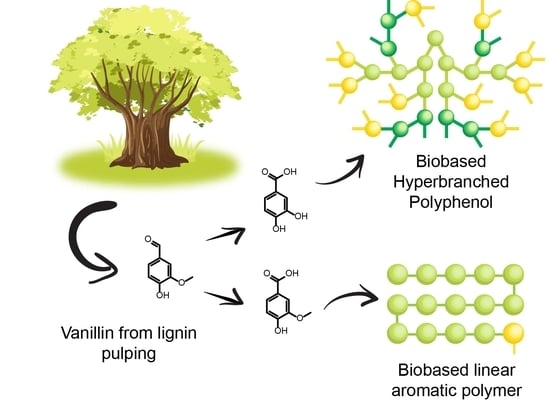
Synthesis of Lignin-based Phenol Terminated Hyperbranched Polymer
Abstract
1. Introduction
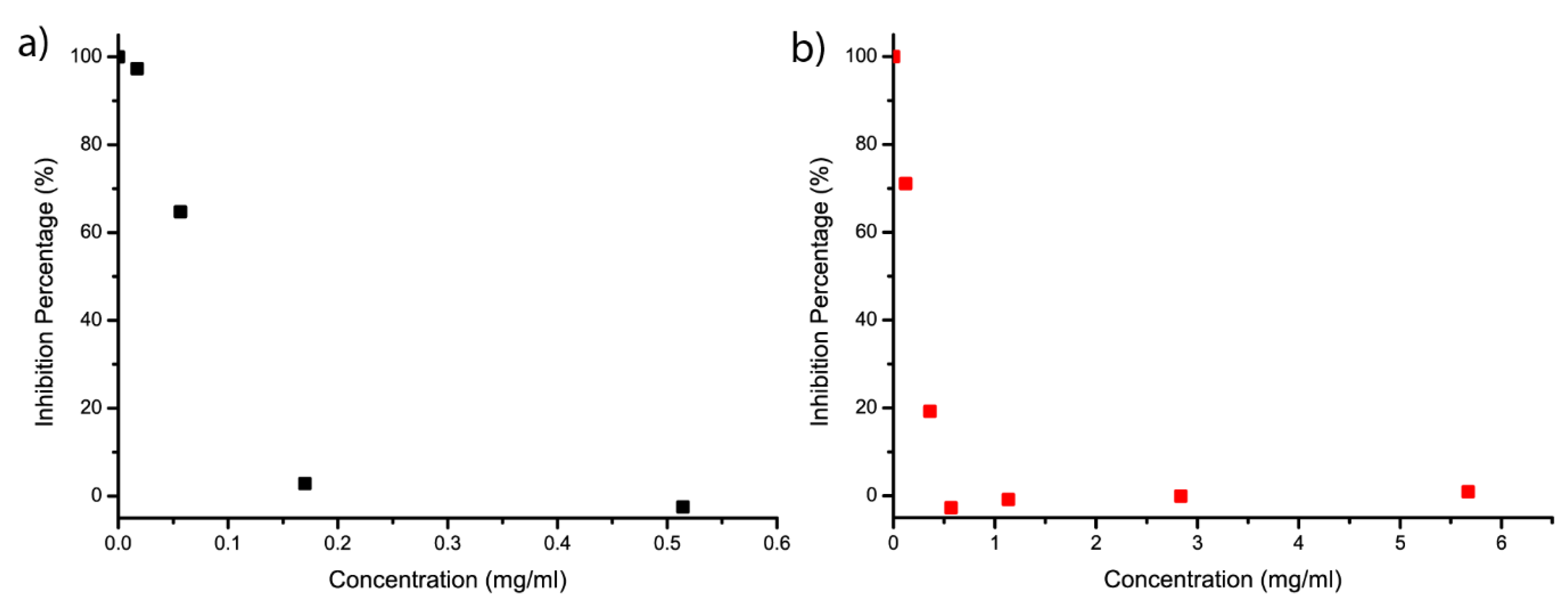
2. Results and Discussion
3. Materials and Methods
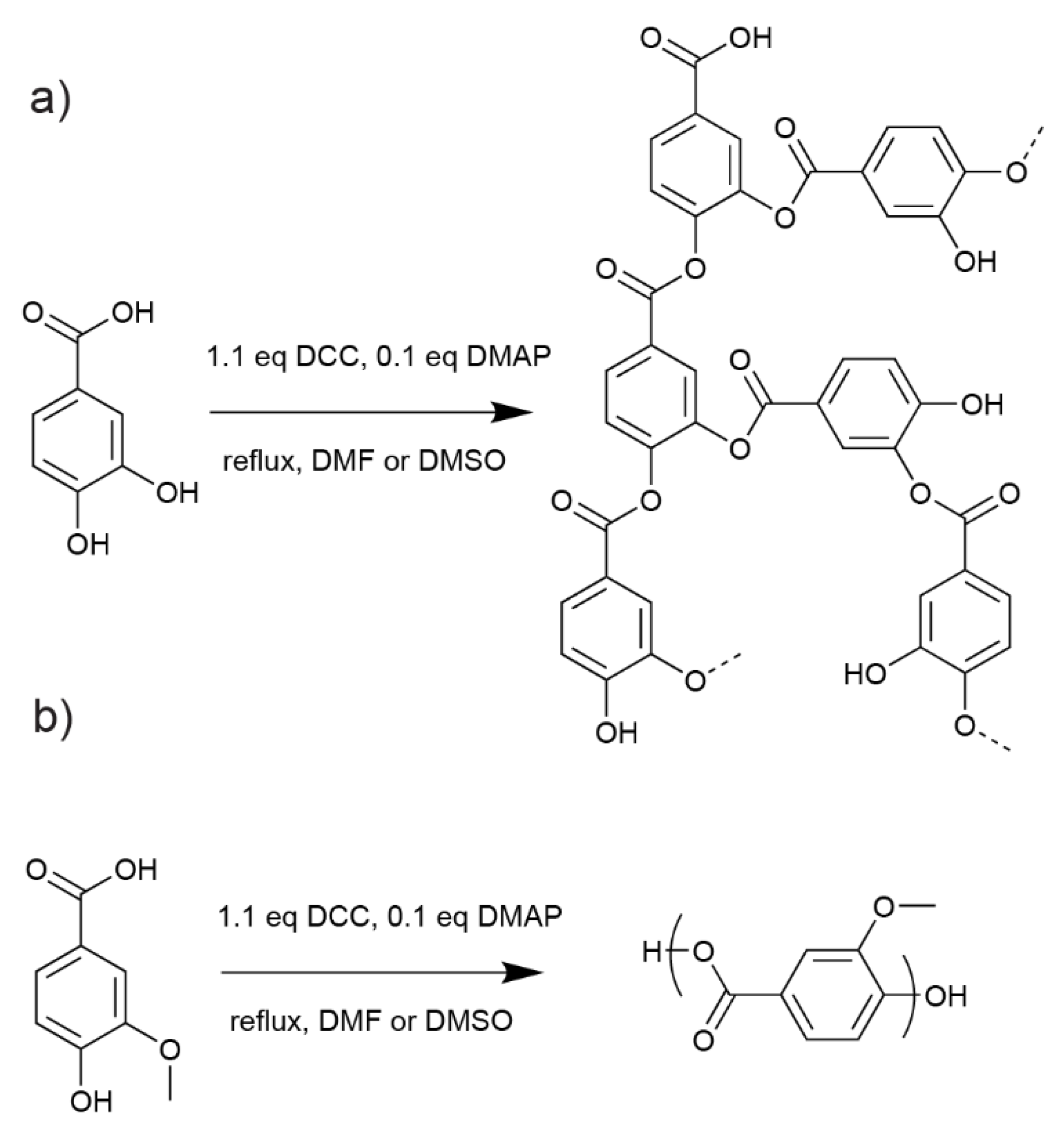
3.1. PA Synthesis
3.2. VA Synthesis
3.3. Polymerisation of VA and PA
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Nabae, Y.; Kakimoto, M.A. Design and synthesis of hyperbranched aromatic polymers for catalysis. Polymers 2018, 12, 1344. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Luo, X.; Kang, L.; Peng, X.; Lu, C. Synthesis of hyperbranched polymers and their applications in analytical chemistry. Polym. Chem. 2015, 6, 1214–1225. [Google Scholar] [CrossRef]
- Yates, C.R.; Hayes, W. Synthesis and applications of hyperbranched polymers. Eur. Polym. J. 2004, 40, 1257–1281. [Google Scholar] [CrossRef]
- Jeon, I.-Y.; Noh, H.-J.; Baek, J.-B. Hyperbranched macromolecules: From synthesis to applications. Molecules 2018, 23, 657. [Google Scholar] [CrossRef]
- Rimondino, G.N.; Oksdath-Mansilla, G.; Brunetti, V.; Strumia, M.C. More than just size: Challenges and opportunities of hybrid dendritic nanocarriers. Curr. Pharm. Des. 2017, 23, 3142–3153. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, S.; Wenga, Z.; Gao, C. Hyperbranched polymers: Advances from synthesis to applications. Chem. Soc. Rev. 2015, 44, 4091–4130. [Google Scholar] [CrossRef]
