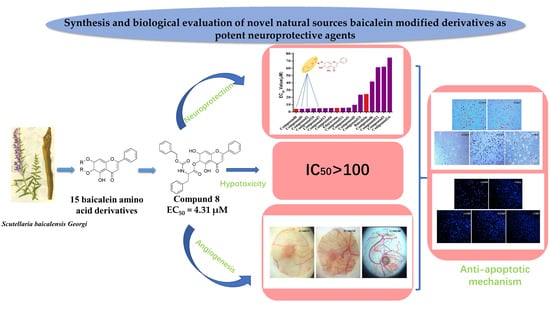
Synthesis of Novel Baicalein Amino Acid Derivatives and Biological Evaluation as Neuroprotective Agents
Abstract
1. Introduction
2. Results
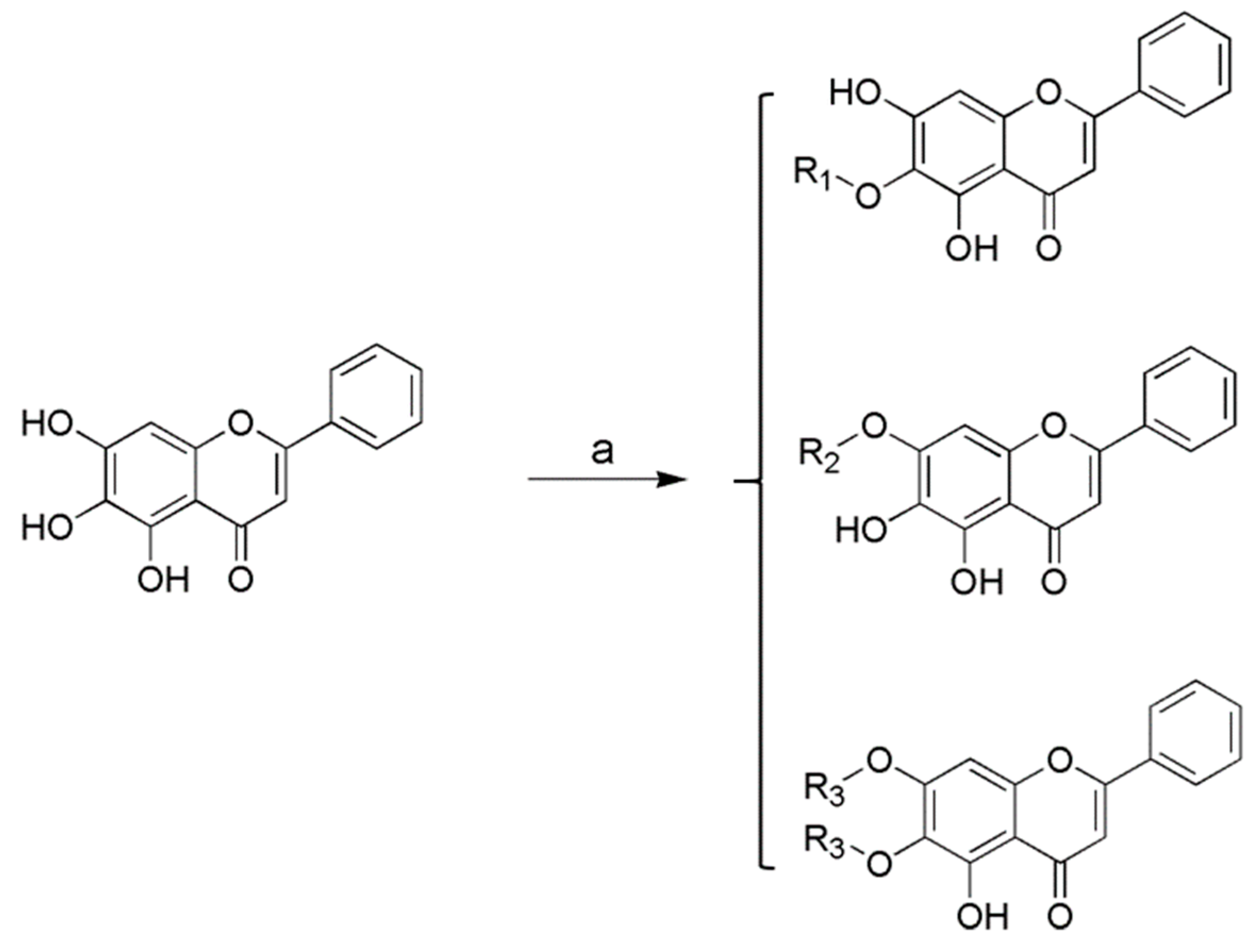
2.1. Chemistry
2.2. Biological Activities
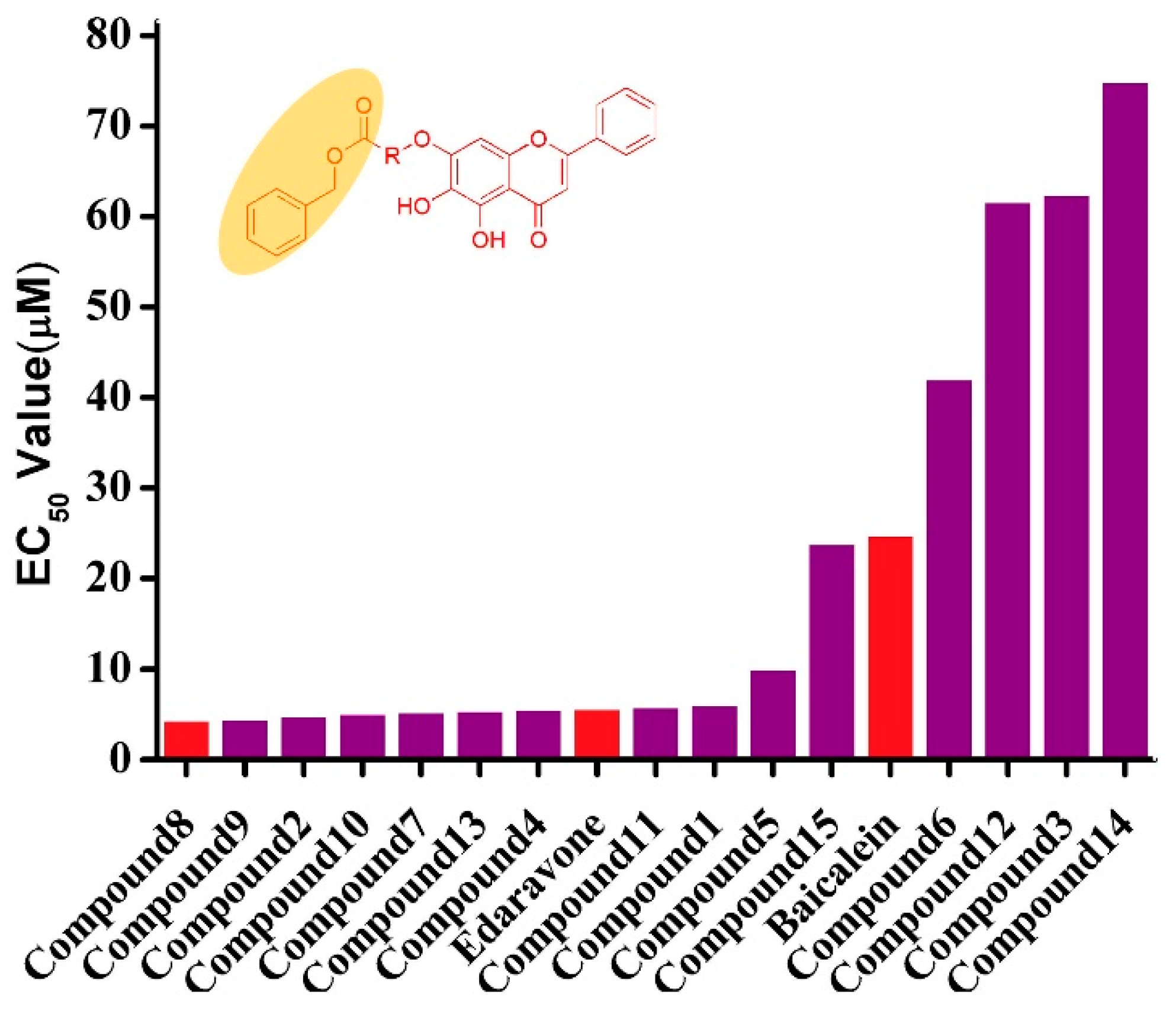
2.2.1. Protective Effects against Tert-Utyl, Hydroperoxide-Induced, SH-SY5Y Neurotoxicity Cells
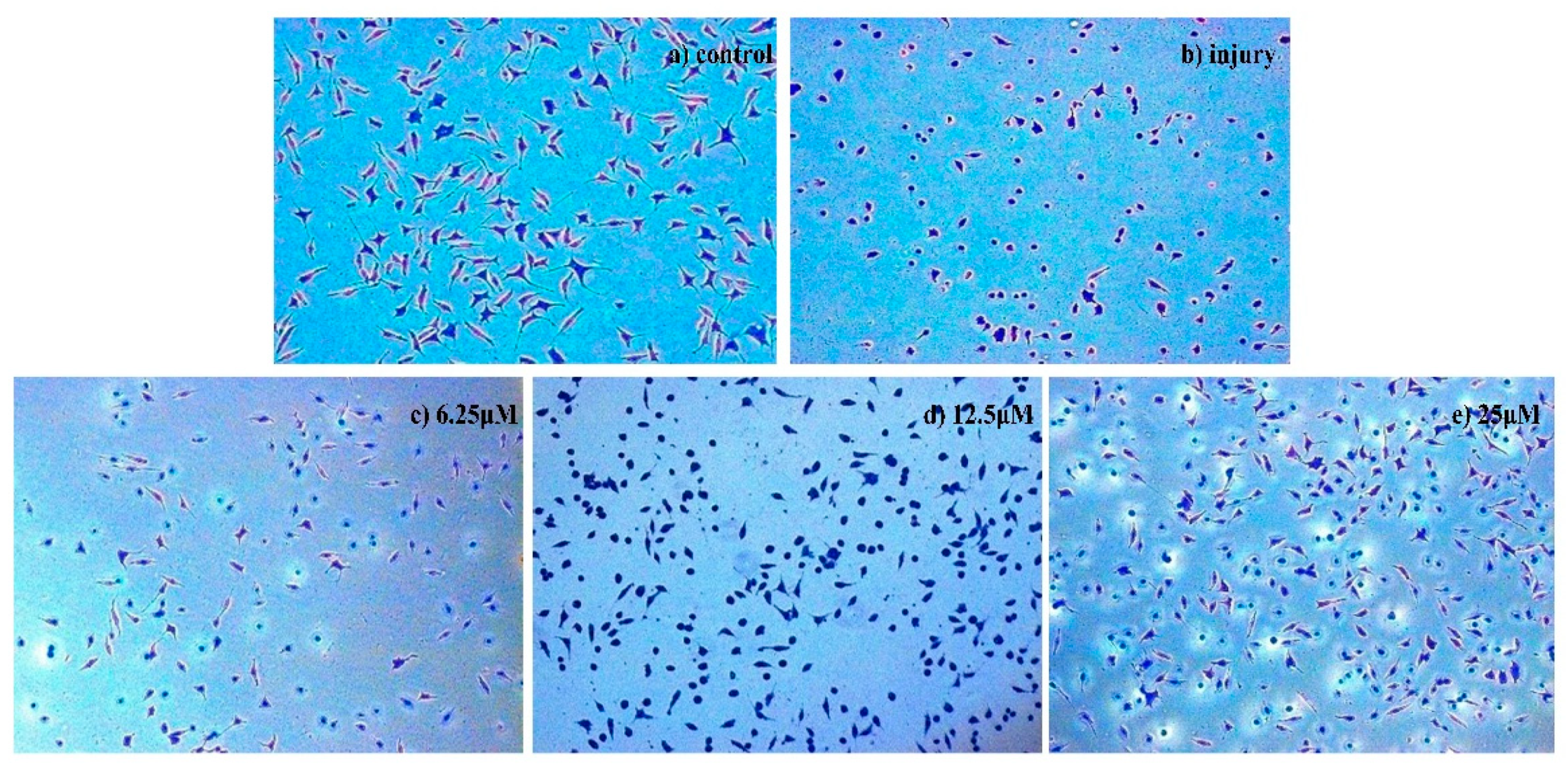
2.2.2. Analyses of Apoptosis
Morphological Detection of Apoptosis Using Giemsa Staining
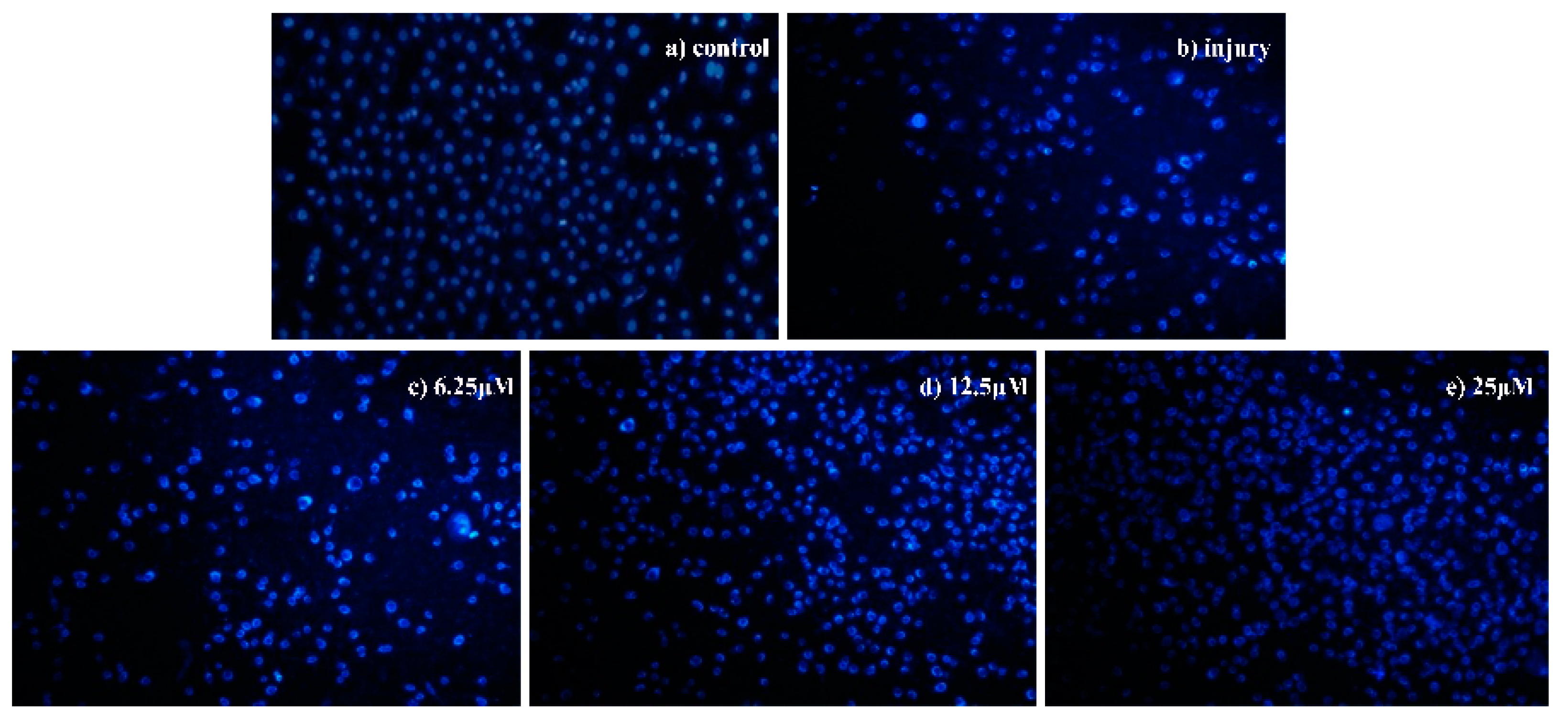
Morphological Detection of Apoptosis Using DAPI Staining
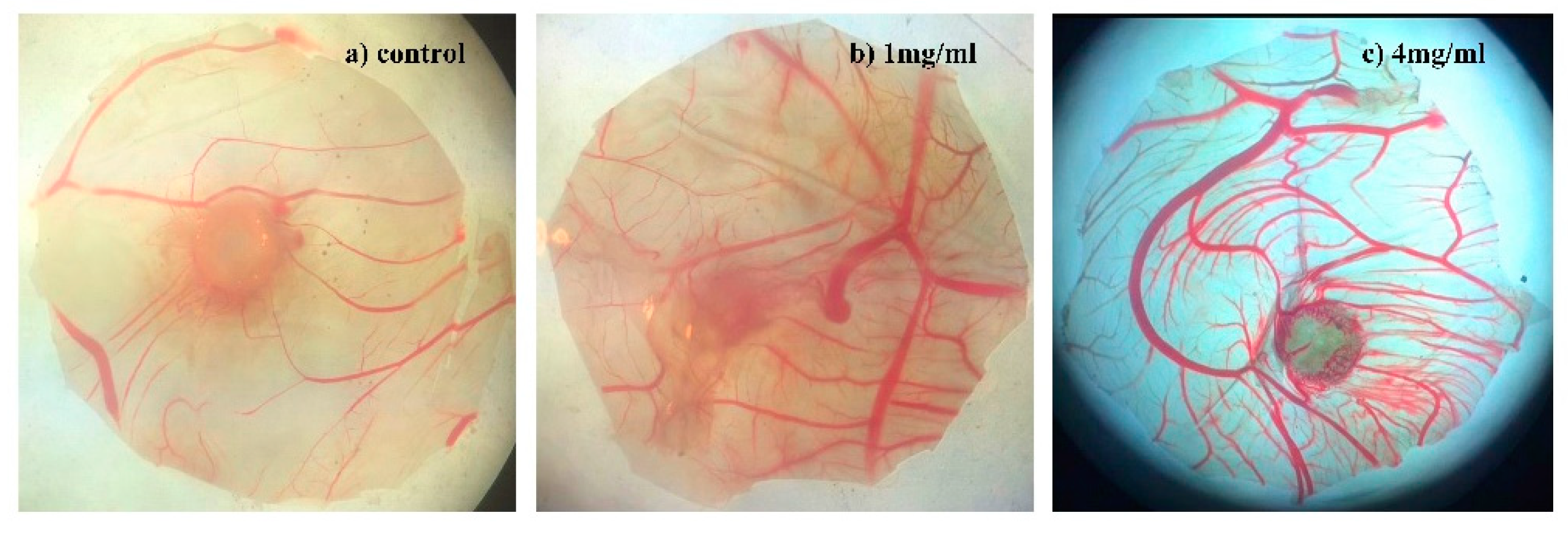
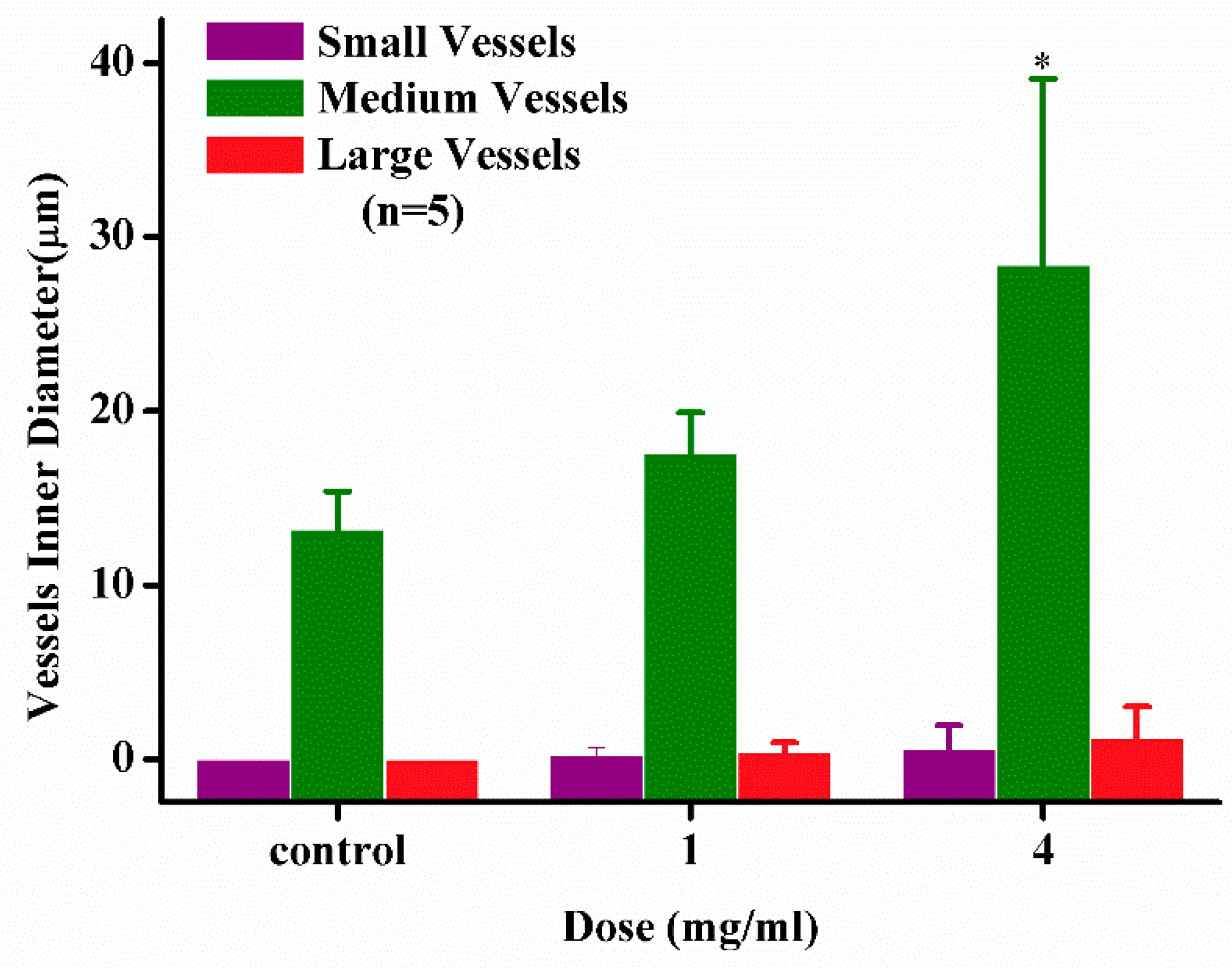
2.2.3. Angiogenesis Activity
3. Experimental Section
3.1. Chemistry
General Procedure for the Preparation of Baicalein Amino Acid Derivatives 1–15
3.2. Bio-Evaluation Methods
3.2.1. Cell Culture
3.2.2. Protective Effect on Injured SH-SY5Y Cells
3.2.3. Giemsa Staining
3.2.4. DAPI Staining
3.2.5. Angiogenesis Assay
3.3. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tejada, S.; Setzer, W.N.; Daglia, M.; Nabavi, S.F.; Sureda, A.; Braidy, N.; Gortzi, O.; Nabavi, S.M. Neuroprotective effects of Ellagitannins: A brief review. Curr. Drug Targets 2016, 18, 1518. [Google Scholar] [CrossRef] [PubMed]
- Losada-Barreiro, S.; Bravo-Dã-Az, C. Free radicals and polyphenols: The redox chemistry of neurodegenerative diseases. Eur. J. Med. Chem. 2017, 133, 379–402. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, H.L.; Yang, C.M. Role of redox signaling in neuroinflammation and neurodegenerative disease. BioMed. Res. Int. 2013, 484613. [Google Scholar] [CrossRef] [PubMed]
