Synthesis and Gelling Abilities of Polyfunctional Cyclohexane-1,2-dicarboxylic Acid Bisamides: Influence of the Hydroxyl Groups
Abstract
1. Introduction
2. Results and Discussion
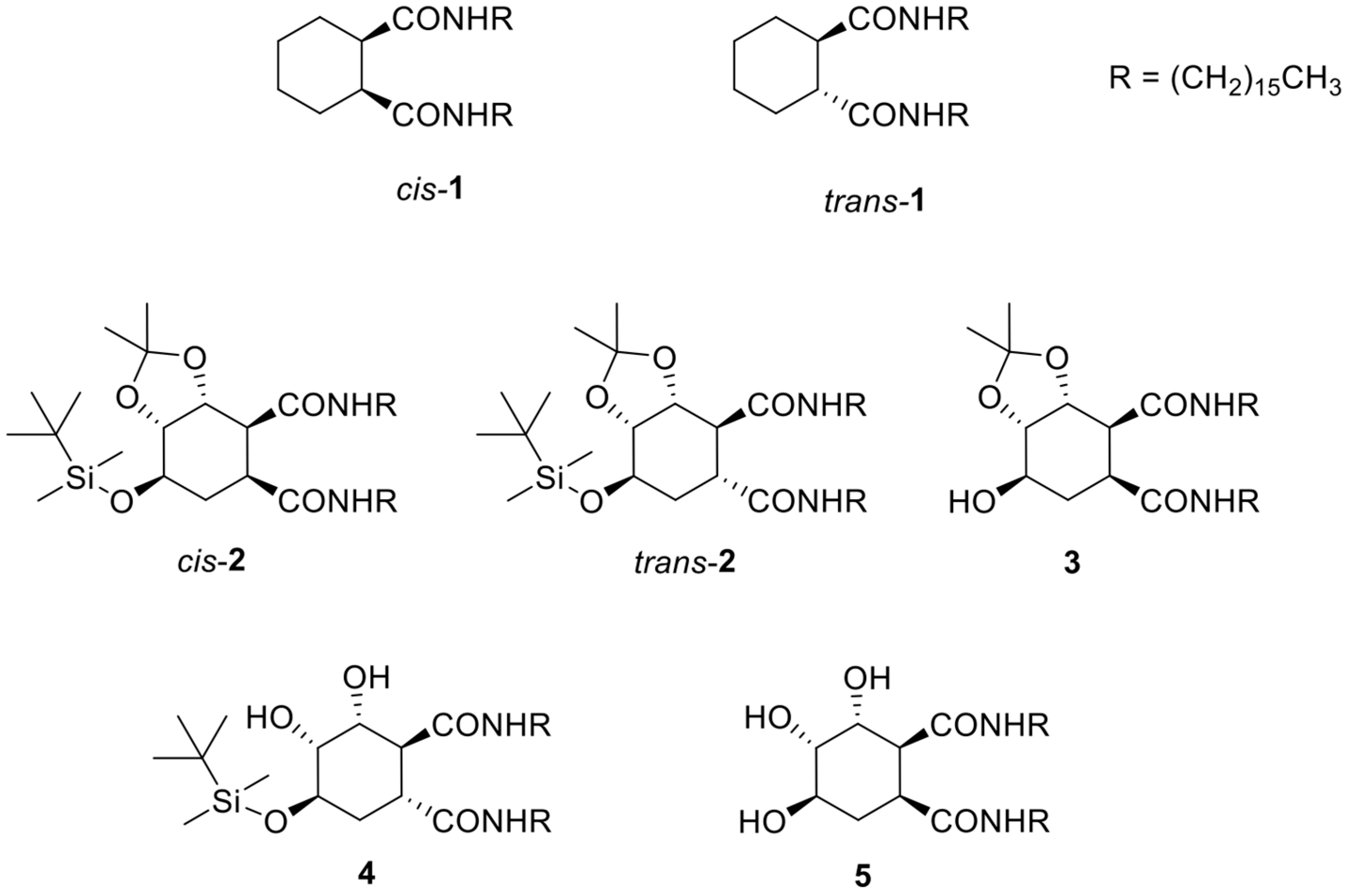
2.1. Synthesis of Organogelators 2–5
2.2. Gelation Studies
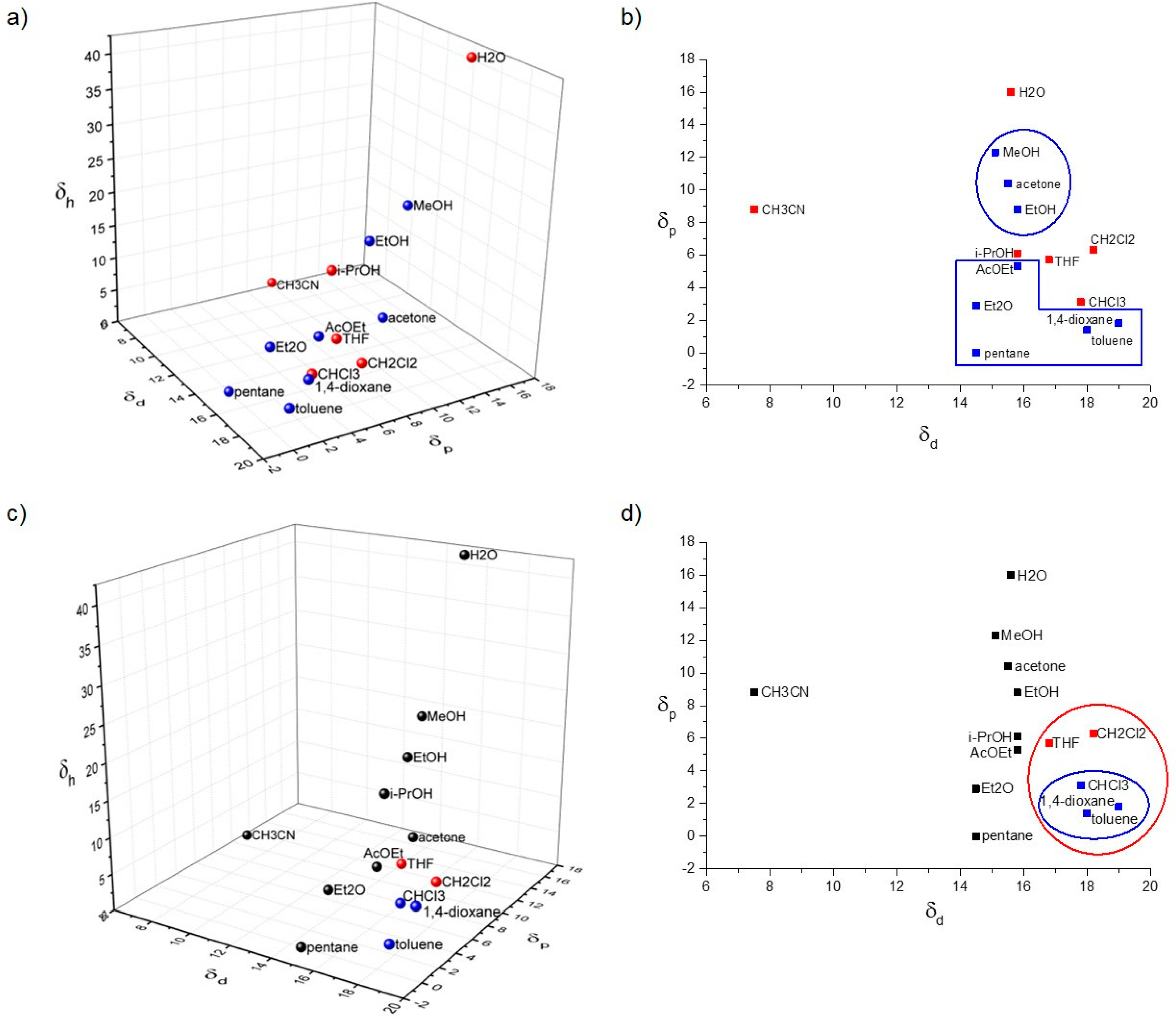
2.3. Hansen Solubility Parameters (HSPs)
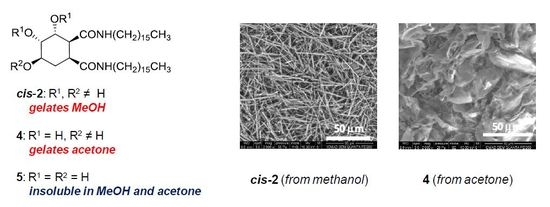
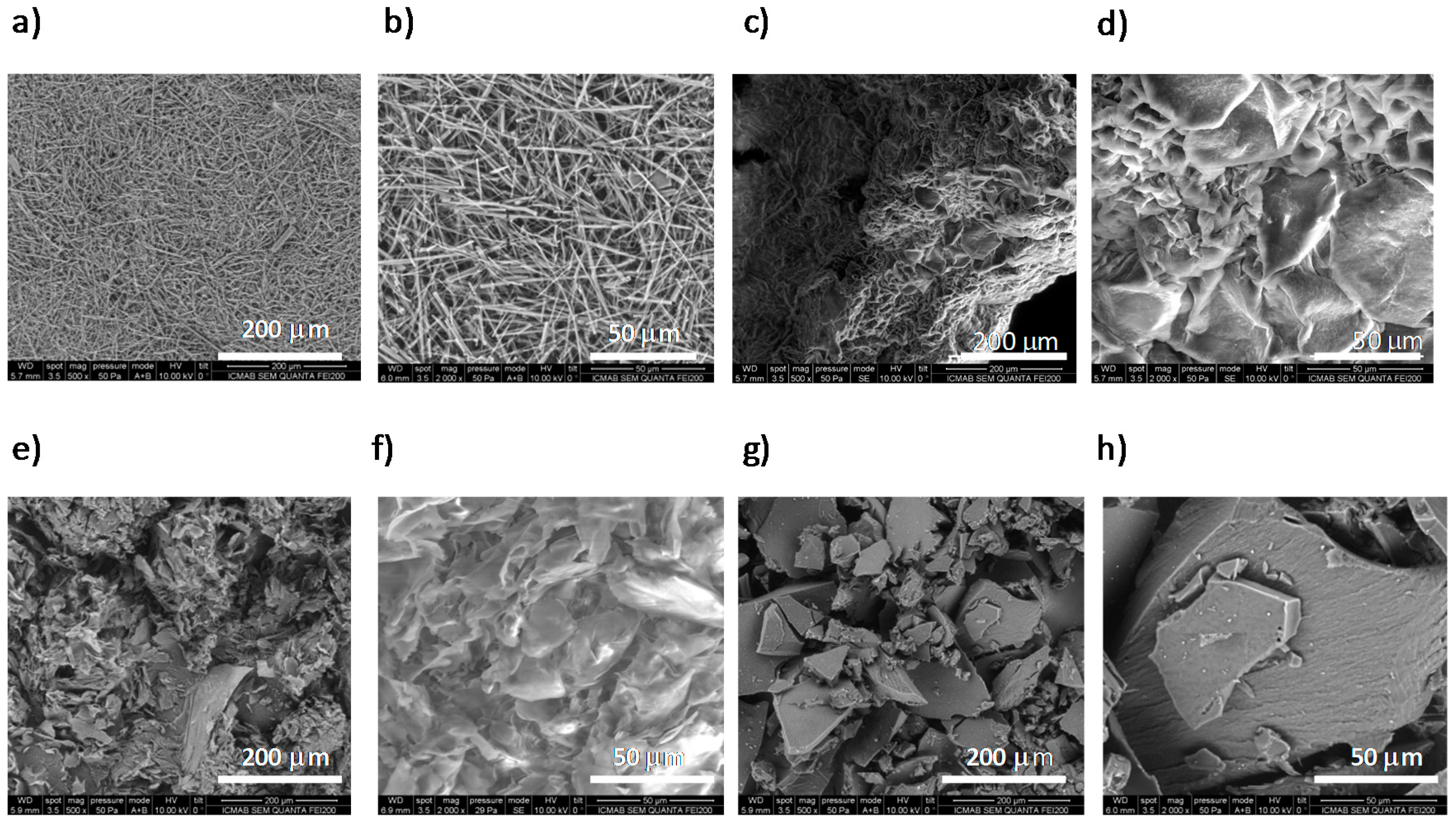
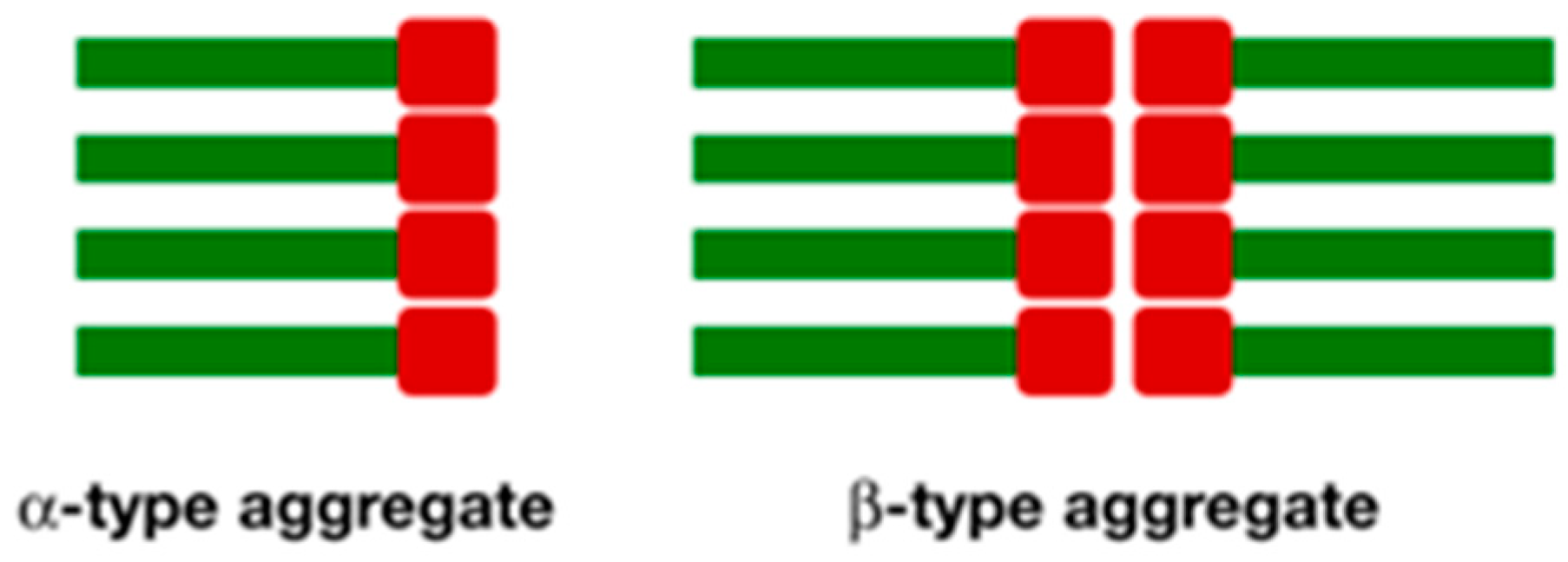
2.4. Scanning Electron Microscopy
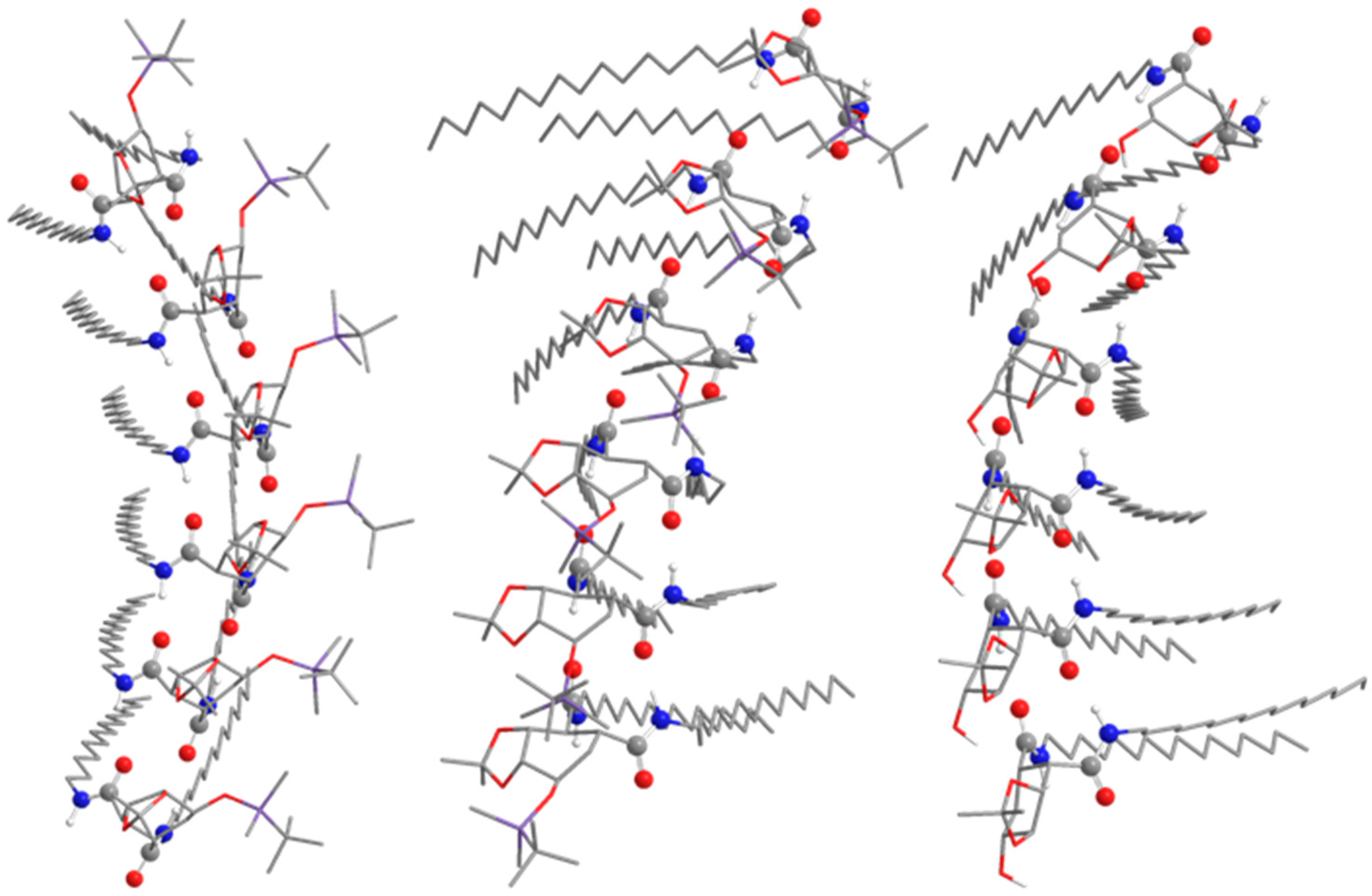
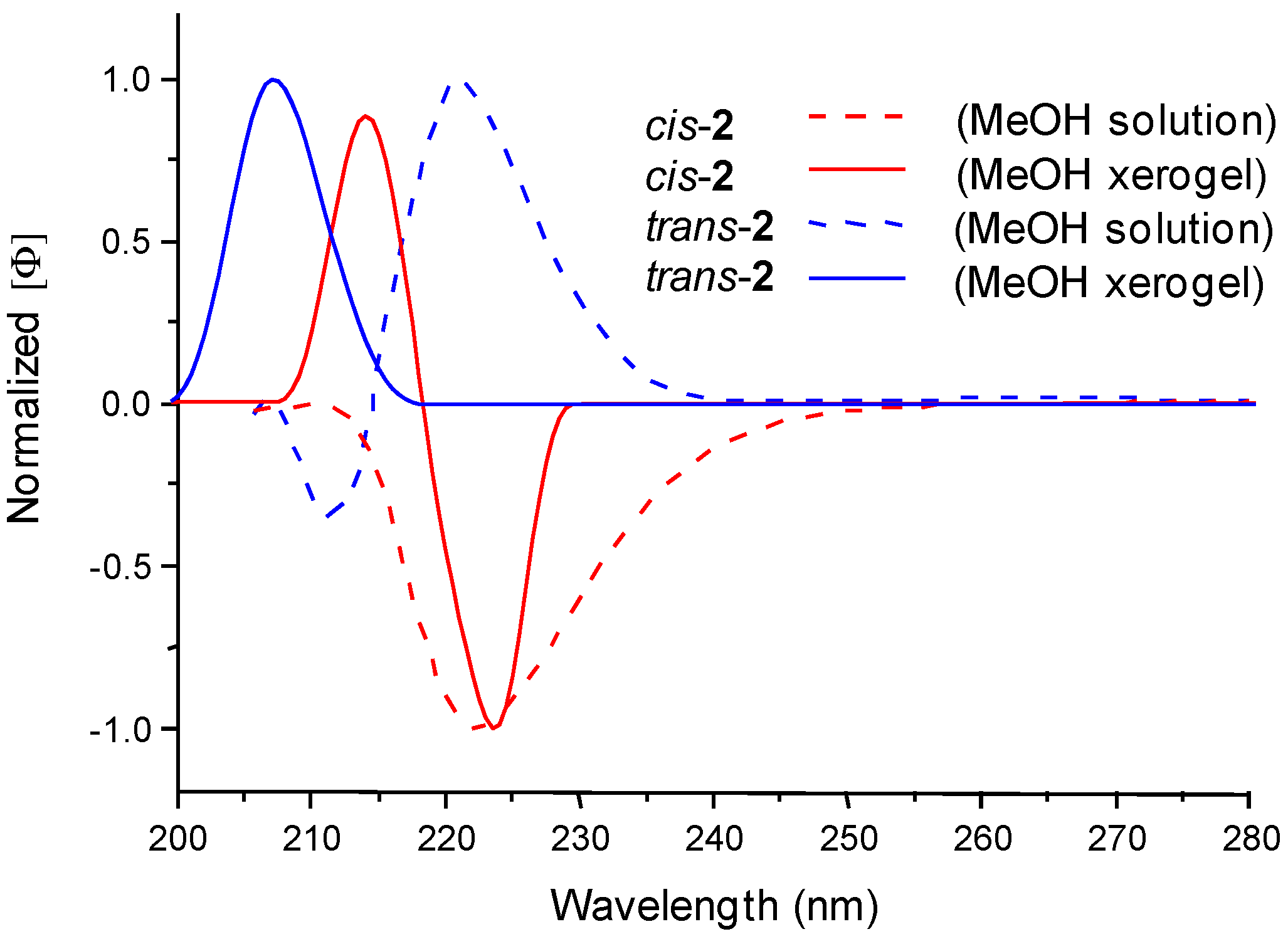
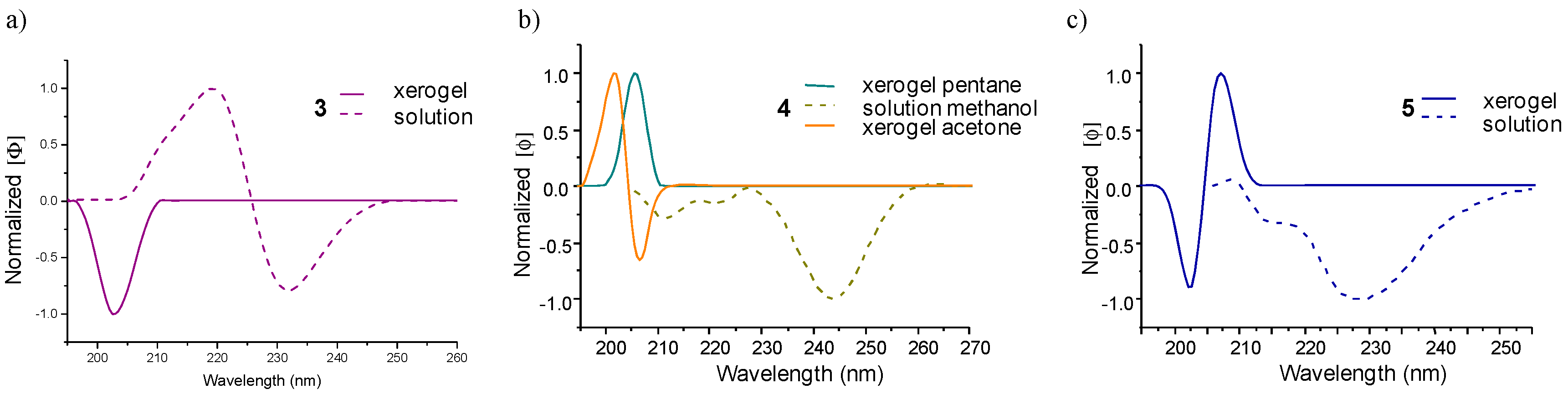
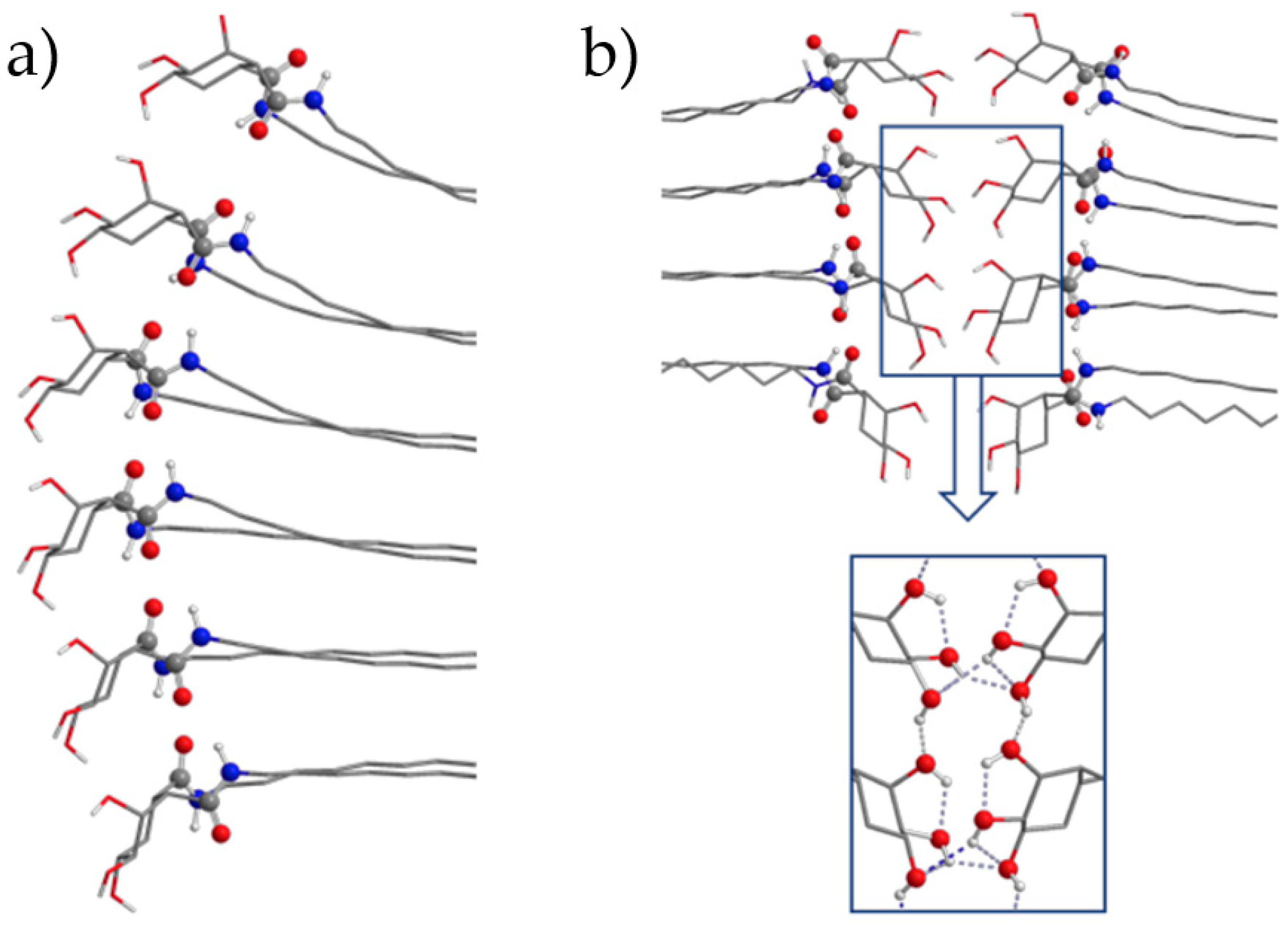
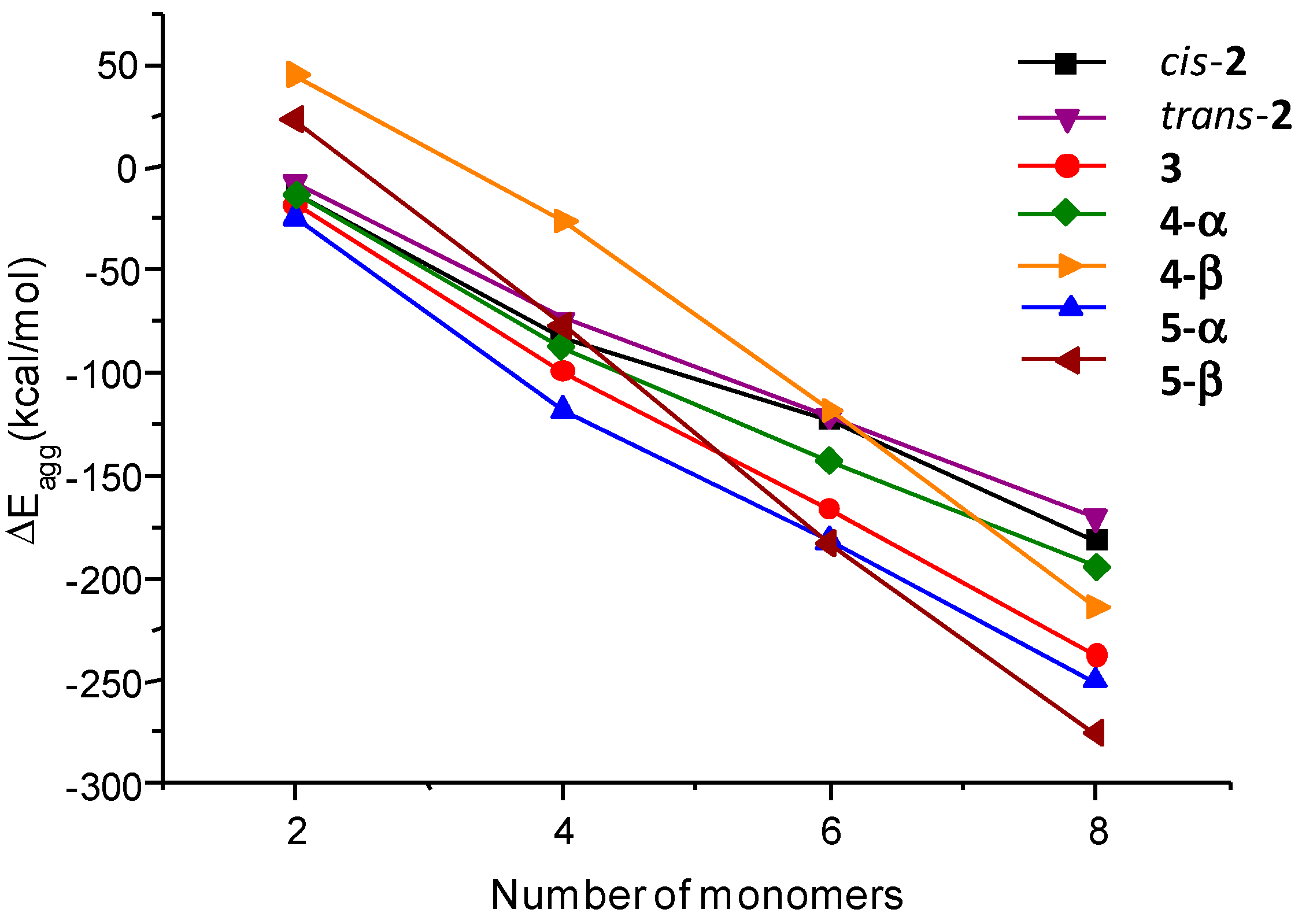
2.5. Computational Calculations and Circular Dichroism
3. Materials and Methods
3.1. General Procedures
3.2. Experimental Section
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Van Esch, J.H.; Feringa, B.L. New Functional Materials Based on Self-Assembling Organogels: From Serendipity towards Design. Angew. Chem. Int. Ed. 2000, 39, 2263–2266. [Google Scholar] [CrossRef]
- Steed, W. Supramolecular gel chemistry: Developments over the last decade. Chem. Commun. 2011, 47, 1379–1383. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Barbero, A.; Suárez, I.J.; Sierra-Martín, B.; Fernández-Nieves, A.; de las Nieves, F.J.; Marquez, M.; Rubio-Retama, J.; López-Cabarcos, E. Gels and microgels for nanotechnological applications. Adv. Colloid Interface Sci. 2009, 147, 88–108. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.K. Lost in translation Chirality effects in the self-assembly of nanostructured gel-phase materials. Chem. Soc. Rev. 2009, 38, 684–694. [Google Scholar] [CrossRef] [PubMed]
