Synthesis, Docking Studies, and In Vitro Evaluation of Some Novel Thienopyridines and Fused Thienopyridine–Quinolines as Antibacterial Agents and DNA Gyrase Inhibitors
Abstract
1. Introduction
2. Results and Discussion
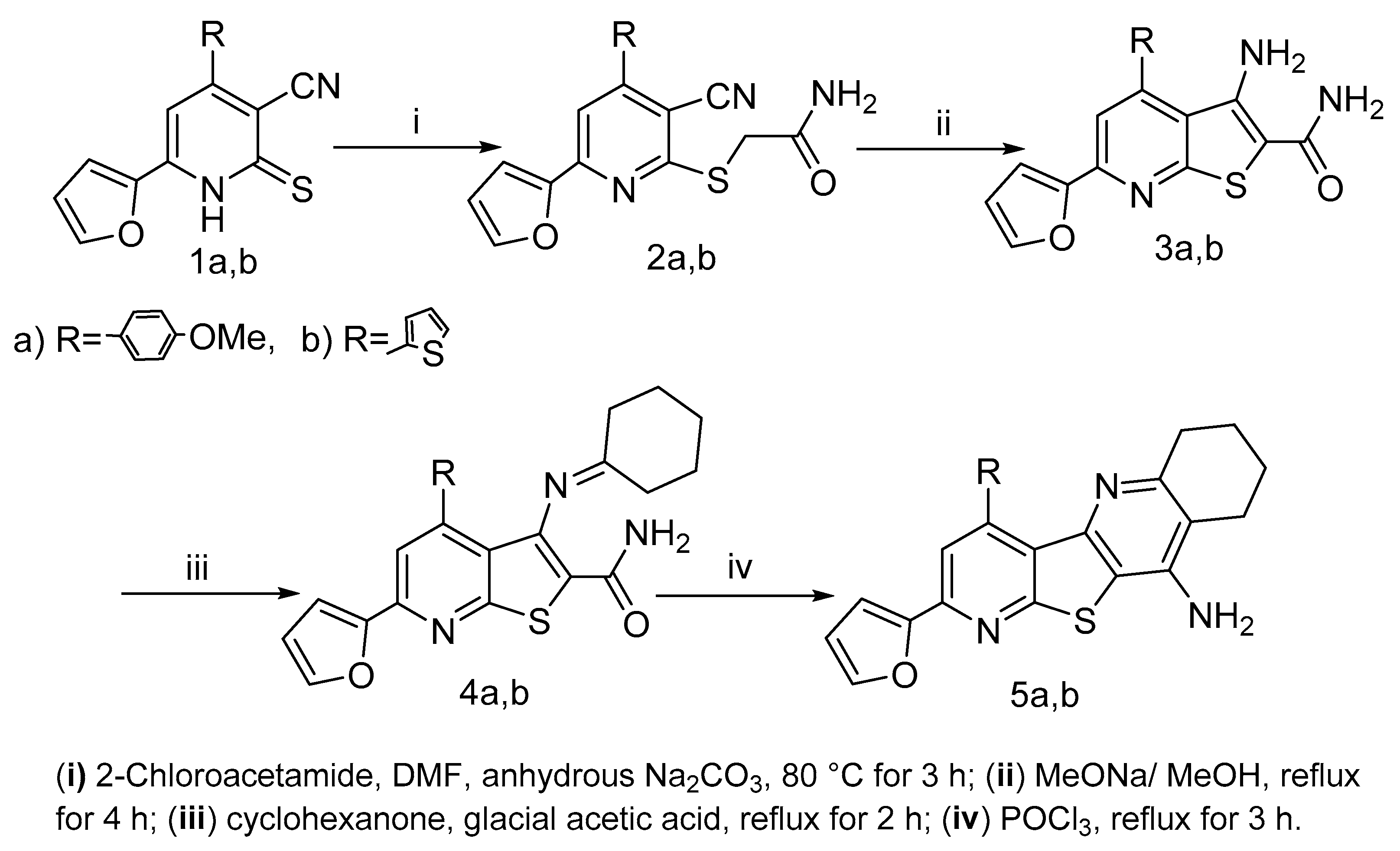
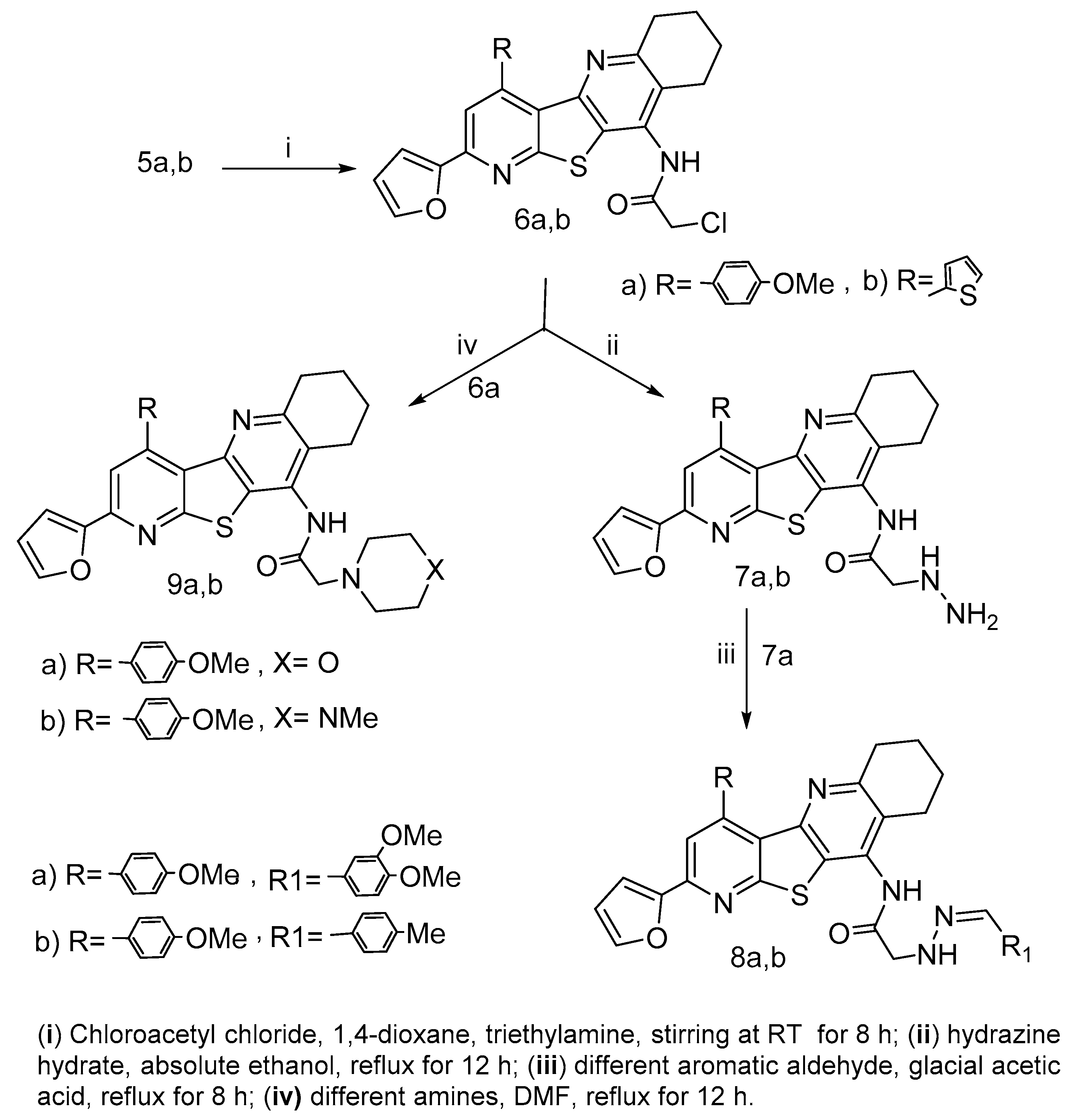
2.1. Chemistry
2.2. Antimicrobial Activity Evaluation
2.2.1. Antibacterial Activity
2.2.2. Antimicrobial Resistance Activity against MRSA
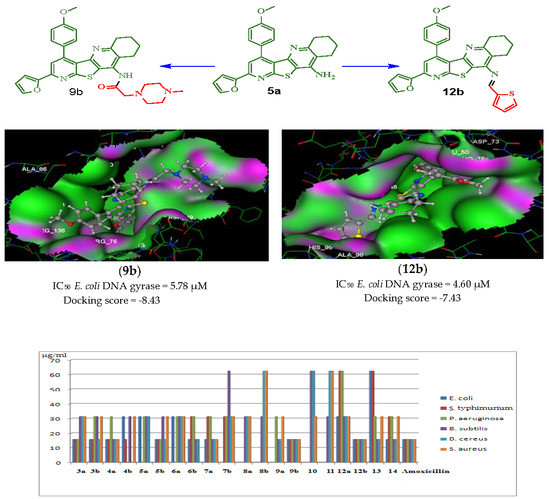
2.2.3. Escherichia coli DNA gyrase Inhibition Activity
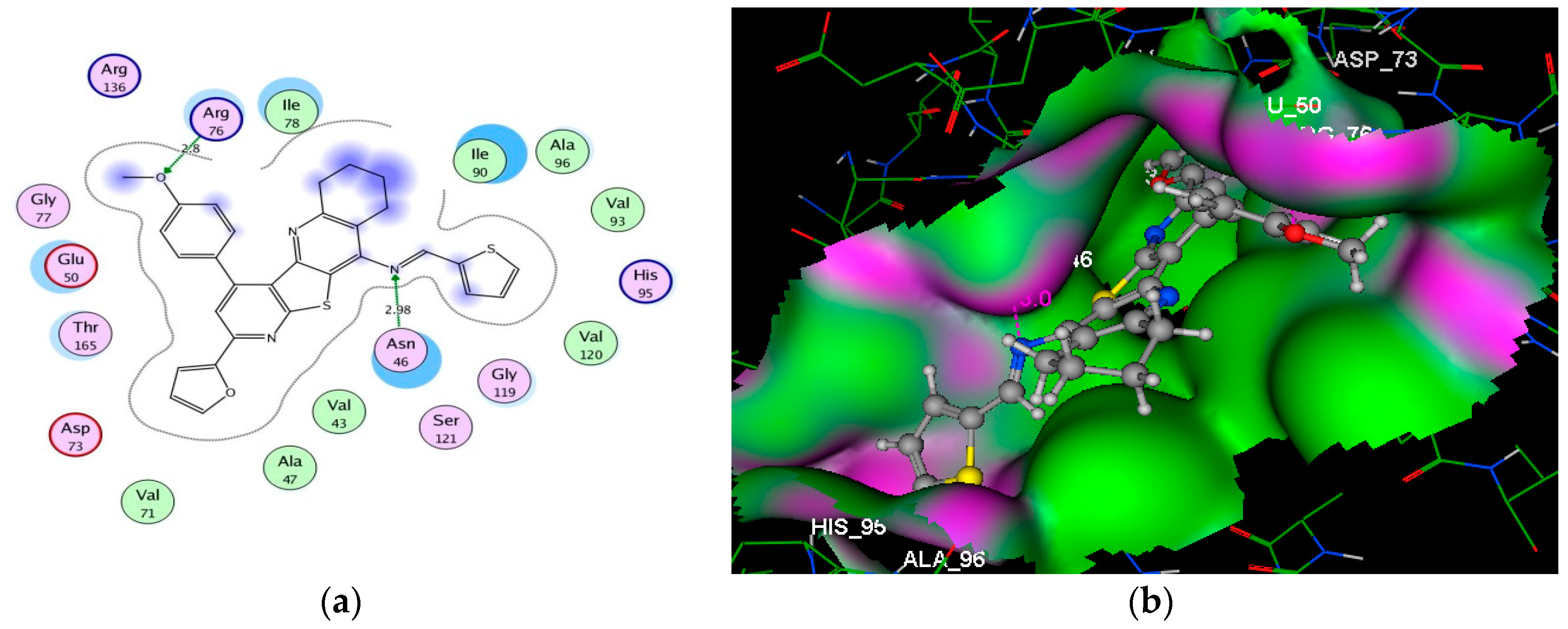
2.2.4. Molecular Docking Studies
3. Experimental
3.1. Chemistry
3.1.1. General Information
3.1.2. Synthesis of 2-((3-Cyanopyridin-2-yl)thio)acetamides 2a,b
3.1.3. Synthesis of 3-Amino-thieno[2,3-b]pyridine-2-carboxamides 3a,b
3.1.4. Synthesis of 3-(Cyclohexylideneamino)-thieno[2,3-b]pyridine-2-carboxamides 4a,b
3.1.5. Synthesis of 6,7,8,9-Tetrahydropyrido[3′,2′:4,5]thieno[3,2-b]quinolin-10-amines 5a,b
3.1.6. Synthesis of Chloroacetamide Derivatives 6a,b
3.1.7. Synthesis of Hydrazinylacetamide Derivatives 7a,b
3.1.8. Synthesis of Arylidene Derivatives 8a,b
3.1.9. Synthesis of Amine Acetamide Derivatives 9a,b
3.1.10. Synthesis of N-(2-(Furan-2-yl)-4-(4-methoxyphenyl)-6,7,8,9-tetrahydropyrido[3′,2′:4,5]thieno [3,2-b]quinolin-10-yl)benzenesulfonamide 10
3.1.11. Synthesis of 1-(2-(Furan-2-yl)-4-(4-methoxyphenyl)-6,7,8,9-tetrahydropyrido[3′,2′:4,5]thieno [3,2-b]quinolin-10-yl)-3-phenylurea 11
3.1.12. Synthesis of Schiff Bases 12a,b
3.1.13. Synthesis of Oxobutanamide Derivative 13
3.1.14. Synthesis of N-(5-methyl-4H-pyrazol-3-yl)-10-amine Derivative 14
3.2. Antimicrobial Activity
3.2.1. Antibacterial Assay
3.2.2. Escherichia coli DNA Gyrase Supercoiling Inhibition Assay
3.2.3. Molecular Docking
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Frieri, M.; Kumar, K.; Boutin, A. Antibiotic resistance. J. Infect. Public. Heal. 2017, 10, 369–378. [Google Scholar] [CrossRef] [PubMed]
