A Simple Mechanical Method to Modulate the Electrochemical Electrosorption Processes at Metal Surfaces
Abstract
1. Introduction
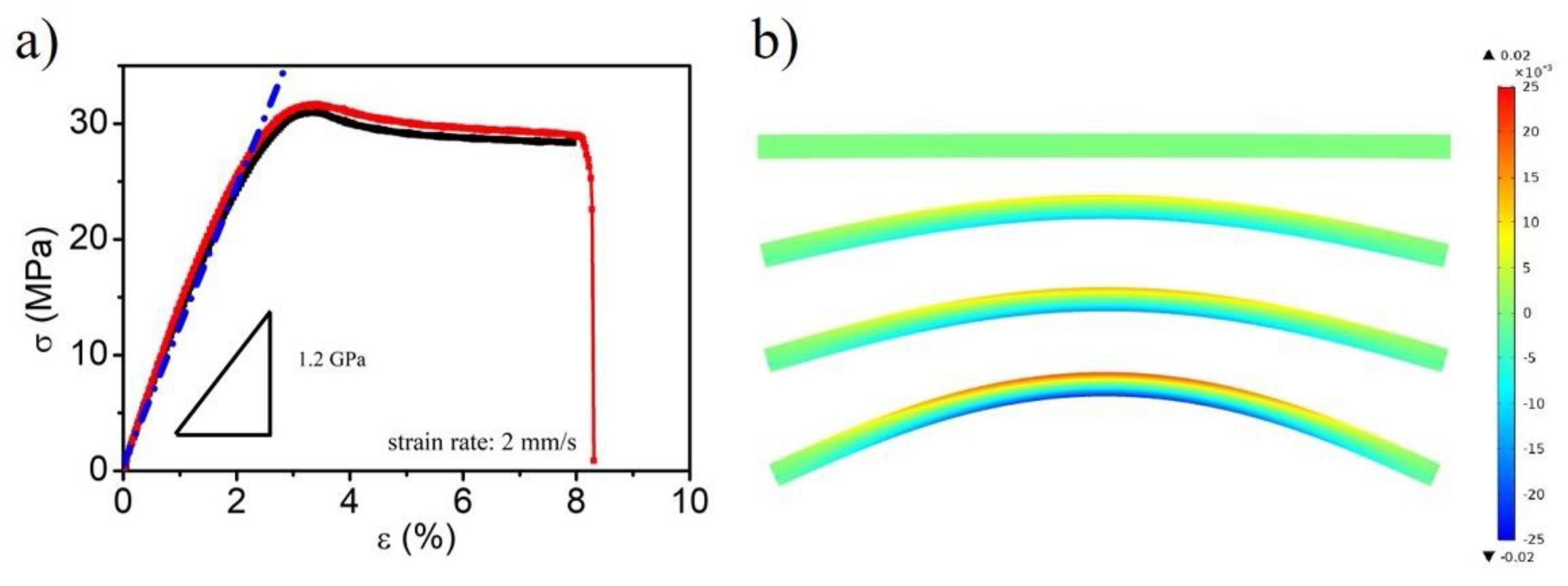
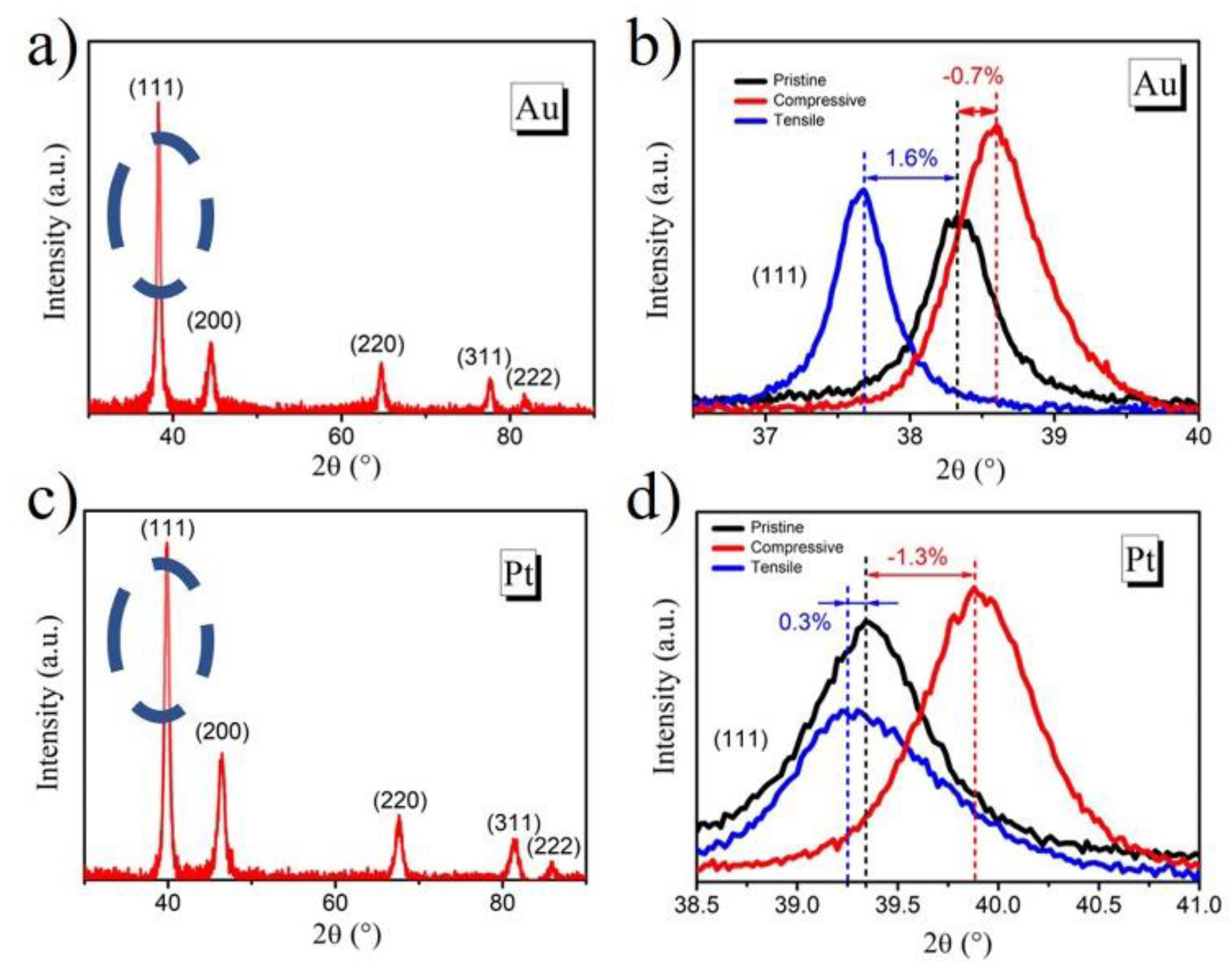
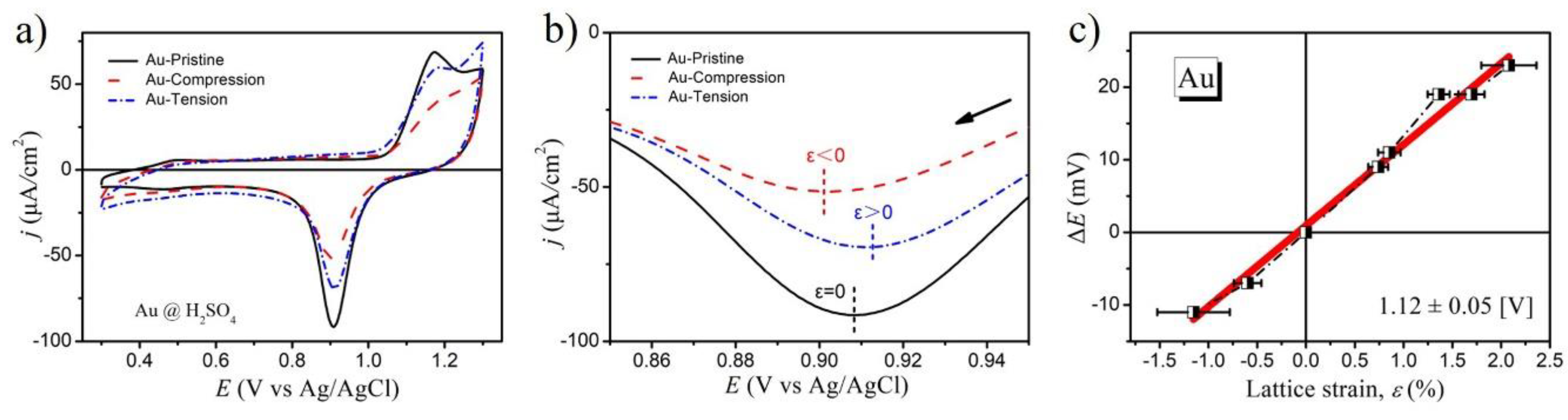
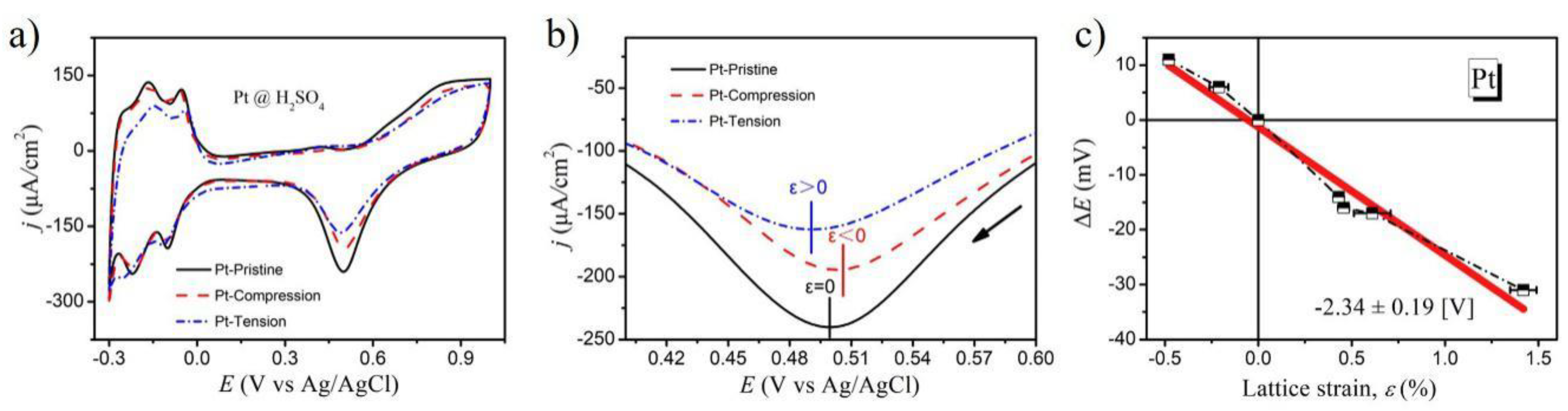
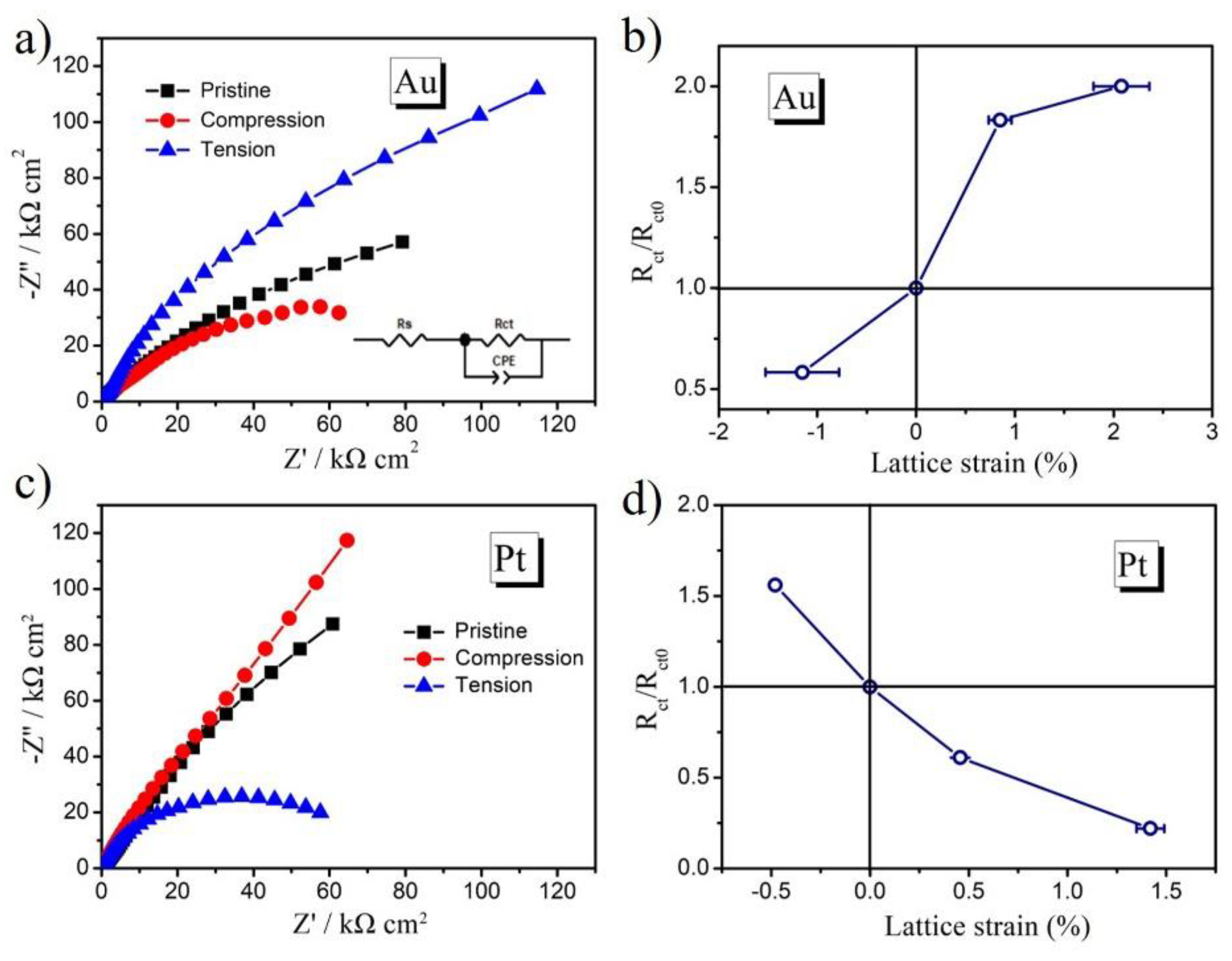
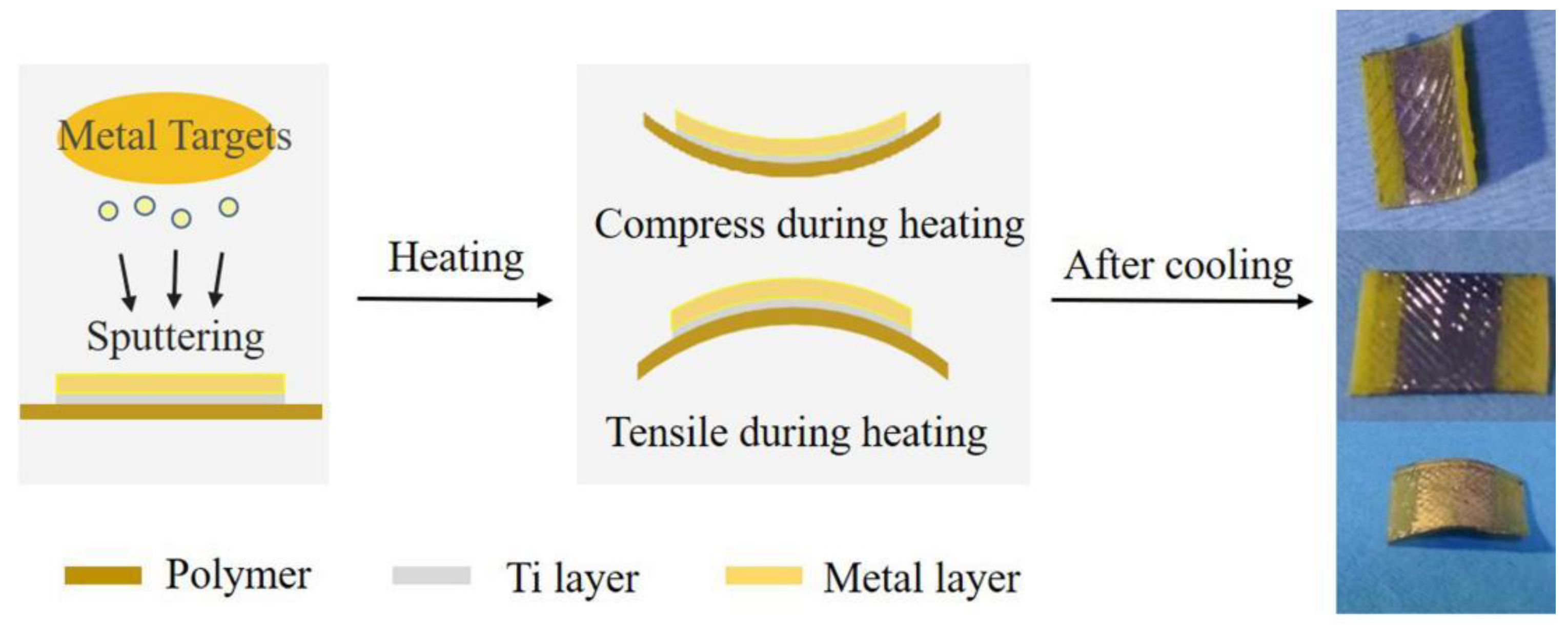
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- She, Z.; Kibsgaard, J.; Dickens, C.; Chorkendorff, I.; Nørskov, K.J.; Jaramilloet, T.F. Combining theory and experiment in electrocatalysis: Insights into materials design. Science 2017, 355, eaad4998. [Google Scholar]
- Wan, Z.; Xu, Q.; Li, H.; Zhang, Y.; Ding, Y.; Wang, J. Efficient Co@CoO core-shell nanocrystals as catalysts for visible-light-driven water oxidation. Appl. Catal. B 2017, 210, 67–76. [Google Scholar] [CrossRef]
- Liu, C.; Ma, Z.; Cui, M.; Zhang, Z.; Zhang, X.; Su, D.; Murray, C.B.; Wang, J.; Zhang, S. Favorable core/shell interface within Co2P/Pt nanorods for oxygen reduction electrocatalysis. Nano Lett. 2018, 12, 7870–7875. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, G.; Shi, L.; Ye, J. Single-atom catalysts: Emerging multifunctional materials in heterogeneous catalysis. Adv. Eng. Mater. 2017, 8, 1701343. [Google Scholar] [CrossRef]
