Voriconazole-Based Salts Are Active against Multidrug-Resistant Human Pathogenic Yeasts
Abstract
1. Introduction
2. Results and Discussion
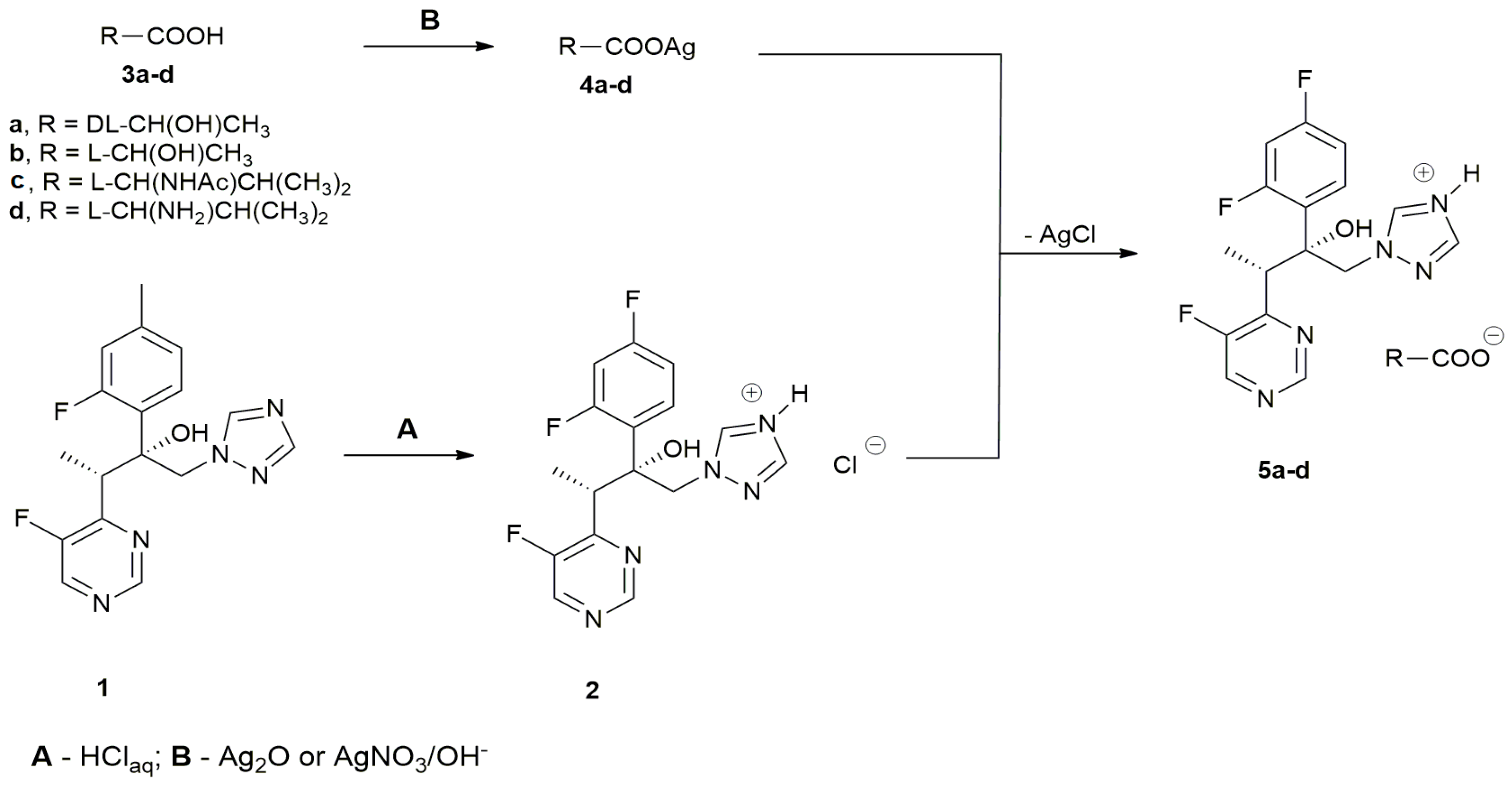
2.1. Chemistry
2.2. Antifungal Activity of VOR Salts
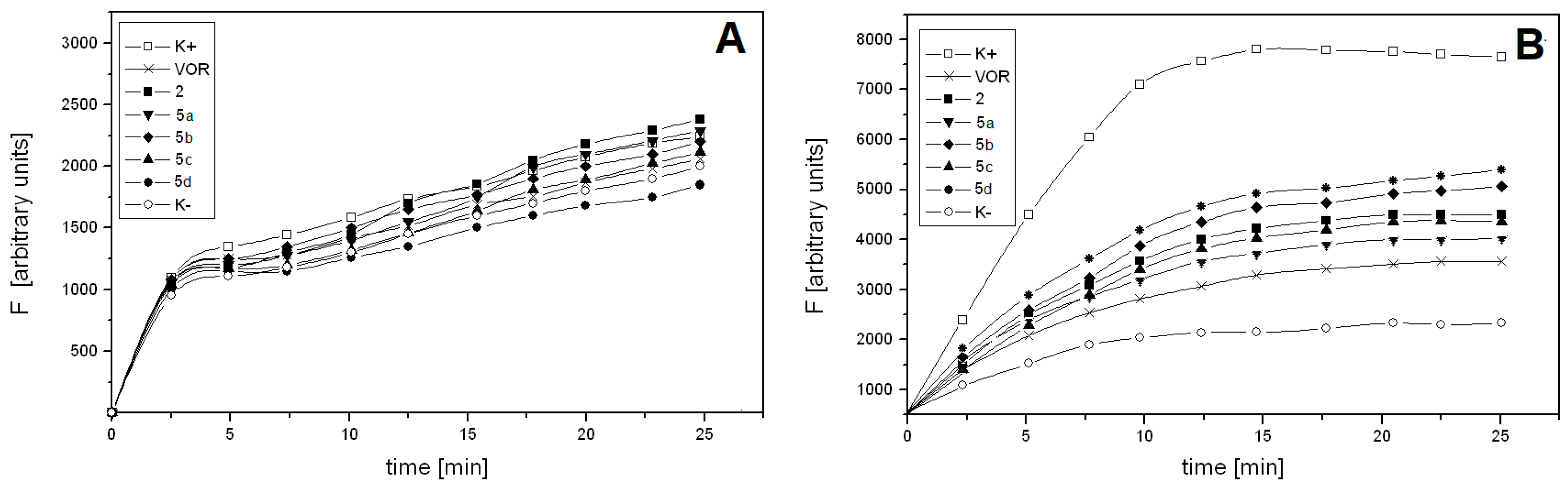
2.3. VOR Salts Are Poorly Effluxed by ABC Drug Transporters of C. albicans
3. Materials and Methods
3.1. Preparation of Voriconazole-Based Salts
3.2. Determination of Physicochemical Properties
3.3. Microorganisms and Growth Conditions
3.4. Determination of Antifungal In Vitro Activity
3.5. Quantification of Rhodamine 6G Efflux from C. albicans Cells
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fisher, M.C.; Hawkins, N.J.; Sanglard, D.; Gurr, N.J. Worldwide emergence of resistance to antifungal drugs challenges human health and food security. Science 2018, 360, 739–742. [Google Scholar] [CrossRef] [PubMed]
- Campoy, S.; Adrio, J.L. Antifungals. Biochem. Pharmacol. 2017, 133, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Peyton, L.R.; Gallagher, S.; Hashemzadeh, M. Triazole antifungals: A review. Drugs Today (Barc) 2015, 51, 705–718. [Google Scholar] [PubMed]
- Thompson, G.R.; Lewis, J.S. Pharmacology and clinical use of voriconazole. Expert Opin. Drug Metab. Toxicol. 2010, 6, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Wiederhold, N.P.; Patterson, T.F. Emergence of azole resistance in Aspergillus. Semin. Respir. Crit. Care Med. 2015, 36, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Wakieć, R.; Prasad, R.; Morschhäuser, J.; Barchiesi, F.; Borowski, E.; Milewski, S. Voriconazole and multidrug resistance in Candida albicans. Mycoses 2007, 50, 109–115. [Google Scholar]