- Duan, P.; Cao, H.; Zhang, L.; Liu, M. Gelation induced supramolecular chirality: Chirality transfer, amplification and application. Soft Matter 2014, 10, 5428–5448. [Google Scholar] [CrossRef]
- Okesola, B.O.; Smith, D.K. Applying low-molecular weight supramolecular gelators in an environmental setting-self-assembled gels as smart materials for pollutant removal. Chem. Soc. Rev. 2016, 45, 4226–4251. [Google Scholar] [CrossRef]
- Prathap, A.; Sureshan, K.M. Organogelator-Cellulose Composite for Practical and Eco-Friendly Marine Oil-Spill Recovery. Angew. Chem. Int. Ed. 2017, 56, 9405–9409. [Google Scholar] [CrossRef]
- Raju, C.S.K.; Pramanik, B.; Ravishankar, R.; Rao, P.V.C.; Sriganesh, G. Xylitol based phase selective organogelators for potential oil spillage recovery. RSC Adv. 2017, 7, 37175–37180. [Google Scholar] [CrossRef]
- Lan, Y.; Corradini, M.G.; Weiss, R.G.; Raghvan, S.R.; Rogers, M.A. To gel or not to gel: Correlating molecular gelation with solvent parameters. Chem. Soc. Rev. 2015, 44, 6035–6058. [Google Scholar] [CrossRef]
- Fages, F. (Ed.) Low Molecular Mass Gelators: Design, Self-Assembly, Function. Topics in Current Chemistry; Springer: New York, NY, USA, 2005; Volume 256. [Google Scholar]
