Biomechanical Stability and Osteogenesis in a Tibial Bone Defect Treated by Autologous Ovine Cord Blood Cells—A Pilot Study
Abstract
:1. Introduction
2. Results
2.1. Characterization of Autologous USSC
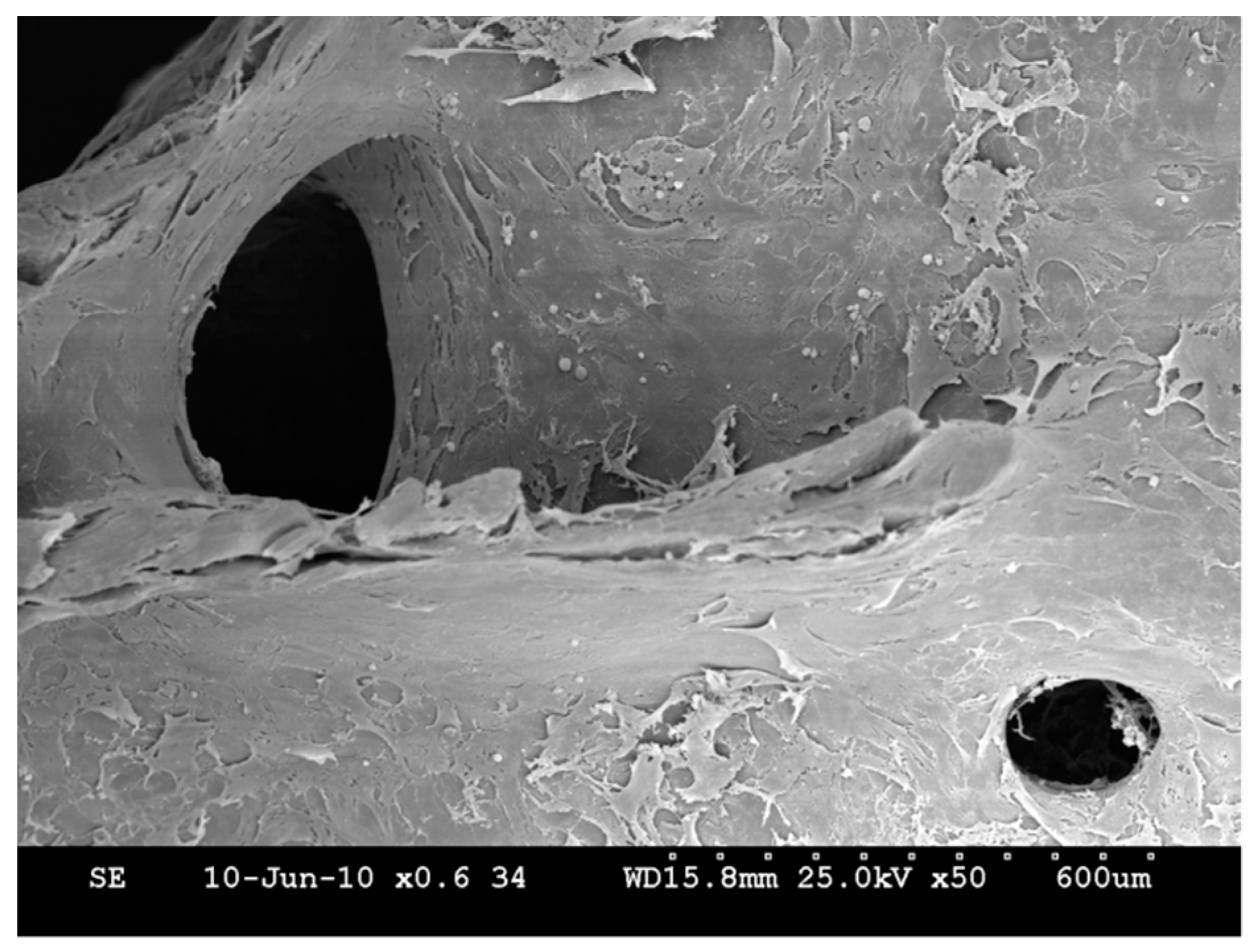
2.2. In Vitro Assessment of the USSC-HA Scaffold
