Anthocyanin Accumulation in the Leaves of the Purple Sweet Potato (Ipomoea batatas L.) Cultivars
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Materials
2.3. Sample Preparation
2.4. Ultra Performance Liquid Chromatography Diode-Array Detection (UPLC-DAD) and UPLC-QTOF-MS Analysis
2.5. Quantitative Real-time PCR ANALYSIS of Anthocyanin Biosynthetic Genes in Sweet Potato Leaves
3. Results and Discussion
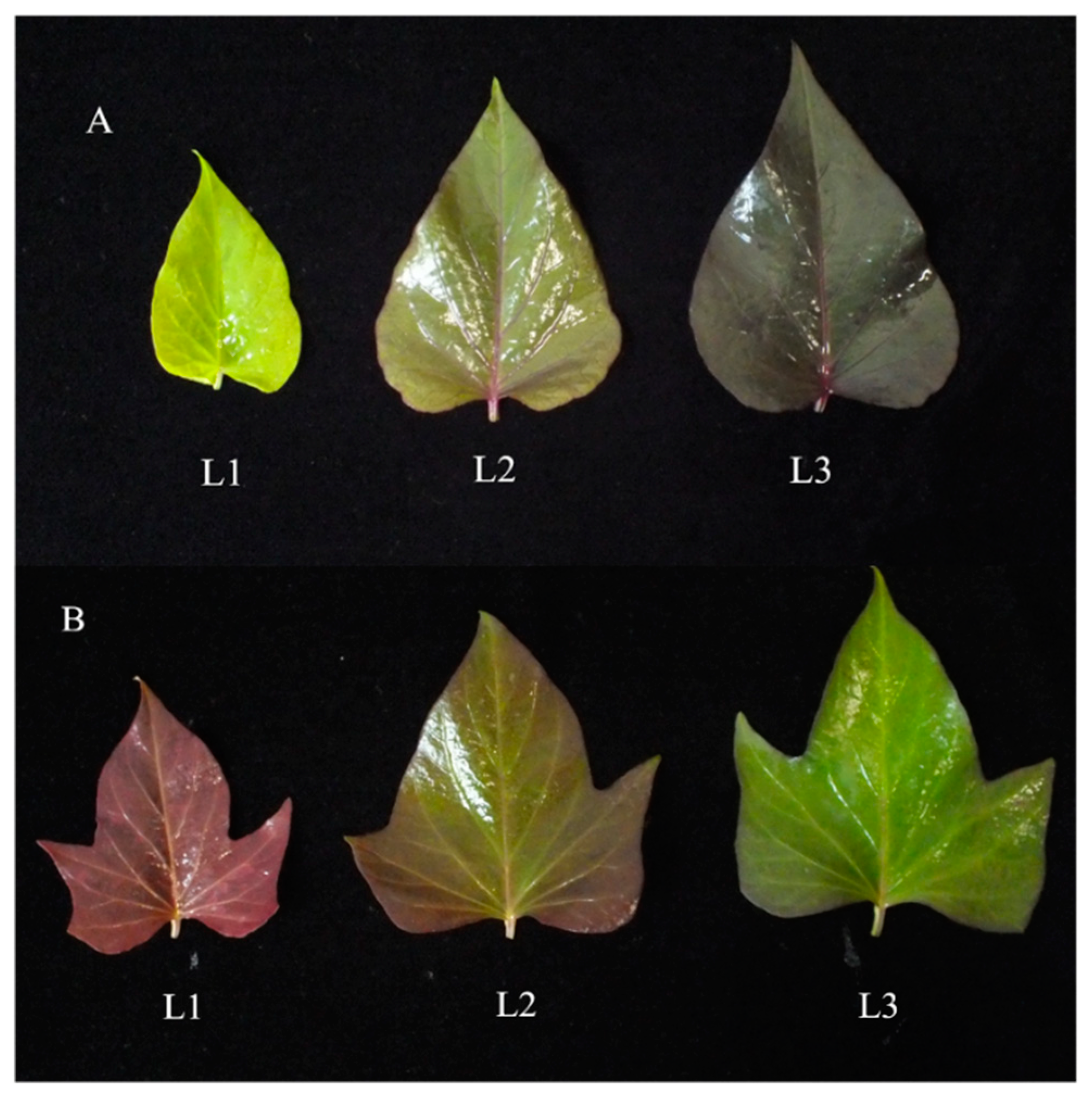
3.1. Total Monomeric Anthocyanin Content in the Leaves of the Cultivars Fushu No. 23 and Fushu No. 317
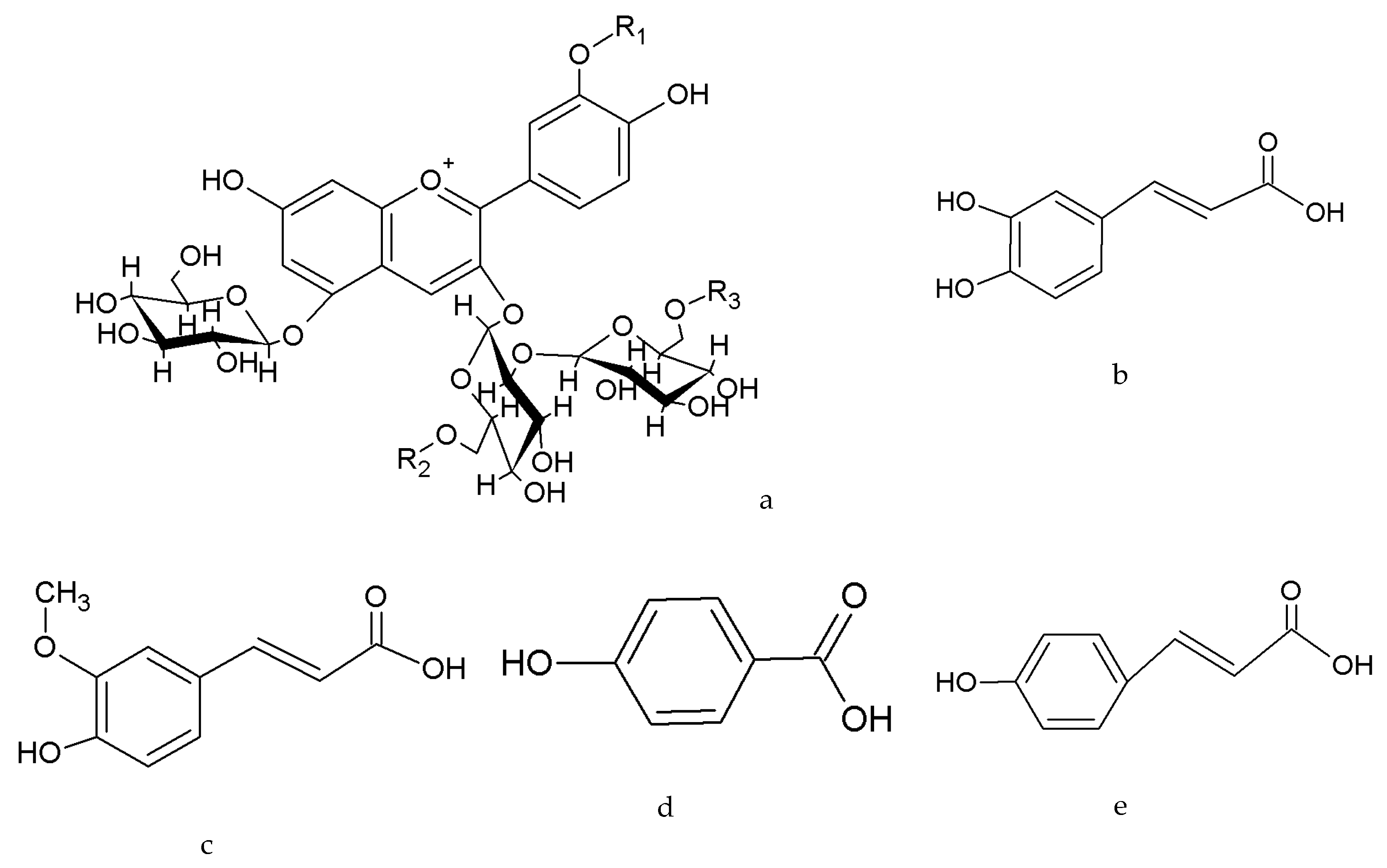
3.2. Differential Accumulation of Anthocyanins in the Leaves of the Cultivars Fushu No. 23 and Fushu No. 317
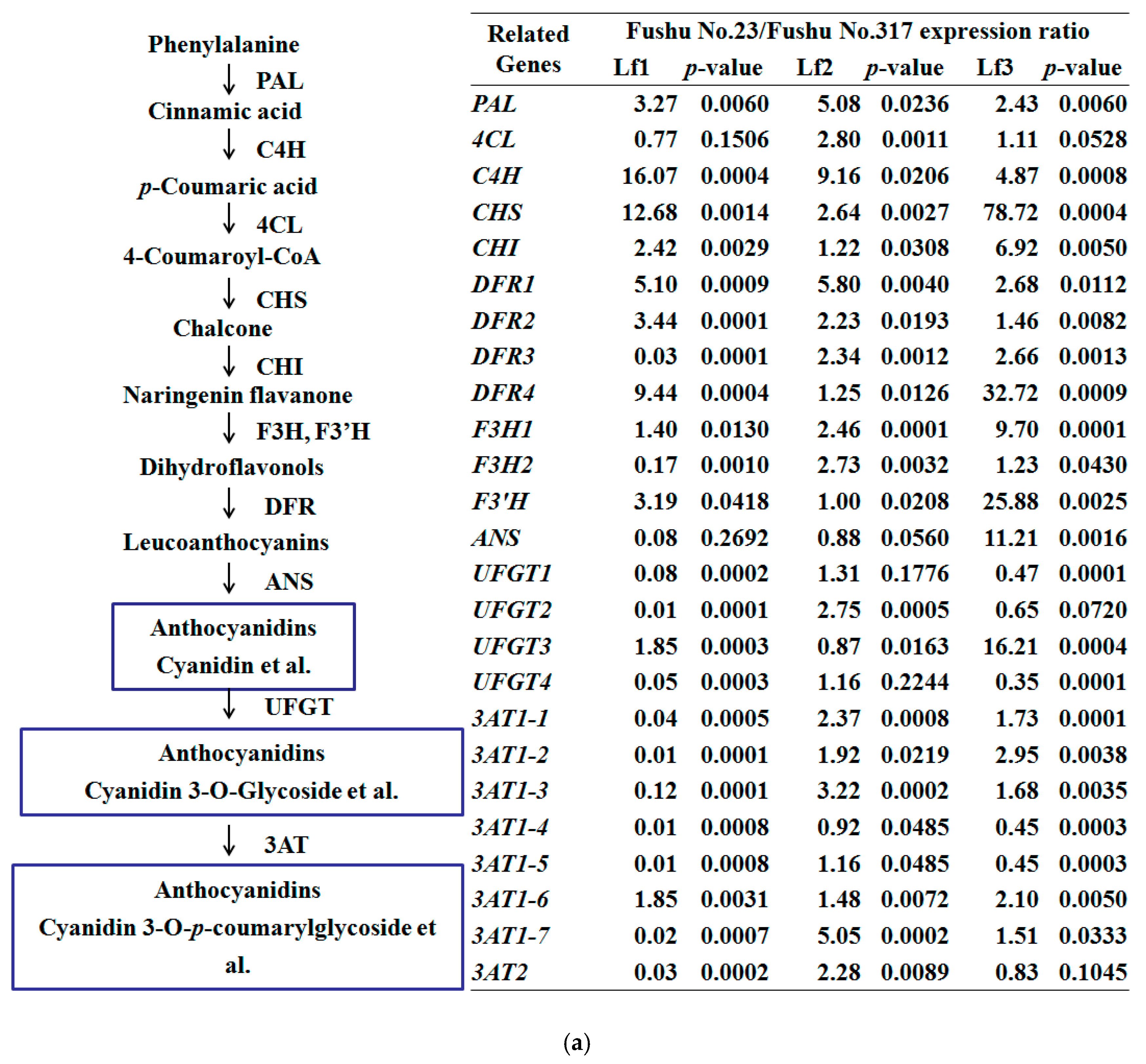
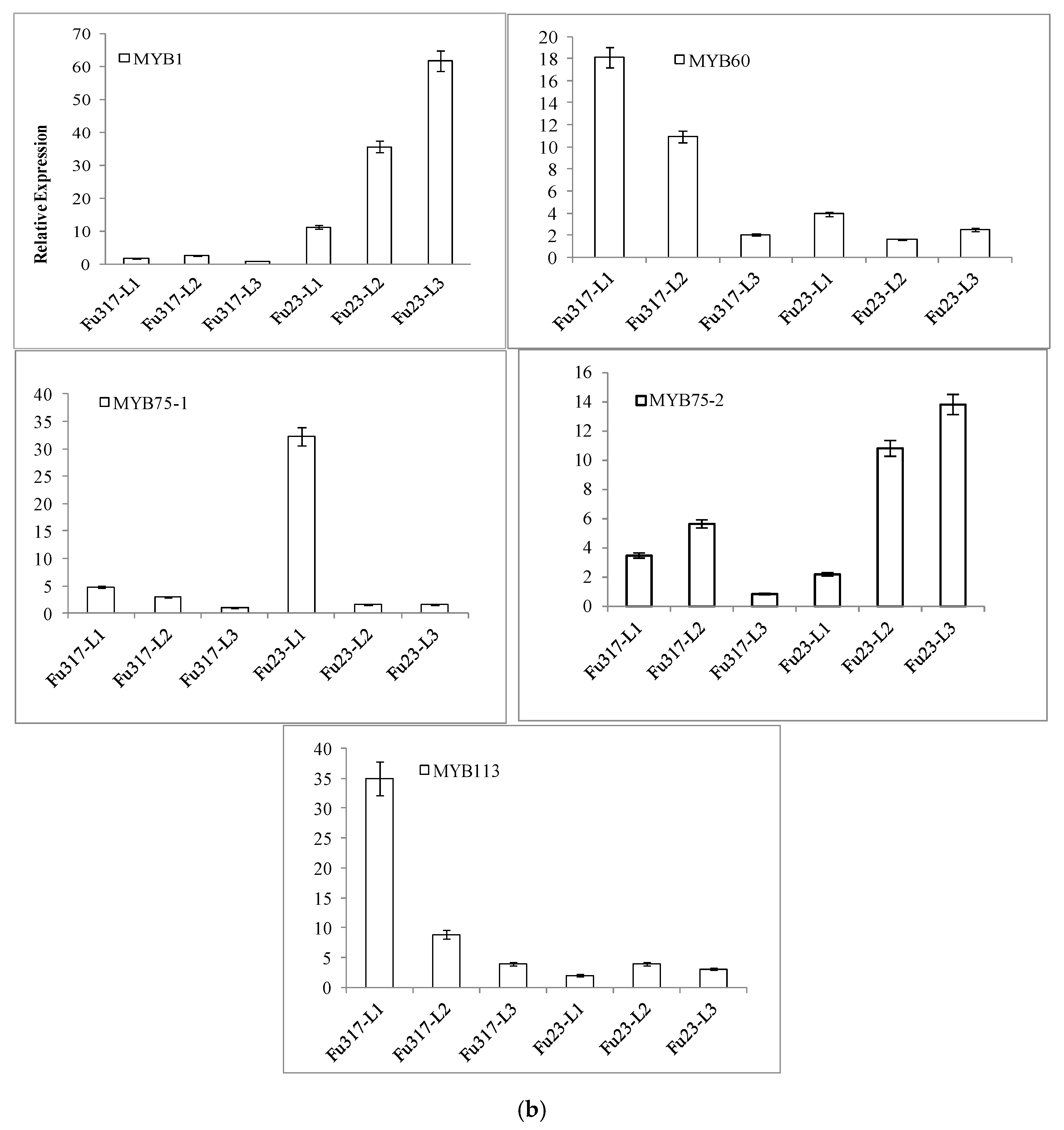
3.3. Expression of Anthocyanidin Biosynthesis Structural Genes and Transcription Factor
3.4. Anthocyanins in Roots of Purple-fleshed Sweet Potato Were Different from the Anthocyanins in Sweet Potato Leaves
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| PAL | phenylalanine ammonialyase |
| C4L | cinnamic acid-4-hydroxylase |
| 4CL | 4-coumarate:CoA ligase |
| CHS | chalconesynthase |
| CHI | chalconeisomerase |
| F3H | flavanone 3-hydroxylase |
| F3′H | flavonoids 3′-hydroxylase |
| DFR | dihydrofavonol 4-reductase |
| ANS | anthocyanin synthase |
| UFGT | UDP-glucose flavonoid 3-O-glucosyltransferase |
| 3AT | anthocyanin 3-O-acyltransferases |
| qRT-PCR | quantitative real time polymerase chain reaction |
| SD | standard deviation |
| UPLC-QTOF-MS | ultra-performance liquid chromatography/quadrupole time-of-flight mass spectrometry |
| UPLC-DAD | ultra-performance liquid chromatography diode-array detection |
| FW | fresh weight |
References
- Tahara, M. Current developments in breeding, genetics, genomics, and molecular biology applied to sweetpotato improvement. Breed. Sci. 2017, 67, 1. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Ishiguro, K.; Oki, T.; Okuno, S. Functional components in sweetpotato and their genetic improvement. Breed. Sci. 2017, 67, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Cai, N.T.; Huang, H.K.; Qiu, Y.X.; Zheng, X.; Wu, Q.Y.; Luo, W.B.; Li, G.X. Breeding and cultivate techniques fo new sweetpotato variety Fushu 7-6 used as leaf vegetable. Fujian J. Agric. Sci. 2006, 1, 12–15. [Google Scholar]
