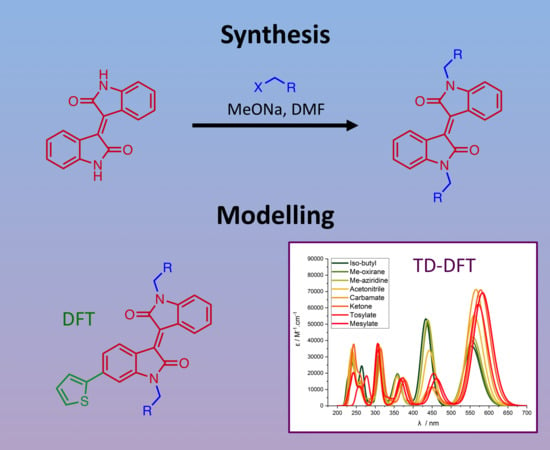
Synthesis and Density Functional Theory Studies of Azirinyl and Oxiranyl Functionalized Isoindigo and (3Z,3’Z)-3,3’-(ethane-1,2-diylidene)bis(indolin-2-one) Derivatives
Abstract
1. Introduction
2. Results
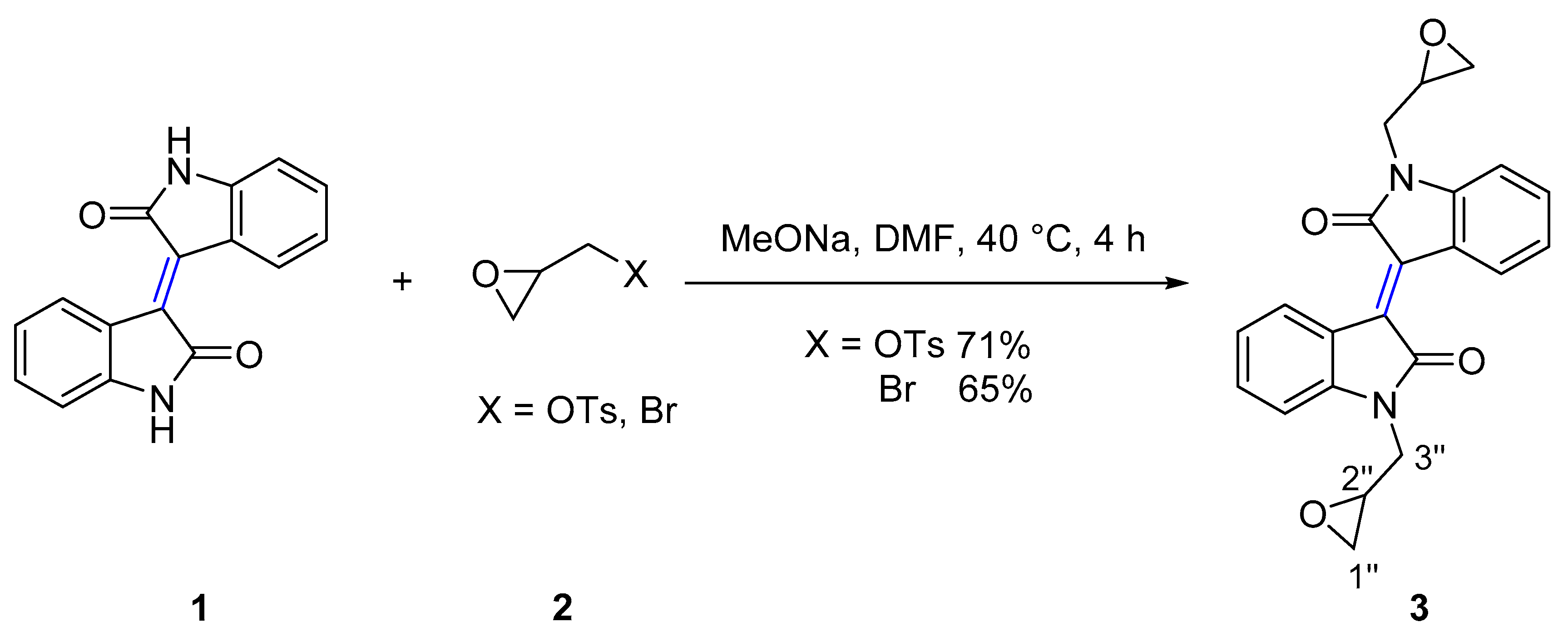
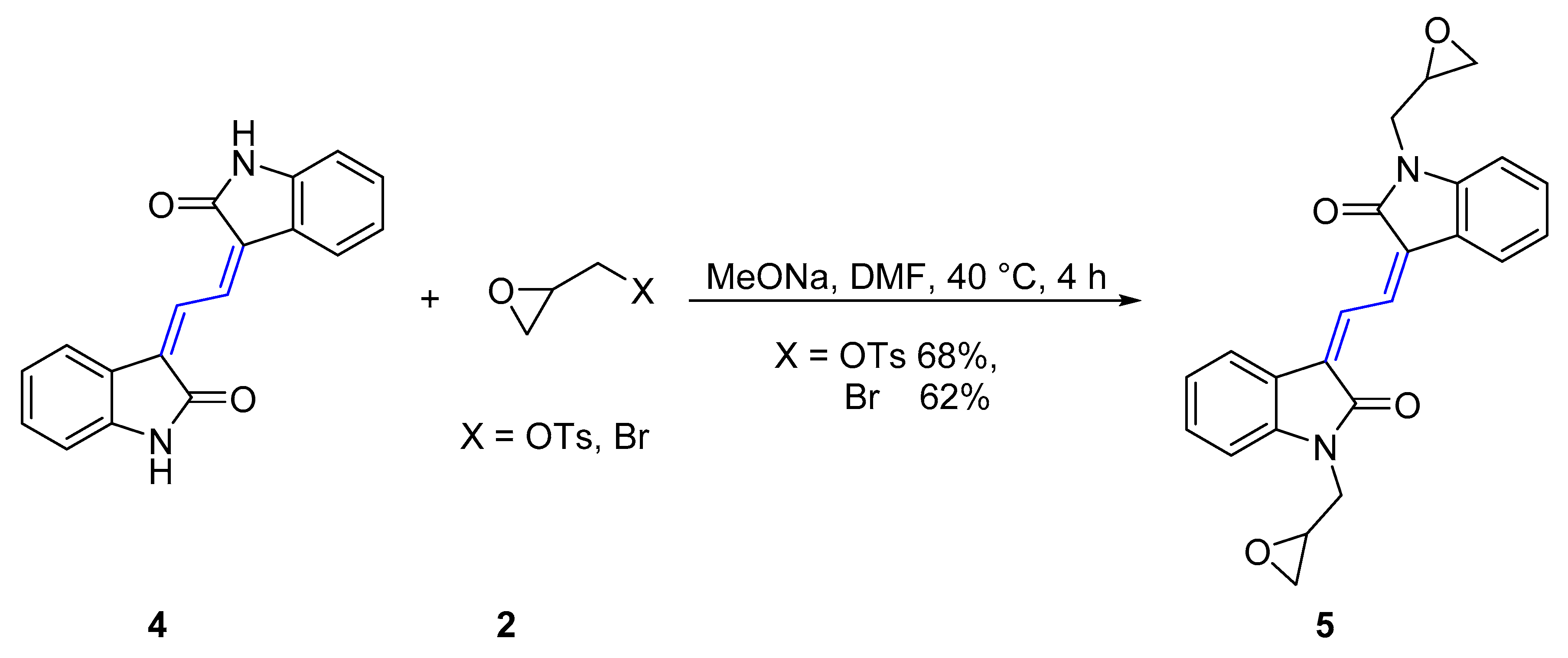
2.1. Chemical Synthesis and Characterisation
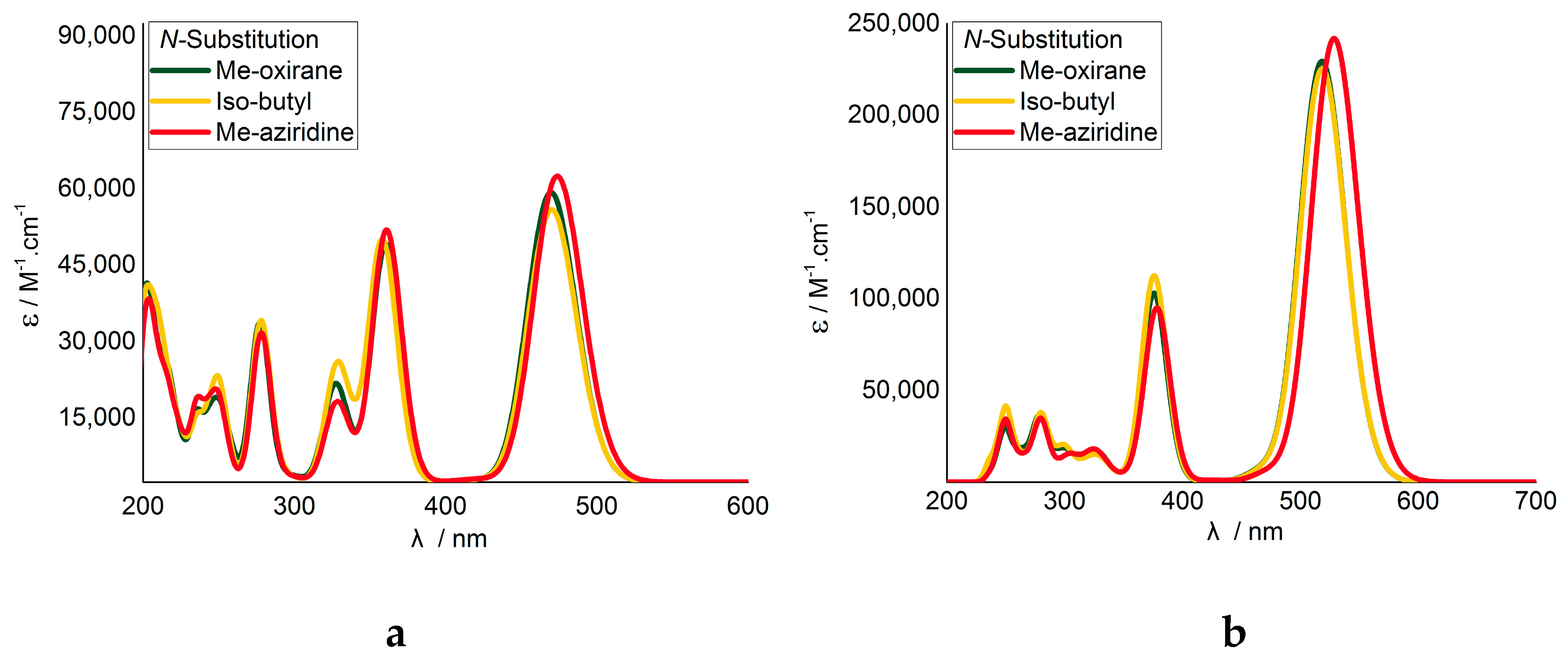
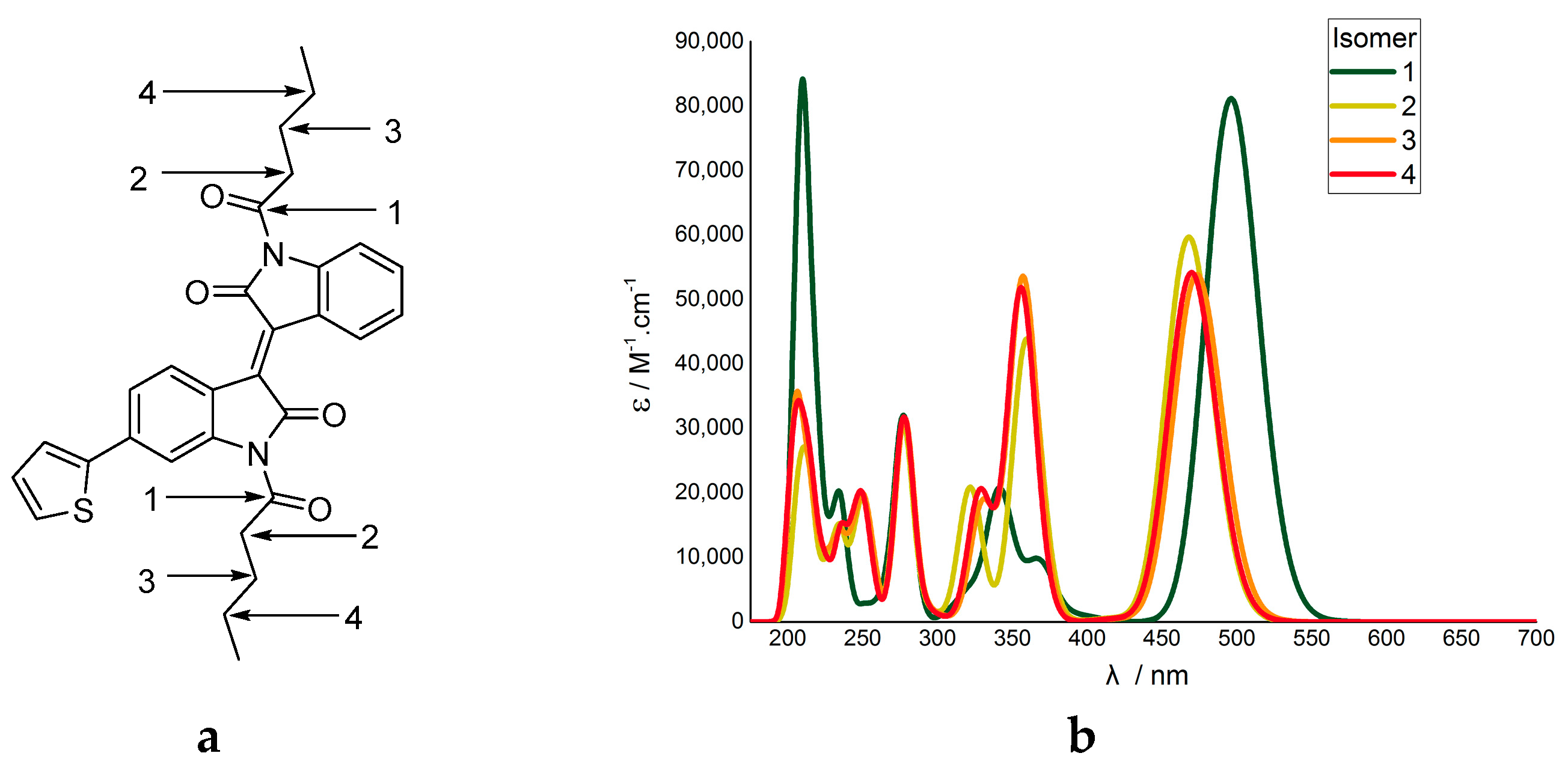
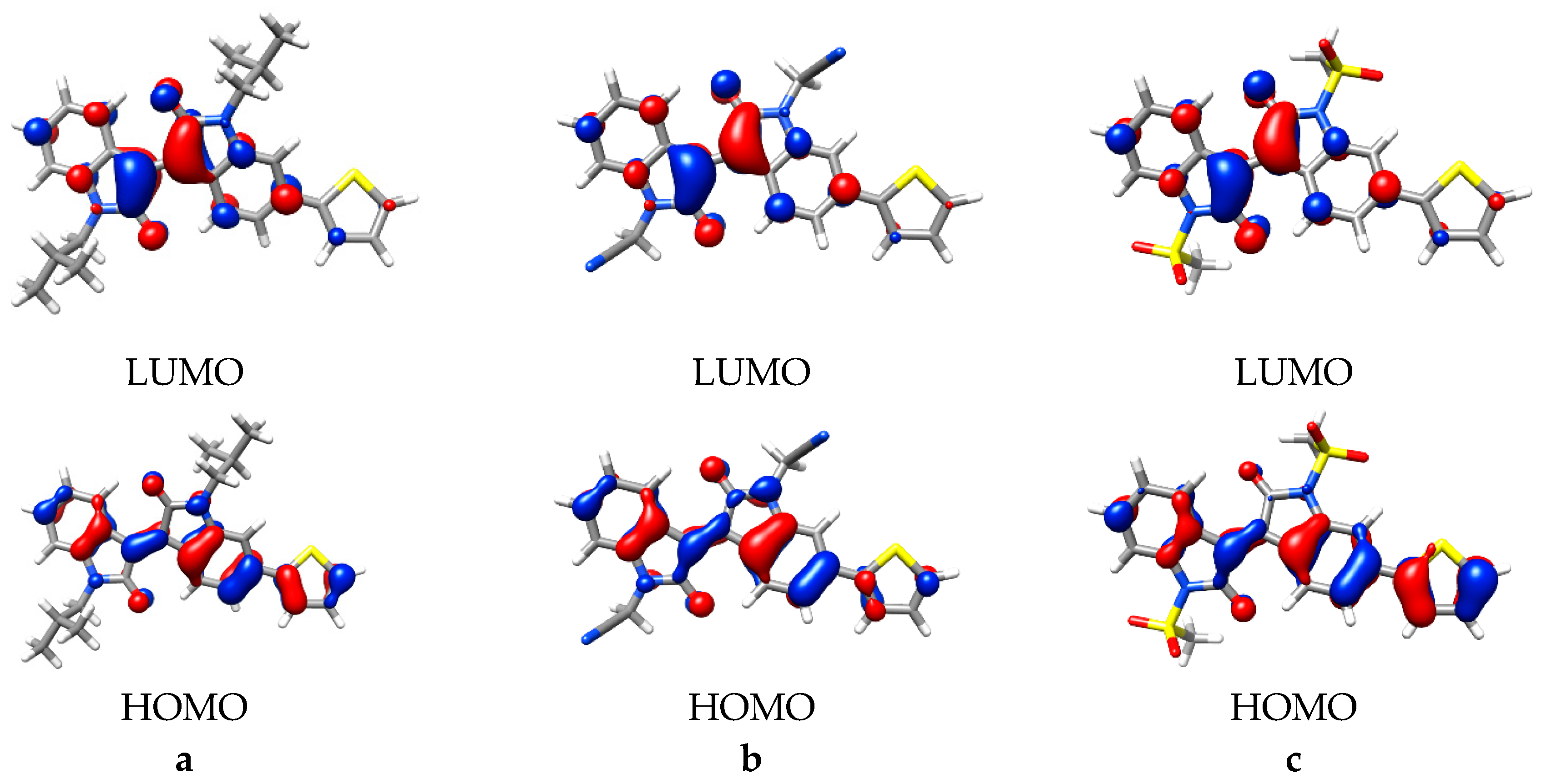
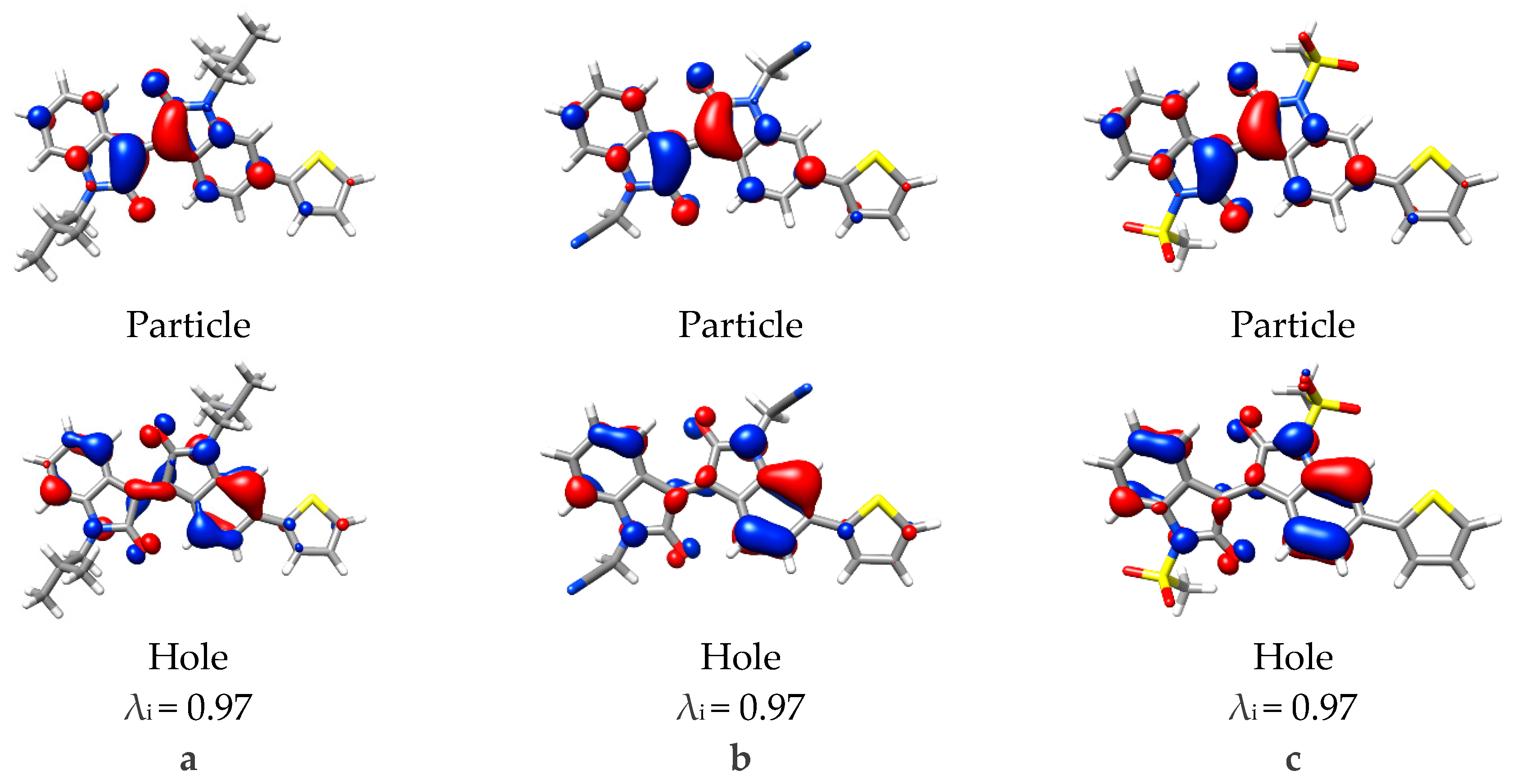
2.2. Computational Studies
3. Conclusions
4. Experimental Section
4.1. General Methods
4.2. Synthesis and Characterisation
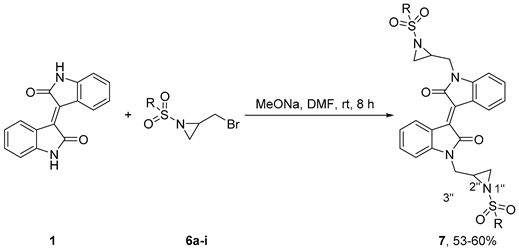
4.2.1. General procedure for the synthesis of 7
4.2.2. General procedure for the synthesis of 6
4.3. Computational Method
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Roncali, J. Molecular Bulk Heterojunctions: An Emerging Approach to Organic Solar Cells. Acc. Chem. Res. 2009, 42, 1719–1730. [Google Scholar] [CrossRef] [PubMed]
- Walker, B.; Kim, C.; Nguyen, T. Small Molecule Solution-Processed Bulk Heterojunction Solar Cells. Chem. Mater. 2011, 23, 470–482. [Google Scholar] [CrossRef]
- Hains, A.; Liang, Z.; Woodhouse, M.; Gregg, B. Molecular Semiconductors in Organic Photovoltaic Cells. Chem. Rev. 2010, 110, 6689–6735. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Hiszpanski, A.M.; Whittaker-Brooks, L.; Loo, Y.-L. Structure–Property Relationship Study of Substitution Effects on Isoindigo-Based Model Compounds as Electron Donors in Organic Solar Cells. ACS Appl. Mater. Interfaces 2014, 6, 14533–14542. [Google Scholar] [CrossRef] [PubMed]