- Niloufar, A.; Fariba, K. Natural Products as Promising Drug Candidates for the Treatment of Alzheimer’s Disease: Molecular Mechanism Aspect. Curr. Neuropharmacol. 2013, 11, 414–429. [Google Scholar]
- Sowndhararajan, K.; Kim, S. Neuroprotective and Cognitive Enhancement Potentials of Angelica gigas Nakai Root: A Review. Sci. Pharm. 2017, 85, 21. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, E.C.; Vyas, S.; Hunot, S. Neuroinflammation in Parkinson’s disease. Parkinsonism Relat. Disord. 2012, 18, S210–S212. [Google Scholar] [CrossRef]
- Chen, W.W.; Zhang, X.; Huang, W.J. Role of neuroinflammation in neurodegenerative diseases. Mol. Med. Rep. 2016, 13, 3391–3396. [Google Scholar] [CrossRef]
- Desai, A.K.; Grossberg, G.T. Diagnosis and treatment of Alzheimer’s disease. Neurology 2005, 64, S34–S39. [Google Scholar] [CrossRef] [PubMed]
- Doody, R.S.; Ferris, S.H.; Salloway, S.; Sun, Y.; Goldman, R.; Watkins, W.E.; Xu, Y.; Murthy, A.K. Donepezil treatment of patients with MCI: A 48-week randomized, placebo-controlled trial. Neurology 2009, 72, 1555–1561. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, Y. Recent research progress in and future perspective on treatment of Parkinson’s disease. Integr. Med. Int. 2014, 1, 67–79. [Google Scholar] [CrossRef]
- Okun, M.S. Deep-brain stimulation—Entering the era of human neural-network modulation. N. Engl. J. Med. 2014, 371, 1369–1373. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Pang, X.; Yan, R.; Lian, W.; Li, C.; Wang, Q.; Du, G.H. Discovery of neuroprotective compounds by machine learning approaches. RSC Adv. 2016, 6, 9857–9871. [Google Scholar] [CrossRef]
- Fang, J.; Liu, C.; Wang, Q.; Lin, P.; Cheng, F. In silico polypharmacology of natural products. Brief. Bioinform. 2017, 19, 1153–1171. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Pan, W. The treatment strategies for neurodegenerative diseases by integrative medicine. Integr. Med. Int. 2014, 1, 223–225. [Google Scholar] [CrossRef]
