The Combination of Whole Cell Lipidomics Analysis and Single Cell Confocal Imaging of Fluidity and Micropolarity Provides Insight into Stress-Induced Lipid Turnover in Subcellular Organelles of Pancreatic Beta Cells
Abstract
1. Introduction
2. Phospholipid Turnover in Cells
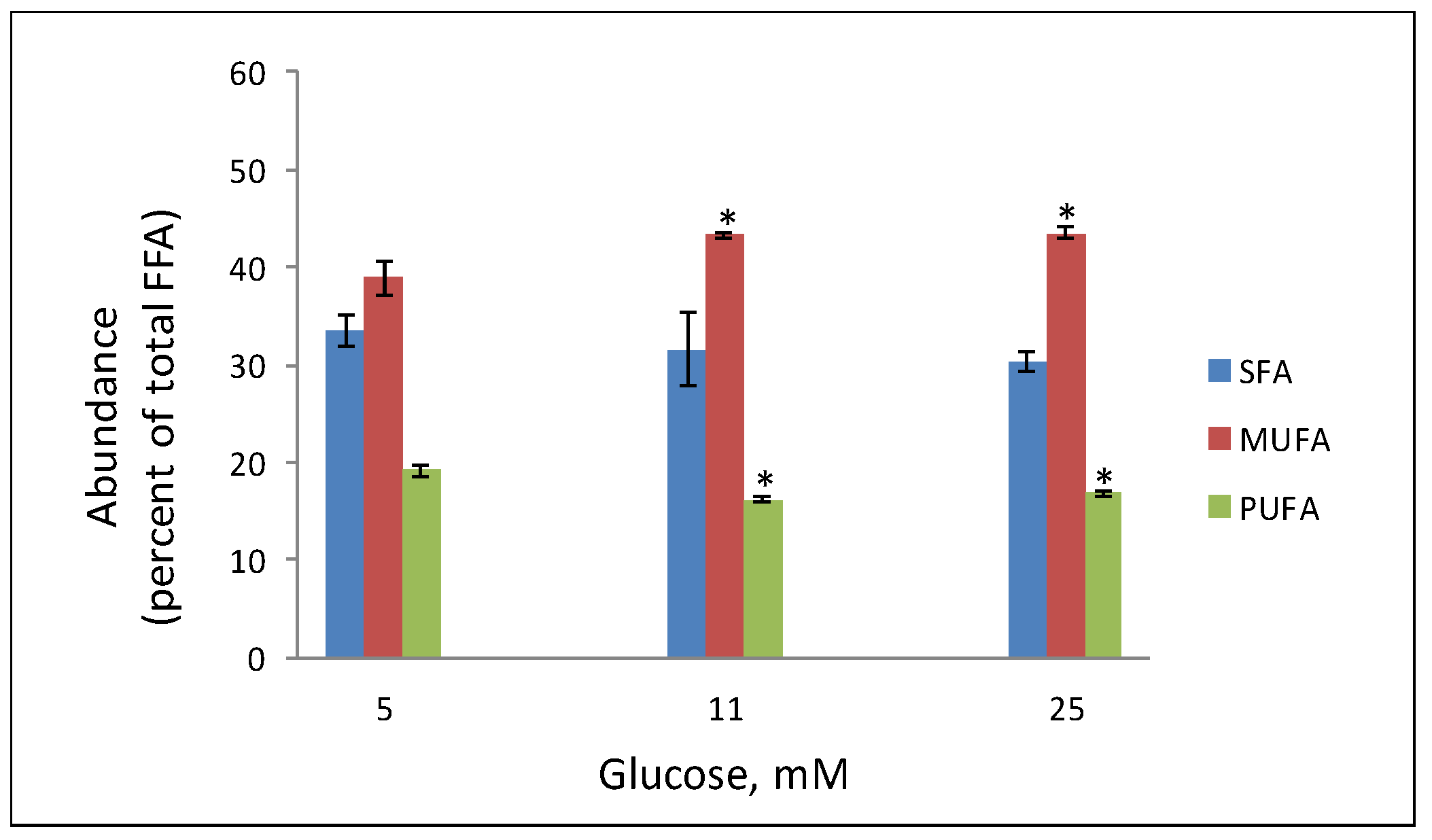
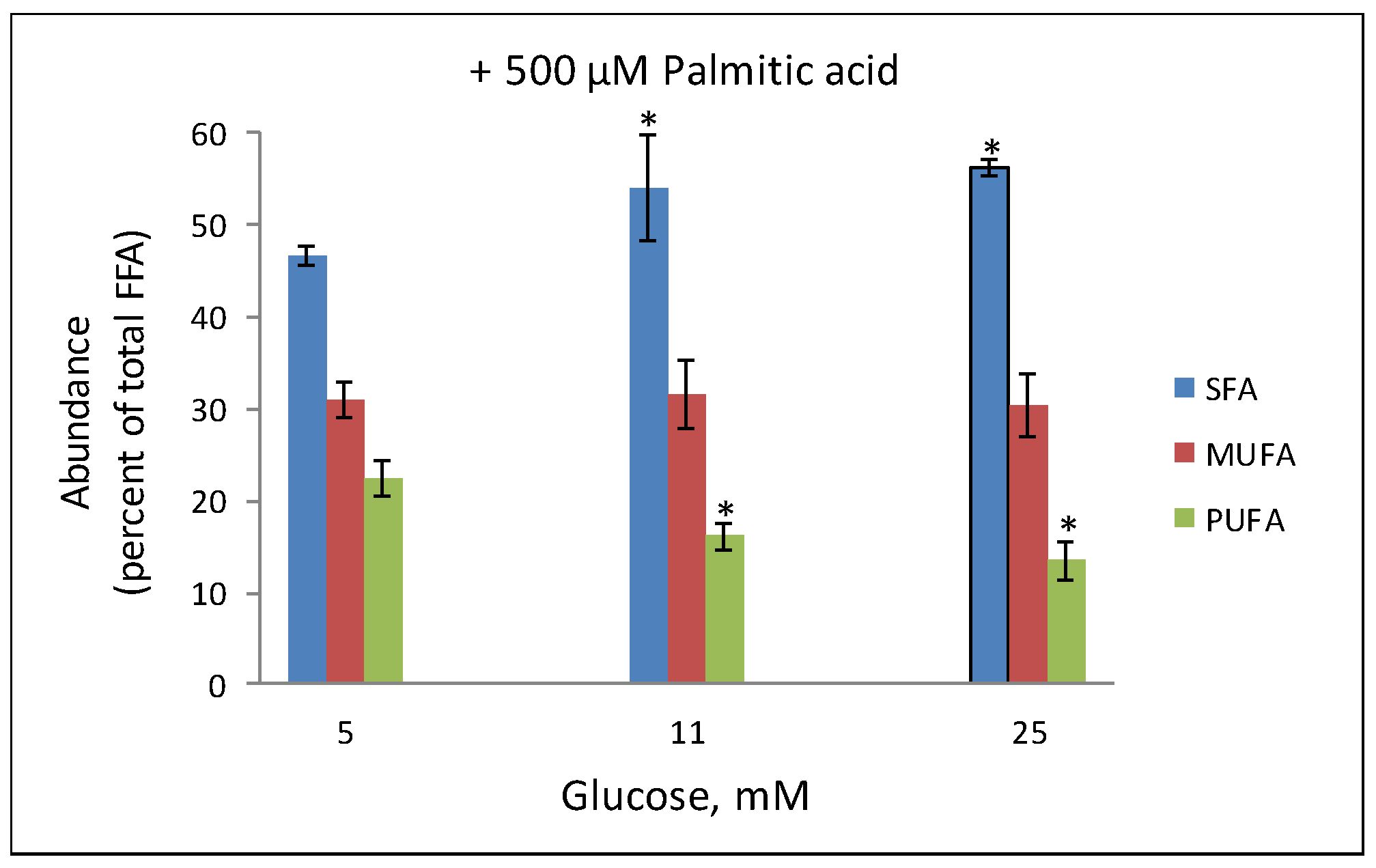
3. Lipidomic Analyses of Beta Cells
4. Confocal Imaging-Based Fluidity and Micropolarity Maps of Single Beta Cells
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Macdonald, M.J.; Ade, L.; Ntambi, J.M.; Ansari, I.-U.H.; Stoker, S.W. Characterization of Phospholipids in Insulin Secretory Granules and Mitochondria in Pancreatic Beta Cells and Their Changes with Glucose Stimulation. J. Boil. Chem. 2015, 290, 11075–11092. [Google Scholar] [CrossRef] [PubMed]
- Pearson, G.L.; Mellett, N.; Chu, K.Y.; Boslem, E.; Meikle, P.J.; Biden, T.J. A comprehensive lipidomic screen of pancreatic beta-cells using mass spectroscopy defines novel features of glucose-stimulated turnover of neutral lipids, sphingolipids and plasmalogens. Mol. Metab. 2016, 5, 404–414. [Google Scholar] [CrossRef] [PubMed]
- Ferreri, C.; Panagiotaki, M.; Chatgilialoglu, C. Trans fatty acids in membranes: The free radical path. Mol. Biotechnol. 2007, 37, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Fruhwirth, G.O.; Loidl, A.; Hermetter, A. Oxidized phospholipids: From molecular properties to disease. Biochim. Biophys. Acta 2007, 1772, 718–736. [Google Scholar] [CrossRef]
- Fex, G.; Lernmark, A. Effects of insulin secretagogues on phospholipid metabolism in pancreatic beta-cells. Biochim. Biophys. Acta 1975, 388, 1–4. [Google Scholar] [CrossRef]
- Cortizo, A.; Paladini, A.; Diaz, G.; Garcia, M.; Gagliardino, J. Changes induced by glucose in the plasma membrane properties of pancreatic islets. Mol. Cell. Endocrinol. 1990, 71, 49–54. [Google Scholar] [CrossRef]
- Best, L.; Dunlop, M.; Malaisse, W.J. Phospholipid metabolism in pancreatic islets. Cell. Mol. Life Sci. 1984, 40, 1085–1091. [Google Scholar] [CrossRef]
- Metz, S.A. Putative roles for lysophospholipids as mediators and lipoxygenase-mediated metabolites of arachidonic acid as potentiators of stimulus-secretion coupling: Dual mechanisms of p-hydroxymercuribenzoic acid-induced insulin release. J. Pharmacol. Exp. Ther. 1986, 238, 819–832. [Google Scholar]
- Neuman, J.C.; Fenske, R.J.; Kimple, M.E. Dietary polyunsaturated fatty acids and their metabolites: Implications for diabetes pathophysiology, prevention, and treatment. Nutr. Heal. Aging 2017, 4, 127–140. [Google Scholar] [CrossRef]