- Zai, H.; Zhao, Y.; Chen, S.; Ge, L.; Chen, C.; Chen, Q.; Li, Y. Heterogeneously supported pseudo-single atom Pt as sustainable hydrosilylation catalyst. Nano Res. 2018, 5, 2544–2552. [Google Scholar] [CrossRef]
- Kim, M.S.; Park, H.; Won, S.O.; Sharma, A.; Kong, J.; Park, H.S.; Sung, Y.M.; Park, T.J.; Moon, M.W.; Hur, K. Copper-halide polymer nanowires as versatile supports for single-atom catalysts. Small 2019, 15, 1903197. [Google Scholar] [CrossRef]
- Kumar, G.; Nikolla, E.; Linic, S.; Medlin, J.W.; Janik, M.J. Multicomponent catalysts: Limitations and prospects. ACS Catal. 2018, 4, 3202–3208. [Google Scholar] [CrossRef]
- Diaz-Marta, A.S.; Tubio, C.R.; Carbajales, C.; Fernandez, C.; Escalante, L.; Sotelo, E.; Guitian, F.; Barrio, V.L.; Gil, A.; Coelho, A. Three-dimensional printing in catalysis: Combining 3D heterogeneous copper and palladium catalysts for multicatalytic multicomponent reactions. ACS Catal. 2018, 1, 392–404. [Google Scholar] [CrossRef]
- Bu, L.; Zhang, N.; Guo, S.; Zhang, X.; Li, J.; Yao, J.; Wu, T.; Lu, G.; Ma, J.; Su, D.; et al. Biaxially strained PtPb/Pt core/shell nanoplate boosts oxygen reduction catalysis. Science 2016, 354, 1410–1414. [Google Scholar] [CrossRef]