- Mangrule, V.; Pore, Y.; Disouza, J. Synthesis and physicochemical studies of fluconazole ionic liquids. J. Appl. Pharm. Sci. 2017, 7, 84–89. [Google Scholar]
- Keramatnia, F.; Jouyban, A.; Valizadeh, H.; Delazar, A. Ketoconazole ionic liquids with citric and tartaric acid: Synthesis, characterization and solubility study. Fluid Phase Equilib. 2016, 425, 108–113. [Google Scholar] [CrossRef]
- Pernak, J.; Markiewicz, B.; Łęgosz, B.; Walkiewicz, F.; Gwiazdowski, R.; Praczyk, T. Known triazole fungicides—A new trick. RSC Adv. 2015, 5, 9695–9702. [Google Scholar] [CrossRef]
- Hartmann, D.O.; Petkovic, M.; Silva Pereira, C. Ionic liquids as unforeseen assets to fight life-threatening mycotic diseases. Front. Microbiol. 2016, 7, e111. [Google Scholar]
- Kalinowska-Lis, U.; Felczak, A.; Chęcińska, L.; Zawadzka, K.; Patyna, E.; Lisowska, K.; Ochocki, J. Synthesis, characterization and antimicrobial activity of water-soluble silver(I) complexes of metronidazole drug and selected counter-ions. Dalton Trans. 2015, 44, 8178–8189. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, R.; Costa-Rodrigues, J.; Fernandes, M.H.; Santos, M.M.; Marrucho, I.M.; Rebelo, L.P.; Prudêncio, C.; Noronha, J.P.; Petrovski, Z.; Branco, L.C. Antitumor activity of ionic liquids based on ampicillin. ChemMedChem 2015, 10, 1480–1483. [Google Scholar] [CrossRef] [PubMed]
- Clinical Laboratory Standards Institute (2008). M27-A3: Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; Approved Standard—2rd ed.; CLSI: Wayne, PA, USA, 2008. [Google Scholar]
- Franz, R.; Kelly, S.L.; Lamb, D.C.; Kelly, D.E.; Ruhnke, M.; Morschhäuser, J. Multiple molecular mechanisms contribute to a stepwise development of fluconazole resistance in clinical Candida albicans strains. Antimicrob. Agents Chemother. 1998, 42, 3065–3072. [Google Scholar] [CrossRef] [PubMed]