- Aggeli, A.; Bell, M.; Boden, N.; Keen, J.N.; Knowles, P.F.; McLeish, T.C.B.; Pitkeathly, M.; Radford, S.E. Responsive gels formed by the spontaneous self-assembly of peptides into polymeric β-sheet tapes. Nature 1997, 386, 259–262. [Google Scholar] [CrossRef]
- Makarevic, J.; Jokic, M.; Peric, B.; Tomisic, V.; Kojic-Prodic, B.; Zinic, M. Bis(Amino Acid) Oxalyl Amides as Ambidextrous Gelators of Water and Organic Solvents: Supramolecular Gels with Temperature Dependent Assembly/Dissolution Equilibrium. Chem. Eur. J. 2001, 7, 3328–3341. [Google Scholar] [CrossRef]
- Hirst, A.R.; Smith, D.K. Solvent Effects on Supramolecular Gel-Phase Materials: Two-Component Dendritic Gel. Langmuir 2004, 20, 10851–10857. [Google Scholar] [CrossRef] [PubMed]
- Frässdorf, W.; Fahrländer, M.; Fuchs, K.; Friedrich, C. Thermorheological properties of self-assembled dibenzylidene sorbitol structures in various polymer matrices: Determination and prediction of characteristic temperatures. J. Rheol. 2003, 47, 1445–1454. [Google Scholar] [CrossRef]
- Edwards, W.; Lagadec, C.A.; Smith, D.K. Solvent–gelator interactions—Using empirical solventparameters to better understand the self-assembly of gel-phase materials. Soft Matter 2011, 7, 110–117. [Google Scholar] [CrossRef]
- Hanabusa, K.; Matsumoto, M.; Kimura, M.; Kakehi, A.; Shirai, H. Low Molecular Weight Gelators for Organic Fluids: Gelation Using a Family of Cyclo(dipeptide)s. J. Colloid Interface Sci. 2000, 224, 231–244. [Google Scholar] [CrossRef]