- Yan, K.; Maark, T.A.; Khorshidi, A.; Khorshidi, A.; Sethuraman, V.A.; Peterson, A.A.; Guduru, P.R. The influence of elastic strain on catalytic activity in the hydrogen evolution reaction. Angew. Chem. Int. Ed. 2016, 55, 6175–6181. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.; Smetanin, M.; Weissmüller, J. Mechanical modulation of reaction rate in electrocatalysis. J. Catal. 2014, 309, 351–361. [Google Scholar] [CrossRef]
- Weissmüller, J. Mechanochemistry breaks with expectations. Nat. Catal. 2018, 4, 238–239. [Google Scholar] [CrossRef]
- Deng, Q.; Gopal, V.; Weissmüller, J. Less noble or more noble: How strain affects the binding of oxygen on gold. Angew. Chem. Int. Ed. 2015, 54, 12981–12985. [Google Scholar] [CrossRef] [PubMed]
- An, C.; Dong, C.; Shao, L.; Deng, Q. Monitoring the length change of Ni@C composite electrodes during the charging/discharging process. Electrochem. Commun. 2019, 103, 94–99. [Google Scholar] [CrossRef]
- Mani, P.; Srivastava, R.; Strasser, P. Dealloyed Pt-Cu core-shell nanoparticle electrocatalysts for use in PEM fuel cell cathodes. J. Phys. Chem. C 2008, 112, 2770–2778. [Google Scholar] [CrossRef]
- Miftah, K.; Daud, W.R.; Majlan, E.H. Study effect of stress in the electrical contact resistance of bipolar plate and membrane electrode assembly in proton exchange membrane fuel cell: A review. Key Eng. Mater. 2010, 447, 775–779. [Google Scholar] [CrossRef]
