
- Vinholes, J.; Silva, B.M.; Silva, L.R. Hydroxycinnamic acids (HCAS): Structure, biological properties and health effects. In Advances in Medicine and Biology; Nova Biomedical: Waltham, MA, USA, 2015; Volume 88, Chapter 8. [Google Scholar]
- El-Seedi, H.R.; Taher, E.A.; Sheikh, B.Y.; Anjum, S.; Saeed, A.; AlAjmi, M.F.; Moustafa, M.S.; Al-Mousawi, S.M.; Farag, M.A.; Hegazy, M.-E.F.; et al. Hydroxycinnamic acids: Natural sources, biosynthesis, possible biological activities, and roles in Islamic medicine. In Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2017; Volume 55, pp. 269–292. [Google Scholar]
- Rocha, O.P.; Felício, R.D.; Rodrigues, A.H.B.; Ambrósio, D.L.; Cicarelli, R.M.B.; Albuquerque, S.D.; Young, M.C.M.; Yokoya, N.S.; Debonsi, H.M. Chemical profile and biological potential of non-polar fractions from Centroceras clavulatum (C. Agardh) Montagne (Ceramiales, Rhodophyta). Molecules 2011, 16, 7105–7114. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, J.; Ballevre, O.; Luo, H.; Zhang, W. Antihypertensive effects and mechanisms of chlorogenic acids. Hypertens. Researc. 2011, 35, 370–374. [Google Scholar] [CrossRef]
- Karthikesan, K.; Pari, L.; Menon, V.P. Combined treatment of tetrahydrocurcumin and chlorogenic acid exerts potential antihyperglycemic effect on streptozotocin-nicotinamide-induced diabetic rats. Gen. Physiol. Biophys. 2010, 29, 23–30. [Google Scholar] [CrossRef]
- Shimoda, H.; Seki, E.; Aitani, M. Inhibitory effect of green coffee bean extract on fat accumulation and body weight gain in mice. Complement. Altern. Med. 2006, 6, 9. [Google Scholar] [CrossRef]
- Upadhyayula, R.; Rao, J.M. An outlook on chlorogenic acids-occurrence, chemistry, technology, and biological activities. Crit. Rev. Food Sci. Nutr. 2013, 53, 968–984. [Google Scholar] [CrossRef]
- Liang, N.; Kitts, D.D. Role of chlorogenic acids in controlling oxidative and inflammatory stress conditions. Nutrients 2016, 8, 16. [Google Scholar] [CrossRef]
- Watanabe, T.; Arai, Y.; Mitsui, Y.; Kusaura, T.; Okawa, W.; Kajihara, Y.; Saito, I. The blood pressure-lowering effect and safety of chlorogenic acid from green coffee bean extract in essential hypertension. Clin. Exp. Hypertens. 2006, 28, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, L.J.; Jiang, F.; Yang, Y.; Wang, X.X.; Zhang, Z.; Li, Z.; Li, L. Caffeic acid improves cell viability and protects against DNA damage: Involvement of reactive oxygen species and extracellular signal-regulated kinase. Braz. J. Med. Biol. Res. 2015, 48, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Koh, K.H.; Kim, M.W.; Hang, S.; Park, Y.H.; Lee, C.H. Polyphenolic compounds and superoxide radical scavenging activity of Moru-Ju. Food Sci. Biotechnol. 2003, 12, 290–297. [Google Scholar]
- Xu, H.; Li, Y.; Che, X.; Tian, H.; Fan, H.; Liu, K. Metabolism of salvianolic acid A and antioxidant activities of its methylated metabolites. Drug Metabol. Dispos. 2014, 42, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Hotta, H.; Nagano, S.; Ueda, M.; Tsujino, Y.; Koyama, J.; Osakai, T. Higher radical scavenging activities of polyphenolic antioxidants can be ascribed to chemical reactions following their oxidation. Biochim. Biophys. Acta 2002, 1572, 123–132. [Google Scholar] [CrossRef]
- Chen, C. Sinapic acid and its derivatives as medicine in oxidative stress-induced diseases and aging. Oxid. Med. Cell. Long. 2016, 2016, 3571614. [Google Scholar] [CrossRef]
- Cha, M.N.; Kim, H.J.; Kim, B.G.; Ahn, J.-H. Synthesis of chlorogenic acid and p-coumaroyl shikimates from glucose using engineered Escherichia coli. J. Microbiol. Biotechnol. 2014, 24, 1109–1117. [Google Scholar] [CrossRef]
- Serafim, T.; Carvalho, S.F.; Marques, M.P.M.; Cruz, R.C.; Silva, T.B.; Garrido, J.M.P.J.; Milhaze, N.; Borges, F.; Roleira, F.M.F.; Silva, E.T.; et al. Lipophilic caffeic and ferulic acid derivatives presenting cytotoxicity against human breast cancer cells. Chem. Res. Toxicol. 2011, 24, 763–774. [Google Scholar] [CrossRef]
- Kumar, N.; Pruthi, V. Potential applications of ferulic acid from natural sources. Biotechnol. Rep. 2014, 4, 86–93. [Google Scholar] [CrossRef]
- Narasimhan, A.; Chinnaiyan, M.; Karundevi, B. Ferulic acid exerts its antidiabetic effect by modulating insulin-signalling molecules in the liver of high-fat diet and fructose-induced type-2 diabetic adult male rat. Appl. Physiol. Nutr. Metab. 2015, 40, 769–781. [Google Scholar] [CrossRef]
