Highly Efficient Synthesis of Substituted 3,4-Dihydropyrimidin-2-(1H)-ones (DHPMs) Catalyzed by Hf(OTf)4: Mechanistic Insights into Reaction Pathways under Metal Lewis Acid Catalysis and Solvent-Free Conditions
Abstract
1. Introduction
2. Results and Discussion
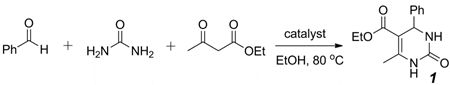
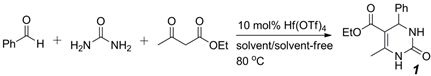
2.1. Optimization of Hf(OTf)4-Catalyzed Biginelli Reaction Conditions
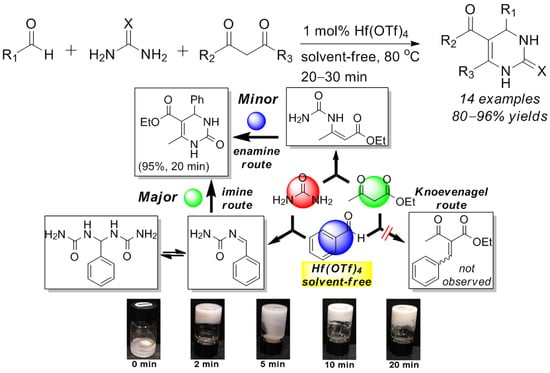
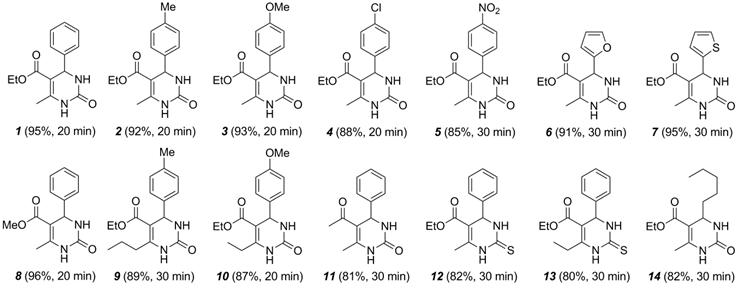
2.2. Scope of Hf(OTf)4-Catalyzed Biginelli Reaction under Solvent-Free Conditions
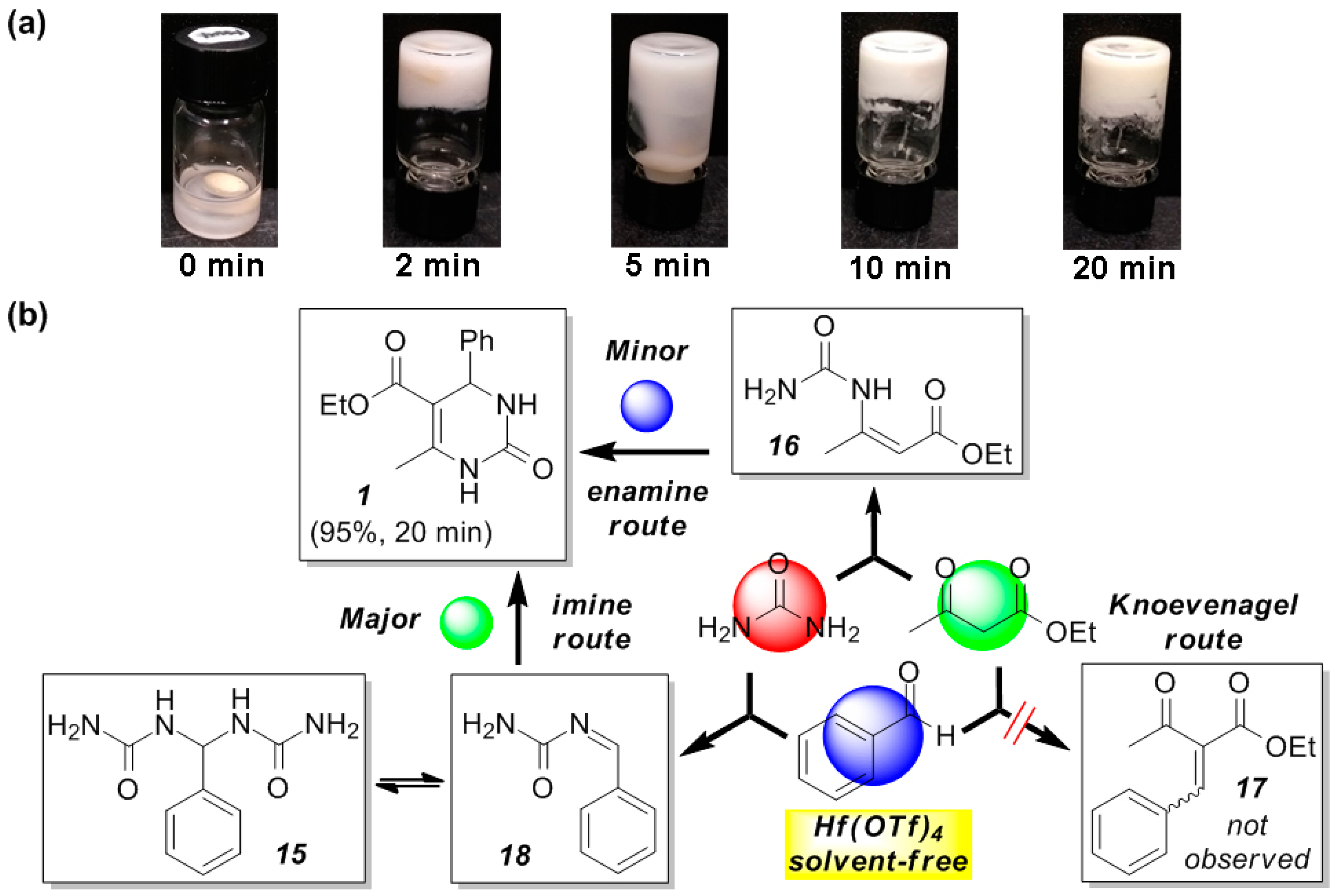
2.3. Mechanistic Investigation on the Reaction Pathways of the Hf(OTf)4-Catalyzed Biginelli Reaction under Solvent-Free Conditions
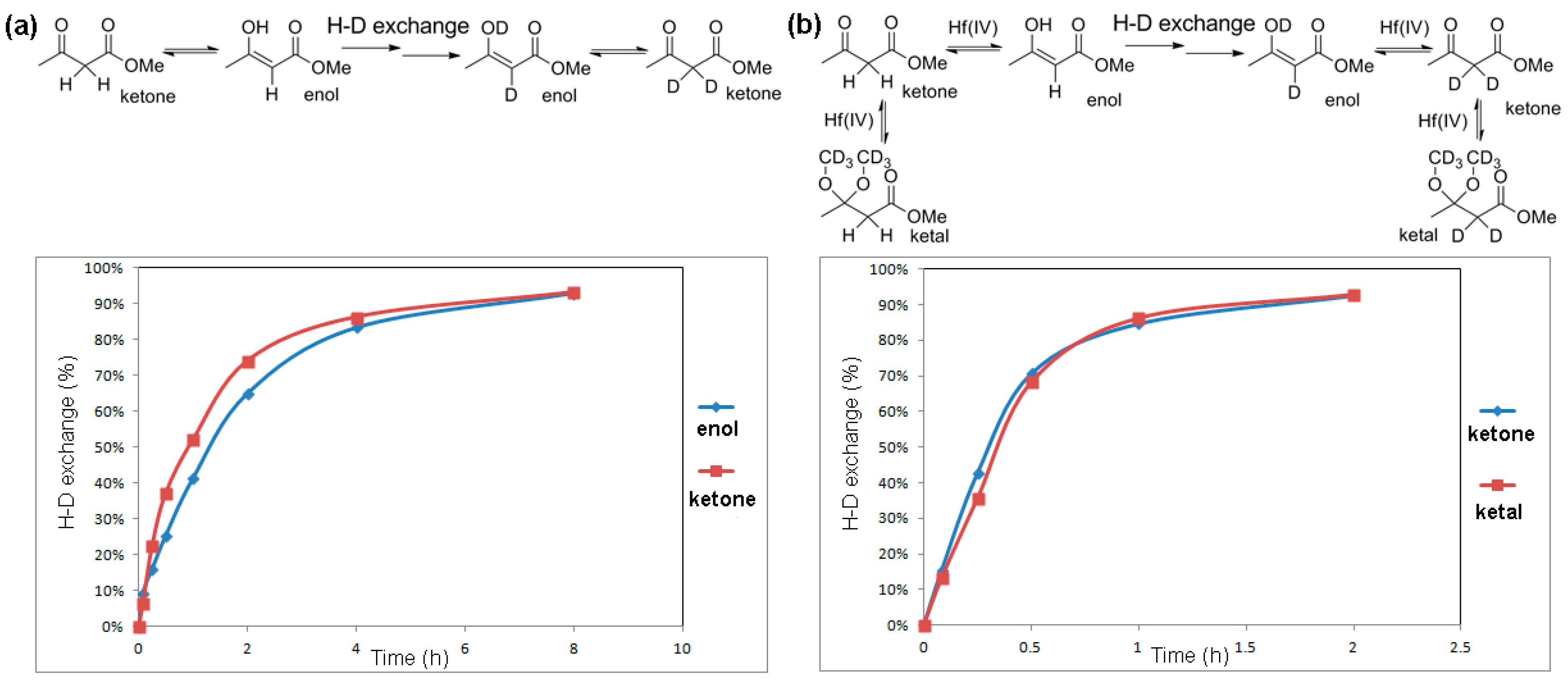
2.4. Activation Effect of Hf(OTf)4 on β-Ketoester
3. Materials and Methods
3.1. General Methods
3.2. General Synthetic Procedure and Characterization of DHPMs 1–14 and 20
3.3. Synthetic Procedures and Characterization of 15–17, 19, and 21
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Biginelli, P. Aldehyde-urea derivatives of aceto- and oxaloacetic acids. Gazz. Chim. Ital. 1893, 23, 360–413. [Google Scholar]
- Kappe, C.O. 100 Years of the Biginelli dihydropyridine synthesis. Tetrahedron 1993, 49, 6937–6963. [Google Scholar] [CrossRef]
- Kappe, C.O. Recent advances in the Biginelli dihydropyrimidine synthesis. New tricks from an old dog. Acc. Chem. Res. 2000, 33, 879–888. [Google Scholar] [CrossRef]
- Nagarajaiah, H.; Mukhopadhyay, A.; Moorthy, J.N. Biginelli reaction: An overview. Tetrahedron Lett. 2016, 57, 5135–5149. [Google Scholar] [CrossRef]
- Aron, Z.D.; Overman, L.E. Total synthesis and properties of the crambescidin core zwitterionic acid and crambescidin 359. J. Am. Chem. Soc. 2005, 127, 3380–3390. [Google Scholar] [CrossRef]
- Arnold, M.A.; Day, K.A.; Duron, S.G.; Gin, D.Y. Total synthesis of (+)-batzelladine A and (-)-batzelladine D via [4+2]-annulation of vinyl carbodiimides with N-alkyl imines. J. Am. Chem. Soc. 2006, 128, 13255–13260. [Google Scholar] [CrossRef] [PubMed]