- Estrada, L.A.; Stalder, R.; Abboud, K.A.; Risko, C.; Bredas, J.-L.; Reynolds, J.R. Understanding the Electronic Structure of Isoindigo in Conjugated Systems: A Combined Theoretical and Experimental Approach. Macromolecules 2013, 46, 8832–8844. [Google Scholar] [CrossRef]
- Stalder, R.; Mei, J.; Graham, K.R.; Estrada, L.A.; Reynolds, J.R. Isoindigo, a Versatile Electron-Deficient Unit For High-Performance Organic Electronics. Chem. Mater. 2014, 26, 664–678. [Google Scholar] [CrossRef]
- Mei, J.; Graham, K.R.; Stalder, R.; Reynolds, J.R. Synthesis of Isoindigo-Based Oligothiophenes for Molecular Bulk Heterojunction Solar Cells. Org. Lett. 2010, 12, 660–663. [Google Scholar] [CrossRef] [PubMed]
- Estrada, L.A.; Liu, D.Y.; Salazar, D.H.; Dyer, A.L.; Reynolds, J.R. Poly[Bis-EDOTIsoindigo]: An Electroactive Polymer Applied to Electrochemical Supercapacitors. Macromolecules 2012, 45, 8211–8220. [Google Scholar] [CrossRef]
- Wang, E.; Mammo, W.; Andersson, M.R. 25th Anniversary Article: Isoindigo-Based Polymers and Small Molecules for Bulk Heterojunction Solar Cells and Field Effect Transistors. Adv. Mater. 2014, 26, 1801–1826. [Google Scholar] [CrossRef]