- Fang, J.; Li, Y.; Liu, R.; Pang, W.; Li, C.; Yang, R.; He, Y.; Lian, W.; Liu, A.L.; Du, G.H. Discovery of multitarget-directed ligands against Alzheimer’s disease through systematic prediction of chemical–protein interactions. J. Chem. Inf. Model. 2015, 55, 149–164. [Google Scholar] [CrossRef] [PubMed]
- Pak, M.E.; Kim, Y.R.; Kim, H.N.; Ahn, S.M.; Shin, H.K.; Baek, J.U.; Choi, B.T. Studies on medicinal herbs for cognitive enhancement based on the text mining of Dongeuibogam and preliminary evaluation of its effects. J. Ethnopharmacol. 2016, 179, 383–390. [Google Scholar] [CrossRef]
- Grossberg, G.T. Diagnosis and treatment of Alzheimer’s disease. J. Clin. Psychiatry 2003, 64, 3–6. [Google Scholar]
- Crane, P.K.; Doody, R.S. Donepezil treatment of patients with MCI: A 48-week randomized, placebo-controlled trial. Neurology 2009, 1514–1516. [Google Scholar] [CrossRef]
- Xiao, J.R.; Do, C.W.; To, C.H. Potential therapeutic effects of baicalein, baicalin and wogonin in ocular disorders. J. Ocul. Pharmacol. Ther. 2014, 30, 605–614. [Google Scholar] [CrossRef]
- de Oliveira, M.R.; Nabavi, S.F.; Habtemariam, S.; Orhan, I.E.; Daglia, M.; Nabavi, S.M. The effects of baicalein and baicalin on mitochondrial function and dynamics: A review. Pharmacol. Res. 2015, 100, 296–308. [Google Scholar] [CrossRef]
- Gao, Y.; Snyder, S.A.; Smith, J.N.; Chen, Y.C. Anticancer properties of baicalein: A review. Med. Chem. Res. 2016, 25, 1515–1523. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhao, J.; Hölscher, C. Therapeutic Potential of baicalein in Alzheimer’s disease and Parkinson’s disease. CNS Drugs 2017, 31, 639–652. [Google Scholar] [CrossRef] [PubMed]