- Makarieva, T.N.; Tabakmaher, K.M.; Guzii, A.G.; Denisenko, V.A.; Dmitrenok, P.S.; Shubina, L.K.; Stonik, V.A. Monanchocidins B–E: Polycyclic guanidine alkaloids with potent antileukemic activities from the sponge monanchora pulchra. J. Nat. Prod. 2011, 74, 1952–1958. [Google Scholar] [CrossRef] [PubMed]
- Kappe, C.O. Biologically active dihydropyrimidones of the Biginelli-type–A literature survey. Eur. J. Med. Chem. 2000, 35, 1043–1052. [Google Scholar] [CrossRef]
- Patil, S.R.; Choudhary, A.S.; Patil, V.S.; Sekar, N. Synthesis, optical properties, dyeing study of dihydropyrimidones (DHPMs) skeleton: Green and regioselectivity of novel Biginelli scaffold from lawsone. Fibers Polym. 2015, 16, 2349–2358. [Google Scholar] [CrossRef]
- Boukis, A.C.; Llevot, A.; Meier, M.A.R. High glass transition temperature renewable polymers via Biginelli multicomponent polymerization. Macromol. Rapid Commun. 2016, 37, 643–649. [Google Scholar] [CrossRef]
- Zhao, Y.; Yu, Y.; Zhang, Y.; Wang, X.; Yang, B.; Zhang, Y.; Zhang, Q.; Fu, C.; Weia, Y.; Tao, L. From drug to adhesive: A new application of poly(dihydropyrimidin-2(1H)-one)s via the Biginelli polycondensation. Polym. Chem. 2015, 6, 4940–4945. [Google Scholar] [CrossRef]
- Lu, J.; Ma, H.R. Iron(III)-catalyzed synthesis of dihydropyrimidinones. Improved conditions for the Biginelli reaction. Synlett 2000, 2000, 63–64. [Google Scholar]
- Ma, Y.; Qian, C.T.; Wang, L.M.; Yang, M. Lanthanide triflate catalyzed Biginelli reaction. One-pot synthesis of dihydropyrimidinones under solvent-free conditions. J. Org. Chem. 2000, 65, 3864–3868. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.A.; Kasthuraiah, M.; Reddy, C.S.; Reddy, C.D. Mn(OAc)3·2H2O-mediated three-component, one-pot, condensation reaction: An efficient synthesis of 4-aryl-substituted 3,4-dihydropyrimidin-2-ones. Tetrahedron Lett. 2001, 42, 7873–7875. [Google Scholar] [CrossRef]
- Ranu, B.C.; Hajra, A.; Jana, U. Indium(III) chloride-catalyzed one-pot synthesis of dihydropyrimidinones by a three-component coupling of 1,3-dicarbonyl compounds, aldehydes, and urea: An improved procedure for the Biginelli reaction. J. Org. Chem. 2000, 65, 6270–6272. [Google Scholar] [CrossRef] [PubMed]
