Nematicidal Activities of Three Naphthoquinones against the Pine Wood Nematode, Bursaphelenchus xylophilus
Abstract
:1. Introduction
2. Results
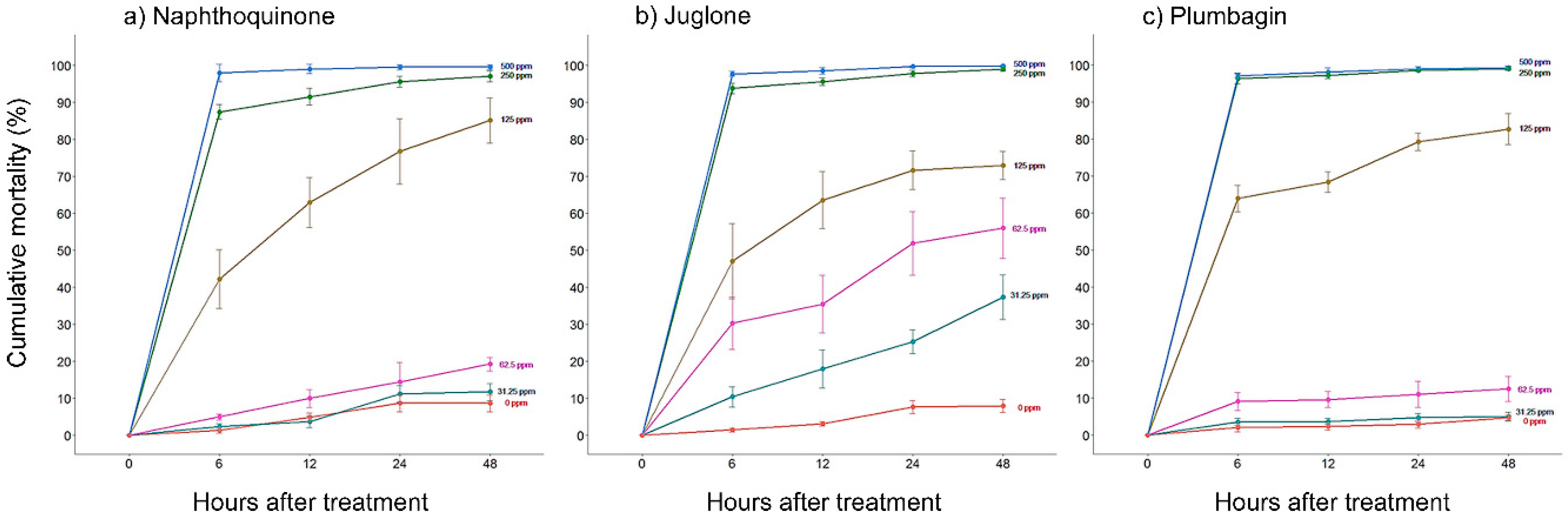
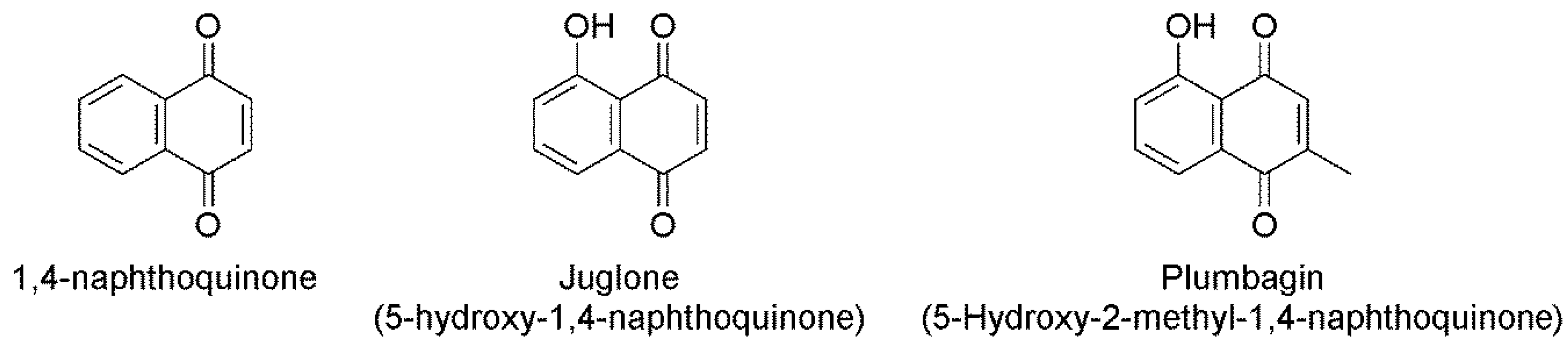
2.1. In Vitro Nematicidal Activities of Three Naphthoquinones on B. xylophilus
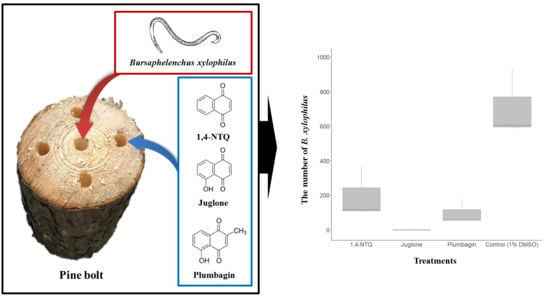
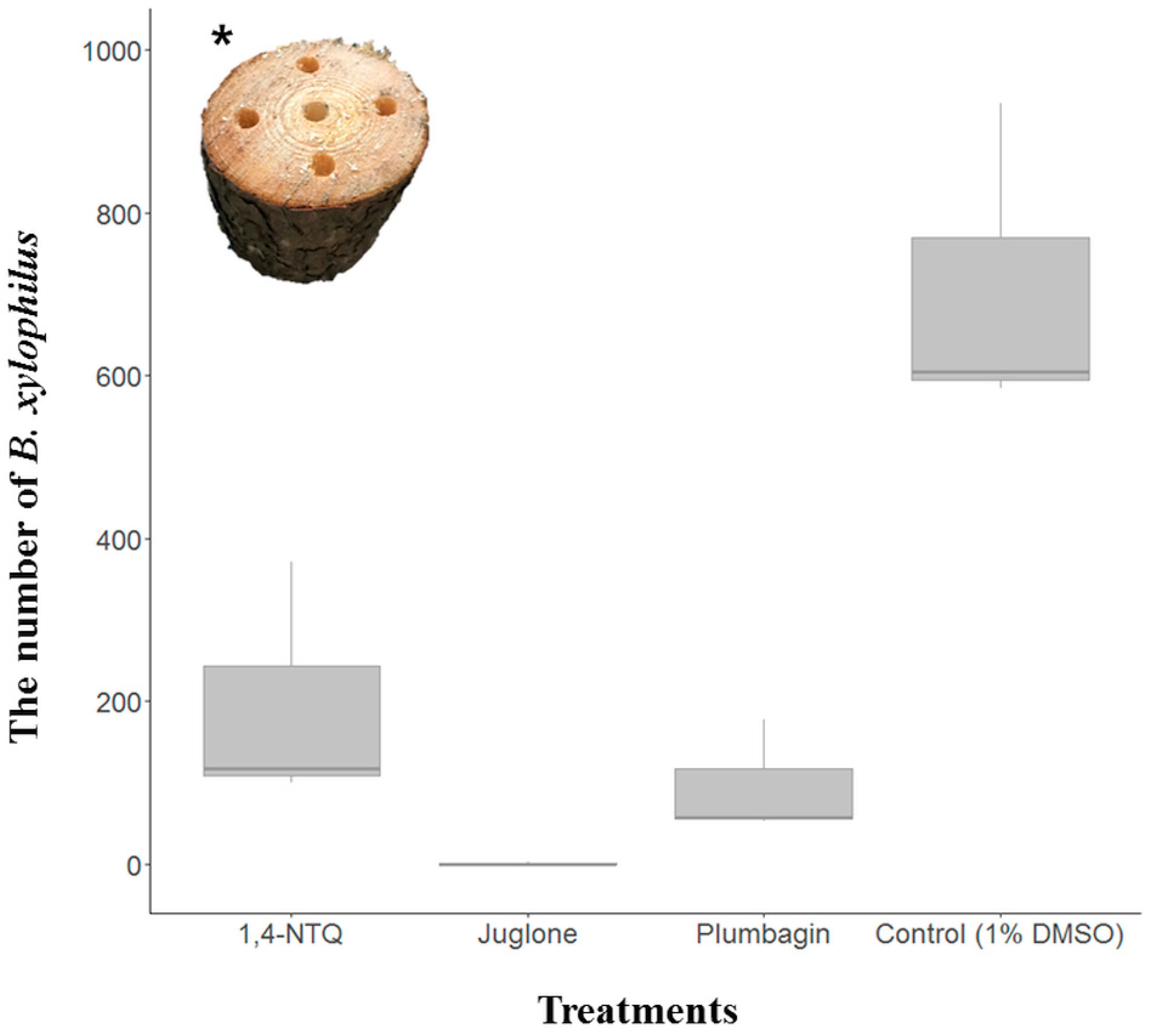
2.2. Semi-In Vivo Nematicidal Activities of Three Naphthoquinones on B. xylophilus
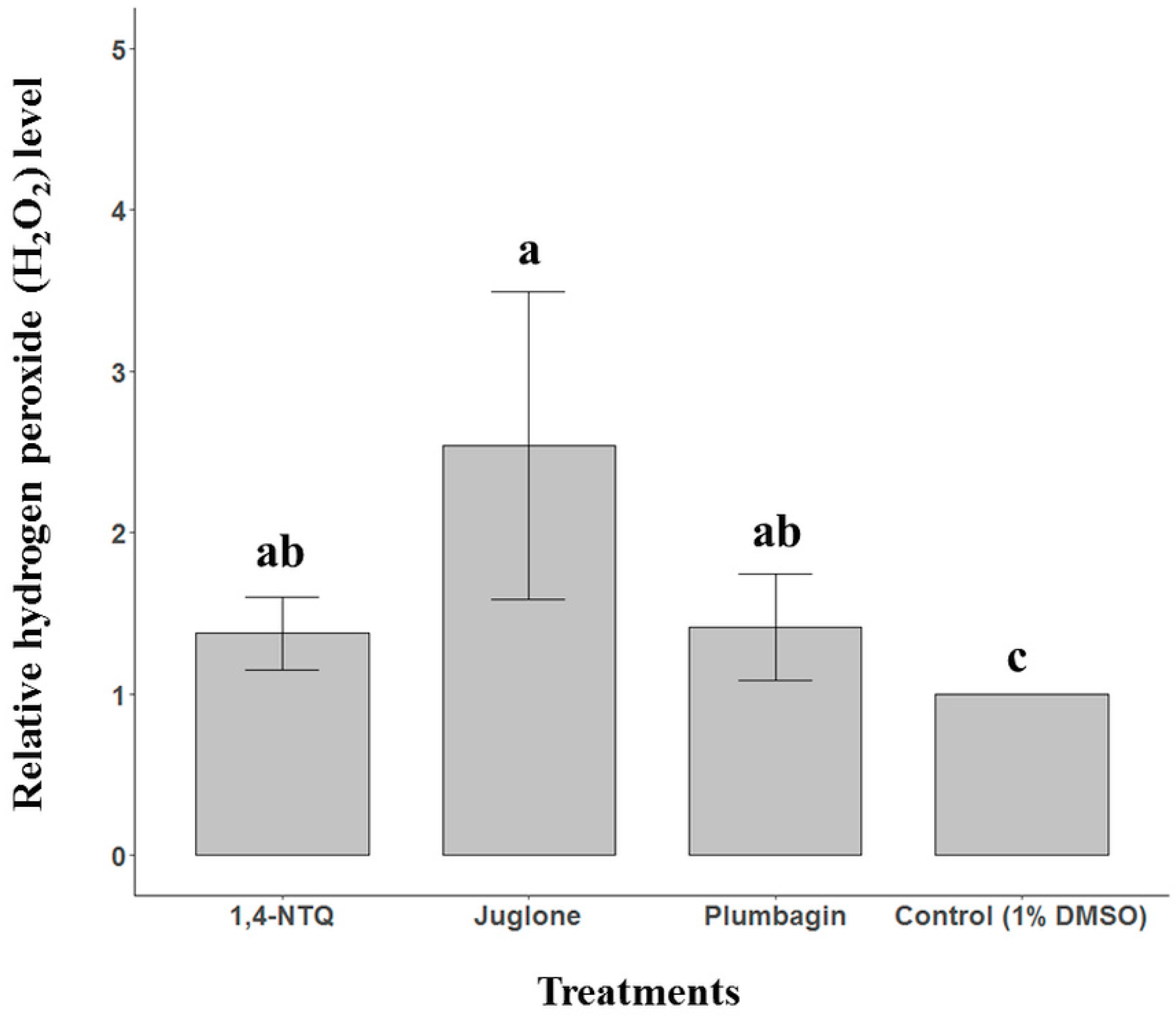
2.3. Naphthoquinone-Generated ROS Detection
3. Discussion
4. Materials and Methods
4.1. Nematodes
4.2. Nematicidal Activities of Three Naphthoquinones on B. xylophilus In Vitro
4.3. Semi-In Vivo Bioassay
4.4. Naphthoquinone-Generated ROS Detection
4.5. Statistics
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mamiya, Y. History of pine wilt disease in Japan. J. Nematol. 1988, 20, 219–226. [Google Scholar] [PubMed]
- Mota, M.; Braasch, H.; Bravo, M.A.; Penas, A.C.; Burgermeister, W.; Metge, K.; Sousa, E. First report of Bursaphelenchus xylophilus in Portugal and in Europe. Nematology 1999, 1, 727–734. [Google Scholar]
- Yi, C.K.; Byun, B.H.; Park, J.D.; Yang, S.I.; Chang, K.H. First finding of the pine wood nematode, Bursaphelenchus xylophilus (Steiner et Buhrer) Nickle and its insect vector in Korea. Res. Rep. For. Res. Inst. 1989, 38, 141–149. [Google Scholar]