- Carboneau, B.A.; Breyer, R.M.; Gannon, M. Regulation of pancreatic beta-cell function and mass dynamics by prostaglandin signaling. J. Cell Commun. 2017, 11, 105–116. [Google Scholar]
- Cohen, G.; Shamni, O.; Avrahami, Y.; Cohen, O.; Broner, E.C.; Filippov-Levy, N.; Chatgilialoglu, C.; Ferreri, C.; Kaiser, N.; Sasson, S. Beta cell response to nutrient overload involves phospholipid remodelling and lipid peroxidation. Diabetologia 2015, 58, 1333–1343. [Google Scholar] [CrossRef] [PubMed]
- Cohen, G.; Riahi, Y.; Sunda, V.; Deplano, S.; Chatgilialoglu, C.; Ferreri, C.; Kaiser, N.; Sasson, S. Signaling properties of 4-hydroxyalkenals formed by lipid peroxidation in diabetes. Free. Radic. Boil. Med. 2013, 65, 978–987. [Google Scholar] [CrossRef] [PubMed]
- Cohen, G.; Riahi, Y.; Shamni, O.; Guichardant, M.; Chatgilialoglu, C.; Ferreri, C.; Kaiser, N.; Sasson, S. Role of lipid peroxidation and PPAR-delta in amplifying glucose-stimulated insulin secretion. Diabetes 2011, 60, 2830–2842. [Google Scholar] [CrossRef]
- Luo, P.; Wang, M.H. Eicosanoids, beta-cell function, and diabetes. Prostaglandins Other Lipid Mediat. 2011, 95, 1–10. [Google Scholar] [CrossRef]
- Ma, K.; Nunemaker, C.S.; Wu, R.; Chakrabarti, S.K.; Taylor-Fishwick, D.A.; Nadler, J.L. 12-Lipoxygenase Products Reduce Insulin Secretion and {beta}-Cell Viability in Human Islets. J. Clin. Endocrinol. Metab. 2010, 95, 887–893. [Google Scholar] [CrossRef]
- Keane, D.; Newsholme, P. Saturated and unsaturated (including arachidonic acid) non-esterified fatty acid modulation of insulin secretion from pancreatic beta-cells. Biochem Soc. Trans. 2008, 36, 955–958. [Google Scholar] [CrossRef]
- Imai, Y.; Cousins, R.S.; Liu, S.; Phelps, B.M.; Promes, J.A. Connecting pancreatic islet lipid metabolism with insulin secretion and the development of type 2 diabetes. Ann. N. Y. Acad. Sci. 2019. [Google Scholar] [CrossRef]
- Ye, R.; Onodera, T.; Scherer, P.E. Lipotoxicity and beta Cell Maintenance in Obesity and Type 2 Diabetes. J. Endocr. Soc. 2019, 3, 617–631. [Google Scholar] [CrossRef]
- Oh, Y.S.; Bae, G.D.; Baek, D.J.; Park, E.-Y.; Jun, H.-S. Fatty Acid-Induced Lipotoxicity in Pancreatic Beta-Cells During Development of Type 2 Diabetes. Front. Endocrinol. 2018, 9, 384. [Google Scholar] [CrossRef]
- Janikiewicz, J.; Hanzelka, K.; Kozinski, K.; Kolczynska, K.; Dobrzyn, A. Islet beta-cell failure in type 2 diabetes—Within the network of toxic lipids. Biochem. Biophys. Res. Commun. 2015, 460, 491–496. [Google Scholar] [CrossRef]