- Drãgan, M.; Stan, C.D.; Pânzariu, A.; Profire, L. Evaluation of anti-inflammatory potential of some new ferullic acid derivatives. Farmacia 2016, 64, 194–197. [Google Scholar]
- Ren, Z.; Zhang, R.; Li, Y.; Li, Y.; Yang, Z.; Yang, H. Ferulic acid exerts neuroprotective effects against cerebral ischemia/reperfusion-induced injury via antioxidant and anti-apoptotic mechanisms in vitro and in vivo. Int. J. Mol. Med. 2017, 40, 1444–1456. [Google Scholar] [CrossRef] [PubMed]
- Devi, J.A.I.; Muthu, A.K. Isolation and characterization of active components derived from whole plant of Saccharum spontaneum (Linn.). Pharm. Lett. 2015, 7, 197–203. [Google Scholar]
- Karthik, D.; Ravikumar, S. Proteome and phytochemical analysis of Cynodon dactylon leaves extract and its biological activity in diabetic rats. Int. J. Prev. Med. 2011, 1, 49–56. [Google Scholar] [CrossRef]
- Harborne, J.B.; Williams, C.A. Flavonoid patterns in leaves of the Gramineae. Biochem. Syst. Ecol. 1976, 4, 267–280. [Google Scholar] [CrossRef]
- Mabry, T.J. The ultraviolet spectra of flavones and flavonols. In The Systematic Identification of Flavonoids; Mabtry, T.J., Markham, K.R., Thomas, M.B., Eds.; Springer: Berlin, Germany, 1970; pp. 41–164. [Google Scholar]
- Li, M.; Pu, Y.; Yoo, C.G.; Ragauskas, A.J. The occurrence of tricin and its derivatives in plants. Green Chem. 2016, 18, 1439–1454. [Google Scholar] [CrossRef]
- Duarte-Almeida, J.M.; Negri, G.; Salatino, A.; Carvalho, J.E.; Lajolo, F.M. Antiproliferative and antioxidant activities of a tricin acylated glycoside from sugarcane (Saccharum officinarum) juice. Phytochemistry 2007, 68, 1165–1171. [Google Scholar] [CrossRef]
- Jiao, J.; Zhang, Y.; Liu, C.; Liu, J.; Wu, X.; Zhang, Y. Separation and purification of tricin from an antioxidant product derived from bamboo leaves. J. Agric. Food Chem. 2007, 55, 10086–10092. [Google Scholar] [CrossRef]
- Zhang, Z.; Chui, B.; Zhang, Y. Electrical penetration graphs indicate that tricin is a key secondary metabolite of rice, inhibiting phloem feeding of brown planthopper, Nilaparvata lugens. Entomol. Exp. Appl. 2015, 156, 14–27. [Google Scholar] [CrossRef]
- Kuwabara, H.; Mouri, K.; Otsuka, H.; Kasai, R.; Yamasaki, K. Tricin from a malagasy connaraceous plant with potent antihistaminic activity. J. Nat. Prod. 2003, 66, 1273–1275. [Google Scholar] [CrossRef]
- Kassem, M.E.S.; Shoela, S.; Marzouk, M.M.; Sleem, A.A. A sulphated flavone glycoside from Livistona australis and its antioxidant and cytotoxic activity. Nat. Prod. Res. 2012, 26, 1381–1387. [Google Scholar] [CrossRef] [PubMed]
- Hsu, F.F.; Turk, J.; Shi, Y.; Groisman, E.A. Characterization of acylphosphatidylglycerols from Salmonella typhimurium by tandem mass spectrometry with electrospray ionization. J. Am. Soc. Mass Spectrom. 2004, 15, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Colombo, R.; Yariwake, J.H.; Queiroz, E.F.; Ndjoko, K.; Hostettmann, K. Lc-Ms/Ms Analysis of sugarcane extracts and differentiation of monosaccharides moieties of flavone C-glycosides. J. Liq. Chromatogr. Relat. Technol. 2013, 36, 239–248. [Google Scholar] [CrossRef]
- Eklund, P.C.; Backman, M.J.; Kronberg, L.A.; Smeds, A.I.; Sjoholm, R.E. Identification of lignans by liquid chromatography-electrospray ionization ion-trap mass spectrometry. J. Mass Spectrom. 2008, 43, 97–107. [Google Scholar] [CrossRef]
- Vuković, N.; Vukić, M.; Đelić, G.; Kacaniova, M.; Cvijović, M. The investigation of bioactive secondary metabolites of the methanol extract of Eryngium amethystinum. J. Sci. 2018, 40, 113–129. [Google Scholar] [CrossRef]
- Levsen, K.; Schiebel, H.M.; Behnke, B.; Dotzer, R.; Dreher, W.; Elend, M.; Thiele, H. Structure elucidation of phase II metabolites by tandem mass spectrometry: An overview. J. Chromatogr. A 2005, 1067, 55–72. [Google Scholar] [CrossRef]
- Negri, G.; Tabach, R. Saponins, tannins and flavonols found in hydroethanolic extract from Periandra dulcis roots. Rev. Bras. Farm. 2013, 23, 851–860. [Google Scholar] [CrossRef]
- Mohanlal, S.; Parvathy, R.; Shalini, V.; Helen, A.; Jayalekshmy, A. Isolation, characterization and quantification of tricin and flavonolignans in the medicinal rice njavara (Oryza sativa L.), as compared to staple varieties. Plant Foods Hum. Nut. 2011, 66, 91–96. [Google Scholar] [CrossRef]