- Deng, Y.; Liu, J.; Wang, J.; Liu, L.; Li, W.; Tian, H.; Zhang, X.; Xie, Z.; Geng, Y.; Wang, F. Dithienocarbazole and Isoindigo based Amorphous Low Bandgap Conjugated Polymers for Efficient Polymer Solar Cells. Adv. Mater. 2014, 26, 471–476. [Google Scholar] [CrossRef]
- Nishinaga, S.; Mori, H.; Nishihara, Y. Impact of Alkyl Side Chains on Thin-film Transistor Performances in Phenanthrodithiophene - Isoindigo Copolymers. Chem. Lett. 2015, 44, 998–1000. [Google Scholar] [CrossRef]
- Wang, E.; Ma, Z.; Zhang, Z.; Henriksson, P.; Inganas, O.; Zhang, F.; Andersson, M.R. An isoindigo-based low band gap polymer for efficient polymer solar cells with high photovoltage. Chem. Commun. 2011, 47, 4908–4910. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Zheng, T.; Wu, Q.; Schneider, A.M.; Zhao, D.; Yu, L. Recent Advances in Bulk Heterojunction Polymer Solar Cells. Chem. Rev. 2015, 115, 12666–12731. [Google Scholar] [CrossRef] [PubMed]
- Khalili, G.; Willis, A.; Keller, P. Design and synthesis of new functionalized isoindigo and (3Z,3Z)-3,3-(ethane-1,2-diylidene)bis(indolin-2-one) derivatives. Mon. Fur Chem.-Chem. 2018, 149, 2103–2111. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, Z.; Hou, R.; Huang, M.; Zhao, B.; Tan, S. The effect of the length of alkyl side-chains on the molecular aggregation and photovoltaic performance of the isoindigo based polymers. Dye. Pigment. 2017, 139, 403–411. [Google Scholar] [CrossRef]
- Grand, C.; Baek, S.; Lai, T.-H.; Deb, N.; Zajaczkowski, W.; Stalder, R.; Mullen, K.; Pisula, W.; Bucknall, D.G.; So, F.; et al. Structure–Property Relationships Directing Transport and Charge Separation in Isoindigo Polymers. Macromolecules 2016, 49, 4008–4022. [Google Scholar] [CrossRef]