- Fu, N.Y.; Yuan, Y.F.; Cao, Z.; Wang, S.W.; Wang, J.T.; Peppe, C. Indium(III) bromide-catalyzed preparation of dihydropyrimidinones: Improved protocol conditions for the Biginelli reaction. Tetrahedron 2002, 58, 4801–4807. [Google Scholar] [CrossRef]
- Ghosh, R.; Maiti, S.; Chakraborty, A. In(OTf)3-catalysed one-pot synthesis of 3,4-dihydropyrimidin-2(lH)- ones. J. Mol. Catal. A Chem. 2004, 217, 47–50. [Google Scholar] [CrossRef]
- Reddy, C.V.; Mahesh, M.; Raju, P.V.; Babu, T.R.; Reddy, V.V.N. Zirconium(IV) chloride catalyzed one-pot synthesis of 3,4-dihydropyrimidin-2(1H)-ones. Tetrahedron Lett. 2002, 43, 2657–2659. [Google Scholar] [CrossRef]
- Rodríguez-Domínguez, J.C.; Bernardi, D.; Kirsch, G. ZrCl4 or ZrOCl2 under neat conditions: optimized green alternatives for the Biginelli reaction. Tetrahedron Lett. 2007, 48, 5777–5780. [Google Scholar] [CrossRef]
- Gohain, M.; Prajapati, D.; Sandhu, J.S. A novel Cu-catalyzed three-component one-pot synthesis of dihydro-pyrimidin-2(1H)-ones using microwaves under solvent-free conditions. Synlett 2004, 2004, 235–238. [Google Scholar]
- Pasunooti, K.K.; Chai, H.; Jensen, C.N.; Gorityala, B.K.; Wang, S.; Liu, X.W. A microwave-assisted, copper-catalyzed three-component synthesis of dihydropyrimidinones under mild conditions. Tetrahedron Lett. 2011, 52, 80–84. [Google Scholar] [CrossRef]
- Han, X.Y.; Xu, F.; Luo, Y.Q.; Shen, Q. An efficient one-pot synthesis of dihydropyrimidinones by a samarium diiodide catalyzed Biginelli reaction under solvent-free conditions. Eur. J. Org. Chem. 2005, 2005, 1500–1503. [Google Scholar] [CrossRef]
- De, S.K.; Gibbs, R.A. Ruthenium(III) chloride catalyzed one-pot synthesis of 3,4-dihydropyrimidin-2-(1H)-ones under solvent-free conditions. Synthesis 2005, 11, 1748–1750. [Google Scholar] [CrossRef]
- Su, W.K.; Li, J.J.; Zheng, Z.K.; Shen, Y.C. One-pot synthesis of dihydropyrimidiones catalyzed by strontium(II) triflate under solvent-free conditions. Tetrahedron Lett. 2005, 46, 6037–6040. [Google Scholar] [CrossRef]
- Adib, M.; Ghanbary, K.; Mostofi, M.; Ganjali, M. Efficient Ce(NO3)3·6H2O-catalyzed solvent-free synthesis of 3,4-dihydropyrimidin-2(1H)-ones. Molecules 2006, 11, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; van Lier, J.E. TaBr5-catalyzed Biginelli reaction: One-pot synthesis of 3,4-dihydropyrimidin-2-(1H)-ones/thiones under solvent-free conditions. Tetrahedron Lett. 2007, 48, 5407–5409. [Google Scholar] [CrossRef]