- Strasser, P.; Koh, S.; Anniyev, T.; Greeley, J.; More, K.; Yu, C.; Liu, Z.; Kaya, S.; Nordlund, D.; Ogasawara, H.; et al. Lattice-strain control of the activity in dealloyed core-shell fuel cell catalysts. Nature Chem. 2010, 2, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Choi, S.I.; Roling, L.T.; Luo, M.; Ma, C.; Zhang, L.; Chi, M.; Liu, J.; Xie, Z.; Herron, J.A.; et al. Palladium-platinum core-shell icosahedra with substantially enhanced activity and durability towards oxygen reduction. Nat. Commun. 2015, 6, 7594. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xu, S.; Tsai, C.; Li, Y.; Liu, C.; Zhao, J.; Liu, Y.; Yuan, H.; Abild-Pedersen, F.; Prinz, F.B.; et al. Direct and continuous strain control of catalysts with tunable battery electrode materials. Science 2016, 354, 1031–1036. [Google Scholar] [CrossRef] [PubMed]
- Khorshidi, A.; Violet, J.; Hashemi, J.; Peterson, A.A. How strain can break the scaling relations of catalysis. Nat. Catal. 2018, 1, 263–268. [Google Scholar] [CrossRef]
- Zhang, H.; An, C.; Yuan, A.; Deng, Q.; Ning, J. A non-conventional way to modulate the capacitive process on carbon cloth by mechanical stretching. Electrochem. Commun. 2018, 89, 43–47. [Google Scholar] [CrossRef]