- Yang, L.; Zhou, H.; You, W. Quantitatively Analyzing the Influence of Side Chains on Photovoltaic Properties of PolymerFullerene Solar Cells. J. Phys. Chem. C 2010, 114, 16793–16800. [Google Scholar] [CrossRef]
- Mei, J.; Bao, Z. Side Chain Engineering in Solution-Processable Conjugated Polymers. Chem. Mater. 2014, 26, 604–615. [Google Scholar] [CrossRef]
- Ma, Z.; Geng, H.; Wang, D.; Shuai, Z. Influence of alkyl side-chain length on the carrier mobility in organic semiconductors: Herringbone vs. pi–pi stacking. J. Mater. Chem. C 2016, 4, 4546–4555. [Google Scholar] [CrossRef]
- Dang, D.; Chen, W.; Himmelberger, S.; Tao, Q.; Lundin, A.; Yang, R.; Zhu, W.; Salleo, A.; Muller, C.; Wang, E. Enhanced Photovoltaic Performance of Indacenodithiophene-Quinoxaline Copolymers by Side-Chain Modulation. Adv. Energy Mater. 2014, 4, 1400680. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, J.; Li, Z.; Mu, C.; Ma, W.; Hu, H.; Jiang, K.; Lin, H.; Ade, H.; Yan, H. Aggregation and morphology control enables multiple cases of high-efficiency polymer solar cells. Nat. Commun. 2014, 5, 5293. [Google Scholar] [CrossRef] [PubMed]
- Constantinou, I.; Lai, T.-H.; Klump, E.D.; Goswami, S.; Schanze, K.S.; So, F. Effect of Polymer Side Chains on Charge Generation and Disorder in PBDTTPD Solar Cells. ACS Appl. Mater. Interfaces 2015, 7, 26999–27005. [Google Scholar] [CrossRef] [PubMed]