- Maulucci, G.; Di Giacinto, F.; De Angelis, C.; Cohen, O.; Daniel, B.; Ferreri, C.; De Spirito, M.; Sasson, S.; Cohen, O. Real time quantitative analysis of lipid storage and lipolysis pathways by confocal spectral imaging of intracellular micropolarity. Biochim. Biophys. Acta 2018, 1863, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Maulucci, G.; Cohen, O.; Daniel, B.; Sansone, A.; Petropoulou, P.I.; Filou, S.; Spyridonidis, A.; Pani, G.; De Spirito, M.; Chatgilialoglu, C.; et al. Fatty acid-related modulations of membrane fluidity in cells: Detection and implications. Free. Radic. Res. 2016, 50, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Maulucci, G.; Daniel, B.; Cohen, O.; Avrahami, Y.; Sasson, S. Hormetic and regulatory effects of lipid peroxidation mediators in pancreatic beta cells. Mol. Asp. Med. 2016, 49, 49–77. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Tontonoz, P. Phospholipid Remodeling in Physiology and Disease. Annu. Rev. Physiol. 2019, 81, 165–188. [Google Scholar] [CrossRef] [PubMed]
- Das, U.N. Arachidonic acid in health and disease with focus on hypertension and diabetes mellitus: A review. J. Adv. Res. 2018, 11, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Hanna, V.S.; Hafez, E.A.A. Synopsis of arachidonic acid metabolism: A review. J. Adv. Res. 2018, 11, 23–32. [Google Scholar] [CrossRef]
- Powell, W.S.; Rokach, J. Biosynthesis, biological effects, and receptors of hydroxyeicosatetraenoic acids (HETEs) and oxoeicosatetraenoic acids (oxo-ETEs) derived from arachidonic acid. Biochim. Biophys. Acta 2015, 1851, 340–355. [Google Scholar] [CrossRef]
- Brash, A.R. Arachidonic acid as a bioactive molecule. J. Clin. Investig. 2001, 107, 1339–1345. [Google Scholar] [CrossRef]
- Hauke, S.; Keutler, K.; Phapale, P.; Yushchenko, D.A.; Schultz, C. Endogenous Fatty Acids Are Essential Signaling Factors of Pancreatic beta-Cells and Insulin Secretion. Diabetes 2018, 67, 1986–1998. [Google Scholar] [CrossRef]
- Tunaru, S.; Bonnavion, R.; Brandenburger, I.; Preussner, J.; Thomas, D.; Scholich, K.; Offermanns, S. 20-HETE promotes glucose-stimulated insulin secretion in an autocrine manner through FFAR1. Nat. Commun. 2018, 9, 177. [Google Scholar] [CrossRef]
- Zhao, Y.; Fang, Q.; Straub, S.G.; Lindau, M.; Sharp, G.W. Prostaglandin E1 inhibits endocytosis in the beta-cell endocytosis. J. Endocrinol. 2016, 229, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Jourdan, T.; Godlewski, G.; Kunos, G. Endocannabinoid regulation of beta-cell functions: Implications for glycaemic control and diabetes. Diabetes Obes. Metab. 2016, 18, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Batchu, S.N.; Majumder, S.; Bowskill, B.B.; White, K.E.; Advani, S.L.; Liu, Y.; Thai, K.; Lee, W.L.; Advani, A. Prostaglandin I2 Receptor Agonism Preserves beta-Cell Function and Attenuates Albuminuria Through Nephrin-Dependent Mechanisms. Diabetes 2016, 65, 1398–1409. [Google Scholar] [CrossRef] [PubMed]