- Lee, S.S.; Baek, Y.S.; Eun, C.S.; Yu, M.H.; Baek, N.I.; Chung, D.; Bang, M.H.; Yang, S.A. Tricin derivatives as anti-inflammatory and anti-allergic constituents from the aerial part of Zizania latifolia. Biosci. Biotechnol. Biochem. 2015, 79, 700–706. [Google Scholar] [CrossRef]
- Chang, C.L.; Zhang, L.J.; Chen, R.Y.; Wu, C.C.; Huang, H.C.; Roy, M.C.; Huang, J.P.; Wu, Y.C.; Kuo, Y.H. Quiquelignan A–H, eight new lignoids from the rattan palm Calamus quiquesetinervius and their antiradical, anti-inflammatory and antiplatelet aggregation activities. Bioorg. Med. Chem. 2010, 18, 518–525. [Google Scholar] [CrossRef]
- Mohanlal, S.; Maney, S.K.; Santhoshkumar, T.R.; Jayalekshmy, A. Tricin 4′-O-(erythro-β-guaiacylglyceryl) ether and tricin 4′-O-(threo-β-guaiacylglyceryl) ether isolated from Njavara (Oryza sativa L. var. Njavara), induce apoptosis in multiple tumor cells by mitochondrial pathway. J. Nat. Med. 2013, 67, 528–533. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.L.; Wang, G.J.; Zhang, L.J.; Tsai, W.J.; Chen, R.Y.; Wu, Y.C.; Kuo, Y.H. Cardiovascular protective flavonolignans and flavonoids from Calamus quiquesetinervius. Phytochemistry 2010, 71, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Robinson, D.H.; Birt, D.F. Evaluation of properties of apigenin and [G-3H]apigenin and analytic method development. J. Pharm. Sci. 1997, 86, 721–725. [Google Scholar] [CrossRef] [PubMed]
- Tahir, N.I.; Shaari, K.; Abas, F.; Parveez, G.K.A.; Ishak, Z.; Ramli, U.S. Characterization of apigenin and luteolin derivatives from oil palm (Elaeis guineensis Jacq.) leaf using LC–ESI-MS/MS. J. Agric. Food Chem. 2012, 60, 11201–11210. [Google Scholar] [CrossRef]
- Pikulski, M.; Brodbelt, J.S. Differentiation of flavonoid glycoside isomers by using metal complexation and electrospray ionization mass spectrometry. J. Am. Soc. Mass Spectrom. 2003, 14, 1437–1453. [Google Scholar] [CrossRef]
- Benayad, Z.; Gómez-Cordové, C.; Es-Safi, N.E. Characterization of flavonoid glycosides from Fenugreek (Trigonella foenum-graecum) crude seeds by HPLC–DAD–ESI/MS analysis. Int. J. Mol. Sci. 2014, 15, 20668–20685. [Google Scholar] [CrossRef]
- Morikawa, T. Biological Activity of Natural Secondary Metabolite Products; MDPI: Basel, Switzerland, 2018. [Google Scholar]
- Bamawa, C.M.; Ndjele, L.M.; Foma, F.M. Characterization of leaf phenolic compounds of Hymenocardia ulmoides (Wild plant tea) by HPLC-MSn. Int. J. Pharm. Biol. Res. 2013, 4, 170–174. [Google Scholar]
- Chen, H.; Sang, S. Identification of phase II metabolites of thiol-conjugated [6]-shogaol in mouse urine using high-performance liquid chromatography tandem mass spectrometry. J. Chromatogr. 2012, 907, 126–139. [Google Scholar] [CrossRef]
- Zhao, H.-Y.; Fan, M.-X.; Wu, X.; Wang, H.-J.; Yang, J.; Si, N.; Bian, B.-L. Chemical profiling of the Chinese herb formula Xiao-Cheng-Qi decoction using liquid chromatography coupled with electrospray ionization mass spectrometry. J. Chromatogr. Sci. 2013, 51, 273–285. [Google Scholar] [CrossRef]
- Yao, H.; Chen, B.; Zhang, Y.; Ou, H.; Li, Y.; Li, S.; Shi, P.; Lin, X. Analysis of the total biflavonoids extract from Selaginella doederleinii by HPLC-QTOF-MS and its in vitro and in vivo anticancer effects. Molecules 2017, 22, 325. [Google Scholar] [CrossRef]
- Keskes, H.; Belhadj, S.; Jlail, L.; Feki, A.E.; Damak, M.; Sayadi, S.; Allouche, N. LC-MS–MS and GC-MS analyses of biologically active extracts and fractions from Tunisian Juniperus phoenice leaves. Pharm. Biol. 2017, 55, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Smiljkovic, M.; Stanisavljevic, D.; Stojkovic, D.; Petrovic, I.; Vicentic, J.M.; Popovic, J.; Grdadolnik, S.G.; Markovic, D.; Sanković-Babić, S.; Glamoclija, J.; et al. Apigenin-7-O-glucoside versus apigenin: Insight into the modes of anticandidal and cytotoxic actions. EXCLI J. 2017, 16, 1611–2156. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, H.; Yuan, M.; Zhou, J.; Tu, Q.; Liu, J.-J.; Wang, J. Synthesis and biological evaluation of apigenin derivatives as antibacterial and antiproliferative agents. Molecules 2013, 18, 11496–11511. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Yu, L.; Yang, J.; Yao, X.; Yan, W.; Bo, S.; Liu, Y.; Wei, Y.; Wu, Z.; Wang, G. Synthesis and anti-cancer activities of apigenin derivatives. Med. Chem. 2014, 10, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.Z.; Harnly, J.M. Phenolic compounds and chromatographic profiles of pear skins (Pyrus spp.). J. Agric. Food Chem. 2008, 56, 9094–9101. [Google Scholar] [CrossRef]