- Han, A.-R.; Lee, J.; Lee, H.R.; Lee, J.; Kang, S.-H.; Ahn, H.; Shin, T.J.; Oh, J.H.; Yang, C. Siloxane Side Chains: A Universal Tool for Practical Applications of Organic Field Effect Transistors. Macromolecules 2016, 49, 3739–3748. [Google Scholar] [CrossRef]
- Wang, P.-I.; Pisula, W.; Mullen, K.; Liaw, D.-J. Structurally defined nanographene-containing conjugated polymers for high quality dispersions and optoelectronic applications. Polym. Chem. 2016, 7, 6211–6219. [Google Scholar] [CrossRef]
- Salvatori, P.; Mosconi, E.; Wang, E.; Andersson, M.; Muccini, M.; De Angelis, F. Computational Modeling of Isoindigo-Based Polymers Used in Organic Solar Cells. J. Phys. Chem. C 2013, 117, 17940–17954. [Google Scholar] [CrossRef]
- Adamo, C.; Jacquemin, D. The calculations of excited-state properties with Time-Dependent Density Functional Theory. Chem. Soc. Rev. 2013, 42, 845–856. [Google Scholar] [CrossRef] [PubMed]
- Pastore, M.; Mosconi, E.; De Angelis, F.; Gratzel, M. A Computational Investigation of¨ Organic Dyes for Dye-Sensitized Solar Cells: Benchmark, Strategies, and Open Issues. J. Phys. Chem. C 2010, 114, 7205–7212. [Google Scholar] [CrossRef]
- Perpète, E.A.; Preat, J.; André, J.-M.; Jacquemin, D. An Ab Initio Study of the Absorption Spectra of Indirubin, Isoindigo, and Related Derivatives. J. Phys. Chem. A 2006, 110, 5629–5635. [Google Scholar] [CrossRef]
- Yanai, T.; Tew, D.P.; Handy, N.C. A new hybrid exchange–correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. [Google Scholar] [CrossRef]