- Banik, B.K.; Reddy, A.T.; Datta, A.; Mukhopadhyay, C. Microwave-induced bismuth nitrate-catalyzed synthesis of dihydropyrimidones via Biginelli condensation under solventless conditions. Tetrahedron Lett. 2007, 48, 7392–7394. [Google Scholar] [CrossRef]
- Cepanec, I.; Litvić, M.; Filipan-Litvić, M.; Grüngold, I. Antimony(III) chloride-catalysed Biginelli reaction: A versatile method for the synthesis of dihydropyrimidinones through a different reaction mechanism. Tetrahedron 2007, 63, 11822–11827. [Google Scholar] [CrossRef]
- Li, D.P.; Mao, H.F.; An, L.T.; Huang, Z.H.; Zou, J.P. Gallium(III) iodide-catalyzed Biginelli-type reaction under solvent-free conditions: Efficient synthesis of dihydropyrimidine-2(1H)-one derivatives. Chin. J. Chem. 2010, 28, 2025–2032. [Google Scholar] [CrossRef]
- Xia, J.J.; Zhang, K.H. Ga(OTf)3 catalyzed synthesis of 3,4-dihydropyrimidin-2(1H)-ones. Heterocycles 2015, 91, 105–112. [Google Scholar] [CrossRef]
- Zheng, S.H.; Jian, Y.J.; Xu, S.; Wu, Y.; Sun, H.; Zhang, G.F.; Zheng, W.Q.; Gao, Z. N-Donor ligand activation of titanocene for the Biginelli reaction via the imine mechanism. RSC Adv. 2018, 8, 8657–8661. [Google Scholar] [CrossRef]
- Debache, A.; Chouguiat, L.; Boulcina, R.; Carboni, B. A one-pot multi-component synthesis of dihydropyrimidinone/thione and dihydropyridine derivatives via Biginelli and Hantzsch condensations using t-BuOK as a catalyst under solvent-free conditions. Open. Org. Chem. J. 2012, 6, 12–20. [Google Scholar] [CrossRef]
- Wang, P.H.; Wang, J.Y.; Au, C.T.; Qiu, R.H.; Xu, X.H.; Yin, S.F. Air-stable organobismuth(V) bisperfluorooctanesulfonate as an efficient catalyst for the synthesis of N-containing compounds. Adv. Synth. Catal. 2016, 358, 1302–1308. [Google Scholar] [CrossRef]
- Pawłowski, R.; Zaorska, E.; Staszko, S.; Szadkowska, A. Copper(I)-catalyzed multicomponent reactions in sustainable media. Appl. Organomet. Chem. 2018, 32, 4256–4266. [Google Scholar] [CrossRef]