- Raynal, M.; Bouteiller, L. Organogel formation rationalized by Hansen solubility parameters. Chem. Commun. 2011, 47, 8271–8273. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Gao, J.; Emge, T.J.; Rogers, M.A. Influence of solvent on the supramolecular architectures in molecular gels. Soft Matter 2013, 9, 5942–5950. [Google Scholar] [CrossRef]
- Yan, N.; Xu, Z.; Diehn, K.K.; Raghavan, S.R.; Fang, Y.; Weiss, R.G. How Do Liquid Mixtures Solubilize Insoluble Gelators? Self-Assembly Properties of Pyrenyl-Linker-Glucono Gelators in Tetrahydrofuran–Water Mixtures. J. Am. Chem. Soc. 2013, 135, 8989–8999. [Google Scholar] [CrossRef]
- Anuradha, D.D.L.; Al-Kobaisi, M.; Bhosale, S.V. Right handed chiral superstructures from achiral molecules: Self-assembly with a twist. Sci. Rep. 2015, 5, 15652. [Google Scholar] [CrossRef]
- Rúa, F.; Boussert, S.; Parella, T.; Diez-Pérez, I.; Branchadell, V.; Giralt, E.; Ortuño, R.M. Self-Assembly of a Cyclobutane β-Tetrapeptide to Form Nanosized Structures. Org. Lett. 2007, 9, 3643–3645. [Google Scholar] [CrossRef]
- Gorrea, E.; Nolis, P.; Torres, E.; Da Silva, E.; Amabilino, D.B.; Branchadell, V.; Ortuño, R.M. Self-Assembly of Chiral trans-Cyclobutane-Containing β-Dipeptides into Ordered Aggregates. Chem. Eur. J. 2011, 17, 4588–4597. [Google Scholar] [CrossRef] [PubMed]
- Celis, S.; Nolis, P.; Illa, O.; Branchadell, V.; Ortuño, R.M. Low-molecular-weight gelators consisting of hybrid cyclobutane-based peptides. Org. Biomol. Chem. 2013, 11, 2839–2846. [Google Scholar] [CrossRef] [PubMed]
- Pi-Boleda, B.; Sans, M.; Campos, M.; Nolis, P.; Illa, O.; Estévez, J.C.; Branchadell, V.; Ortuño, R.M. Studies on cycloalkane-based bisamide organogelators: A new example of stochastic chiral symmetry breaking induced by sonication. Chem. Eur. J. 2017, 23, 3357–3365. [Google Scholar] [CrossRef] [PubMed]