- Testud, B.; Pintori, D.; Grau, E.; Taton, D.; Cramail, H. Hyperbranched polyesters by polycondensation of fatty acid-based ABn-type monomers. Green Chem. 2017, 19, 259–269. [Google Scholar] [CrossRef]
- Pramanik, S.; Hazarika, J.; Kumar, A.; Karak, N. Castor oil based hyperbranched poly(ester amide)/polyaniline nanofiber nanocomposites as antistatic materials. Ind. Eng. Chem. Res. 2013, 52, 5700–5707. [Google Scholar] [CrossRef]
- Thakur, S.; Karak, N. Ultratough, ductile, castor oil-based, hyperbranched, polyurethane nanocomposite using functionalized reduced graphene oxide. ACS Sustainable Chem. Eng. 2014, 2, 1195–1202. [Google Scholar] [CrossRef]
- Zhang, T.; Howell, B.A.; Dumitrascu, A.; Martin, S.J.; Smith, P.B. Synthesis and characterization of glycerol-adipic acid hyperbranched polyesters. Polymer 2014, 55, 5065–5072. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, D.; Zhu, X.; Yana, D.; Chen, R. Synthesis and therapeutic applications of biocompatible or biodegradable hyperbranched polymers. Polym. Chem. 2015, 6, 2794–2812. [Google Scholar] [CrossRef]
- Žagara, E.; Žigon, M. Aliphatic hyperbranched polyesters based on 2,2-bis(methylol)propionic acid—Determination of structure, solution and bulk properties. Prog. Polym. Sci. 2011, 36, 53–88. [Google Scholar] [CrossRef]