- Gurgul-Convey, E.; Hanzelka, K.; Lenzen, S. Mechanism of Prostacyclin-Induced Potentiation of Glucose-Induced Insulin Secretion. Endocrinology 2012, 153, 2612–2622. [Google Scholar] [CrossRef] [PubMed]
- Persaud, S.J.; Muller, D.; Belin, V.D.; Papadimitriou, A.; Huang, G.C.; Amiel, S.A.; Jones, P.M. Expression and function of cyclooxygenase and lipoxygenase enzymes in human islets of Langerhans. Arch. Physiol. Biochem. 2007, 113, 104–109. [Google Scholar] [CrossRef]
- Chambers, K.T.; Weber, S.M.; Corbett, J.A. PGJ2-stimulated beta-cell apoptosis is associated with prolonged UPR activation. Am. J. Physiol. Endocrinol. Metab. 2007, 292, 1052–1061. [Google Scholar] [CrossRef]
- Meng, Z.X.; Sun, J.X.; Ling, J.J.; Lv, J.H.; Zhu, D.Y.; Chen, Q.; Sun, Y.J.; Han, X. Prostaglandin E2 regulates Foxo activity via the Akt pathway: Implications for pancreatic islet beta cell dysfunction. Diabetology 2006, 49, 2959–2968. [Google Scholar] [CrossRef]
- Franca, T.; Pinto, E.; Nogueira, F.; Velho, H.V. Malignant localized fibrous tumor of the pleura. Acta Med Port 2001, 14, 435–440. [Google Scholar]
- Jones, P.M.; Persaud, S.J. Arachidonic acid as a second messenger in glucose-induced insulin secretion from pancreatic beta-cells. J. Endocrinol. 1993, 137, 7–14. [Google Scholar] [CrossRef]
- Oliveira, V.; Marinho, R.; Vitorino, D.; Santos, G.; Moraes, J.; Dragano, N.; Sartori-Cintra, A.; Pereira, L.; Catharino, R.; Da Silva, A.; et al. Diets containing alpha-linolenic (omega 3) or oleic (omega 9) fatty acids rescues obese mice from insulin resistance. Endocrinology 2015, 156, 4033–4046. [Google Scholar] [CrossRef]
- Bhaswant, M.; Poudyal, H.; Brown, L. Mechanisms of enhanced insulin secretion and sensitivity with n-3 unsaturated fatty acids. J. Nutr. Biochem. 2015, 26, 571–584. [Google Scholar] [CrossRef] [PubMed]
- Jezek, P.; Jaburek, M.; Holendova, B.; Plecita-Hlavata, L. Fatty Acid-Stimulated Insulin Secretion vs. Lipotoxicity. Molecules 2018, 23, 1483. [Google Scholar] [CrossRef] [PubMed]
- Veprik, A.; Laufer, D.; Weiss, S.; Rubins, N.; Walker, M.D. GPR41 modulates insulin secretion and gene expression in pancreatic beta-cells and modifies metabolic homeostasis in fed and fasting states. FASEB J. 2016, 30, 3860–3869. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.S. Mechanistic insights into pancreatic beta-cell mass regulation by glucose and free fatty acids. Anat. Cell Boil. 2015, 48, 16–24. [Google Scholar] [CrossRef]