- Shin, W.S.; Jung, Y.H.; Lee, S.M.; Lee, C.M.; Lee, C.J.; Kim, D.S.; Mun, L.S.; Lee, D.W. Development of effective screening method for efficacy test of trunk injection agents against pine wood nematode, Bursaphelenchus xylophilus in Japanese black pine, Pinus thunbergii. Korean J. Pestic. Sci. 2015, 19, 440–449. [Google Scholar] [CrossRef]
- Barbosa, P.; Lima, A.S.; Vieira, P.; Dias, L.S.; Tinoco, M.T.; Barroso, J.G.; Pedro, L.G.; Figueiredo, A.C.; Mota, M. Nematicidal activity of essential oils and volatiles derived from Portuguese aromatic flora against the pinewood nematode, Bursaphelenchus xylophilus. J. Nematol. 2010, 42, 8–16. [Google Scholar] [PubMed]
- Park, I.K.; Kim, J.; Lee, S.G.; Shin, S.C. Nematicidal activity of plant essential oils and components from ajowan (Trachyspermum ammi), allspice (Pimenta dioica) and litsea (Litsea cubeba) essential oils against pine wood nematode (Bursaphelenchus xylophilus). J. Nematol. 2007, 39, 275–279. [Google Scholar]
- Kim, J.; Seo, S.-M.; Park, I.-K. Nematicidal activity of plant essential oils and components from Gaultheria fragrantissima and Zanthoxylum alatum against pine wood nematode (Bursaphelenchus xylophilus). Nematology 2011, 13, 87–93. [Google Scholar]
- Bi, Z.; Gong, Y.; Huang, X.; Yu, H.; Bai, L.; Hu, J. Efficacy of four nematicides against the reproduction and development of pinewood nematode, Bursaphelenchus xylophilus. J. Nematol. 2015, 47, 126–132. [Google Scholar]