- Tian, Q.; Konczak, I.; Schwartz, S.J. Probing anthocyanin profiles in purple sweetpotato cell line (Ipomoea batatas L. Cv. Ayamurasaki) by high-performance liquid chromatography and electrospray ionization tandem mass spectrometry. J. Agric. Food Chem. 2005, 53, 6503–6509. [Google Scholar] [CrossRef] [PubMed]
- Truong, V.-D.; Deighton, N.; Thompson, R.T.; McFeeters, R.F.; Dean, L.O.; Pecota, K.V.; Yencho, G.C. Characterization of Anthocyanins and Anthocyanidins in Purple-Fleshed Sweetpotatoes by HPLC-DAD/ESI-MS/MS. J. Agric. Food Chem. 2010, 58, 404–410. [Google Scholar] [CrossRef]
- Lee, M.J.; Park, J.S.; Choi, D.S.; Jung, M.Y. Characterization and quantitation of anthocyanins in purple-fleshed sweetpotatoes cultivated in Korea by HPLC-DAD and HPLC-ESI-QTOF-MS/MS. J. Agric. Food Chem. 2013, 61, 3148–3158. [Google Scholar] [CrossRef]
- Xu, J.; Su, X.; Lim, S.; Griffin, J.; Carey, E.; Katz, B.; Tomich, J.; Smith, J.S.; Wang, W. Characterisation and stability of anthocyanins in purple-fleshed sweetpotato P40. Food Chem. 2015, 186, 90–96. [Google Scholar] [CrossRef]
- He, W.; Zeng, M.; Chen, J.; Jiao, Y.; Niu, F.; Tao, G.; Zhang, S.; Qin, F.; He, Z. Identification and quantitation of anthocyanins in purple-fleshed sweetpotatoes cultivated in china by UPLC-PDA and UPLC-QTOF-MS/MS. J. Agric. Food Chem. 2016, 64, 171–177. [Google Scholar] [CrossRef]
- Terahara, N.; Shimizu, T.; Kato, Y.; Nakamura, M.; Maitani, T.; Yamaguchi, M.-A.; Goda, Y. Six diacylated anthocyanins from the storage roots of purple sweetpotato, (Ipomoea batatas). Biosci. Biotechnol. Biochem. 1999, 63, 1420–1424. [Google Scholar] [CrossRef][Green Version]
- Islam, M.S.; Yoshimoto, M.; Terahara, N.; Yamakawa, O. Anthocyanin compositions in sweetpotato (Ipomoea batatas L.) leaves. Biosci. Biotechnol. Biochem. 2002, 66, 2483–2486. [Google Scholar] [CrossRef]
- Su, X.Y.; Griffin, J.; Xu, J.W.; Ouyan, P.; Zhao, Z.H.; Wang, W.Q. Identification and quantification of anthocyanins in purple-fleshed sweet potato leaves. Heliyon 2019, 5, e01964. [Google Scholar] [CrossRef] [PubMed]