2.3. Labeling Control
2.4. In Vivo Assessment of Bone Healing
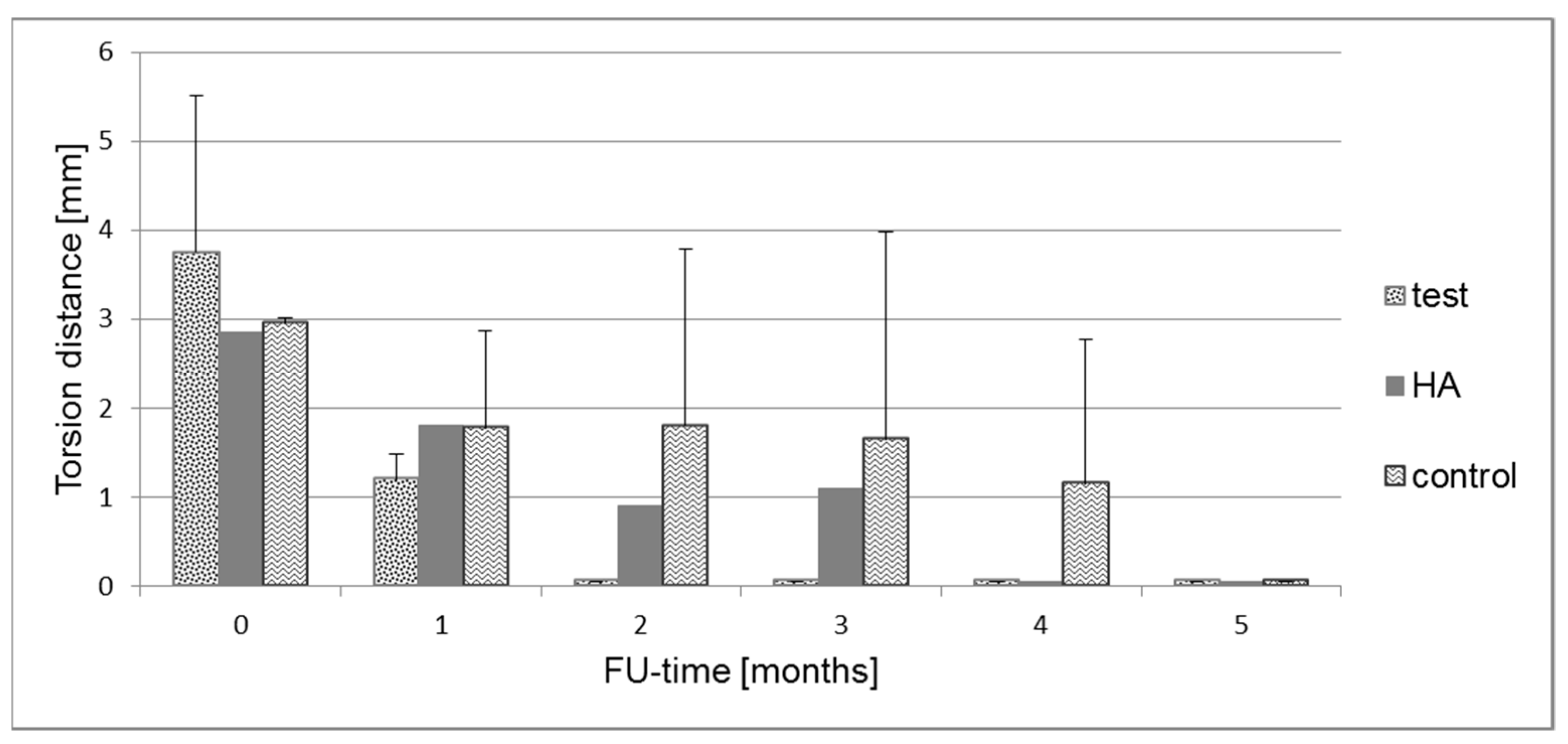
2.5. Biomechanical Measurements