- Bayan, R.; Karak, N. Renewable resource modified polyol derived aliphatic hyperbranched polyurethane as a biodegradable and UV-resistant smart material. Polym. Int. 2017, 66, 839–850. [Google Scholar] [CrossRef]
- Abbina, S.; Vappala, S.; Kumar, P.; Siren, E.M.J.; La, C.C.; Abbasi, U.; Brooks, D.E.; Kizhakkedathu, J.N. Hyperbranched polyglycerols: Recent advances in synthesis, biocompatibility and biomedical applications. J. Mater. Chem. B 2017, 5, 9249–9277. [Google Scholar] [CrossRef]
- Pu, S.; Li, J.; Sun, L.; Zhong, L.; Ma, Q. An in vitro comparison of the antioxidant activities of chitosan and green synthesized gold nanoparticles. Carbohydr. Polym. 2019, 211, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Omidi, S.; Kakanejadifard, A. Modification of chitosan and chitosan nanoparticle by long chain pyridinium compounds: Synthesis, characterization, antibacterial, and antioxidant activities. Carbohydr. Polym. 2019, 15, 477–485. [Google Scholar] [CrossRef] [PubMed]
- İlyasoğlu, H.; Nadzieja, M.; Guo, Z. Caffeic acid grafted chitosan as a novel dual-functional stabilizer for food-grade emulsions and additive antioxidant property. Food Hydrocoll. 2019, 95, 168–176. [Google Scholar] [CrossRef]
- Jing, Y.; Diao, Y.; Yu, X. Free radical-mediated conjugation of chitosan with tannic acid: Characterization and antioxidant capacity. React. Funct. Polym. 2019, 135, 16–22. [Google Scholar] [CrossRef]