- Vishnu, V.R.; Renjith, R.S.; Mukherjee, A.; Anil, S.R.; Sreekumar, J.; Jyothi, A.N. Comparative study on the chemical structure and in vitro antiproliferative activity of anthocyanins in purple root tubers and leaves of sweetpotato (Ipomoea batatas). J. Agric. Food Chem. 2019, 67, 2467–2475. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Zhang, P.; Zhu, Y.; Lou, Q.; He, S. Antioxidant and prebiotic activity of five peonidin-based anthocyanins extracted from purple sweetpotato (Ipomoea batatas (L.) Lam.). Sci. Rep. 2018, 8, 5018. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, J.; Wang, Z.; Gao, H.; Su, L.; Xie, J.; Chen, X.; Liang, H.; Wang, C.; Han, Y. Oral hepatoprotective ability evaluation of purple sweetpotato anthocyanins on acute and chronic chemical liver injuries. Cell Biochem. Biophys. 2014, 69, 539–548. [Google Scholar] [CrossRef]
- Hwang, Y.P.; Choi, J.H.; Yun, H.J.; Han, E.H.; Kim, H.G.; Kim, J.Y.; Park, B.H.; Khanal, T.; Choi, J.M.; Chung, Y.C.; et al. Anthocyanins from purple sweetpotato attenuate dimethylnitrosamine-induced liver injury in rats by inducing Nrf2-mediated antioxidant enzymes and reducing COX-2 and iNOS expression. Food Chem. Toxicol. 2011, 49, 93–99. [Google Scholar] [CrossRef]
- Zhao, J.G.; Yan, Q.Q.; Lu, L.Z.; Zhang, Y.Q. In vivo antioxidant, hypoglycemic, and anti-tumor activities of anthocyanin extracts from purple sweetpotato. Nutr. Res. Pract. 2013, 7, 359–365. [Google Scholar] [CrossRef]
- Zhu, Y.; Bo, Y.; Wang, X.; Lu, W.; Wang, X.; Han, Z.; Qiu, C. The effect of anthocyanins on blood pressure: A prisma-compliant meta-analysis of randomized clinical trials. Medince (Baltim.) 2016, 95, e3380. [Google Scholar] [CrossRef]
- Zhuang, J.; Lu, J.; Wang, X.; Wang, X.; Hu, W.; Hong, F.; Zhao, X.X.; Zheng, Y.L. Purple sweet potato color protects against high-fat diet-induced cognitive deficits through AMPK-mediated autophagy in mouse hippocampus. J. Nutr. Biochem. 2019, 65, 35–45. [Google Scholar] [CrossRef]
- Zhang, Z.C.; Su, G.H.; Luo, C.L.; Pang, Y.L.; Wang, L.; Li, X.; Wen, J.H.; Zhang, J.L. Effects of anthocyanins from purple sweet potato (Ipomoea batatas L. cultivar Eshu No. 8) on the serum uric acid level and xanthine oxidase activity in hyperuricemic mice. Food Funct. 2015, 6, 3045–3055. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 6, 1101–1108. [Google Scholar] [CrossRef]
- Dixon, R.A.; Liu, C.; Jun, J.H. Metabolic engineering of anthocyanins and condensed tannins in plants. Curr. Opin. Biotechnol. 2013, 24, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Gong, Y.; Jin, S.; Zhu, Q. Molecular analysis of a UDP-glucose: Flavonoid 3-O-glucosyltransferase (UFGT) gene from purple potato (Solanum tuberosum). Mol. Biol. Rep. 2011, 38, 561–567. [Google Scholar] [CrossRef]