- Lin, L.Z.; Harnly, J.M. Identification of the phenolic components of Chrysanthemum flower (Chrysanthemum morifolium Ramat). Food Chem. 2010, 120, 319–326. [Google Scholar] [CrossRef]
- Brito, A.; Ramirez, J.E.; Areche, C.; Sepúlveda, B.; Simirgiotis, M.J. HPLC-UV-MS profiles of phenolic compounds and antioxidant activity of fruits from three citrus species consumed in Northern Chile. Molecules 2014, 19, 17400–17421. [Google Scholar] [CrossRef]
- Oszmianski, J.; Kolniak-Ostek, J.; Wojdyło, A. Application of ultra-performance liquid chromatographyphotodiode detector-quadrupole/time of flight-mass spectrometry (UPLC-PDA-Q/TOF-MS) method for the characterization of phenolic compounds of Lepidium sativum L. sprouts. Eur. Food Res. Technol. 2013, 236, 699–706. [Google Scholar] [CrossRef]
- Geng, P.; Sun, J.; Zhang, M.; Li, X.; Harnly, J.M.; Chen, P. Comprehensive characterization of C-glycosyl flavones in wheat (Triticum aestivum L.) germ using UPLC-PDA-ESI/HRMSn and mass defect filtering. J. Mass Spectrom. 2016, 51, 914–930. [Google Scholar] [CrossRef]
- Omosa, L.K.; Amugune, B.; Ndunda, B.; Milugo, T.K.; Heydenreich, M.; Yenesew, A.; Midiwo, J.O. Antimicrobial flavonoids and diterpenoids from Dodonaea angustifolia. S. Afr. J. Bot. 2014, 91, 58–62. [Google Scholar] [CrossRef]
- Roy, S.; Pawar, S.; Chowdhary, A. Evaluation of in vitro cytotoxic and antioxidant activity of Datura metel Linn. and Cynodon dactylon Linn. extracts. Pharm. Res. 2016, 8, 123–127. [Google Scholar] [CrossRef]
- Lopez-Lazaro, M. Distribution and biological activities of the flavonoid luteolin. Mini-Rev. Med. Chem. 2009, 9, 31–59. [Google Scholar] [CrossRef] [PubMed]
- Aziz, N.; Kim, M.Y.; Cho, J.Y. Anti-inflammatory effects of luteolin: A review of in vitro, in vivo, and in silico studies. J. Ethnopharmacol. 2018, 225, 342–358. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Jayaprakasha, G.K.; Patil, B.S. Rapid ultra-high-performance liquid chromatography/quadrupole time-of-flight tandem mass spectrometry and selected reaction monitoring strategy for the identification and quantification of minor spinacetin derivatives in spinach. Rap. Commun. Mass Spectrom. 2017, 31, 1803–1812. [Google Scholar] [CrossRef] [PubMed]
- Silva, N.A.; Rodrigues, E.; Mercadante, A.Z.; Rosso, V.V. Phenolic compounds and carotenoids from four fruits native from the Brazilian atlantic forest. J. Agric. Food Chem. 2014, 62, 5072–5084. [Google Scholar] [CrossRef] [PubMed]
- Saldanha, L.L.; Vilegas, W.; Dokkedal, A.L. Characterization of flavonoids and phenolic acids in Myrcia bella Cambess. using FIA-ESI-IT-MSn and HPLC-PAD-ESI-IT-MS combined with NMR. Molecules 2013, 18, 8402–8416. [Google Scholar] [CrossRef]
- Roriz, C.L.; Barros, L.; Carvalho, A.M.; Santos-Buelga, C.; Ferreira, I.C.F.R. Pterospartum tridentatum, Gomphrena globosa and Cymbopogon citratus: A phytochemical study focused on antioxidant compounds. Food Res. Int. 2014, 62, 684–693. [Google Scholar] [CrossRef]
- Barron, D.; Varin, L.; Ibrahim, R.K.; Harborne, J.B.; Williams, C.A. Sulphated flavonoids—An update. Phytochemistry 1988, 27, 2375–2395. [Google Scholar] [CrossRef]
- Tatsimo, S.J.N.; Tamokou, J.D.; Havyarimana, L.; Csupor, D.; Forgo, P.; Hohmann, J.; Kuiate, J.-R.; Tane, P. Antimicrobial and antioxidant activity of kaempferol rhamnoside derivatives from Bryophyllum pinnatum. BMC Res. Notes 2012, 5, 158–161. [Google Scholar] [CrossRef]
- Cho, H.J.; Park, J.H.Y. Kaempferol induces cell cycle arrest in HT-29 human colon cancer cells. J. Cancer Prev. 2013, 18, 257–263. [Google Scholar] [CrossRef]
- Kadioglu, O.; Nass, J.; Saeed, M.E.M.; Schuler, B.; Efferth, T. Kaempferol is an anti-inflammatory compound with activity towards NF-κB pathway proteins. Anticancer Res. 2015, 35, 2645–2650. [Google Scholar]