- De Socio, G.V.; Rubbioni, P.; Botta, D.; Cenci, E.; Belati, A.; Paggi, R.; Pasticci, M.B.; Mencacci, A. Measurement and prediction of antimicrobial resistance in blood stream infections by ESKAPE and Escherichia coli pathogens. J. Glob. Antimicrob. Resist. 2019, 19, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Webster, T.J. Bacteria antibiotic resistance: New challenges and opportunities for implant-associated orthopaedic infections. J. Orthop. Res. 2018, 36, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Kaur, D.C.; Chate, S.S. Study of antibiotic resistance pattern in Methicillin resistant staphylococcus aureus with special reference to newer antibiotic. J. Glob. Infect. Dis. 2015, 7, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Teitzel, G. Responding to antimicrobial resistance with novel therapeutics. Trends Microbiol. 2019, 27, 285–286. [Google Scholar] [CrossRef] [PubMed]
- Sissi, C.; Palumbo, M. In front of and behind the replication fork: Bacterial type IIa topoisomerases. Cell. Mol. Life Sci. 2010, 67, 2001–2024. [Google Scholar] [CrossRef] [PubMed]
- Klostermeier, D. Why Two? On the Role of (A-)Symmetry in Negative Supercoiling of DNA by Gyrase. Int. J. Mol. Sci. 2018, 19, 1489. [Google Scholar] [CrossRef] [PubMed]
- Hooper, D.C.; Jacoby, G.A. Topoisomerase inhibitors: Fluoroquinolone mechanisms of action and resistance. Cold Spring Harbor Perspect. Med. 2016, 6, a025320. [Google Scholar] [CrossRef]
- Kumar, R.; Madhumathi, B.S.; Nagaraja, V. Molecular basis for the differential quinolone susceptibility of mycobacterial dna gyrase. Antimicrob. Agents Chemother. 2014, 58, 2013–2020. [Google Scholar] [CrossRef]
- Mayer, C.; Janin, Y.L. Non-quinolone inhibitors of bacterial type IIA topoisomerases: A feat of bioisosterism. Chem. Rev. 2014, 114, 2313–2342. [Google Scholar] [CrossRef]
- Tomasic, T.; Masic, L.P. Prospects for developing new antibacterials targeting bacterial type IIA topoisomerases. Curr. Top. Med. Chem. 2014, 14, 130–151. [Google Scholar] [CrossRef] [PubMed]
- Manchester, J.I.; Dussault, D.D.; Rose, J.A.; Boriack-Sjodin, P.A.; Uria-Nickelsen, M.; Ioannidis, G.; Bist, S.; Fleming, P.; Hull, K.G. Discovery of a novel azaindole class of antibacterial agents targeting the ATPase domains of DNA gyrase and topoisomerase IV. Bioorg. Med. Chem. Lett. 2012, 22, 5150–5156. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.; Lu, X. New strategy on antimicrobial-resistance: Inhibitors of DNA replication enzymes. Curr. Med. Chem. 2019, 26, 1761–1787. [Google Scholar] [CrossRef] [PubMed]