- Kołaczkowski, M.; van der Rest, M.; Cebularz-Kołaczkowska, A.; Soumillion, J.P.; Konings, W.N.; Goffeau, A. Anticancer drugs, ionophoric peptides, and steroids as substrates of yeast multidrug transporter Pdr5. J. Biol. Chem. 1996, 271, 31543–31548. [Google Scholar] [CrossRef] [PubMed]
- Puri, N.; Prakash, O.; Manoharlal, R.; Sharma, M.; Ghosh, I.; Prasad, R. Analysis of physico-chemical properties of substrates of ABC and MFS multidrug transporters of pathogenic Candida albicans. Eur. J. Med. Chem. 2010, 45, 4813–4826. [Google Scholar] [CrossRef] [PubMed]
- Simons, C.; van Leeuwen, J.G.E.; Stemmer, R.; Arends, I.W.C.E.; Maschmeyer, T.; Sheldon, R.A.; Hanefeld, U. Enzyme-catalysed deprotection of N-acetyl and N-formyl amino acids. J. Mol. Catal. B Enzym. 2008, 54, 67–71. [Google Scholar] [CrossRef]
- Nakamura, K.; Niimi, M.; Niimi, K.; Holmes, A.R.; Yates, J.E.; Decottignies, A.; Monk, B.C.; Goffeau, A.; Cannon, R.D. Functional expression of Candida albicans drug efflux pump Cdr1p in Saccharomyces cerevisiae strain deficient in membrane transporters. Antimicrob. Agents Chemother. 2001, 45, 3366–3374. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |
| Comp. | Tg [°C] | mp [°C] | Tonset [°C] |
|---|---|---|---|
| [VOR][Cl] 2 | 38 | - | >250 |
| [VOR][dl-Lac] 5a | - | 203 | >250 |
| [VOR][l-Lac] 5b | - | 119 | >250 |
| [VOR][Ac-l-Val] 5c | - | 130 | >250 |
| [VOR][l-Val] 5d | - | 176 | >250 |
| Comp. | MIC (μg mL−1) | ||||
|---|---|---|---|---|---|
| C. albicans | C. glabrata | C. krusei | C. tropicalis | C. lusitaniae | |