- Robinson, D. Comparison of the Transition Dipole Moments Calculated by TDDFT with High Level Wave Function Theory. J. Chem. Theory Comput. 2018, 14, 5303–5309. [Google Scholar] [CrossRef]
- Martin, R.L. Natural transition orbitals. J. Chem. Phys. 2003, 118, 4775–4777. [Google Scholar] [CrossRef]
- Lowden, P. Aziridines and Epoxides in Organic Synthesis; Yudin, A., Ed.; Wiley-VCH: Weinheim, Germany, 2006; p. 399. [Google Scholar]
- Ismail, F.; Levitsky, D.; Dembitsky, V. Aziridine alkaloids as potential therapeutic agents. Eur. J. Med. Chem. 2009, 44, 3373–3387. [Google Scholar] [CrossRef] [PubMed]
- Bailey, S.J.; Wales, S.M.; Willis, A.C.; Keller, P.A. Ring-Opening and -Expansion of 2,2’-Biaziridine: Access to Diverse Enantiopure Linear and Bicyclic Vicinal Diamines. Org. Lett. 2014, 16, 4344–4347. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Sun, B.; Guo, C.; Hong, W.; Meng, Y.; Li, Y. 3,3-(Ethane-1,2-diylidene)bis(indolin2-one) based conjugated polymers for organic thin film transistors. Chem. Commun. 2014, 50, 6509–6512. [Google Scholar] [CrossRef] [PubMed]
- Thakur, V.; Sudalai, A. N-Bromoamides as versatile catalysts for aziridination of olefins using chloramine-T. Tetrahedron Lett. 2003, 44, 989–992. [Google Scholar] [CrossRef]
- Ali, S.; Nikalje, M.; Sudalai, A. Pyridinium Hydrobromide Perbromide: A Versatile Catalyst for Aziridination of Olefins Using Chloramine-T. Org. Lett. 1999, 1, 705–707. [Google Scholar] [CrossRef] [PubMed]