- Van Eijk, E.; Wittekoek, B.; Kuijper, E.J.; Smits, W.K. MRSA DNA replication proteins as potential targets for antimicrobials in drug-resistant bacterial pathogens. J. Antimicrob. Chemother. 2017, 72, 1275–1284. [Google Scholar] [CrossRef] [PubMed]
- Madhusudana, K.; Shireesha, B.; Naidu, V.G.M.; Ramakrishna, S.; Narsaiah, B.; Rao, A.R.; Diwan, P.V. Anti-inflammatory potential of thienopyridines as possible alternative to NSAIDs. Eur. J. Pharmacol. 2012, 678, 48–54. [Google Scholar] [CrossRef]
- Pevet, I.; Brule, C.I.; Tizot, A.; Gohier, A.; Cruzalegui, F.; Boutin, A.; Goldstein, S. Synthesis and pharmacological evaluation of thieno[2,3-b]pyridine derivatives as novel c-Src inhibitors. Bioorg. Med. Chem. 2011, 19, 2517–2528. [Google Scholar] [CrossRef]
- Said, S.A.; El-Sayed, H.A.; Amr, A.E.; Abdalla, M.M. Selective and orally bioavailable chk1 inhibitors of some synthesized substituted thieno[2,3-b]pyridine candidates. Int. J. Pharmacol. 2015, 11, 659–671. [Google Scholar] [CrossRef]
- Elansary, A.K.; Moneer, A.A.; Kadry, H.H.; Gedawy, E.M. Synthesis and anticancer activity of some novel fused pyridine ring system. Arch. Pharm. Res. 2012, 35, 1909–1917. [Google Scholar] [CrossRef]
- Mohareb, R.M.; Wardakhan, W.W.; Elmegeed, G.A.; Ashour, R.M. Heterocyclizations of pregnenolone: Novel synthesis of thiosemicarbazone, thiophene, thiazole, thieno[2,3-b]pyridine derivatives and their cytotoxicity evaluations. Steroids 2012, 77, 1560–1569. [Google Scholar] [CrossRef]
- Abdelaziz, M.E.; El-Miligy, M.M.M.; Fahmy, S.M.; Mahran, M.A.; Hazzaa, A.A. Design, synthesis and docking study of pyridine and thieno[2,3-b] pyridine derivatives as anticancer PIM-1 kinase inhibitors. Bioorg. Chem. 2018, 80, 674–692. [Google Scholar] [CrossRef]
- Ghattas, A.A.G.; Khodairy, A.; Hassan, M.; Moustafa, H.M.; Bahgat, R.M.; Hussein, B.R.M. Synthesis and Biological Evaluation of Some Novel Thienopyridines. J. Pharm. Appl. Chem. 2015, 1, 21–26. [Google Scholar] [CrossRef]