- Luo, J.; Nishiyama, Y.; Fuell, C.; Taguchi, G.; Elliott, K.; Hill, L.; Tanaka, Y.; Kitayama, M.; Yamazaki, M.; Bailey, P.; et al. Convergent evolution in the BAHD family of acyl transferases: Identification and characterization of anthocyanin acyl transferases from Arabidopsis thaliana. Plant J. Cell Mol. Biol. 2007, 50, 678–695. [Google Scholar] [CrossRef] [PubMed]
- Unno, H.; Ichimaida, F.; Suzuki, H.; Takahashi, S.; Tanaka, Y.; Saito, A.; Nishino, T.; Kusunoki, M.; Nakayama, T. Structural and mutational studies of anthocyanin malonyltransferases establish the features of BAHD enzyme catalysis. J. Biol. Chem. 2007, 282, 15812–15822. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Fan, W.; Wu, Y.; Zhang, P.; Wang, C.; Yang, J.; Appelhagen, I. A novel glycosyltransferase catalyses the transfer of glucose to glucosylated anthocyanins in purple sweetpotato. J. Exp. Bot. 2018, 69, 5444–5459. [Google Scholar] [PubMed]
- Xie, F.; Burklew, C.E.; Yang, Y.; Liu, M.; Xiao, P.; Zhang, B.; Qiu, D. De novo sequencing and a comprehensive analysis of purple sweetpotato (Ipomoea batatas L.) transcriptome. Planta 2012, 236, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Mano, H.; Ogasawara, F.; Sato, K.; Higo, H.; Minobe, Y. Isolation of a regulatory gene of anthocyanin biosynthesis in tuberous roots of purple-fleshed sweetpotato. Plant Physiol. 2007, 143, 1252–1268. [Google Scholar] [CrossRef]
- Park, J.S.; Kim, J.B.; Cho, K.J.; Cheon, C.I.; Sung, M.K.; Choung, M.G.; Roh, K.H. Arabidopsis R2R3-MYB transcription factor AtMYB60 functions as a transcriptional repressor of anthocyanin biosynthesis in lettuce (Lactuca sativa). Plant Cell Rep. 2008, 27, 985–994. [Google Scholar] [CrossRef]
- Teng, S.; Keurentjes, J.; Bentsink, L.; Koornneef, M.; Smeekens, S. Sucrose-specific induction of anthocyanin biosynthesis in Arabidopsis requires the MYB75/PAP1 gene. Plant Physiol. 2005, 139, 1840–1852. [Google Scholar] [CrossRef]
- Li, X.; Gao, M.J.; Pan, H.Y.; Cui, D.J.; Gruber, M.Y. Purple canola: Arabidopsis PAP1 increases antioxidants and phenolics in Brassica napus leaves. J. Agric. Food Chem. 2010, 58, 1639–1645. [Google Scholar] [CrossRef]
- Gonzalez, A.; Zhao, M.; Leavitt, J.M.; Lloyd, A.M. Regulation of the anthocyanin biosynthetic pathway by the TTG1/bHLH/Myb transcriptional complex in Arabidopsis seedlings. Plant J. Cell Mol. Biol. 2008, 53, 814–827. [Google Scholar] [CrossRef] [PubMed]