- Muto, T.; Motozuka, M.; Nakano, Y.; Tatsumi, F. The chemical structure of new substance as the metabolite of baicalin and time profiles for the plasma concentration after oral administration of Sho-saiko-to in human. J. Pharm. Soc. Jpn. 1998, 18, 79–87. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, L.; Lin, G.; Zuo, Z. High-performance liquid chromatographic method for simultaneous determination of baicalein and baicalein 7-glucuronide in rat plasma. J. Pharm. Biomed. Anal. 2004, 36, 637–641. [Google Scholar] [CrossRef] [PubMed]
- Taiming, L.; Xuehua, J. Investigation of the absorption mechanisms of baicalin and baicalein in rats. J. Pharm. Sci. 2006, 95, 1326–1333. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.Y.; Hsiu, S.L.; Tsai, S.Y.; Hou, Y.C.; Chao, P.D. Comparison of metabolic pharmacokinetics of baicalin and baicalein in rats. J. Pharm. Pharmacol. 2010, 55, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Smolders, I.; Khan, G.M.; Lindekens, H.; Prikken, S.; Marvin, C.A.; Manil, J.; Ebinger, G.; Michotte, Y. Effectiveness of vigabatrin against focally evoked pilocarpine-induced seizures and concomitant changes in extracellular hippocampal and cerebellar glutamate, gamma-aminobutyric acid and dopamine levels, a microdialysis-electrocorticography study in freely moving rats. J. Pharmacol. Exp. Ther. 1997, 283, 1239. [Google Scholar] [PubMed]
- Heales, S.J.; Bolaños, J.P.; Stewart, V.C.; Brookes, P.S.; Land, J.M.; Clark, J.B. Nitric oxide, mitochondria and neurological disease. Biochim. Et Biophys. Acta (BBA)-Bioenerg. 1999, 1410, 215–228. [Google Scholar] [CrossRef]