- Chelinho, S.; Maleita, C.M.N.; Francisco, R.; Braga, M.E.M.; da Cunha, M.J.M.; Abrantes, I.; Sousa, H.C.; Morais, P.V.; Sousa, J.P. Toxicity of the bionematicide 1,4-naphthoquinone on non-target soil organisms. Chemosphere 2017, 181, 579–588. [Google Scholar] [CrossRef]
- Hu, W.; Du, W.; Bai, S.; Lv, S.; Chen, G. Phenoloxidase, an effective bioactivity target for botanical insecticide screening from green walnut husks. Nat. Prod. Res. 2018, 32, 2848–2851. [Google Scholar] [CrossRef]
- Maleita, C.M.N.; Esteves, I.; Chim, R.; Fonseca, L.; Braga, M.E.M.; Abrantes, I.; Sousa, H.C. Naphthoquinones from walnut husk residues show strong nematicidal activities against the root-knot nematode Meloidogyne hispanica. ACS Sustain. Chem. Eng. 2007, 5, 3390–3398. [Google Scholar] [CrossRef]
- Pavela, R. Efficacy of naphthoquinones as insecticides against the house fly, Musca domestica L. Ind. Crops Prod. 2013, 43, 745–750. [Google Scholar] [CrossRef]
- Ravichandiran, P.; Sheet, S.; Premnath, D.; Kim, A.R.; Yoo, D.J. 1,4-Naphthoquinone analogues: Potent antibacterial agents and mode of action evaluation. Molecules 2019, 24, 1437. [Google Scholar] [CrossRef] [PubMed]
- Shang, X.F.; Liu, Y.Q.; Guo, X.; Miao, X.L.; Chen, C.; Zhang, J.X.; Xu, X.S.; Yang, G.Z.; Yang, C.J.; Li, J.C.; et al. Application of sustainable natural resources in agriculture: Acaricidal and enzyme inhibitory activities of naphthoquinones and their analogs against Psoroptes cuniculi. Sci. Rep. 2018, 8, 1609. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.D.; Zou, Y.P.; Wang, P.; Yao, X.H.; Sun, Y.; Duan, M.H.; Fu, Y.J.; Yu, B. Chimaphilin induces apoptosis in human breast cancer MCF-7 cells through a ROS-mediated mitochondrial pathway. Food Chem. Toxicol. 2014, 70, 1–8. [Google Scholar] [CrossRef]