- Rateb, N.M.; Abdelaziz, S.H.; Zohdi, H.F. Synthesis and antimicrobial evaluation of some new thienopyridine, pyrazolopyridine and pyridothienopyrimidine derivatives. J. Sulfur Chem. 2011, 32, 345–354. [Google Scholar] [CrossRef]
- Altalbawy, F.M.A. Synthesis and antimicrobial evaluation of some novel bis-α,β-unsaturated ketones, nicotinonitrile-1,2-dihydro pyridine-3-carbonitrile, fused thieno[2,3-b]pyridine and pyrazolo[3,4-b]pyridine derivatives. Int. J. Mol. Sci. 2013, 14, 2967–2979. [Google Scholar] [CrossRef] [PubMed]
- Mohi El-Deen, E.M.; Abd El-Hameed, E.K. synthesis and in vitro biological evaluation of new tetracyclic pyridothienoquinolines as potential antimicrobial agents. Acta Pol. Pharm. 2017, 74, 837–847. [Google Scholar] [PubMed]
- Sweidan, N.I.; Nazer, M.Z.; El-Abadelah, M.M.; Voelter, W. Thienopyridone antibacterials. Part IV [1]. Synthesis of some N(7)-heteroaryl-4-oxothieno[2,3-b]pyridine-5-carboxylic acids and esters. Lett. Org. Chem. 2010, 7, 79–84. [Google Scholar] [CrossRef]
- Chokkar, N.; Kalra, S.; Chauhan, M.; Kumar, R. A review on quinoline derived scaffolds as anti-hiv agents. Mini Rev. Med. Chem. 2019, 19, 510–526. [Google Scholar] [CrossRef] [PubMed]
- Strekowski, L.; Mokrosz, J.L.; Honkan, V.A.; Czarny, A.; Cegla, M.T.; Patterson, S.E.; Wydra, R.L.; Schinazi, R.F. Synthesis and quantitative structure-activity relationship analysis of 2-(aryl or heteroaryl)quinolin-4-amines, a new class of anti-HIV-1 agents. J. Med. Chem. 1991, 34, 1739–1746. [Google Scholar] [CrossRef] [PubMed]
- Nqoro, X.; Tobeka, N.; Aderibigbe, B.A. Quinoline-based hybrid compounds with antimalarial activity. Molecules 2017, 22, 2268. [Google Scholar] [CrossRef] [PubMed]
- Muruganantham, N.; Sivakumar, R.; Anbalagan, N.; Gunasekaran, V.; Leonard, J.T. Synthesis, anticonvulsant and antihypertensive activities of 8-substituted quinoline derivatives. Biol. Pharm. Bull. 2004, 27, 1683–1687. [Google Scholar] [CrossRef]
- Eswaran, S.; Adhikari, A.V.; Kumar, R.A. New 1,3-oxazolo[4,5-c]quinoline derivatives: Synthesis and evaluation of antibacterial and antituberculosis properties. Eur. J. Med. Chem. 2010, 45, 957–966. [Google Scholar] [CrossRef] [PubMed]