- Hanabusa, K.; Yamada, M.; Kimura, M.; Shirai, H. Prominent Gelation and Chiral Aggregation of Alkylamides Derived from trans-1,2-Diaminocyclohexane. Angew. Chem. Int. Ed. 1996, 35, 1949–1951. [Google Scholar] [CrossRef]
- Zweep, N.; Hopkinson, A.; Meetsma, A.; Browne, W.R.; Feringa, B.L.; van Esch, J.H. Balancing Hydrogen Bonding and Van der Waals Interactions in Cyclohexane-Based Bisamide and Bisurea Organogelators. Langmuir 2009, 25, 8802–8809. [Google Scholar] [CrossRef] [PubMed]
- Gronwald, O.; Shinkai, S. Sugar-Integrated Gelators of Organic Solvents. Chem. Eur. J. 2001, 7, 4328–4334. [Google Scholar] [CrossRef]
- Luboradzki, R.; Pakulski, Z.; Sartowska, B. Glucofuranose derivatives as a library for designing and investigating low molecular mass organogelator. Tetrahedron 2005, 61, 10122–10128. [Google Scholar] [CrossRef]
- Edelsztein, V.C.; Mac Cormack, A.S.; Ciarlantini, M.; Di Chenna, P.H. Self-assembly of 2,3-dihydroxycholestane steroids into supramolecular organogels as a soft template for the in-situ generation of silicate nanomaterials. Beilstein J. Org. Chem. 2013, 9, 1826–1836. [Google Scholar] [CrossRef]
- Rajkamal, D.; Pathak, N.P.; Halder, T.; Dhara, S.; Yadav, S. Partially Acetylated or Benzoylated Arabinose Derivatives as Structurally Simple Organogelators: Effect of the Ester Protecting Group on Gel Properties. Chem. Eur. J. 2017, 23, 11323–11329. [Google Scholar] [CrossRef]