- Wang, J.; Zeng, G.; Huang, X.; Wang, Z.; Tan, N. 1,4-naphthoquinone triggers nematode lethality by inducing oxidative stress and activating insulin/IGF signaling pathway in Caenorhabditis elegans. Molecules 2017, 22, 798. [Google Scholar] [CrossRef]
- Esteves, I.; Maleita, C.M.N.; Fonseca, L.; Braga, M.E.M.; Abrantes, I.; Sousa, H.C. In vitro nematicidal activity of naphthoquinones against the root-lesion nematode, Pratylenchus thornei. Phytopathol. Meditter. 2017, 56, 127–132. [Google Scholar]
- Mohanty, J.G.; Jaffe, J.S.; Schulman, E.S.; Raible, D.G. A highly sensitive fluorescent micro-assay of H2O2 release from activated human leukocytes using a dihydroxyphenoxazine derivative. J. Immunol. Methods 1997, 202, 133–141. [Google Scholar] [CrossRef]
- Yoon, D.S.; Lee, M.H.; Cha, D.S. Measurement of intracellular ROS in Caenorhabditis elegans using 2′,7′-dichlorodihydrofluorescein diacetate. Bio. Protoc. 2018, 8, E2774. [Google Scholar] [CrossRef]
- Akhtar, Y.; Isman, M.B.; Lee, C.-H.; Lee, S.-G.; Lee, H.-S. Toxicity of quinones against two-spotted spider mite and three species of aphids in laboratory and greenhouse conditions. Ind. Crops Prod. 2012, 37, 536–541. [Google Scholar] [CrossRef]