- Frugier, G.; Coussen, F.; Giraud, M.F.; Odessa, M.F.; Emerit, M.B.; Boué-Grabot, E.; Garret, M. A gamma 2(R43Q) mutation, linked to epilepsy in humans, alters GABAA receptor assembly and modifies subunit composition on the cell surface. J. Biol. Chem. 2007, 282, 3819–3828. [Google Scholar] [CrossRef]
- Ding, R.; Asada, H.; Obata, K. Changes in extracellular glutamate and GABA levels in the hippocampal CA3 and CA1 areas and the induction of glutamic acid decarboxylase-67 in dentate granule cells of rats treated with kainic acid. Brain Res. 1998, 800, 105–113. [Google Scholar] [CrossRef]
- Xiudao, S.; Yunmei, L.; Jin, M.; Jun, H.; Xing, Z.; Xiaoyong, L.; Guorong, J.; Lurong, Z. Synthesis of novel amino acid derivatives containing chrysin as anti-tumor agents against human gastric carcinoma MGC-803 cells. Med. Chem. Res. 2015, 24, 1789–1798. [Google Scholar]
- Brady, S.F.; Pawluczyk, J.M.; Lumma, P.K.; Feng, D.M.; Wai, J.M.; Jones, R.; Oliff, A. Design and synthesis of a pro-drug of vinblastine targeted at treatment of prostate cancer with enhanced efficacy and reduced systemic toxicity. J. Med. Chem. 2002, 45, 4706–4715. [Google Scholar] [CrossRef] [PubMed]
- DeFeo-Jones, D.; Brady, S.F.; Feng, D.M.; Wong, B.K.; Bolyar, T.; Haskell, K.; Kiefer, D.M.; Leander, K.; McAvoy, E.; Lumma, P.; et al. A prostate-specific antigen (PSA)-activated vinblastine prodrug selectively kills PSA-secreting cells in vivo. Mol. Cancer Ther. 2002, 1, 451–459. [Google Scholar] [PubMed]
- Fang, K.; Zhang, X.H.; Han, Y.T.; Wu, G.R.; Cai, D.S.; Xue, N.N.; Guo, W.B.; Yang, Y.Q.; Chen, M.; Zhang, X.Y.; et al. Design, Synthesis, and Cytotoxic Analysis of Novel Hederagenin–Pyrazine Derivatives Based on Partial Least Squares Discriminant Analysis. Int. J. Mol. Sci. 2018, 19, 2994. [Google Scholar] [CrossRef]