- Ji, N.; Pan, S.; Shao, C.; Chen, Y.; Zhang, Z.; Wang, R.; Qiu, Y.; Jin, M.; Kong, D. Spinacetin suppresses the mast cell activation and passive cutaneous anaphylaxis in mouse model. Front. Pharmacol. 2018, 9, 824. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Guo, D.; Ye, G.; Huang, C. Analysis of homoisoflavonoids in Ophiopogon japonicus by HPLC-DAD-ESI-MSn1. J. Am. Soc. Mass Spectrom. 2005, 16, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Pereira, O.R.; Peres, A.M.; Silva, A.M.S.; Domingues, M.R.M.; Cardoso, S.M. Simultaneous characterization and quantification of phenolic compounds in Thymus x Citriodorus using a validated HPLC–UV and ESI–MS combined method. Food Res. Int. 2013, 54, 1773–1780. [Google Scholar] [CrossRef]
- Mitra, S.P. UV-Vis spectrophotometry plus HPLC to measure the level of catechin/poly–phenolics and to understand its oxidized conditions in commercially available green and black teas. Ind. J. Chem. 2014, 8, 1255–1262. [Google Scholar]
- Lehner, A.F.; Duringer, J.M.; Estill, C.T.; Tobin, T.; Craig, A.M. ESI-Mass spectrometric and HPLC elucidation of a new ergot alkaloid from perennial ryegrass hay silage associated with bovine reproductive problems. Toxicol. Mech. Methods 2011, 21, 606–621. [Google Scholar] [CrossRef] [PubMed]
- Slimen, I.B.; Mabrouk, M.; Hanène, C.; Najar, T.; Abderrabba, M. LC-MS analysis of phenolic acids, flavonoids and betanin from spineless Opuntia ficus-indica fruits. Cell Biol. 2017, 5, 17–28. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Alam, M.B.; Quan, K.T.; Kwon, K.-R.; Ju, M.K.; Choi, H.-J.; Lee, J.S.; Yoon, J.-I.; Majumder, R.; Rather, I.A.; et al. Antioxidant efficacy and the upregulation of Nrf2-mediated HO-1 expression by (+)-lariciresinol, a lignan isolated from Rubia philippinensis, through the activation of p38. Sci. Rep. 2017, 7, 46035. [Google Scholar] [CrossRef]
- Lin, X.; Peng, Z.; Su, C. Potential anti-cancer activities and mechanisms of costunolide and dehydrocostuslactone. Int. J. Mol. Sci. 2015, 16, 10888–10906. [Google Scholar] [CrossRef]
- Schweiggert, U.; Kammerer, D.R.; Carle, R.; Schieber, A. Characterization of carotenoids and carotenoid esters in red pepper pods (Capsicum annuum L.) by high-performance liquid chromatography/atmospheric pressure chemical ionization mass spectrometry. Rap. Commun. Mass Spectrom. 2005, 19, 2617–2628. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhang, Y.; Li, Q.; Zou, Y.; Shao, J.; Lan, S. Quantification of lutein and zeaxanthin in marigold (Tagetes erecta L.) and poultry feed by ultra-performance liquid chromatography and high performance liquid chromatography. J. Liq. Chromatogr. Relat. Technol. 2011, 34, 2653–2663. [Google Scholar] [CrossRef]
- Milenković, S.M.; Zvezdanović, J.B.; Anđelković, T.D.; Marković, D.Z. The identification of chlorophyll and its derivatives in the pigment mixtures: HPLC-chromatography, visible and mass spectroscopy studies. Adv. Technol. 2012, 1, 16–24. [Google Scholar]
- Zcezdanovic, J.B.; Petrovic, S.M.; Markovic, D.Z.; Andjelkovic, T.; Andjelkovic, D.H. Electrospray ionization mass spectrometry combined with ultra-high performance liquid chromatography in the analysis of in vitro formation of chlorophyll complexes with copper and zinc. J. Serb. Chem. Soc. 2014, 79, 689–706. [Google Scholar] [CrossRef]
- Clarke, P.A.; Barnes, K.A.; Startiin, J.R.; Ibe, F.I.; Shepherd, M.J. High performance liquid chromatography/atmospheric pressure chemical ionization-mass spectrometry for the determination of carotenoids. Rap. Commun. Mass Spectrom. 1996, 10, 1781–1785. [Google Scholar] [CrossRef]
- Li, W.T.; Tsao, H.W.; Chen, Y.Y.; Cheng, S.W.; Hsu, Y.C. A study on the photodynamic properties of chlorophyll derivatives using human hepatocellular carcinoma cells. Photochem. Photobiol. Sci. 2007, 6, 1341–1348. [Google Scholar] [CrossRef]
- Sindhu, E.R.; Preethi, K.C.; Kuttan, R. Antioxidant activity of carotenoid lutein in vitro and in vivo. Ind. J. Exp. Biol. 2010, 48, 843–848. [Google Scholar]
- Everett, S.A.; Kundu, S.C.; Maddix, S.; Willson, R.L. Mechanisms of free-radical scavenging by the nutritional antioxidant beta-carotene. Biochem. Soc. Transact. 1995, 23, 230S. [Google Scholar] [CrossRef]
- Trevithick-Sutton, C.C.; Foote, C.S.; Collins, M.; Trevithick, J.R. The retinal carotenoids zeaxanthin and lutein scavenge superoxide and hydroxy radicals: A chemiluminesence and ESR study. Mol. Vis. 2006, 12, 1127–1135. [Google Scholar]
- Wojdyło, A.; Oszmiański, J.; Czemerys, R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007, 105, 940–949. [Google Scholar] [CrossRef]