- Jitender, G.D.; Poornachandra, Y.; Ratnakar, K.R.; Naresh, R.K.; Ravikumar, N.; Krishna, D.S.; Ranjithreddy, P.; Shravan, G.K.; Jagadeesh, B.N.; Ganesh, C.K.; et al. Synthesis of novel pyrazolo[3,4-b]quinolinyl acetamide analogs, their evaluation for antimicrobial and anticancer activities, validation by molecular modeling and CoMFA analysis. Eur. J. Med. Chem. 2017, 130, 223–239. [Google Scholar] [CrossRef]
- Shang, X.F.; Morris-Natschke, S.L.; Liu, Y.Q.; Guo, X.; Xu, X.S.; Goto, M.; Li, J.C.; Yang, G.Z.; Lee, K.H. Biologically active quinoline and quinazoline alkaloids part I. Med. Res. Rev. 2018, 38, 775–828. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.S.; Dhivya, D.; Vijayakumar, B. A focus on quinolones and its medicinal importance. Intl. J. Novel. Tr. Pharm. Sci. 2011, 1, 23–29. [Google Scholar]
- Hooper, D.C.; Jacoby, G.A. Mechanisms of drug resistance: Quinolone resistance. Ann. N. Y. Acad. Sci. 2015, 1354, 12–31. [Google Scholar] [CrossRef] [PubMed]
- Rateb, N.M.; Elnagdy, S.M.; Zohdi, H.F. Eco-friendly, green synthesis and antimicrobial evaluation of 4,6-disubstituted-2-(6′-acetylO-β-d-glucopyranosylsulfanyl)-nicotinonitrile. Int. J. Adv. Res. 2014, 2, 355–364. [Google Scholar]
- Eldeab, H. Ecofriendly microwave assisted synthesis of some new pyridine glycosides. Nucleosides Nucleotides Nucleic Acids 2019, 38, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Elzahabi, H.S.A.; Nossier, E.S.; Khalifa, N.M.; Alasfoury, R.A.; El-Manawat, M.A. Anticancer evaluation and molecular modeling of multi-targeted kinase inhibitors based pyrido[2,3-d]pyrimidine scaffold. J. Enzy. Inh. Med. Chem. 2018, 33, 546–557. [Google Scholar] [CrossRef] [PubMed]
- Othman, I.M.M.; Gad-Elkareem, M.A.M.; El-Naggar, M.; Nossier, E.S.; Amr, A.E. Novel phthalimide based analogues: Design, synthesis, biological evaluation, and molecular docking studies. J. Enzy. Inhibit. Med. Chem. 2019, 34, 1259–1270. [Google Scholar] [CrossRef]
- Holdgate, G.A.; Tunnicliffe, A.; Ward, W.H.; Weston, S.A.; Rosenbrock, G.; Barth, P.T.; Taylor, I.W.; Pauptit, R.A.; Timms, D. The entropic penalty of ordered water accounts for weaker binding of the antibiotic novobiocin to a resistant mutant of DNA gyrase: A thermodynamic and crystallographic study. Biochem. 1997, 36, 9663–9673. [Google Scholar] [CrossRef]