- Elhamifar, D.; Shabani, A. Manganese-containing periodic mesoporous organosilica with ionic-liquid framework (Mn@PMO-IL): A powerful, durable, and reusable nanocatalyst for the Biginelli reaction. Chem. Eur. J. 2014, 20, 3212–3217. [Google Scholar] [CrossRef] [PubMed]
- Lima, C.G.S.; Silva, S.; Gonçalves, R.H.; Leite, E.R.; Schwab, R.S.; Correa, A.G.; Paixao, M.W. Highly efficient and magnetically recoverable niobium nanocatalyst for the multicomponent Biginelli reaction. ChemCatChem 2014, 6, 3455–3463. [Google Scholar] [CrossRef]
- Shi, X.L.; Xing, X.L.; Lin, H.K.; Zhang, W.Q. Synthesis of polyacrylonitrile fiber-supported poly(ammonium methanesulfonate)s as active and recyclable heterogeneous Brønsted acid catalysts. Adv. Synth. Catal. 2014, 356, 2349–2354. [Google Scholar] [CrossRef]
- Wang, J.H.; Tang, G.M.; Yan, S.C.; Wang, Y.T.; Zhan, S.J.; Zhang, E.; Sun, Y.; Jiang, Y.; Cui, Y.Z. Cobalt-based metal coordination polymers with 4,4′-bipyridinyl groups: Highly efficient catalysis for one-pot synthesis of 3,4-dihydropyrimidin-2(1H)-ones under solvent-free conditions. Appl. Organometal. Chem. 2016, 30, 1009–1021. [Google Scholar] [CrossRef]
- Lacotte, P.; Puente, C.; Ambroise, Y. Synthesis and evaluation of 3,4-dihydropyrimidin-2(1H)-ones as sodium iodide symporter inhibitors. ChemMedChem 2012, 8, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Crespo, A.; El Maatougui, A.; Biagini, P.; Azuaje, J.; Coelho, A.; Brea, J.; Loza, M.I.; Cadavid, M.I.; Garcia-Mera, X.; Gutierrez-de-Teran, H.; et al. Discovery of 3,4-dihydropyrimidin-2(1H)-ones as a novel class of potent and selective A2B adenosine receptor antagonists. ACS Med. Chem. Lett. 2013, 4, 1031–1036. [Google Scholar] [CrossRef] [PubMed]
- Sari, O.; Roy, V.; Métifiot, M.; Marchand, C.; Pommier, Y.; Bourg, S.; Bonnet, P.; Schinazi, R.F.; Agrofoglio, L.A. Synthesis of dihydropyrimidine α,γ-diketobutanoic acid derivatives targeting HIV integrase. Eur. J. Med. Chem. 2015, 104, 127–138. [Google Scholar] [CrossRef]
- Matias, M.; Campos, G.; Santos, A.O.; Falcao, A.; Silvestre, S.; Alves, G. Potential antitumoral 3,4-dihydropyrimidin-2-(1H)-ones: Synthesis, in vitro biological evaluation and QSAR studies. RSC Adv. 2016, 6, 84943–84958. [Google Scholar] [CrossRef]