- Baudry, A.; Caboche, M.; Lepiniec, L. TT8 controls its own expression in a feedback regulation involving TTG1 and homologous MYB and bHLH factors, allowing a strong and cell-specific accumulation of flavonoids in Arabidopsis thaliana. Plant J. Cell Mol. Biol. 2006, 46, 768–779. [Google Scholar] [CrossRef] [PubMed]
- Goda, Y.; Shimizu, T.; Kato, Y.; Nakamura, M.; Maitani, T.; Yamada, T.; Terahara, N.; Yamaguchi, M. Two acylated anthocyanins from purple sweet-potato. Phytochemistry 1997, 44, 183–186. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
| Peak | Rt a (min) | Fragment Ions (m/z) | [M]+ (m/z) | Identity b | ||
|---|---|---|---|---|---|---|
| 1 | 4.45 | 287 | 449 | 611 | 773.2117 | Cy 3-soph-5glc |
| 2 | 4.99 | 301 | 463 | 625 | 787.2266 | Peo 3-soph-5glc |
| 3 | 5.92 | 287 | 449 | 731 | 893.2325 | Cy 3-p-hydroxybenzoylsoph-5glc |
| 4 | 6.10 | 287 | 449 | 773 | 935.2421 | Cy 3-caffeylsophsoph-5glc |
| 5 | 6.63 | 301 | 625 | 745 | 907.2479 | Peo 3-p-hydroxybenzoylsoph-5glc |
| 6 | 6.77 | 301 | 463 | 787 | 949.2578 | Peo 3-caffeylsophsoph-5glc |
| 7 | 6.86 | 287 | 449 | 757 | 919.2477 | Cy 3-p-coumarylsoph-5glc |
| 8 | 6.98 | 287 | 449 | 787 | 949.2577 | Cy 3-feruloylsoph-5glc |
| 9 | 7.50 | 301 | 463 | 771 | 933.2634 | Peo 3-p-coumarylsoph-5glc |
| 10 | 7.64 | 301 | 463 | 801 | 963.2734 | Peo 3-feruloylsoph-5glc |
| 11a | 7.83 | 287 | 449 | 935 | 1097.2741 | Cy 3-dicaffeylsoph-5glc |
| 11b | 7.89 | 287 | 449 | 893 | 1055.2636 | Cy 3-caffeoyl-p-hydroxybenzoylsoph-5glc |
| 12a | 8.33 | 287 | 449 | 949 | 1081.2794 | Cy 3-caffeoyl-p-coumarylsoph-5glc |
| 12b | 8.35 | 287 | 433 | 919 | 1111.2898 | Cy 3-caffeoyl-feruloylsoph-5glc |
| 13a | 8.52 | 301 | 463 | 907 | 1111.2898 | Peo 3-dicaffeoylsoph-5glc |
| 13b | 8.62 | 301 | 463 | 949 | 1069.2791 | Peo 3-caffeoyl-p-hydroxybenzoylsoph-5glc |
| 14a | 9.05 | 301 | 463 | 933 | 1095.2942 | Peo 3-caffeoyl-p-coumarylsoph-5glc |
| 14b | 9.05 | 301 | 463 | 963 | 1125.3038 | Peo 3-caffeoyl-feruloylsoph-5glc |
| Identity a | Fushu No. 23 | Fushu No. 317 | ||||
|---|---|---|---|---|---|---|
| Lf1 | Lf2 | Lf3 | Lf1 | Lf2 | Lf3 | |
| Cy 3-soph-5glc | 0.93 ± 0.22 | 1.65 ± 0.10 | 0.58 ± 0.06 | 3.47 ± 0.36 | 1.36 ± 0.17 | 0.21 ± 0.02 |
| Peo 3-soph-5glc | 0.68 ± 0.36 | 2.31 ± 0.12 | 1.40 ± 0.21 | 5.88 ± 0.84 | 2.76 ± 0.60 | 0.53 ± 0.05 |