- Wang, A.; Deng, Q.; Deng, L.; Guan, X.; Liu, P.; Luo, J. Eliminating tip dendrite growth by Lorentz force for stable lithium metal anodes. Adv. Funct. Mater. 2019, 29, 1902630. [Google Scholar] [CrossRef]
- Shi, Y.; Zhai, T.; Zhou, Y.; Xu, W.; Yang, D.; Wang, F.; Xia, X. Atomic level tailoring of the electrocatalytic activity of Au-Pt core-shell nanoparticles with controllable Pt layers toward hydrogen evolution reaction. J. Electroanal. Chem. 2018, 819, 442–446. [Google Scholar] [CrossRef]
- Sasaki, K.; Wang, J.; Naohara, H.; Marinkovic, N.; More, K.; Inada, H.; Adzic, R.R. Recent advances in platinum monolayer electrocatalysts for oxygen reduction reaction: Scale-up synthesis, structure and activity of Pt shells on Pd cores. Electrochim. Acta. 2010, 55, 2645–2652. [Google Scholar] [CrossRef]
- Deng, Q.; Yuan, A. Monitoring and modeling the variation of electrochemical current induced by dynamic strain at gold surfaces. J. Electrochem. Soc. 2019, 166, H480–H484. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, H.; Deng, Q. Understanding the copper underpotential deposition process at strained gold surface. Electrochem. Commun. 2017, 82, 125–128. [Google Scholar] [CrossRef]