- González, M.A.; Estévez, A.M.; Campos, M.; Estévez, J.C.; Estévez, R.J. Protocol for the Incorporation of γ-Amino Acids into Peptides: Application to (−)-Shikimic Acid Based 2-Amino-Methylcyclohexanecarboxylic Acids. J. Org. Chem. 2018, 83, 1543–1550. [Google Scholar] [CrossRef]
- Kobayashi, T. J-Aggregates, 1st ed.; Kobayashi, T., Ed.; World Scientific Publishing Co. Pte. Ltd.: Singapore, 1996; Volume 2. [Google Scholar]
- Pescitelli, G.; Di Bari, L.; Berova, N. Application of electronic circular dichroism in the study of supramolecular systems. Chem. Soc. Rev. 2014, 43, 5211–5233. [Google Scholar] [CrossRef] [PubMed]
- Kolossváry, I.; Guida, W.C. Low Mode Search. An Efficient, Automated Computational Method for Conformational Analysis: Application to Cyclic and Acyclic Alkanes and Cyclic Peptides. J. Am. Chem. Soc. 1996, 118, 5011–5019. [Google Scholar] [CrossRef]
- Kaminski, G.A.; Friesner, R.A.; Tirado-Rives, J.; Jorgensen, W.L. Evaluation and Reparametrization of the OPLS-AA Force Field for Proteins via Comparison with Accurate Quantum Chemical Calculations on Peptides. J. Phys. Chem. B 2001, 105, 6474–6487. [Google Scholar] [CrossRef]
- Mohamadi, F.; Richards, N.G.J.; Guida, W.C.; Liskamp, R.; Lipton, M.; Caufield, C.; Chang, G.; Hendrickson, T.; Still, W.C. Macromodel—An integrated software system for modeling organic and bioorganic molecules using molecular mechanics. J. Comput. Chem. 1990, 11, 440–467. [Google Scholar] [CrossRef]