- Reller, L.B.; Weinstein, M.; Jorgensen, J.H.; Ferraro, M.J. Antimicrobial susceptibility testing: A review of general principles and contemporary practices. Clin. Infect. Dis. 2009, 49, 1749–1755. [Google Scholar] [CrossRef]
- Gianecini, R.; Oviedo, C.; Irazu, L.; Rodríguez, M.; GASSP-AR Working Group; Galarza, P. Comparison of disk diffusion and agar dilution methods for gentamicin susceptibility testing of Neisseria gonorrhoeae. Diagn. Microb. Infect. Dis. 2018, 91, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharmaceut. Analysis 2016, 6, 71–79. [Google Scholar] [CrossRef]
- Tomašicˇ, T.; Mirt, M.; ˇoková, M.B.; Ilaš, J.; Zidar, N.; Tammela, P.; Kikelj, D. Design, synthesis and biological evaluation of 4,5-dibromo-N-(thiazol-2-yl)-1H-pyrrole-2-carboxamide derivatives as novel DNA gyrase inhibitors. Bioorg. Med. Chem. 2017, 25, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, A.; Burton, N.P.; O’ Hagen, N. High-throughput assays for DNA gyrase and other topoisomerases. Nucleic Acids Res. 2006, 34, e104. [Google Scholar] [CrossRef] [PubMed]
- Nossier, E.S.; Abd El-Karim, S.S.; Khalifa, N.M.; El-Sayed, A.S.; Hassan, E.S.I.; El-Hallouty, S.M. Kinase inhibitory activities and molecular docking of a novel series of anticancer pyrazole derivatives. Molecules 2018, 23, 3074. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 2a, 3a, 5a and 5b are available from the authors. |

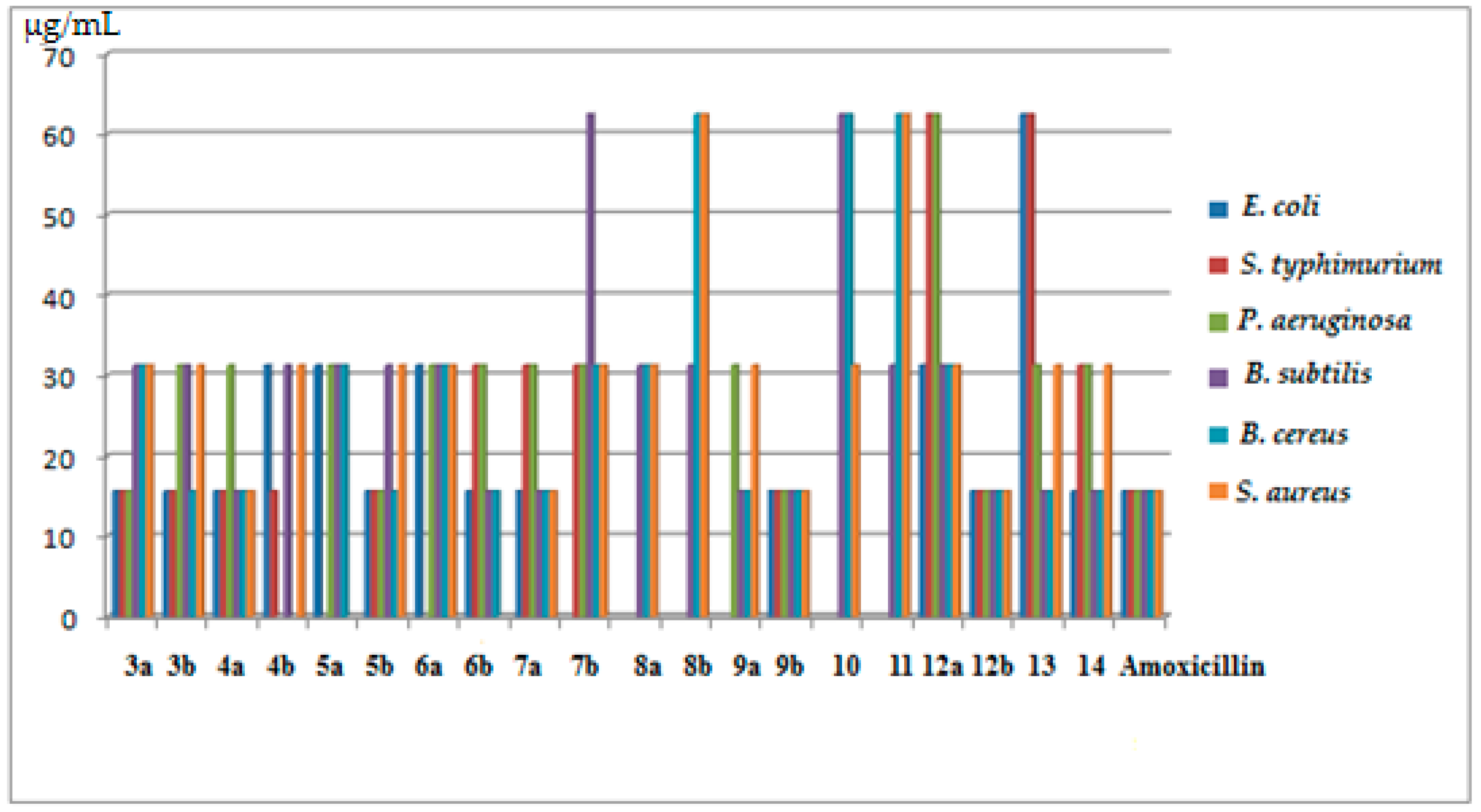
| M.O | E. coli | S. typhimurium | P. aeruginosa | B. subtilis | B. cereus | S. aureus | |
|---|---|---|---|---|---|---|---|
| Compound | |||||||
| 3a | 18 (15.63) | 15 (15.63) | 15 (15.63) | 10 (31.25) | 13 (31.25) | 12 (31.25) | |
| 3b | 19 (15.63) | 18 (15.63) | 13 (31.25) | 12 (31.25) | 18 (15.63) | 13 (31.25) | |
| 4a | 18 (15.63) | 15 (15.63) | 13 (31.25) | 15 (15.63) | 16 (15.63) | 18 (15.63) | |
| 4b | 12 (31.25) | 15 (15.63) | NA | 10 (31.25) | NA | 12 (31.25) | |
| 5a | 12 (31.25) | NA | 12 (31.25) | 10 (31.25) | 13 (31.25) | NA | |