- Prentice, K.J.; Wheeler, M.B. FFAR out new targets for diabetes. Cell Metab. 2015, 21, 353–354. [Google Scholar] [CrossRef]
- Wang, X.; Chan, C.B. n-3 polyunsaturated fatty acids and insulin secretion. J. Endocrinol. 2015, 224, 97–106. [Google Scholar] [CrossRef]
- Hara, T.; Ichimura, A.; Hirasawa, A. Therapeutic Role and Ligands of Medium- to Long-Chain Fatty Acid Receptors. Front. Endocrinol. 2014, 5, 83. [Google Scholar] [CrossRef]
- Nolan, C.J.; Madiraju, M.S.; Delghingaro-Augusto, V.; Peyot, M.L.; Prentki, M. Fatty acid signaling in the beta-cell and insulin secretion. Diabetes 2006, 55, 16–23. [Google Scholar] [CrossRef]
- Bellini, L.; Campana, M.; Rouch, C.; Chacinska, M.; Bugliani, M.; Meneyrol, K.; Hainault, I.; Lenoir, V.; Denom, J.; Veret, J.; et al. Protective role of the ELOVL2/docosahexaenoic acid axis in glucolipotoxicity-induced apoptosis in rodent beta cells and human islets. Diabetology 2018, 61, 1780–1793. [Google Scholar] [CrossRef]
- Johnston, L.W.; Harris, S.B.; Retnakaran, R.; Giacca, A.; Liu, Z.; Bazinet, R.P.; Hanley, A.J. Association of NEFA composition with insulin sensitivity and beta cell function in the Prospective Metabolism and Islet Cell Evaluation (PROMISE) cohort. Diabetologia 2018, 61, 821–830. [Google Scholar] [CrossRef]
- Negre-Salvayre, A.; Auge, N.; Ayala, V.; Basaga, H.; Boada, J.; Brenke, R.; Chapple, S.; Cohen, G.; Fehér, J.; Grune, T.; et al. Pathological aspects of lipid peroxidation. Free. Radic. Res. 2010, 44, 1125–1171. [Google Scholar] [CrossRef] [PubMed]
- Poganik, J.R.; Long, M.J.C.; Aye, Y. Getting the Message? Native Reactive Electrophiles Pass Two Out of Three Thresholds to be Bona Fide Signaling Mediators. Bioessays 2018, 40, e1700240. [Google Scholar] [CrossRef] [PubMed]
- Sasson, S. 4-Hydroxyalkenal-activated PPARdelta mediates hormetic interactions in diabetes. Biochimie 2017, 136, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Sasson, S. Nutrient overload, lipid peroxidation and pancreatic beta cell function. Free. Radic. Boil. Med. 2017, 111, 102–109. [Google Scholar] [CrossRef]
- Davies, K.J. Adaptive homeostasis. Mol. Aspects Med. 2016, 49, 1–7. [Google Scholar] [CrossRef]
- Jaganjac, M.; Tirosh, O.; Cohen, G.; Sasson, S.; Zarkovic, N. Reactive aldehydes—Second messengers of free radicals in diabetes mellitus. Free. Radic. Res. 2013, 47, 39–48. [Google Scholar] [CrossRef]
- Riahi, Y.; Cohen, G.; Shamni, O.; Sasson, S. Signaling and cytotoxic functions of 4-hydroxyalkenals. Am. J. Physiol. Metab. 2010, 299, 879–886. [Google Scholar] [CrossRef]