| VOR 1 | 1.0 | 4.0 | 2.0 | 0.25 | 0.062 |
| [VOR][Cl] 2 | 2.0 | 0.5 | 1.0 | 0.25 | 0.062 |
| [VOR][dl-Lac] 5a | 1.0 | 0.5 | 1.0 | 0.125 | 0.031 |
| [VOR][l-Lac] 5b | 0.5 | 0.5 | 1.0 | 0.25 | 0.031 |
| [VOR][Ac-l-Val] 5c | 0.5 | 0.5 | 0.5 | 0.125 | 0.062 |
| [VOR][l-Val] 5d | 0.5 | 0.5 | 1.0 | 0.125 | 0.062 |
| Comp. | MIC (μg·mL−1) | |||
|---|---|---|---|---|
| Gu4 | Gu5 | B3 | B4 | |
| VOR 1 | 0.125 | 4.0 | 1.0 | 4.0 |
| [VOR][Cl] 2 | 0.031 | 0.5 | 1.0 | 4.0 |
| [VOR][dl-Lac] 5a | 0.062 | 0.5 | 0.5 | 2.0 |
| [VOR][l-Lac] 5b | 0.031 | 0.25 | 0.5 | 2.0 |
| [VOR][Ac-l-Val] 5c | 0.062 | 0.5 | 1.0 | 4.0 |
| [VOR][l-Val] 5d | 0.031 | 0.25 | 0.5 | 2.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szepiński, E.; Martynow, D.; Szweda, P.; Milewska, M.J.; Milewski, S. Voriconazole-Based Salts Are Active against Multidrug-Resistant Human Pathogenic Yeasts. Molecules 2019, 24, 3635. https://doi.org/10.3390/molecules24203635
Szepiński E, Martynow D, Szweda P, Milewska MJ, Milewski S. Voriconazole-Based Salts Are Active against Multidrug-Resistant Human Pathogenic Yeasts. Molecules. 2019; 24(20):3635. https://doi.org/10.3390/molecules24203635
Chicago/Turabian StyleSzepiński, Emil, Dorota Martynow, Piotr Szweda, Maria J. Milewska, and Sławomir Milewski. 2019. "Voriconazole-Based Salts Are Active against Multidrug-Resistant Human Pathogenic Yeasts" Molecules 24, no. 20: 3635. https://doi.org/10.3390/molecules24203635
APA StyleSzepiński, E., Martynow, D., Szweda, P., Milewska, M. J., & Milewski, S. (2019). Voriconazole-Based Salts Are Active against Multidrug-Resistant Human Pathogenic Yeasts. Molecules, 24(20), 3635. https://doi.org/10.3390/molecules24203635