- Butler, N.M.; Hendra, R.; Bremner, J.B.; Willis, A.C.; Lucantoni, L.; Avery, V.M.; Keller, P.A. Cascade Reactions of Indigo with Oxiranes and Aziridines: Efficient Access to Dihydropyrazinodiindoles and Sprio-oxazocinodiindoles. Org. Biomol. Chem. 2018, 16, 6006–6016. [Google Scholar] [CrossRef] [PubMed]
- Tanner, D. Chiral Aziridines—Their Synthesis and Use in Stereoselective Transformations. Angew. Chem. Int. Ed. 1994, 33, 599–619. [Google Scholar] [CrossRef]
- Panda, A.N.; Plasser, F.; Aquino, A.J.A.; Burghardt, I.; Lischka, H. Electronically Excited States in Poly(p-phenylenevinylene): Vertical Excitations and Torsional Potentials from High Level Ab Initio Calculations. J. Phys. Chem. A 2013, 117, 2181–2189. [Google Scholar] [CrossRef]
- Tehrani, K.A.; Van, T.N.; Karikomi, M.; Rottiers, M.; De Kimpe, N. Electron Transfer Induced Ring Opening of 2-(bromomethyl)aziridines by Magnesium in Methanol. Tetrahedron 2002, 58, 7145–7152. [Google Scholar] [CrossRef]
- D’hooghe, M.; Kerkaert, I.; Rottiers, M.; Norbert De Kimpe, N. Ring Opening Reactions of 1-arenesulfonyl-2-(bromomethyl)aziridines. Tetrahedron 2004, 60, 3637–3641. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 3, 5, 7a–d, and 7f–i are available from the authors. |
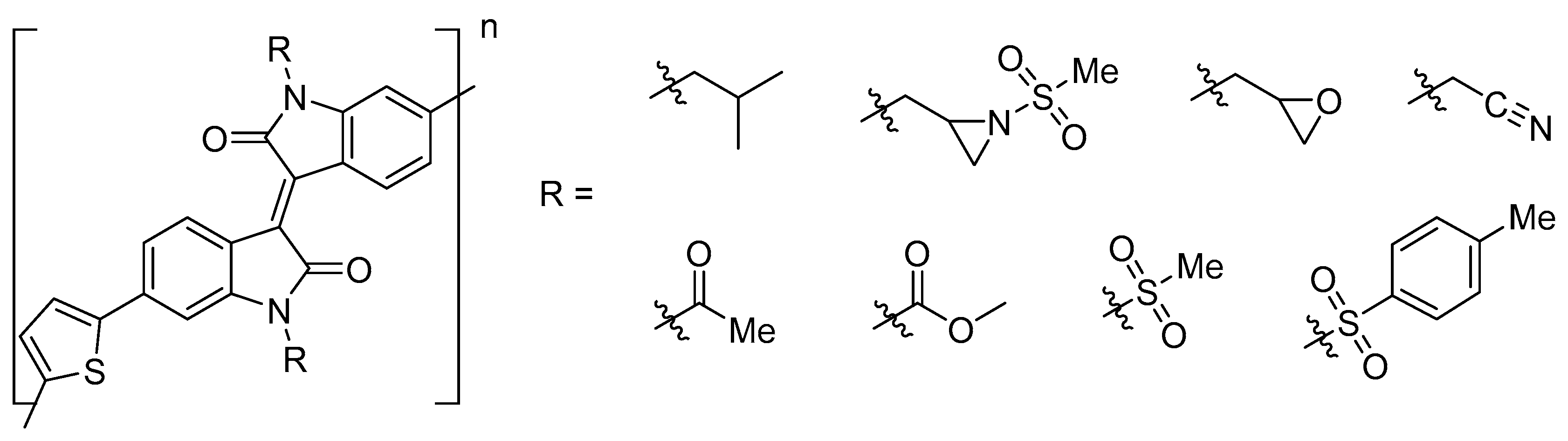

| Entry | Compound | R | Yield % |
|---|---|---|---|
| 1 | 7a | propyl | 58 |
| 2 | 7b | isopropyl | 57 |
| 3 | 7c | ethyl | 59 |