- Greco-Perotto, R.; Wertheimer, E.; Jeanrenaud, B.; Cerasi, E.; Sasson, S. Glucose regulates its transport in L8myocytes by modulating cellular trafficking of the transporter GLUT-1. Biochem. J. 1992, 286, 157–163. [Google Scholar] [CrossRef]
- Hsu, F.-F. Mass spectrometry-based shotgun lipidomics—A critical review from the technical point of view. Anal. Bioanal. Chem. 2018, 410, 6387–6409. [Google Scholar] [CrossRef]
- Jeucken, A.; Brouwers, J.F. High-Throughput Screening of Lipidomic Adaptations in Cultured Cells. Biomolecules 2019, 9, 42. [Google Scholar] [CrossRef]
- Han, X.; Yang, J.; Yang, K.; Zhongdan, Z.; Abendschein, D.R.; Gross, R.W.; Zhao, Z. Alterations in myocardial cardiolipin content and composition occur at the very earliest stages of diabetes: A shotgun lipidomics study. Biochemistry 2007, 46, 6417–6428. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, J.; Wang, M.; Wang, C.; Han, R.H.; Jiang, Z.Y.; Han, X. Lipidomic analysis reveals significant lipogenesis and accumulation of lipotoxic components in ob/ob mouse organs. Prostaglandins Leukot. Essent. Fatty Acids 2018, 136, 161–169. [Google Scholar] [CrossRef]
- Kopprasch, S.; Dheban, S.; Schuhmann, K.; Xu, A.; Schulte, K.-M.; Simeonovic, C.J.; Schwarz, P.E.H.; Bornstein, S.R.; Shevchenko, A.; Graessler, J. Detection of Independent Associations of Plasma Lipidomic Parameters with Insulin Sensitivity Indices Using Data Mining Methodology. PLoS ONE 2016, 11, e0164173. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, H.; Niu, Z.; Zhong, W.; Xue, M.; Wang, J.; Yang, F.; Zhou, Y.; Zhou, Y.; Xu, T.; et al. Temporal Proteomic Analysis of Pancreatic beta-Cells in Response to Lipotoxicity and Glucolipotoxicity. Mol. Cell. Proteomics 2018, 17, 2119–2131. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-C.; Yokomizo, T. Applications of mass spectrometry-based targeted and non-targeted lipidomics. Biochem. Biophys. Res. Commun. 2018, 504, 576–581. [Google Scholar] [CrossRef] [PubMed]
- Ramanadham, S.; Hsu, F.-F.; Zhang, S.; Bohrer, A.; Ma, Z.; Turk, J. Electrospray ionization mass spectrometric analyses of phospholipids from INS-1 insulinoma cells: Comparison to pancreatic islets and effects of fatty acid supplementation on phospholipid composition and insulin secretion. Biochim. Biophys. Acta 2000, 1484, 251–266. [Google Scholar] [CrossRef]
- Prentice, B.M.; Hart, N.J.; Phillips, N.; Haliyur, R.; Judd, A.; Armandala, R.; Spraggins, J.M.; Lowe, C.L.; Boyd, K.L.; Stein, R.W.; et al. Imaging mass spectrometry enables molecular profiling of mouse and human pancreatic tissue. Diabetology 2019, 62, 1036–1047. [Google Scholar] [CrossRef]