- Jeon, J.-H.; Kim, M.-G.; Lee, H.-S. Larvicidal activity of naturally occurring naphthalenedione and its structurally related analogs against three mosquito species. J. Am. Mosq. Control Assoc. 2015, 31, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-G.; Lee, H.-S. Insecticidal toxicities of naphthoquinone and its structural derivatives. Appl. Biol. Chem. 2016, 59, 2–8. [Google Scholar] [CrossRef]
- Michaelakis, A.; Strongilos, A.T.; Bouzas, E.A.; Koliopoulos, G.; Couladouros, E.A. Larvicidal activity of naturally occurring naphthoquinones and derivatives against the West Nile virus vector Culex pipiens. Parasitol. Res. 2009, 104, 657–662. [Google Scholar] [CrossRef] [PubMed]
- Choi, I.-H.; Kim, J.; Shin, S.-C.; Park, I.-K. Nematicidal activity of monoterpenoids against the pine wood nematode (Bursaphelenchus xylophilus). Russ. J. Nematol. 2007, 1, 35–40. [Google Scholar]
- Seo, S.-M.; Kim, J.; Kim, E.; Park, H.-M.; Kim, Y.-J.; Park, I.-K. Structure-activity relationship of aliphatic compounds for nematicidal activity against pine wood nematode (Bursaphelenchus xylophilus). J. Agric. Food Chem. 2010, 58, 1823–1827. [Google Scholar] [CrossRef] [PubMed]
- Han, H.-R.; Han, B.Y.; Chung, Y.J.; Shin, S.C. A simple PCR-RFLP for idenficiation of Bursaphelenchus spp. collected from Korea. Plant Pathol. J. 2008, 24, 150–163. [Google Scholar] [CrossRef]
- Finney, D.J. Probit Analysis, 3rd ed.; Cambridge University Press: Cambridge, UK, 1971; p. 333. [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2008. [Google Scholar]