- Frisch, G.W.; Trucks, H.B.; Schlegel, G.E.; Scuseria, M.A.; Robb, J.R.; Cheeseman, G.; Scalmani, V.; Barone, B.; Mennucci, G.A.; Petersson, H.; et al. Gaussian 09; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2007, 120, 215–241. [Google Scholar]
- Zhao, Y.; Truhlar, D.G. A new local density functional for main-group thermochemistry, transition metal bonding, thermochemical kinetics, and noncovalent interactions. J. Chem. Phys. 2006, 125, 194101. [Google Scholar] [CrossRef] [PubMed]
- O’Boyle, N.M.; Tenderholt, A.L.; Langner, K.M. Cclib: A library for package-independent computational chemistry algorithms. J. Comput. Chem. 2008, 29, 839–845. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |

| Pentane | 1,4-Dioxane | Toluene | Et2O | CHCl3 | EtOAc | THF | CH2Cl2 | iPrOH | Acetone | EtOH | MeOH | CH3CN | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| cis-116 | 30 | 51 | 100 | I | S | I | I | I | I | I | I | I | I |
| (48) | (82) | (161) | |||||||||||
| O | O | O | |||||||||||
| trans-116 | I | 3 | 7 | I | 18 | I | I | I | I | I | I | I | I |
| (5) | (11) | (29) | |||||||||||
| T | T | T | |||||||||||
| cis-2 | 83 | S | S | S | S | 100 | S | S | 100 | S | 21 | 17 | S |
| (101) | (122) | (122) | (26) | (21) | |||||||||
| C | O | O | O | O | |||||||||
| trans-2 | S | S | S | S | S | S | S | S | S | S | 50 | 16 | S |
| (61) | (19) | ||||||||||||
| T | O | ||||||||||||
| 3 | I | 51 | 102 | S | S | S | S | S | S | S | S | S | S |
| (72) | (144) | ||||||||||||
| T | T | ||||||||||||
| 4 | 54 | 61 | 64 | 70 | S | 45 | S | S | S | 22 | 56 | 70 | S |