| 4 | 7d | methyl | 60 |
| 5 | 7e |  | 57 |
| 6 | 7f |  | 53 |
| 7 | 7g | 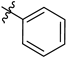 | 54 |
| 8 | 7h | 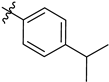 | 56 |
| 9 | 7i |  | 52 1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalili, G.; McCosker, P.M.; Clark, T.; Keller, P.A. Synthesis and Density Functional Theory Studies of Azirinyl and Oxiranyl Functionalized Isoindigo and (3Z,3’Z)-3,3’-(ethane-1,2-diylidene)bis(indolin-2-one) Derivatives. Molecules 2019, 24, 3649. https://doi.org/10.3390/molecules24203649
Khalili G, McCosker PM, Clark T, Keller PA. Synthesis and Density Functional Theory Studies of Azirinyl and Oxiranyl Functionalized Isoindigo and (3Z,3’Z)-3,3’-(ethane-1,2-diylidene)bis(indolin-2-one) Derivatives. Molecules. 2019; 24(20):3649. https://doi.org/10.3390/molecules24203649
Chicago/Turabian StyleKhalili, Gholamhossein, Patrick M. McCosker, Timothy Clark, and Paul A. Keller. 2019. "Synthesis and Density Functional Theory Studies of Azirinyl and Oxiranyl Functionalized Isoindigo and (3Z,3’Z)-3,3’-(ethane-1,2-diylidene)bis(indolin-2-one) Derivatives" Molecules 24, no. 20: 3649. https://doi.org/10.3390/molecules24203649
APA StyleKhalili, G., McCosker, P. M., Clark, T., & Keller, P. A. (2019). Synthesis and Density Functional Theory Studies of Azirinyl and Oxiranyl Functionalized Isoindigo and (3Z,3’Z)-3,3’-(ethane-1,2-diylidene)bis(indolin-2-one) Derivatives. Molecules, 24(20), 3649. https://doi.org/10.3390/molecules24203649