- Biegon, A. Cannabinoids as Neuroprotective Agents in Traumatic Brain Injury. Curr. Pharm. Des. 2004, 10, 2177–2183. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Kim, S.K.; Kim, Y.S.; Son, D.H.; Nam, J.H.; Kim, I.S.; Park, R.W.; Kim, S.Y.; Byun, Y. Suppression of angiogenesis and tumor growth by orally active deoxycholic acid-heparin conjugate. J. Control. Release. 2007, 118, 310–317. [Google Scholar] [CrossRef]
- Li, H.Q.; Wu, X.Z.; Bai, D.; Yang, Y.N.; Lei, H.M. Screening active fraction of compound Sanhuang capsules for inhibition of angiogenesis in tumor. Chin. J. Exp. Tradit. Med. Formulae 2011, 17, 172–175. [Google Scholar]
- Wang, P.; She, G.; Yang, Y.; Li, Q.; Zhang, H.; Liu, J.; Lei, H. Synthesis and Biological Evaluation of New Ligustrazine Derivatives as Anti-Tumor Agents. Molecules 2012, 17, 4972–4985. [Google Scholar] [CrossRef]
- Li, B.; Yan, W.; Zhang, C.; Zhang, Y.; Liang, M.; Chu, F.; Lei, H. New Synthesis Method for Sultone Derivatives: Synthesis, Crystal Structure and Biological Evaluation of S-CA. Molecules 2015, 20, 4307–4318. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 1–15 are available from the authors. |

| Compound | Structure | Compound | Structure |
|---|---|---|---|
| 1 | 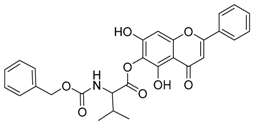 | 2 |  |
| 3 | 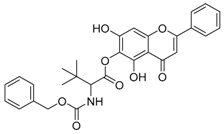 | 4 | 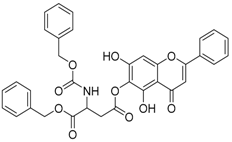 |
| 5 | 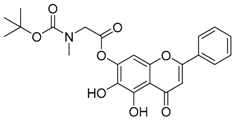 | 6 | 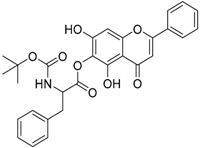 |
| 7 | 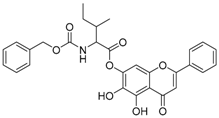 | 8 | 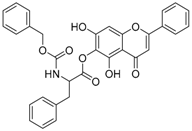 |
| 9 | 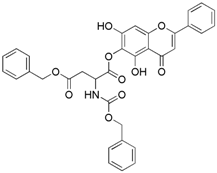 | 10 | 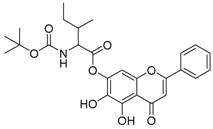 |
| 11 | 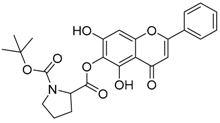 | 12 | 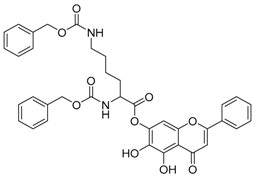 |