- Yin, R.; Kyle, J.; Burnum-Johnson, K.; Bloodsworth, K.J.; Sussel, L.; Ansong, C.; Laskin, J. High Spatial Resolution Imaging of Mouse Pancreatic Islets Using Nanospray Desorption Electrospray Ionization Mass Spectrometry. Anal. Chem. 2018, 90, 6548–6555. [Google Scholar] [CrossRef]
- Yin, L.; Zhang, Z.; Liu, Y.; Gao, Y.; Gu, J. Recent advances in single-cell analysis by mass spectrometry. Analyst 2019, 144, 824–845. [Google Scholar] [CrossRef]
- Pigeau, G.M.; Kolic, J.; Ball, B.J.; Hoppa, M.B.; Wang, Y.W.; Rückle, T.; Woo, M.; Manning Fox, J.E.; MacDonald, P.E. Insulin granule recruitment and exocytosis is dependent on p110gamma in insulinoma and human beta-cells. Diabetes 2009, 58, 2084–2092. [Google Scholar] [CrossRef]
- Bagatoll, L.A.; Parasassi, T.; Fidelio, G.D.; Gratton, E. A Model for the Interaction of 6-Lauroyl-2-(N,N-dimethylamino)naphthalene with Lipid Environments: Implications for Spectral Properties. Photochem. Photobiol. 1999, 70, 557–564. [Google Scholar] [CrossRef]
- Parasassi, T.; De Stasio, G.; Ravagnan, G.; Rusch, R.; Gratton, E. Quantitation of lipid phases in phospholipid vesicles by the generalized polarization of Laurdan fluorescence. Biophys. J. 1991, 60, 179–189. [Google Scholar] [CrossRef]
- Barelli, H.; Antonny, B. Lipid unsaturation and organelle dynamics. Curr. Opin. Cell Boil. 2016, 41, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Greenspan, P.; Mayer, E.P.; Fowler, S.D. Nile red: A selective fluorescent stain for intracellular lipid droplets. J. Cell Boil. 1985, 100, 965–973. [Google Scholar] [CrossRef]
- Bongiovanni, M.N.; Godet, J.; Horrocks, M.H.; Tosatto, L.; Carr, A.R.; Wirthensohn, D.C.; Ranasinghe, R.T.; Lee, J.-E.; Ponjavic, A.; Fritz, J.V.; et al. Multi-dimensional super-resolution imaging enables surface hydrophobicity mapping. Nat. Commun. 2016, 7, 13544. [Google Scholar] [CrossRef]
- Diaz, G.; Melis, M.; Batetta, B.; Angius, F.; Falchi, A.M. Hydrophobic characterization of intracellular lipids in situ by Nile Red red/yellow emission ratio. Micron 2008, 39, 819–824. [Google Scholar] [CrossRef]
- Cutrale, F.; Trivedi, V.; Trinh, L.A.; Chiu, C.-L.; Choi, J.M.; Artiga, M.S.; Fraser, S.E. Hyperspectral phasor analysis enables multiplexed 5D in vivo imaging. Nat. Methods 2017, 14, 149–152. [Google Scholar] [CrossRef]
- Stringari, C.; Nourse, J.L.; Flanagan, L.A.; Gratton, E. Phasor Fluorescence Lifetime Microscopy of Free and Protein-Bound NADH Reveals Neural Stem Cell Differentiation Potential. PLoS ONE 2012, 7, e48014. [Google Scholar] [CrossRef]
- Gao, M.; Huang, X.; Song, B.-L.; Yang, H. The biogenesis of lipid droplets: Lipids take center stage. Prog. Lipid Res. 2019, 75, 100989. [Google Scholar] [CrossRef]