| 5b | 15 (15.63) | 17 (15.63) | 14 (15.63) | 12 (31.25) | 16 (15.63) | 13 (31.25) | |
| 6a | 13 (31.25) | NA | 13 (31.25) | 10 (31.25) | 13 (31.25) | 12 (31.25) | |
| 6b | 16 (15.63) | 12 (31.25) | 12 (31.25) | 15 (15.63) | 15 (15.63) | NA | |
| 7a | 16 (15.63) | 11 (31.25) | 11 (31.25) | 17 (15.63) | 15 (15.63) | 19 (15.63) | |
| 7b | NA | 10 (31.25) | 12 (31.25) | 8 (62.5) | 11 (31.25) | 10 (31.25) | |
| 8a | NA | NA | NA | 11 (31.25) | 9 (31.25) | 11 (31.25) | |
| 8b | NA | NA | NA | 9 (31.25) | 9 (62.5) | 8 (62.5) | |
| 9a | NA | NA | 11 (31.25) | 14 (15.63) | 16 (15.63) | 12 (31.25) | |
| 9b | 20 (15.63) | 19 (15.63) | 18 (15.63) | 15 (15.63) | 18 (15.63) | 16 (15.63) | |
| 10 | NA | NA | NA | 8 (62.5) | 9 (62.5) | 9 (31.25) | |
| 11 | NA | NA | NA | 9 (31.25) | 8 (62.5) | 8 (62.5) | |
| 12a | 11 (31.25) | 8 (62.5) | 9 (62.5) | 13 (31.25) | 11 (31.25) | 10 (31.25) | |
| 12b | 18 (15.63) | 16 (15.63) | 16 (15.63) | 15 (15.63) | 15 (15.63) | 16 (15.63) | |
| 13 | 8 (62.5) | 7 (62.5) | 11 (31.25) | 15 (15.63) | 16 (15.63) | 12 (31.25) | |
| 14 | 15 (15.63) | 12 (31.25) | 10 (31.25) | 16 (15.63) | 17 (15.63) | 13 (31.25) | |
| Amoxicillin | 20 (15.63) | 21 (15.63) | 18 (15.63) | 17 (15.63) | 19 (15.63) | 19 (15.63) | |
| M.O: microorganism; NA: no activity; Clear zone: mm; MIC ( ): µg/mL | |||||||
| M.O. | 4a | 7a | 9b | 12b | Gentamicin |
|---|---|---|---|---|---|
| MRSA | 13 | 18 | 14 | 15 | 15 |
| 3a | 3b | 4a | 5b | 6a | 6b | 7a | 9b | 12b | 14 | Novobiocin | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| IC50 µM | 2.26 ± 0.19 | 4.50 ± 0.32 | 3.69 ± 0.15 | 16.70 ± 0.88 | 13.74 ± 0.65 | 5.95 ± 0.34 | 8.66 ± 0.61 | 5.78 ± 0.46 | 4.60 ± 0.28 | 11.72 ± 0.93 | 4.17 ± 0.32 |
| Compd. NO. | Docking Score (Kcal/mol) | Amino Acid Residues (Bond Length A°) | Atoms of Compound | Type of Bond |
|---|---|---|---|---|
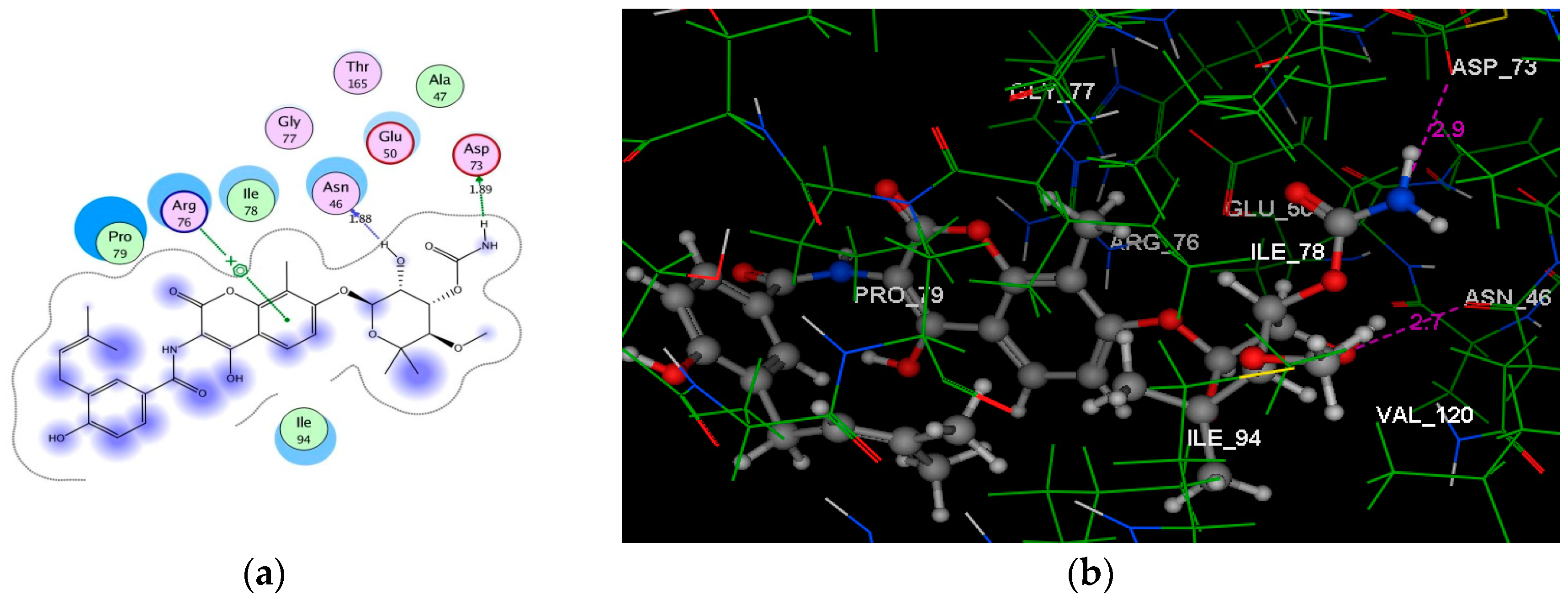
| Novobiocin | −6.30 | Asn46 (1.88); Asp73 (1.89); Arg76 | H(OH)(oxan-4-yl); H(OCONH2); C6H2(coumarin) | H–don H–don Arene–cation |
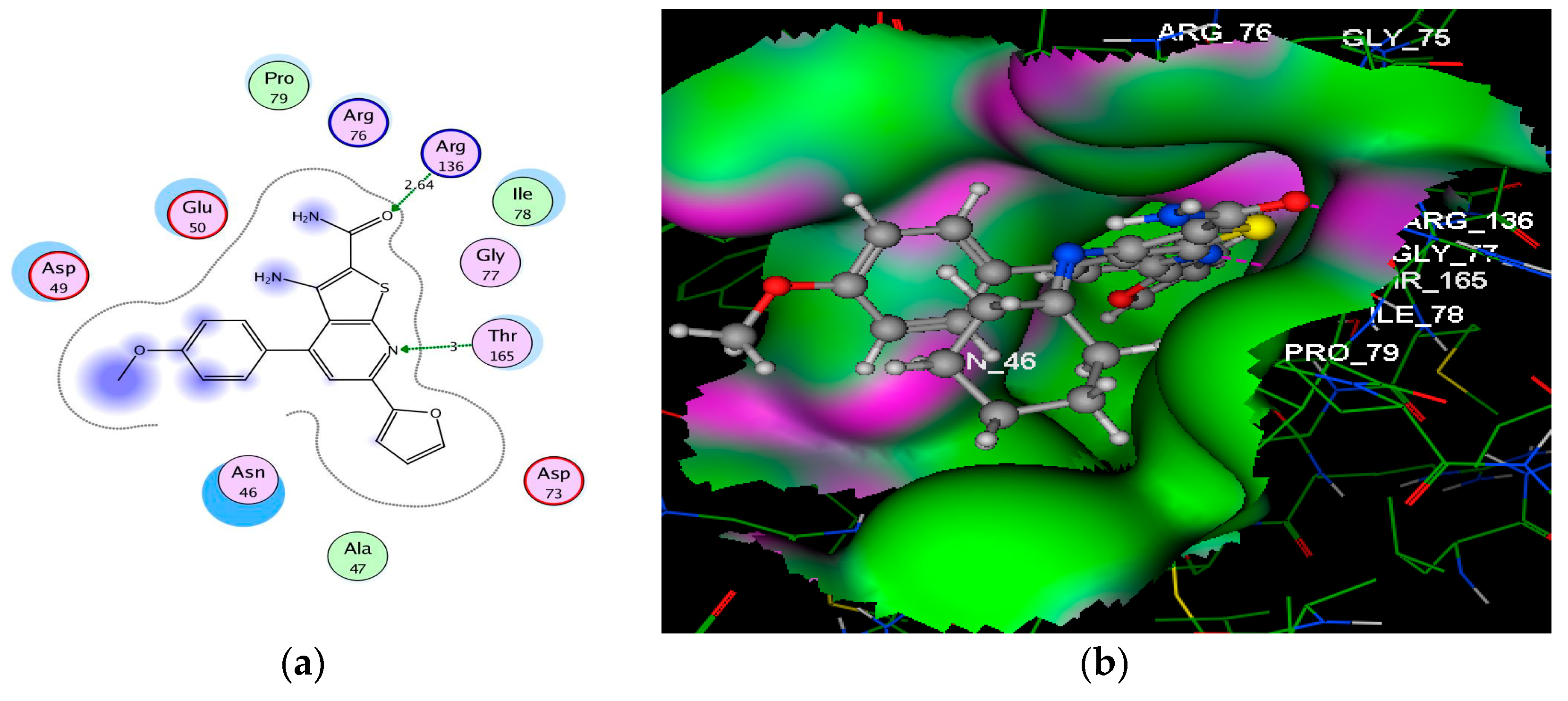
| 3a | −7.32 | Arg136 (2.64); Thr165 (3.00) | O(CONH2); N(pyridine) | H–acc H–acc |
| 3b | −6.83 | Arg136 (2.52); Thr165 (3.10) | O(CONH2); N(pyridine) | H–acc H–acc |