| 13 |  | 14 | 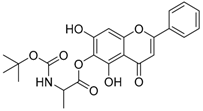 |
| 15 |  |
| Compound | Proliferation Rate (%) | EC50 (µM) | ||||
|---|---|---|---|---|---|---|
| 50 µM | 25 µM | 12.5 µM | 6.25 µM | 3.125 µM | ||
| 1 | 54.92 | 67.35 | 81.81 | 84.35 | 55.99 | 6.07 |
| 2 | 94.24 | 106.84 | 81.03 | 48.22 | 45.07 | 4.79 |
| 3 | −0.18 | 0.15 | 8.10 | 14.82 | 15.27 | 62.36 |
| 4 | 54.80 | 70.23 | 77.70 | 94.11 | 59.08 | 5.51 |
| 5 | 60.70 | 78.11 | 76.37 | 37.56 | 28.36 | 9.96 |
| 6 | 16.74 | 21.58 | 24.75 | 19.99 | 8.61 | 42.04 |
| 7 | 72.17 | 79.76 | 79.76 | 69.72 | 63.62 | 5.24 |
| 8 | 94.03 | 87.31 | 83.08 | 75.12 | 52.49 | 4.31 |
| 9 | 84.15 | 101.00 | 79.58 | 98.20 | 28.13 | 4.47 |
| 10 | 63.22 | 74.55 | 79.27 | 77.21 | 75.23 | 5.07 |
| 11 | 64.80 | 74.48 | 78.16 | 86.25 | 47.94 | 5.82 |
| 12 | 7.34 | 20.81 | 7.83 | 2.35 | 1.10 | 61.60 |
| 13 | 65.86 | 72.99 | 91.54 | 92.10 | 40.12 | 5.42 |
| 14 | 0.10 | 4.09 | 7.09 | 3.34 | 0.35 | 74.86 |
| 15 | 22.31 | 41.27 | 42.51 | 42.76 | 17.81 | 23.85 |
| Baicalein | 11.69 | 28.36 | 29.85 | 35.07 | 54.73 | 24.77 |
| Edaravone | 60.70 | 69.90 | 76.12 | 85.82 | 61.44 | 5.62 |
| Compound | Survival Rate (%) | IC50 (µM) | ||||
|---|---|---|---|---|---|---|
| 100 µM | 50 µM | 25 µM | 12.5 µM | 6.25 µM | ||
| 1 | 204.12 | 215.52 | 231.16 | 260.35 | 269.76 | >100.00 |
| 2 | 107.88 | 112.46 | 133.06 | 149.84 | 213.19 | >100.00 |
| 3 | 104.18 | 132.42 | 134.97 | 137.27 | 144.06 | >100.00 |
| 4 | 125.68 | 130.55 | 140.46 | 140.39 | 155.60 | >100.00 |
| 5 | 76.93 | 95.13 | 105.32 | 115.71 | 115.66 | >100.00 |
| 6 | 121.68 | 137.47 | 153.61 | 154.46 | 157.32 | >100.000 |
| 7 | 123.82 | 138.56 | 144.70 | 146.75 | 152.03 | >100.00 |
| 8 | 167.58 | 168.17 | 169.08 | 182.13 | 195.31 | >100.00 |