- Li, C.; Wei, Y.; Shi, W.; Wang, J.; Wang, B. Antioxidant capacity and kinetics of dendritic hindered phenols using DPPH assay. Prog. React. Kinet. Mec. 2015, 40, 279–290. [Google Scholar] [CrossRef]
- Wei, L.; Li, Q.; Chen, Y.; Zhang, J.; Mi, Y.; Dong, F.; Guo, Z. Enhanced antioxidant and antifungal activity of chitosan derivatives bearing 6-O-imidazole-based quaternary ammonium salts. Carbohydr. Polym. 2019, 206, 493–503. [Google Scholar] [CrossRef]
- Wang, S.; Kaneko, D.; Kan, K.; Jin, X.; Kaneko, T. Syntheses of hyperbranched liquid-crystalline biopolymers with strong adhesion from phenolic phytomonomers. Pure Appl. Chem. 2012, 84, 2559–2568. [Google Scholar] [CrossRef]
- Wang, S.; Tateyama, S.; Kaneko, D.; Ohki, S.Y.; Kaneko, T. Synthesis of well-defined hyperbranched polymers bio-based on multifunctional phenolic acids and their structure–thermal property relationships. Polym. Degrad. Stab. 2011, 96, 2048–2054. [Google Scholar] [CrossRef]
- Kaneko, D.; Kinugawa, S.; Matsumoto, K.; Kaneko, T. Terminally-catecholized hyper-branched polymers with high performance adhesive characteristics. Plant Biotechnol. 2010, 27, 293–296. [Google Scholar] [CrossRef][Green Version]
- Hocking, M.B. Vanillin: Synthetic flavoring from spent sulfite liquor. J. Chem. Educ. 1997, 74, 1055–1059. [Google Scholar] [CrossRef]