| (69) | (78) | (82) | (90) | (58) | (28) | (72) | (90) | ||||||
| O | O | T | O | O | O | O | O | ||||||
| 5 | I | 102 | 82 | I | 82 | I | S | S | I | I | I | I | I |
| (193) | (156) | (156) | |||||||||||
| O | T | T |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pi-Boleda, B.; Campos, M.; Sans, M.; Basavilbaso, A.; Illa, O.; Branchadell, V.; Estévez, J.C.; Ortuño, R.M. Synthesis and Gelling Abilities of Polyfunctional Cyclohexane-1,2-dicarboxylic Acid Bisamides: Influence of the Hydroxyl Groups. Molecules 2019, 24, 352. https://doi.org/10.3390/molecules24020352
Pi-Boleda B, Campos M, Sans M, Basavilbaso A, Illa O, Branchadell V, Estévez JC, Ortuño RM. Synthesis and Gelling Abilities of Polyfunctional Cyclohexane-1,2-dicarboxylic Acid Bisamides: Influence of the Hydroxyl Groups. Molecules. 2019; 24(2):352. https://doi.org/10.3390/molecules24020352
Chicago/Turabian StylePi-Boleda, Bernat, María Campos, Marta Sans, Antonio Basavilbaso, Ona Illa, Vicenç Branchadell, Juan Carlos Estévez, and Rosa M. Ortuño. 2019. "Synthesis and Gelling Abilities of Polyfunctional Cyclohexane-1,2-dicarboxylic Acid Bisamides: Influence of the Hydroxyl Groups" Molecules 24, no. 2: 352. https://doi.org/10.3390/molecules24020352
APA StylePi-Boleda, B., Campos, M., Sans, M., Basavilbaso, A., Illa, O., Branchadell, V., Estévez, J. C., & Ortuño, R. M. (2019). Synthesis and Gelling Abilities of Polyfunctional Cyclohexane-1,2-dicarboxylic Acid Bisamides: Influence of the Hydroxyl Groups. Molecules, 24(2), 352. https://doi.org/10.3390/molecules24020352