| 9 | 107.43 | 114.84 | 129.50 | 133.10 | 152.78 | >100.00 |
| 10 | 141.70 | 152.13 | 169.33 | 179.84 | 194.73 | >100.00 |
| 11 | 45.75 | 60.67 | 61.98 | 67.32 | 70.98 | 95.21 |
| 12 | 183.88 | 187.47 | 200.19 | 206.64 | 216.11 | >100.00 |
| 13 | 123.43 | 132.48 | 151.84 | 166.23 | 215.46 | >100.00 |
| 14 | 97.10 | 129.44 | 137.29 | 166.30 | 184.85 | >100.00 |
| 15 | 159.21 | 172.74 | 177.39 | 193.04 | 196.35 | >100.00 |
| Baicalein | 168.20 | 169.76 | 178.56 | 198.36 | 212.09 | >100.00 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, X.; Jia, M.; Yang, Y.; Wang, D.; Zhou, F.; Zhang, W.; Huang, X.; Guo, W.; Cai, D.; Chen, H.; et al. Synthesis of Novel Baicalein Amino Acid Derivatives and Biological Evaluation as Neuroprotective Agents. Molecules 2019, 24, 3647. https://doi.org/10.3390/molecules24203647
Jia X, Jia M, Yang Y, Wang D, Zhou F, Zhang W, Huang X, Guo W, Cai D, Chen H, et al. Synthesis of Novel Baicalein Amino Acid Derivatives and Biological Evaluation as Neuroprotective Agents. Molecules. 2019; 24(20):3647. https://doi.org/10.3390/molecules24203647
Chicago/Turabian StyleJia, Xiaohui, Menglu Jia, Yuqin Yang, Di Wang, Fei Zhou, Wenxi Zhang, Xuemei Huang, Wenbo Guo, Desheng Cai, Hongshan Chen, and et al. 2019. "Synthesis of Novel Baicalein Amino Acid Derivatives and Biological Evaluation as Neuroprotective Agents" Molecules 24, no. 20: 3647. https://doi.org/10.3390/molecules24203647
APA StyleJia, X., Jia, M., Yang, Y., Wang, D., Zhou, F., Zhang, W., Huang, X., Guo, W., Cai, D., Chen, H., Qi, J., Zhou, S., Ren, H., Xu, B., Ma, T., Wang, P., & Lei, H. (2019). Synthesis of Novel Baicalein Amino Acid Derivatives and Biological Evaluation as Neuroprotective Agents. Molecules, 24(20), 3647. https://doi.org/10.3390/molecules24203647