- Fargues, C.; Mathias, Á.; Rodrigues, A. Kinetics of vanillin production from kraft lignin oxidation. Ind. Eng. Chem. Res. 1996, 35, 28–36. [Google Scholar] [CrossRef]
- Collis, B.C. Manufacture of vanillin from lignin. U.S. Patent No. 2692291, 1954. [Google Scholar]
- Schulz, L. Manufacture of vanillin. U.S. Patent No. 2187366, 1940. [Google Scholar]
- Gaborieau, M.; Castignolles, P. Size-exclusion chromatography (SEC) of branched polymers and polysaccharides. Anal. Bioanal. Chem. 2011, 4, 1413–1423. [Google Scholar] [CrossRef]
- Burdon, M.G.; Moffatt, J.G. Sulfoxide-carbodiimide reactions. IV. acid-catalyzed reactions of phenols with sulfoxides and carbodiimides. J. Am. Chem. Soc. 1966, 88, 5855–8564. [Google Scholar] [CrossRef]
- Lerch, U.; Moffatt, J.G. Carbodiimide-sulfoxide reactions. XIII. Reactions of amines and hydrazine derivatives. J. Org. Chem. 1971, 36, 3861–3869. [Google Scholar]
- Kedare, S.B.; Singh, R.P. Genesis and development of DPPH method of antioxidant assay. J. Food Sci. Technol. 2011, 48, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Avelino, F.; Silva, K.T.; Mazzetto, S.E.; Lomonaco, D. Tailor-made organosolv lignins from coconut wastes: Effects of green solvents in microwave-assisted processes upon their structure and antioxidant activities. Bioresour. Technol. Repor. 2019, 7. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds PA, VA, PA-polymer and VA-polymer both polymerised in DMSO are available from the authors. |