| Cy 3-p-hydroxybenzoylsoph-5glc | 2.46 ± 0.50 | 6.66 ± 0.39 | 5.71 ± 0.43 | 10.41 ± 0.10 | 4.06 ± 1.11 | 0.63 ± 0.06 |
| Cy 3-caffeylsophsoph-5glc | 0.14 ± 0.00 | 0.40 ± 0.07 | 0.79 ± 0.11 | 11.09 ± 1.14 | 4.55 ± 1.08 | 0.31 ± 0.02 |
| Peo 3-p-hydroxybenzoylsoph-5glc | 0.98 ± 0.49 | 3.55 ± 0.11 | 2.85 ± 0.22 | 5.72 ± 0.05 | 2.76 ± 1.00 | 0.45 ± 0.08 |
| Peo 3-caffeylsophsoph-5glc | 0.02 ± 0.00 | 0.11 ± 0.02 | 0.18 ± 0.01 | 5.45 ± 1.16 | 2.00 ± 0.67 | 0.14 ± 0.01 |
| Cy 3-p-coumarylsoph-5glc | 0.06 ± 0.01 | 0.17 ± 0.04 | 0.12 ± 0.01 | 21.23 ± 2.04 | 7.42 ± 1.91 | 0.55 ± 0.05 |
| Cy 3-feruloylsoph-5glc | 0.21 ± 0.01 | 0.38 ± 0.03 | 0.45 ± 0.08 | 4.65 ± 0.72 | 1.63 ± 0.19 | 0.31 ± 0.05 |
| Peo 3-p-coumarylsoph-5glc | 0.11 ± 0.01 | 0.42 ± 0.07 | 0.31 ± 0.01 | 15.42 ± 0.79 | 6.84 ± 2.22 | 0.35 ± 0.04 |
| Peo 3-feruloylsoph-5glc | 0.05 ± 0.03 | 0.18 ± 0.04 | 0.20 ± 0.01 | 4.50 ± 0.85 | 1.45 ± 0.35 | 0.33 ± 0.06 |
| Cy 3-dicaffeylsoph-5glc | 0.24 ± 0.07 | 0.71 ± 0.13 | 3.27 ± 0.66 | 11.08 ± 0.82 | 6.34 ± 2.36 | 0.11 ± 0.01 |
| Cy 3-caffeoyl-p-hydroxybenzoylsoph-5glc | 1.43 ± 0.28 | 4.04 ± 0.61 | 8.57 ± 1.54 | 4.52 ± 0.40 | 2.61 ± 0.95 | 0.09 ± 0.01 |
| Cy 3-caffeoyl-p-coumarylsoph-5glc | 0.02 ± 0.00 | 0.10 ± 0.03 | 0.16 ± 0.04 | 7.24 ± 0.53 | 4.18±1.27 | 0.05 ± 0.00 |
| Cy 3-caffeoyl-feruloylsoph-5glc | 0.06 ± 0.03 | 0.21 ± 0.02 | 0.70 ± 0.22 | 5.07 ± 0.69 | 2.14 ± 0.40 | 0.10 ± 0.00 |
| Peo 3-dicaffeoylsoph-5glc | 0.02 ± 0.00 | 0.09 ± 0.01 | 0.40 ± 0.03 | 4.81 ± 0.35 | 2.72 ± 1.11 | 0.04 ± 0.00 |
| Peo 3-caffeoyl-p-hydroxybenzoylsoph-5glc | 0.20 ± 0.09 | 0.88 ± 0.08 | 1.56 ± 0.06 | 2.31 ± 0.14 | 1.42 ± 0.61 | 0.04 ± 0.00 |
| Peo 3-caffeoyl-p-coumarylsoph-5glc | - | 0.02 ± 0.00 | 0.02 ± 0.00 | 2.57 ± 0.28 | 2.12 ± 0.67 | 0.01 ± 0.00 |
| Peo 3-caffeoyl-feruloylsoph-5glc | - | 0.04 ± 0.01 | 0.14 ± 0.02 | 1.92 ± 0.45 | 0.85 ± 0.24 | 0.03 ± 0.00 |
| Total anthocyanin | 7.62 ± 1.43 | 21.92 ± 1.25 | 27.41 ± 2.69 | 127.33 ± 7.55 | 57.20 ± 6.75 | 4.28 ± 0.25 |
| non-acylated anthocyanin | 0.98 ± 0.24 | 1.84 ± 0.14 | 0.78 ± 0.06 | 7.97 ± 1.21 | 2.81 ± 0.51 | 0.53 ± 0.09 |
| monoacylated anthocyanin | 6.01 ± 1.75 | 17.98 ± 1.48 | 23.46 ± 3.42 | 60.99 ± 4.58 | 29.39 ± 8.78 | 2.28 ± 0.24 |
| diacylated anthocyanin | 0.63 ± 0.13 | 2.10 ± 0.25 | 3.17 ± 0.21 | 58.37 ± 5.93 | 25.00 ± 7.62 | 1.48 ± 0.17 |