- Razzaghi-Asl, N.; Garrido, J.; Khazraei, H.; Borges, F.; Firuzi, O. Antioxidant properties of hydroxycinnamic acids: A review of structure-activity relationships. Curr. Med. Chem. 2013, 20, 4436–4450. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Scorzoni, L.; Benaducci, T.; Almeida, A.M.F.; Silva, D.H.S.; Bolzani, V.S.; Mendes-Giannini, M.J.S. Comparative study of disk diffusion and microdilution methods for evaluation of antifungal activity of natural compounds against medical yeasts Candida spp. and Cryptococcus sp. Ciênc. Farm. Básica Apl. 2007, 28, 25–34. [Google Scholar]
- Pinho, E.; Ferreira, I.C.F.R.; Barros, L.; Carvalho, A.M.; Soares, G.; Henriques, M. Antibacterial potential of northeastern Portugal wild plant extracts and respective phenolic compounds. BioMed Res. Int. 2014, 2014, 814590. [Google Scholar] [CrossRef]
- Ansari, M.A.; Anurag, A.; Fatima, Z.; Hameed, S. Natural phenolic compounds: A potential antifungal agent. In Microbial Pathogens and Strategies for Combating Them: Science, Technology and Education; Méndez-Villas, A., Ed.; Formatex Research Center: Badajoz, Spain, 2013; pp. 1189–1195. [Google Scholar]
- Barreto, M.C.; Arruda, M.; Rego, E.; Medeiros, J.S.; Rainha, N. Cell-free assays. In Determination of Biological Activities: A Laboratory Manual; Barreto, M.C., Simões, N., Eds.; Universidade dos Açores: Ponta Delgada, Portugal, 2012; pp. 65–81. [Google Scholar]
- Catarino, M.D.; Silva, A.M.S.; Cruz, M.T.; Cardoso, S.M. Antioxidant and anti-inflammatory activities of Geranium robertianum L. decoctions. Food Funct. 2017, 8, 3355–3365. [Google Scholar] [CrossRef] [PubMed]
- Katschnig, D.; Broekman, R.; Rozema, J. Salt tolerance in the halophyte Salicornia dolichostachya Moss: Growth, morphology and physiology. Environ. Exp. Bot. 2013, 92, 32–42. [Google Scholar] [CrossRef]
- Rozema, J.; Schat, H. Salt tolerance of halophytes, research questions reviewed in the perspective of saline agriculture. Environ. Exp. Bot. 2013, 92, 83–95. [Google Scholar] [CrossRef]
- Kumari, A.; Das, P.; Parida, A.K.; Agarwal, P.K. Proteomics, metabolomics, and ionomics perspectives of salinity tolerance in halophytes. Front. Plant Sci. 2015, 6, 537. [Google Scholar] [CrossRef]
- Kumari, A.; Parida, A.K. Metabolomics and network analysis reveal the potential metabolites and biological pathways involved in salinity tolerance of the halophyte Salvadora persica. Environ. Exp. Bot. 2018, 148, 85–99. [Google Scholar] [CrossRef]
- Vermue, E.; Metselaar, K.; van der Zee, S.E.A.T.M. Modelling of soil salinity and halophyte crop production. Environ. Exp. Bot. 2013, 92, 186–196. [Google Scholar] [CrossRef]
- Nikalje, G.C.; Variyar, P.S.; Joshi, M.V.; Nikam, T.D.; Suprasanna, P. Temporal and special changes in ion homeostasis, antioxidant defense and accumulation of flavonoids and glycolipid in a halophyte Sesuvium portulacastrum (L.) L. PLoS ONE 2018, 13, e0193394. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
| No. | Rt | [M-H]− | MS2 (m/z) | Quantification (mg/100 mg of Extract) | Assigned Identification § | ||
|---|---|---|---|---|---|---|---|
| EPm | ESm | ESp | |||||
| Hydroxycinnamic Acid Derivatives | |||||||
| 1 | 5.2 | 353 | 191 (100), 179 (48), 135 (10) | 0.19 ± 0.01 | - | - | 3-O-Caffeoylquinic acid |
| 2 | 7.4 | 247 | 179 (100), 161 (40), 135 (15) | - | 0.22 ± 0.01 | - | Caffeic acid isoprenyl ester |
| 3 | 7.6 | 341 | 179 (100), 161(60), 135(51) | - | - | 0.20 ± 0.01 | Caffeic acid hexoside |
| 4 | 7.9 | 353 | 191 (100), 179 (8), 161 (2) | 0.20 ± 0.01 | - | - | 5-O-Caffeoylquinic acid |
| 5 | 8.4 | 367 | 193 (100), 191 (2), 134 (4) | 0.20 ± 0.01 | 0.22 ± 0.01 | 0.23 ± 0.01 | 3-O-Feruloylquinic acid |
| 6 | 8.5 | 429 | 429 (100), 235 (20), 193 (4), 161 (10) | 0.20 ± 0.01 | 0.22 ± 0.01 | 0.20 ± 0.01 | Isomeric form of feruloyl-caffeoylglycerol |
| 7 | 8.7 | 429 | 429 (100), 235 (20), 193 (4), 161 (10) | 0.20 ± 0.01 | - | - | Isomeric form of feruloyl-caffeoylglycerol |
| 8 | 9.4 | 655 | 493 (100), 359 (32), 179 (41) | 0.20 ± 0.01 | - | - | Salvianolic acid A hexoside |
| 9 | 9.8 | 431 | 385 (100), 223 (10), 205 (23) | 0.20 ± 0.01 | - | - | Sinapic acid hexoside derivative |
| 10 | 10.4 | 367 | 193 (80), 173 (100) | 0.41 ± 0.14 | 0.22 ± 0.01 | 0.24 ± 0.01 | 4-O-Feroluoylquinic acid |