| 4a | −7.46 | Arg136 (2.67); Thr165 (3.01) | O(CONH2); N(pyridine) | H–acc H–acc |
| 5b | −6.22 | Asn46 (3.29) | N(pyridine) | H–acc |
| 6a | −6.24 | Asn46 (3.00); Val120 (3.2) | N(pyridine); O(NHCOCH2) | H–acc H–acc |
| 6b | −6.75 | Asn46 (2.25); Val120 (2.72); Arg76 | N(pyridine); O(NHCOCH2) thiophene | H–acc H–acc Arene–cation |
| 7a | −6.25 | Asn46 (3.28); Val120 (2.95) | N(pyridine); O(NHCOCH2) | H–acc H–acc |
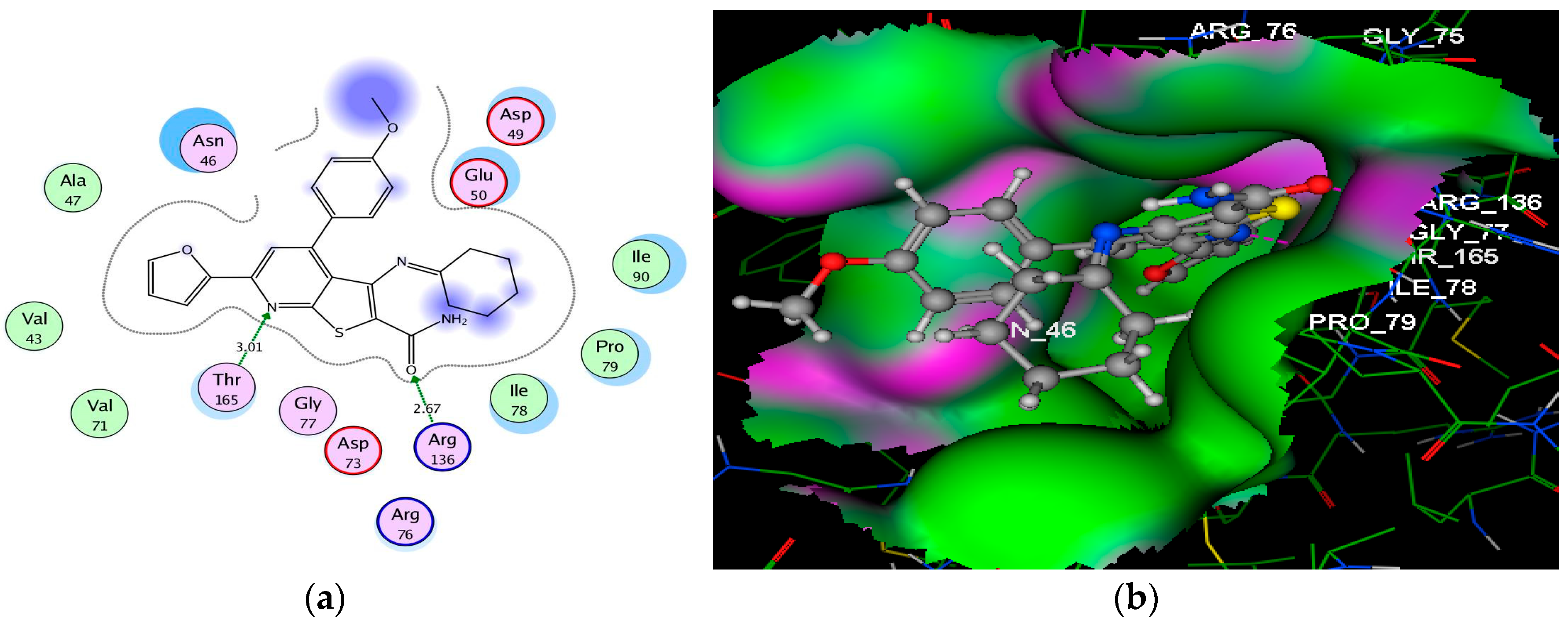
| 9b | −8.43 | Asn46 (2.46); Asn46 (2.79) | O(NHCOCH2); N(pyridine) | H–acc H–acc |
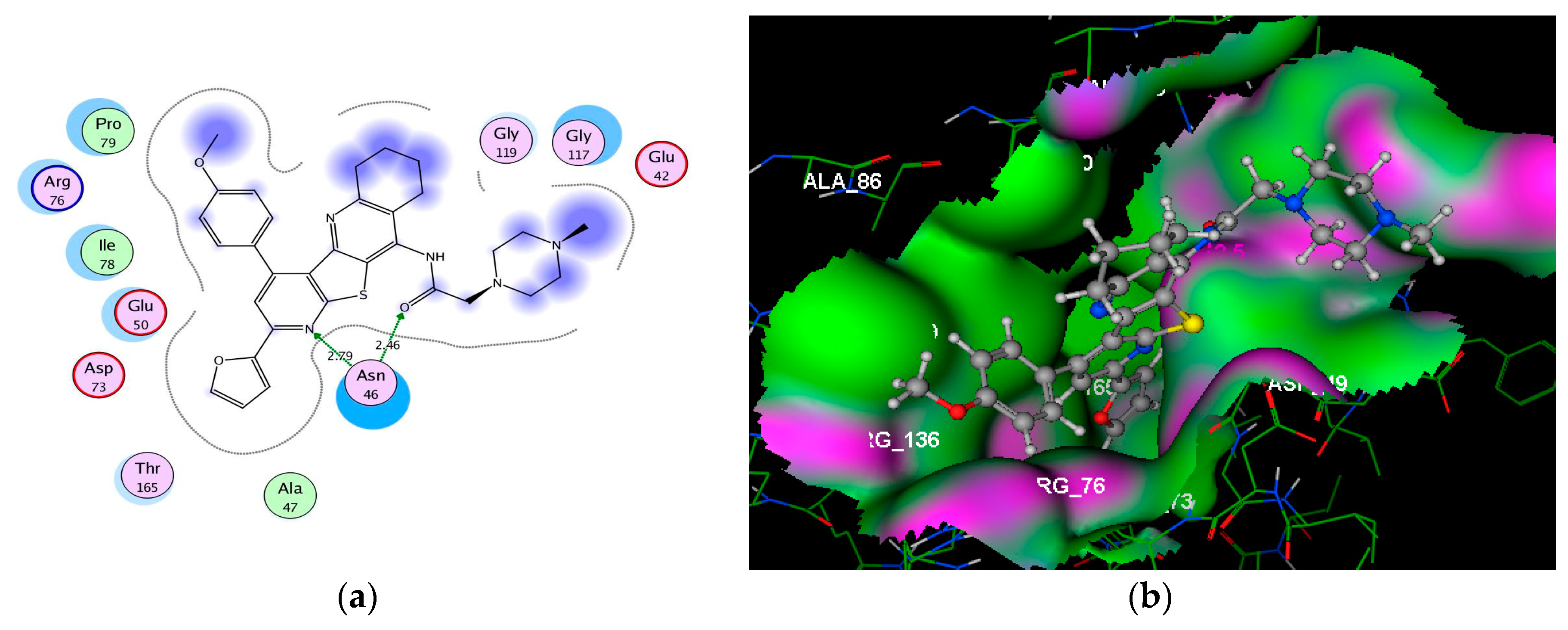
| 12b | −7.43 | Asn46 (2.98); Arg76 (2.80) | N(N=CH); O(OCH3) | H–acc H–acc |
| 14 | −5.45 | Asn46 (2.23); Arg76 | N(pyridine); thiophene | H–acc Arene–cation |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohi El-Deen, E.M.; Abd El-Meguid, E.A.; Hasabelnaby, S.; Karam, E.A.; Nossier, E.S. Synthesis, Docking Studies, and In Vitro Evaluation of Some Novel Thienopyridines and Fused Thienopyridine–Quinolines as Antibacterial Agents and DNA Gyrase Inhibitors. Molecules 2019, 24, 3650. https://doi.org/10.3390/molecules24203650
Mohi El-Deen EM, Abd El-Meguid EA, Hasabelnaby S, Karam EA, Nossier ES. Synthesis, Docking Studies, and In Vitro Evaluation of Some Novel Thienopyridines and Fused Thienopyridine–Quinolines as Antibacterial Agents and DNA Gyrase Inhibitors. Molecules. 2019; 24(20):3650. https://doi.org/10.3390/molecules24203650
Chicago/Turabian StyleMohi El-Deen, Eman M., Eman A. Abd El-Meguid, Sherifa Hasabelnaby, Eman A. Karam, and Eman S. Nossier. 2019. "Synthesis, Docking Studies, and In Vitro Evaluation of Some Novel Thienopyridines and Fused Thienopyridine–Quinolines as Antibacterial Agents and DNA Gyrase Inhibitors" Molecules 24, no. 20: 3650. https://doi.org/10.3390/molecules24203650
APA StyleMohi El-Deen, E. M., Abd El-Meguid, E. A., Hasabelnaby, S., Karam, E. A., & Nossier, E. S. (2019). Synthesis, Docking Studies, and In Vitro Evaluation of Some Novel Thienopyridines and Fused Thienopyridine–Quinolines as Antibacterial Agents and DNA Gyrase Inhibitors. Molecules, 24(20), 3650. https://doi.org/10.3390/molecules24203650