| cyanidin-based anthocyanin | 5.59 ± 1.59 | 15.66 ± 0.93 | 12.38 ± 1.11 | 83.32 ± 7.19 | 33.37 ± 8.95 | 3.47 ± 0.38 |
| peonidin-based anthocyanin | 2.03 ± 0.52 | 6.26 ± 0.94 | 15.03 ± 2.58 | 44.01 ± 4.52 | 23.83 ± 7.96 | 0.81 ± 0.11 |
| Identity a | Leaves | Roots | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fushu No. 23 | Fushu No. 317 | Fushu No. 25 | OP | Yanshu No. 5 | Fushu No. 9 | Fushu No. 24 | Longzishu No. 4 | Longzishu No. 6 | Fushu No. 317 | |
| Cy 3-soph-5glc | 3.16 ± 0.27 | 5.04 ± 0.62 | 4.27 ± 0.32 | 7.51 ± 0.46 | 3.75 ± 0.26 | 2.82 ± 0.16 | 3.60 ± 0.25 | 1.30 ± 0.09 | 3.21 ± 0.32 | 42.02 ± 2.54 |
| Peo 3-soph-5glc | 4.38 ± 0.46 | 9.16 ± 0.94 | 2.62 ± 0.15 | 5.38 ± 0.32 | 8.15 ± 0.52 | 4.82 ± 0.25 | 4.02 ± 0.35 | 5.13 ± 0.69 | 27.41 ± 1.86 | 57.40 ± 3.68 |
| Cy 3-p-hydroxybenzoylsoph-5glc | 14.83 ± 1.98 | 15.09 ± 2.21 | 3.58 ± 0.16 | 2.55 ± 0.15 | 1.27 ± 0.08 | 2.87 ± 0.12 | 3.41 ± 0.27 | 3.24 ± 0.73 | 12.62 ± 0.79 | 112.39 ± 8.52 |
| Cy 3-caffeylsophsoph-5glc | 1.33 ± 0.15 | 15.94 ± 2.66 | 14.31 ± 0.86 | 16.64 ± 1.12 | 14.19 ± 1.03 | 1.72 ± 0.08 | 7.51 ± 0.43 | 16.15 ± 0.89 | 27.93 ± 1.56 | 19.97 ± 1.64 |
| Peo 3-p-hydroxybenzoylsoph-5glc | 7.39 ± 0.66 | 8.93 ± 1.04 | 5.10 ± 0.35 | 12.33 ± 0.84 | 6.17 ± 0.46 | 1.12 ± 0.07 | 13.84 ± 0.95 | 1.65 ± 0.06 | 4.91 ± 0.23 | 135.07 ± 9.36 |
| Peo 3-caffeylsophsoph-5glc | 0.31 ± 0.06 | 7.58 ± 0.98 | 3.70 ± 0.21 | 10.47 ± 0.76 | 6.75 ± 0.81 | 0.99 ± 0.11 | 0.77 ± 0.06 | - | 3.67 ± 0.28 | 13.28 ± 1.12 |
| Cy 3-p-coumarylsoph-5glc | 0.34 ± 0.07 | 29.20±1.45 | 22.26 ± 2.00 | 40.33 ± 2.38 | 20.16 ± 1.74 | - | - | - | - | - |
| Cy 3-feruloylsoph-5glc | 1.04 ± 0.15 | 6.59 ± 0.73 | 8.08 ± 0.19 | 9.10 ± 0.55 | 5.87 ± 0.44 | 11.46 ± 1.34 | 1.21 ± 0.08 | 8.53 ± 0.77 | 19.29 ± 1.64 | 11.32 ± 0.88 |
| Peo 3-p-coumarylsoph-5glc | 0.84 ± 0.11 | 22.61 ± 1.56 | 27.73 ± 1.87 | 31.23 ± 2.53 | 15.62 ± 1.15 | - | 4.15 ± 0.41 | 2.81 ± 0.16 | 8.47 ± 0.53 | 51.15 ± 3.63 |
| Peo 3-feruloylsoph-5glc | 0.43 ± 0.05 | 6.27 ± 0.42 | 7.69 ± 0.23 | 8.67 ± 0.79 | 5.58 ± 0.65 | 19.71 ± 1.74 | 3.47 ± 0.26 | 7.84 ± 0.63 | 61.44 ± 3.86 | 17.28 ± 1.41 |
| Cy 3-dicaffeylsoph-5glc | 4.23 ± 0.51 | 17.53 ± 2.08 | 21.49 ± 1.68 | 24.21 ± 1.82 | 12.1 ± 1.04 | 1.04 ± 0.07 | 3.11 ± 0.22 | 16.72 ± 0.92 | 14.07 ± 1.12 | 41.56 ± 3.76 |
| Cy 3-caffeoyl-p-hydroxybenzoylsoph-5glc | 14.04 ± 2.11 | 7.22 ± 0.55 | 8.85 ± 0.66 | 9.97 ± 0.73 | 6.42 ± 0.47 | 3.27 ± 0.19 | - | 3.04 ± 0.23 | 3.99 ± 0.24 | 32.23 ± 2.09 |