- Ferri, G.; Digiacomo, L.; Lavagnino, Z.; Occhipinti, M.; Bugliani, M.; Cappello, V.; Caracciolo, G.; Marchetti, P.; Piston, D.W.; Cardarelli, F. Insulin secretory granules labelled with phogrin-fluorescent proteins show alterations in size, mobility and responsiveness to glucose stimulation in living beta-cells. Sci Rep. 2019, 9, 2890. [Google Scholar] [CrossRef]
- Makhmutova, M.; Liang, T.; Gaisano, H.; Caicedo, A.; Almaça, J. Confocal Imaging of Neuropeptide Y-pHluorin: A Technique to Visualize Insulin Granule Exocytosis in Intact Murine and Human Islets. J. Vis. Exp. 2017. [Google Scholar] [CrossRef] [PubMed]
- Sparvero, L.J.; Amoscato, A.A.; Kochanek, P.M.; Pitt, B.R.; Kagan, V.E.; Bayir, H.; Bayır, H. Mass-spectrometry based oxidative lipidomics and lipid imaging: Applications in traumatic brain injury. J. Neurochem. 2010, 115, 1322–1336. [Google Scholar] [CrossRef] [PubMed]
- Avrahami, D.; Klochendler, A.; Dor, Y.; Glaser, B. Beta cell heterogeneity: An evolving concept. Diabetology 2017, 60, 1363–1369. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Cell lines and probes are commercially available. |


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maulucci, G.; Cohen, O.; Daniel, B.; Ferreri, C.; Sasson, S. The Combination of Whole Cell Lipidomics Analysis and Single Cell Confocal Imaging of Fluidity and Micropolarity Provides Insight into Stress-Induced Lipid Turnover in Subcellular Organelles of Pancreatic Beta Cells. Molecules 2019, 24, 3742. https://doi.org/10.3390/molecules24203742
Maulucci G, Cohen O, Daniel B, Ferreri C, Sasson S. The Combination of Whole Cell Lipidomics Analysis and Single Cell Confocal Imaging of Fluidity and Micropolarity Provides Insight into Stress-Induced Lipid Turnover in Subcellular Organelles of Pancreatic Beta Cells. Molecules. 2019; 24(20):3742. https://doi.org/10.3390/molecules24203742
Chicago/Turabian StyleMaulucci, Giuseppe, Ofir Cohen, Bareket Daniel, Carla Ferreri, and Shlomo Sasson. 2019. "The Combination of Whole Cell Lipidomics Analysis and Single Cell Confocal Imaging of Fluidity and Micropolarity Provides Insight into Stress-Induced Lipid Turnover in Subcellular Organelles of Pancreatic Beta Cells" Molecules 24, no. 20: 3742. https://doi.org/10.3390/molecules24203742
APA StyleMaulucci, G., Cohen, O., Daniel, B., Ferreri, C., & Sasson, S. (2019). The Combination of Whole Cell Lipidomics Analysis and Single Cell Confocal Imaging of Fluidity and Micropolarity Provides Insight into Stress-Induced Lipid Turnover in Subcellular Organelles of Pancreatic Beta Cells. Molecules, 24(20), 3742. https://doi.org/10.3390/molecules24203742