| 11 | 11.9 | 319 | 163 (100), 145 (60), 119 (20) | - | - | 0.68 ± 0.01 | p-Coumaroylshikimic acid |
| 12 | 13.0 | 565 | 519 (100) | - | 0.69 ± 0.01 | - | Coumaroylferulic acid hexoside derivative |
| 13 | 13.2 | 565 | 519 (100) | 0.62 ± 0.01 | - | 0.64 ± 0.01 | Coumaroylferulic acid hexoside derivative |
| 14 | 18.7 | 443 | 235 (100), 207 (60), 193 (66), 161 (9), 135 (4) | - | - | 0.20 ± 0.01 | Diferuloylglycerol |
| 15 | 20.0 | 613 | 569 (100), 417 (34), 193 (59) | - | - | 0.21 ± 0.01 | Ferulic acid guaiacylglyceryl derivative |
| Flavonoids | |||||||
| 16 | 10.2 | 653 | 635 (65), 445 (100), 313 (8) | 0.46 ± 0.01 | 0.50 ± 0.01 | - | Trihydroxymethylenedioxyflavone-O-pentosyl-O-hydroxyferuloyl |
| 17 | 10.5 | 447 | 357 (64), 327 (100) | 0.60 ± 0.02 | 0.65 ± 0.02 | 0.74 ± 0.04 | Luteolin-8-C-hexoside |
| 18 | 10.8 | 563 | 545 (2), 473 (100), 443 (69), 383 (22), 353 (28) | 0.50 ± 0.01 | 0.55 ± 0.01 | - | Apigenin-6(8)-C-hexoside-8(6)-C-pentoside |
| 19 | 10.9 | 461 | 371 (42), 341 (100), 313 (32), 299(4) | - | - | 0.80 ± 0.05 | Trihydroxymethoxy flavone C-hexoside (isomer I) |
| 20 | 11.1 | 593 | 473 (44), 383 (100), 353 (70) | - | 1.09 ± 0.13 | 0.48 ± 0.01 | Apigenin di-C-hexoside |
| 21 | 11.4 | 623 | 503 (10), 443 (100), 353 (4), 323 (26) | - | 0.79 ± 0.08 | - | Dihydroxymethoxy flavone caffeoyl C-hexoside |
| 22 | 11.5 | 533 | 515 (24), 473 (63), 443 (100), 383 (14), 353 (14) | 0.46 ± 0.01 | - | - | Apigenin-di-C-pentoside |
| 23 | 11.6 | 431 | 269 (100) | 0.50 ± 0.01 | 3.14 ± 0.24 | 0.56 ± 0.02 | Apigenin-O-hexoside |
| 24 | 11.8 | 607 | 487 (41), 443 (100), 353 (40), 323 (26) | - | - | 0.50 ± 0.01 | Dihydroxymethoxyflavone coumaroyl-C-hexoside |
| 25 | 12.0 | 461 | 443 (6), 371 (21), 341 (100), | 0.47 ± 0.01 | 1.54 ± 0.14 | 0.51 ± 0.01 | Trihydroxymethoxy flavone C-hexoside (isomer II) |
| 26 | 12.2 | 447 | 429 (24), 357 (80), 327 (100), 285 (10) | 0.48 ± 0.01 | - | - | Luteolin C-hexoside |
| 27 | 12.3 | 461 | 371 (8), 341 (100) | - | 0.53 ± 0.01 | - | Trihydroxymethoxy flavone C-hexoside (isomer III) |
| 28 | 12.5 | 815 | 507 (100) | - | - | 0.47 ± 0.01 | Hydroxydimethoxydimethyl homoisoflavone I ♣ |
| 29 | 12.6 | 769 | 593 (70), 413 (100), 323 (6), 293 (52) | - | 0.51 ± 0.01 | - | Apigenin C-hexoside-O-caffeoylglucoronide |
| 30 | 12.8 | 537 | 493 (10), 375 (100), 331 (6) | 0.49 ± 0.01 | - | 0.48 ± 0.01 | Biapigenin (Amentoflavone) |
| 31 | 13.4 | 491 | 476 (9), 329 (100) | 0.47 ± 0.01 | - | - | Tricin-7-O-hexoside |
| 32 | 13.5 | 431 | 341 (28), 311 (100) | - | 0.55 ± 0.01 | - | Apigenin-6-C-hexoside |
| 33 | 13.9 | 461 | 371 (8), 341 (100) | - | 0.52 ± 0.01 | - | Trihydroxymethoxy flavone C-hexoside (isomer IV) |
| 34 | 14.1 | 687 | 525 (100), 329 (8) | 0.46 ± 0.01 | - | - | Tricin guaiacylglyceryl hexoside (isomer I) |
| 35 | 14.3 | 431 | 341 (10), 311 (100) | - | 0.55 ± 0.01 | - | Apigenin-8-C-hexoside |
| 36 | 14.6 | 687 | 525 (100), 329 (8) | 0.46 ± 0.01 | - | - | Tricin guaiacylglyceryl hexoside (isomer II) |
| 37 | 14.7 | 461 | 371 (8), 341 (100) | - | 0.51 ± 0.01 | - | Trihydroxymethoxy flavone C-hexoside (isomer V) |
| 38 | 15.0 | 373 | 329 (100) [tricin-H]− | - | 0.51 ± 0.01 | - | 3-O-Acetyl tricin |
| 39 | 15.1 | 611 | 593 (18), 449 (100) | - | - | 0.47 ± 0.01 | Erioctioyl di-O-hexoside |
| 40 | 15.2 | 409 | 329 (100) | - | 0.51 ± 0.01 | - | Tricin sulfate |
| 41 | 15.6 | 629 | 611 (6), 467 (100) | - | - | 0.47 ± 0.01 | Trihydroxymethylenedioxyflavone derivative ♥ |
| 42 | 16.0 | 605 | 329 (100), 314 (10) | - | 0.51 ± 0.01 | 0.47 ± 0.01 | Tricin guaiacylglycerylsulfate |
| 43 | 16.4 | 403 | 388 (9), 329 (100) | - | 0.54 ± 0.01 | 0.49 ± 0.01 | Tricin glyceryl |
| 44 | 16.5 | 507 | 492 (100), 339 (12), 311 (8) | - | 0.50 ± 0.01 | 0.48 ± 0.01 | Hydroxydimethoxydimethyl homoisoflavone II ♦ |
| 45 | 16.7 | 691 | 497 (100), 329 (60), 314 (10) | 0.58 ± 0.01 | 0.58 ± 0.01 | - | Tricin C-methylgalloyl glucuronide |
| 46 | 16.8 | 417 | 373 (10), 354 (21), 329 (100) | Tr | - | - | Tricin derivative |
| 47 | 16.9 | 643 | 481 (100), 329 (20), 314 (12), 299 (10) | - | - | 0.48 ± 0.01 | Tricin-C-galloyl-O-hexoside |
| 48 | 17.6 | 329 | 314 (100), 299 (7) | 0.48 ± 0.01 | 0.79 ± 0.02 | 0.62 ± 0.02 | Tricin |
| 49 | 17.9 | 587 | 571 (87), 439 (100), 359 (16) | 0.47 ± 0.01 | 0.77 ± 0.02 | - | Quercetagetin trimethyl O-sulfate pentoside |