| Cy 3-caffeoyl-p-coumarylsoph-5glc | 0.28 ± 0.05 | 11.48 ± 0.92 | 14.07 ± 1.12 | 15.85 ± 1.75 | 7.92 ± 0.78 | - | - | - | - | - |
| Cy 3-caffeoyl-feruloylsoph-5glc | 0.97 ± 0.10 | 7.30 ± 0.67 | 5.06 ± 0.36 | 10.09 ± 0.95 | 6.50 ± 0.47 | 9.15 ± 1.22 | 0.91 ± 0.04 | 17.33 ± 0.81 | 3.77 ± 0.13 | 82.11 ± 5.49 |
| Peo 3-dicaffeoylsoph-5glc | 0.50 ± 0.07 | 7.58 ± 0.72 | 6.37 ± 0.43 | 10.47 ± 1.07 | 5.23 ± 0.32 | 13.07 ± 1.68 | 10.63 ± 0.78 | 40.75 ± 2.58 | 63.36 ± 4.75 | 37.02 ± 1.83 |
| Peo 3-caffeoyl-p-hydroxybenzoylsoph-5glc | 2.64 ± 0.33 | 3.77 ± 0.21 | 8.26 ± 0.61 | 5.21 ± 0.33 | 3.36 ± 0.23 | 1.52 ± 0.13 | 1.00 ± 0.03 | 18.17 ± 0.94 | 1.62 ± 0.08 | 166.51 ± 10.25 |
| Peo 3-caffeoyl-p-coumarylsoph-5glc | 0.04 ± 0.01 | 4.70 ± 0.37 | 5.76 ± 0.16 | 6.49 ± 0.49 | 3.25 ± 0.28 | - | - | - | - | 181.75 ± 9.58 |
| Peo 3-caffeoyl-feruloylsoph-5glc | 0.18 ± 0.02 | 2.80 ± 0.22 | 3.43 ± 0.04 | 3.87 ± 0.22 | 2.49 ± 0.17 | 3.75 ± 0.26 | - | - | - | 8.22 ± 0.63 |
| Total anthocyanin | 56.95 ± 7.10 | 188.81 ± 18.35 | 172.65 ± 11.41 | 230.36 ± 17.26 | 134.8 ± 9.65 | 77.32 ± 7.42 | 57.64 ± 4.13 | 142.66 ± 9.50 | 255.75 ± 17.39 | 1009.29 ± 66.41 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, G.; Lin, Z.; Zhang, H.; Liu, Z.; Xu, Y.; Xu, G.; Li, H.; Ji, R.; Luo, W.; Qiu, Y.; et al. Anthocyanin Accumulation in the Leaves of the Purple Sweet Potato (Ipomoea batatas L.) Cultivars. Molecules 2019, 24, 3743. https://doi.org/10.3390/molecules24203743
Li G, Lin Z, Zhang H, Liu Z, Xu Y, Xu G, Li H, Ji R, Luo W, Qiu Y, et al. Anthocyanin Accumulation in the Leaves of the Purple Sweet Potato (Ipomoea batatas L.) Cultivars. Molecules. 2019; 24(20):3743. https://doi.org/10.3390/molecules24203743
Chicago/Turabian StyleLi, GuoLiang, Zhaomiao Lin, Hong Zhang, Zhonghua Liu, Yongqing Xu, Guochun Xu, Huawei Li, Rongchang Ji, Wenbin Luo, Yongxiang Qiu, and et al. 2019. "Anthocyanin Accumulation in the Leaves of the Purple Sweet Potato (Ipomoea batatas L.) Cultivars" Molecules 24, no. 20: 3743. https://doi.org/10.3390/molecules24203743
APA StyleLi, G., Lin, Z., Zhang, H., Liu, Z., Xu, Y., Xu, G., Li, H., Ji, R., Luo, W., Qiu, Y., Qiu, S., & Tang, H. (2019). Anthocyanin Accumulation in the Leaves of the Purple Sweet Potato (Ipomoea batatas L.) Cultivars. Molecules, 24(20), 3743. https://doi.org/10.3390/molecules24203743