- Deng, Q.; Gosslar, D.H.; Smetanin, M.; Weissmüller, J. Electrocapillary coupling at rough surfaces. Phys. Chem. Chem. Phys. 2015, 17, 11725–11731. [Google Scholar] [CrossRef]
- Muralidharan, N.; Brock, C.N.; Cohn, A.P.; Schauben, D.; Carter, R.E.; Oakes, L.; Walker, D.G.; Pint, C.L. Tunable mechanochemistry of lithium battery electrodes. ACS Nano 2017, 11, 6243–6251. [Google Scholar] [CrossRef] [PubMed]
- Kibler, L.A.; El-Aziz, A.M.; Hoyer, R.; Kolb, D.M. Tuning reaction rates by lateral strain in a palladium monolayer. Angew Chem Int Edit. 2005, 14, 2080–2084. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.; Weissmüller, J. Electrocapillary coupling during electrosorption. Langmuir 2014, 30, 10522–10530. [Google Scholar] [CrossRef]
- Anuar, N.S.; Basirun, W.J.; Ladan, M.; Shalauddin, M.; Mehmood, M.S. Fabrication of platinum nitrogen-doped graphene nanocomposite modified electrode for the electrochemical detection of acetaminophen. Sens. Actuators B Chem. 2018, 266, 375–383. [Google Scholar] [CrossRef]
- Wang, C.; Fan, H.; Ren, X.; Wen, Y.; Wang, W. Highly dispersed PtO nanodots as efficient co-catalyst for photocatalytic hydrogen evolution. Appl. Surf. Sci. 2018, 462, 423–431. [Google Scholar] [CrossRef]