| 50 | 18.0 | 525 | 329 (100) | - | - | 0.58 ± 0.02 | Tricin-4-O-guaiacylglyceryl |
| 51 | 18.2 | 541 | 495 (100), 345 (19) | - | 0.55 ± 0.01 | 0.50 ± 0.01 | Spinacetin guaiacylglyceryl |
| 52 | 19.1 | 569 | 551 (34), 345 (100) | - | 0.49 ± 0.01 | 0.48 ± 0.01 | Spinacetin sinapoyl |
| 53 | 19.9 | 853 | 805 (12), 493 (100), 329 (54), 314 (13), 299 (12) | - | 0.50 ± 0.01 | 0.48 ± 0.01 | Tricin secoisolariciresinol coumaroyl |
| 54 | 20.1 | 817 | 577 (60), 559 (88), 537 (42), 451 (46), 407 (100) | - | - | 0.52 ± 0.01 | Procyanidyn dimer derivative |
| 55 | 20.4 | 599 | 447 (100), 285 (47) | - | 0.52 ± 0.01 | 0.51 ± 0.01 | Kaempferol galloyl hexoside |
| 56 | 20.5 | 599 | 584 (100), 419 (16), 283 (18) | 0.46 ± 0.01 | 0.53 ± 0.01 | 0.52 ± 0.01 | Methylgnistein caffeoyl derivative |
| 57 | 21.0 | 641 | 623 (100), 445 (32), 293 (30), 255 (21) | - | - | 0.49 ± 0.01 | Dihydroxyflavanone Acetyl guaiacylglyceryl galloyl |
| 58 | 21.7 | 577 | 415 (22), 299 (100) | 0.48 ± 0.01 | - | 0.48 ± 0.01 | Trihydroxymethoxyflavone-O-hexose-O-malloyl |
| 59 | 22.1 | 649 | 603 (10), 487 (51), 413 (82), 299 (100) | 0.45 ± 0.01 | - | - | Trihydroxymethoxy flavone derivative ♠ |
| 60 | 22.4 | 540 | 480 (100), 304 (9) | - | 0.50 ± 0.01 | - | O-Methylcatechin acetate glucuronide |
| Others | |||||||
| 61 | 2.8 | 241 | 197 (100) | 2.36 ± 0.35 | 2.23 ± 0.12 | 0.53 ± 0.02 | Syringic acid derivative |
| 62 | 9.5 | 535 | 197 (21), 163 (8), 129 (10), 85 (100) | 0.19 ± 0.01 | - | - | Coumaroylsyringylglucarate acid |
| 63 | 12.9 | 683 | 521 (42), 359(27), 329 (100) | Tr | - | Tr | Lariciresinol dihexoside |
| 64 | 14.8 | 231 | 213 (100), 187(10) | - | - | Tr | Costunolide |
| 65 | 18.4 | 571 | 525 (100) | Tr | 0.09 ± 0.01 | 0.06 ± 0.01 | Dehydrated oleanolic acid pentoside |
| Rt | [M+H]+ | UV–Vis | PmMW * | PmUS * | SmMW * | SmST * | SmUS * | SpMW * | SpST * | SpUS * | Assigned Identification |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 8.8 | 569 | 431, 443, 473 | 57.2 | 14.8 | 9.3 | 23.9 | 9.9 | 27.4 | 15.5 | 1.2 | Lutein |
| 17.5 | 885 | 420, 435, 460sh, 652 | 15.8 | 12.5 | 12.4 | 13.1 | 18.8 | 6.5 | 0.6 | 7.0 | Chlorophyll b I |
| 18.0 | 889 | 435, 651 | - | - | 3.4 | 2.5 | 3.6 | - | - | - | Chlorophyll b II |
| 18.4 | 569 | 438sh, 455, 476 | 17.4 | 3.7 | - | - | - | - | - | - | Zeaxanthin |
| 18.8 | 537 | 273, 453 | - | - | 18.5 | 12.6 | 6.3 | 13.8 | 36.8 | - | Carotene |
| 19.8 | 887 | 407, 655 | - | - | - | - | - | 15.0 | 35.4 | - | Chlorophyll b III |
| 20.5 | 871 | 410, 653, 665 | 7.6 | 59.8 | 39.6 | 40.7 | 51.6 | 32.4 | 10.4 | 91.8 | Pheophytin a I |
| 21.2 | 871 | 420, 438, 455, 663 | 1.9 | 9.6 | 16.9 | 7.2 | 9.7 | 4.8 | 1.4 | - | Pheophytin a II |
| Extract | P. maritima | S. maritima | S. patens |
|---|---|---|---|
| C. albicans | 31.06 ± 6.56 * | 30.54 ± 6.17 * | 46.90 ± 8.30 * |
| L. innocua | 44.59 ± 0.12 * | 41.59 ± 3.76 * | 31.89 ± 1.94 * |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faustino, M.V.; Faustino, M.A.F.; Silva, H.; Cunha, Â.; Silva, A.M.S.; Pinto, D.C.G.A. Puccinellia maritima, Spartina maritime, and Spartina patens Halophytic Grasses: Characterization of Polyphenolic and Chlorophyll Profiles and Evaluation of Their Biological Activities. Molecules 2019, 24, 3796. https://doi.org/10.3390/molecules24203796
Faustino MV, Faustino MAF, Silva H, Cunha Â, Silva AMS, Pinto DCGA. Puccinellia maritima, Spartina maritime, and Spartina patens Halophytic Grasses: Characterization of Polyphenolic and Chlorophyll Profiles and Evaluation of Their Biological Activities. Molecules. 2019; 24(20):3796. https://doi.org/10.3390/molecules24203796
Chicago/Turabian StyleFaustino, Maria V., Maria A. F. Faustino, Helena Silva, Ângela Cunha, Artur M. S. Silva, and Diana C. G. A. Pinto. 2019. "Puccinellia maritima, Spartina maritime, and Spartina patens Halophytic Grasses: Characterization of Polyphenolic and Chlorophyll Profiles and Evaluation of Their Biological Activities" Molecules 24, no. 20: 3796. https://doi.org/10.3390/molecules24203796
APA StyleFaustino, M. V., Faustino, M. A. F., Silva, H., Cunha, Â., Silva, A. M. S., & Pinto, D. C. G. A. (2019). Puccinellia maritima, Spartina maritime, and Spartina patens Halophytic Grasses: Characterization of Polyphenolic and Chlorophyll Profiles and Evaluation of Their Biological Activities. Molecules, 24(20), 3796. https://doi.org/10.3390/molecules24203796