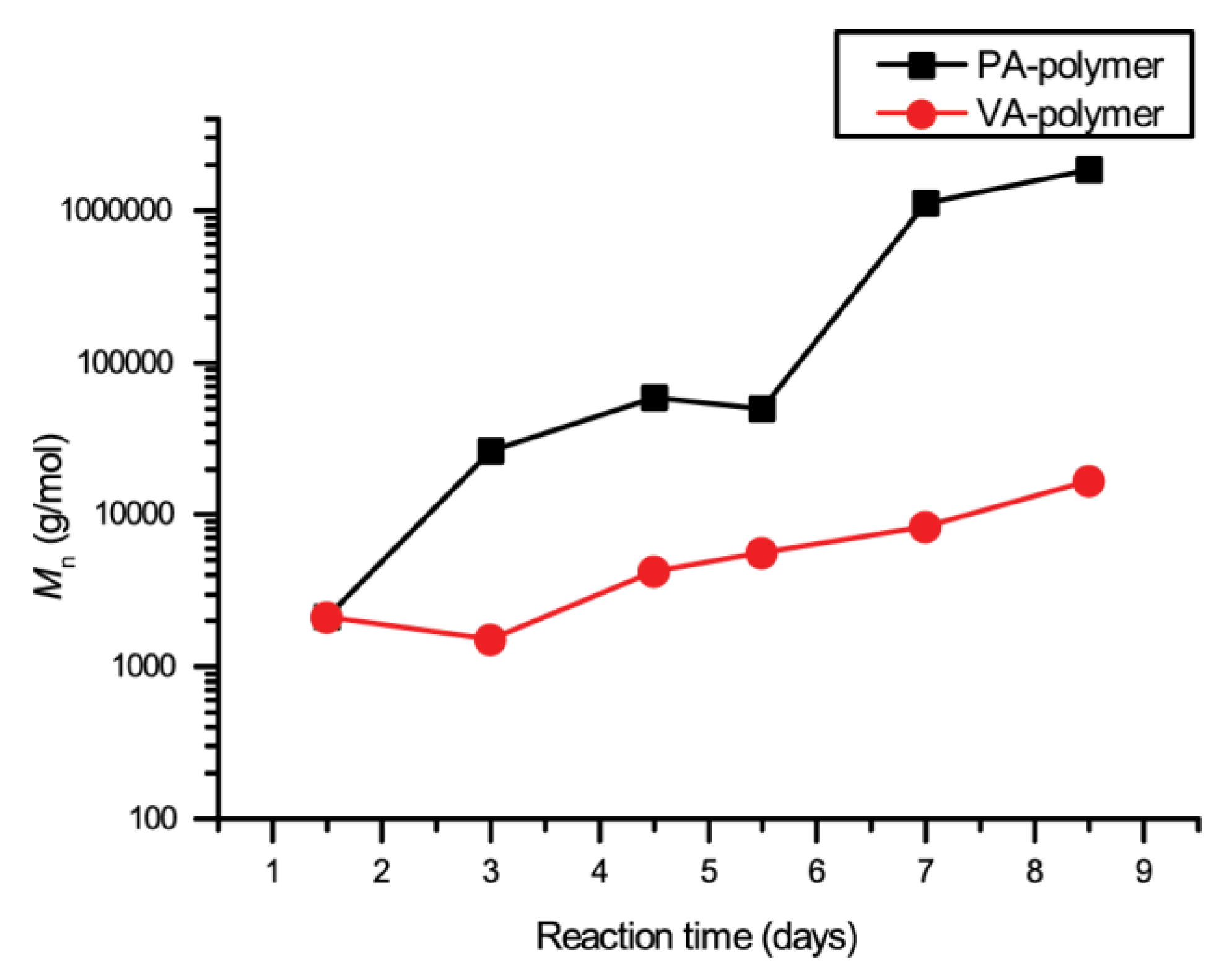
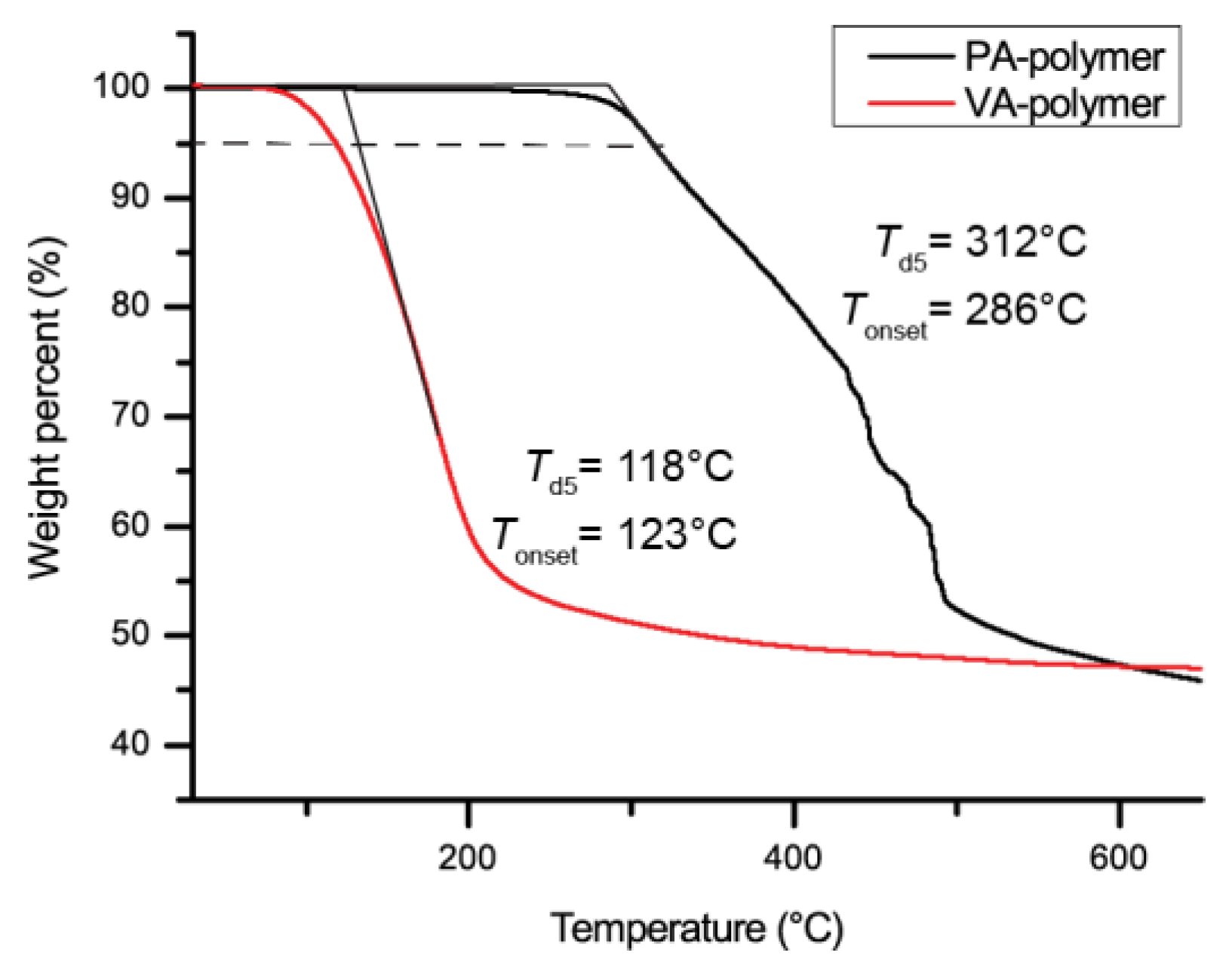
| Mn (g/mol) | Đ | Tonset | Td5 | |
|---|---|---|---|---|
| PA-based polymer | 7900 | 3.1 | 180 °C | 164 °C |
| VA-based polymer | 6400 | 2.6 | 118 °C | 108 °C |
| EC50 (mg/mL) | Antiradical Efficacy (mmol) a | |
|---|---|---|
| PA-polymer | 0.08 | 0.76 |
| VA-polymer | 0.22 | 0.30 |
| Chitosan | / | n.d. [17] |
| Chitosan graft caffeic acid | / | 0.16 [17] |
| Chitosan graft tannic acid | / | 5.8 [18] |
| Dendritic phenol | / | 0.36 [19] |
| Irganox® 1010 | / | 0.18 [32] |
| Irganox® 1098 | / | 0.36 [19] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Longe, L.; Garnier, G.; Saito, K. Synthesis of Lignin-based Phenol Terminated Hyperbranched Polymer. Molecules 2019, 24, 3717. https://doi.org/10.3390/molecules24203717
Longe L, Garnier G, Saito K. Synthesis of Lignin-based Phenol Terminated Hyperbranched Polymer. Molecules. 2019; 24(20):3717. https://doi.org/10.3390/molecules24203717
Chicago/Turabian StyleLonge, Lionel, Gil Garnier, and Kei Saito. 2019. "Synthesis of Lignin-based Phenol Terminated Hyperbranched Polymer" Molecules 24, no. 20: 3717. https://doi.org/10.3390/molecules24203717
APA StyleLonge, L., Garnier, G., & Saito, K. (2019). Synthesis of Lignin-based Phenol Terminated Hyperbranched Polymer. Molecules, 24(20), 3717. https://doi.org/10.3390/molecules24203717