- Wahl, P.; Traussnig, T.; Landgraf, S.; Jin, H.; Weissmuller, J.; Wurschum, R. Adsorption-driven tuning of the electrical resistance of nanoporous gold. J. Appl. Phys. 2010, 108, 073706. [Google Scholar] [CrossRef]
- Steyskal, E.M.; Besenhard, M.; Landgraf, S.; Zhong, Y.; Weissmuller, J.; Polt, P.; Albu, M.; Wurschum, R. Sign-inversion of charging-induced variation of electrical resistance of nanoporous platinum. J. Appl. Phys. 2012, 112, 73703. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, A.; Zhang, H.; Deng, Q. A Simple Mechanical Method to Modulate the Electrochemical Electrosorption Processes at Metal Surfaces. Molecules 2019, 24, 3662. https://doi.org/10.3390/molecules24203662
Yuan A, Zhang H, Deng Q. A Simple Mechanical Method to Modulate the Electrochemical Electrosorption Processes at Metal Surfaces. Molecules. 2019; 24(20):3662. https://doi.org/10.3390/molecules24203662
Chicago/Turabian StyleYuan, Aiting, Haixia Zhang, and Qibo Deng. 2019. "A Simple Mechanical Method to Modulate the Electrochemical Electrosorption Processes at Metal Surfaces" Molecules 24, no. 20: 3662. https://doi.org/10.3390/molecules24203662
APA StyleYuan, A., Zhang, H., & Deng, Q. (2019). A Simple Mechanical Method to Modulate the Electrochemical Electrosorption Processes at Metal Surfaces. Molecules, 24(20), 3662. https://doi.org/10.3390/molecules24203662