Sample Availability: Sample of the compounds 1,4-naphthoquinone, juglone, and plumbagin are available from the authors. |

| Compounds | LC50 (ppm) 1 | 95% CI (ppm) 2 | R Square | Regression Line |
|---|---|---|---|---|
| 1,4-NTQ (1,4-Naphthoquinone) | 100 | 60–140 | R2 = 0.8489 | y = 92.086x − 134.17 |
| Juglone (5-Hydroxy-1,4-naphthoquinone) | 56.98 | 16.98–96.98 | R2 = 0.9238 | y = 60.502x − 56.227 |
| Plumbagin (5-Hydroxy-2-methyl-1,4-naphthoquinone) | 103.86 | 63.86–143.86 | R2 = 0.8512 | y = 95.707x − 142.99 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cha, D.J.; Kim, J.; Kim, D.S. Nematicidal Activities of Three Naphthoquinones against the Pine Wood Nematode, Bursaphelenchus xylophilus. Molecules 2019, 24, 3634. https://doi.org/10.3390/molecules24203634
Cha DJ, Kim J, Kim DS. Nematicidal Activities of Three Naphthoquinones against the Pine Wood Nematode, Bursaphelenchus xylophilus. Molecules. 2019; 24(20):3634. https://doi.org/10.3390/molecules24203634
Chicago/Turabian StyleCha, Deok Jea, Junheon Kim, and Dong Soo Kim. 2019. "Nematicidal Activities of Three Naphthoquinones against the Pine Wood Nematode, Bursaphelenchus xylophilus" Molecules 24, no. 20: 3634. https://doi.org/10.3390/molecules24203634
APA StyleCha, D. J., Kim, J., & Kim, D. S. (2019). Nematicidal Activities of Three Naphthoquinones against the Pine Wood Nematode, Bursaphelenchus xylophilus. Molecules, 24(20), 3634. https://doi.org/10.3390/molecules24203634