- Kappe, C.O. A reexamination of the mechanism of the Biginelli dihydropyrimidine synthesis. Support for an N-acyliminium ion intermediate. J. Org. Chem. 1997, 62, 7201–7204. [Google Scholar] [CrossRef]
- De Souza, R.; Penha, E.T.; Milagre, H.M.S.; Garden, S.J.; Esteves, P.M.; Eberlin, M.N.; Antunes, O.A.C. The three-component Biginelli reaction: A combined experimental and theoretical mechanistic investigation. Chem. Eur. J. 2009, 15, 9799–9804. [Google Scholar] [CrossRef] [PubMed]
- Ramos, L.M.; Tobio, A.Y.P.L.; Santos, M.R.; Oliveira, H.C.B.; Gomes, A.F.; Gozzo, F.C.; Oliveira, A.L.; Neto, B.A.D. Mechanistic studies on Lewis acid catalyzed Biginelli reactions in ionic liquids: Evidence for the reactive intermediates and the role of the reagents. J. Org. Chem. 2012, 77, 10184–10193. [Google Scholar] [CrossRef]
- Puripat, M.; Ramozzi, R.; Hatanaka, M.; Parasuk, W.; Parasuk, V.; Morokuma, K. The Biginelli reaction is a urea-catalyzed organocatalytic multicomponent reaction. J. Org. Chem. 2015, 80, 6959–6967. [Google Scholar] [CrossRef] [PubMed]
- Jorres, M.; Chen, X.; Acena, J.L.; Merkens, C.; Bolm, C.; Liu, H.; Soloshonok, V.A. Asymmetric synthesis of α-amino acids under operationally convenient conditions. Adv. Synth. Catal. 2014, 356, 2203–2208. [Google Scholar] [CrossRef]
- Jorres, M.; Acena, J.L.; Soloshonok, V.A.; Bolm, C. Asymmetric carbon-carbon bond formation under solventless conditions in ball mills. ChemCatChem 2015, 7, 1265–1269. [Google Scholar] [CrossRef]
- Wang, R.; Chen, J.-Z.; Zheng, X.-A.; Kong, R.; Gong, S.-S.; Sun, Q. Hafnium(IV) triflate as a potent catalyst for selective 1-O-deacetylation of peracetylated saccharides. Carbohydr. Res. 2018, 455, 114–118. [Google Scholar] [CrossRef]
- Li, X.-C.; Gong, S.-S.; Zeng, D.-Y.; You, Y.-H.; Sun, Q. Highly efficient synthesis of α-aminophosphonates catalyzed by hafnium(IV) chloride. Tetrahedron Lett. 2016, 57, 1782–1785. [Google Scholar] [CrossRef]
- Chen, P.R.; Tu, M.D. Synthesis of 2-selenoxo DHPMs by Biginelli reaction with Hf(OTf)4 as catalyst. Tetrahedron Lett. 2018, 59, 987–990. [Google Scholar] [CrossRef]
- Ranu, B.C.; Hajra, A.; Dey, S.S. A practical and green approach towards synthesis of dihydropyrimidinones without any solvent or catalyst. Org. Process Res. Dev. 2002, 6, 817–818. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |


| Catalyst | Reaction Time (h) | Yield of 1 (%) | |
|---|---|---|---|
| 1 | no | 24 | no reaction |
| 2 | conc. HCl | 18 | 65 |
| 3 | ZrCl4 (10 mol%) | 12 | 80 |
| 4 | ZrCp2Cl2 (10 mol%) | 12 | 62 1 |
| 5 | ZrOCl2·8H2O (10 mol%) | 9 | 78 |
| 6 | HfCp2Cl2 (10 mol%) | 12 | 73 1 |
| 7 | HfCl4 (10 mol%) | 9 | 81 |
| 8 | Hf(OTf)4 (10 mol%) | 9 | 88 |
| Solvent | Reaction Time (h) | Yield of 1 (%) | |
|---|---|---|---|
| 1 | toluene | 12 | 52 1 |
| 2 | 1,2-dichloroethane | 9 | 56 1 |
| 3 | THF | 9 | 63 1 |
| 4 | CH3CN | 9 | 65 1 |
| 5 | EtOH | 9 | 88 |
| 6 | solvent-free | 5 min | 96 |
 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, R.; Han, S.-B.; Wei, J.-Y.; Peng, X.-C.; Xie, Z.-B.; Gong, S.-S.; Sun, Q. Highly Efficient Synthesis of Substituted 3,4-Dihydropyrimidin-2-(1H)-ones (DHPMs) Catalyzed by Hf(OTf)4: Mechanistic Insights into Reaction Pathways under Metal Lewis Acid Catalysis and Solvent-Free Conditions. Molecules 2019, 24, 364. https://doi.org/10.3390/molecules24020364
Kong R, Han S-B, Wei J-Y, Peng X-C, Xie Z-B, Gong S-S, Sun Q. Highly Efficient Synthesis of Substituted 3,4-Dihydropyrimidin-2-(1H)-ones (DHPMs) Catalyzed by Hf(OTf)4: Mechanistic Insights into Reaction Pathways under Metal Lewis Acid Catalysis and Solvent-Free Conditions. Molecules. 2019; 24(2):364. https://doi.org/10.3390/molecules24020364
Chicago/Turabian StyleKong, Rui, Shuai-Bo Han, Jing-Ying Wei, Xiao-Chong Peng, Zhen-Biao Xie, Shan-Shan Gong, and Qi Sun. 2019. "Highly Efficient Synthesis of Substituted 3,4-Dihydropyrimidin-2-(1H)-ones (DHPMs) Catalyzed by Hf(OTf)4: Mechanistic Insights into Reaction Pathways under Metal Lewis Acid Catalysis and Solvent-Free Conditions" Molecules 24, no. 2: 364. https://doi.org/10.3390/molecules24020364
APA StyleKong, R., Han, S.-B., Wei, J.-Y., Peng, X.-C., Xie, Z.-B., Gong, S.-S., & Sun, Q. (2019). Highly Efficient Synthesis of Substituted 3,4-Dihydropyrimidin-2-(1H)-ones (DHPMs) Catalyzed by Hf(OTf)4: Mechanistic Insights into Reaction Pathways under Metal Lewis Acid Catalysis and Solvent-Free Conditions. Molecules, 24(2), 364. https://doi.org/10.3390/molecules24020364