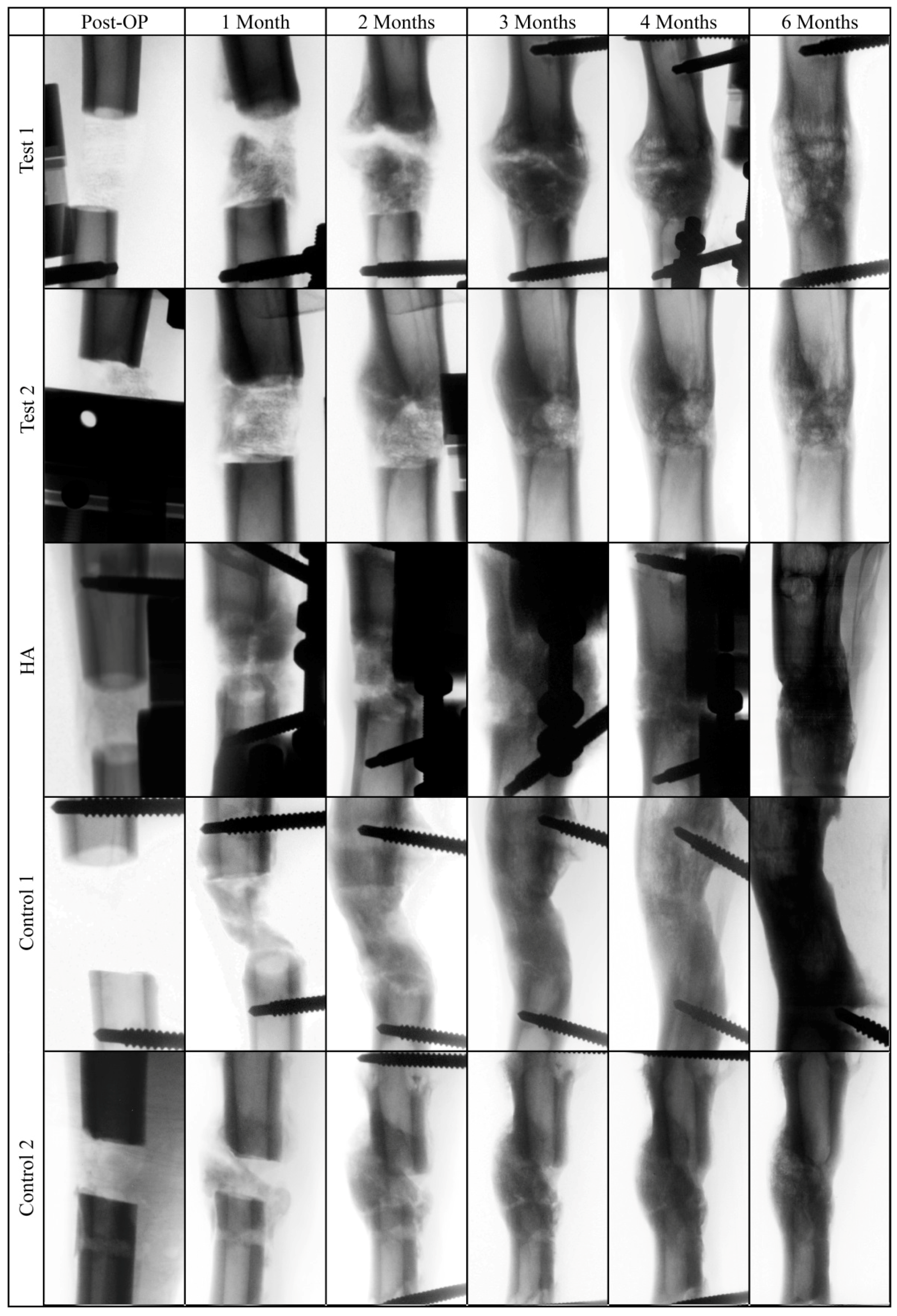
2.6. X-Ray Analysis
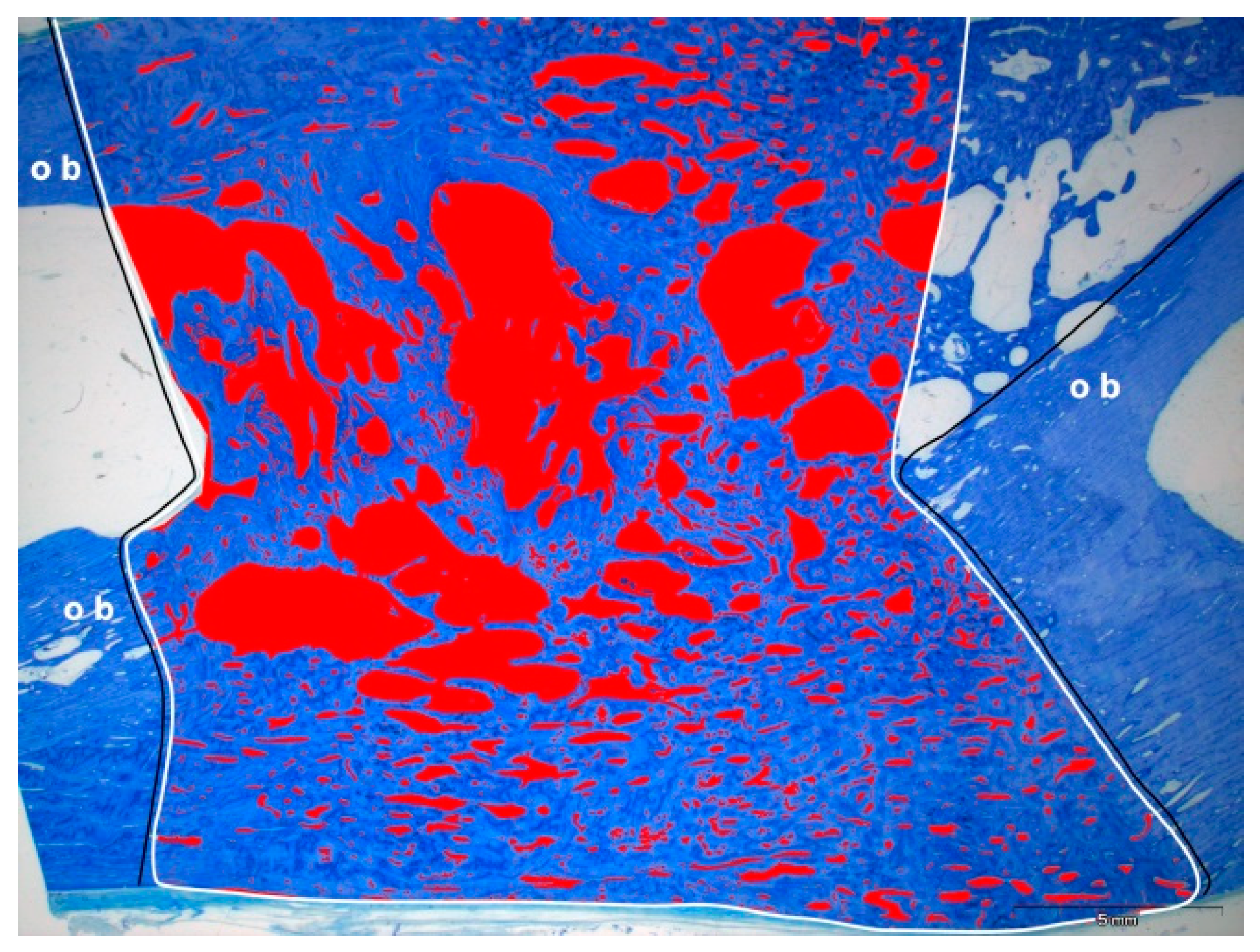
2.7. Histological Results of Bone Formation
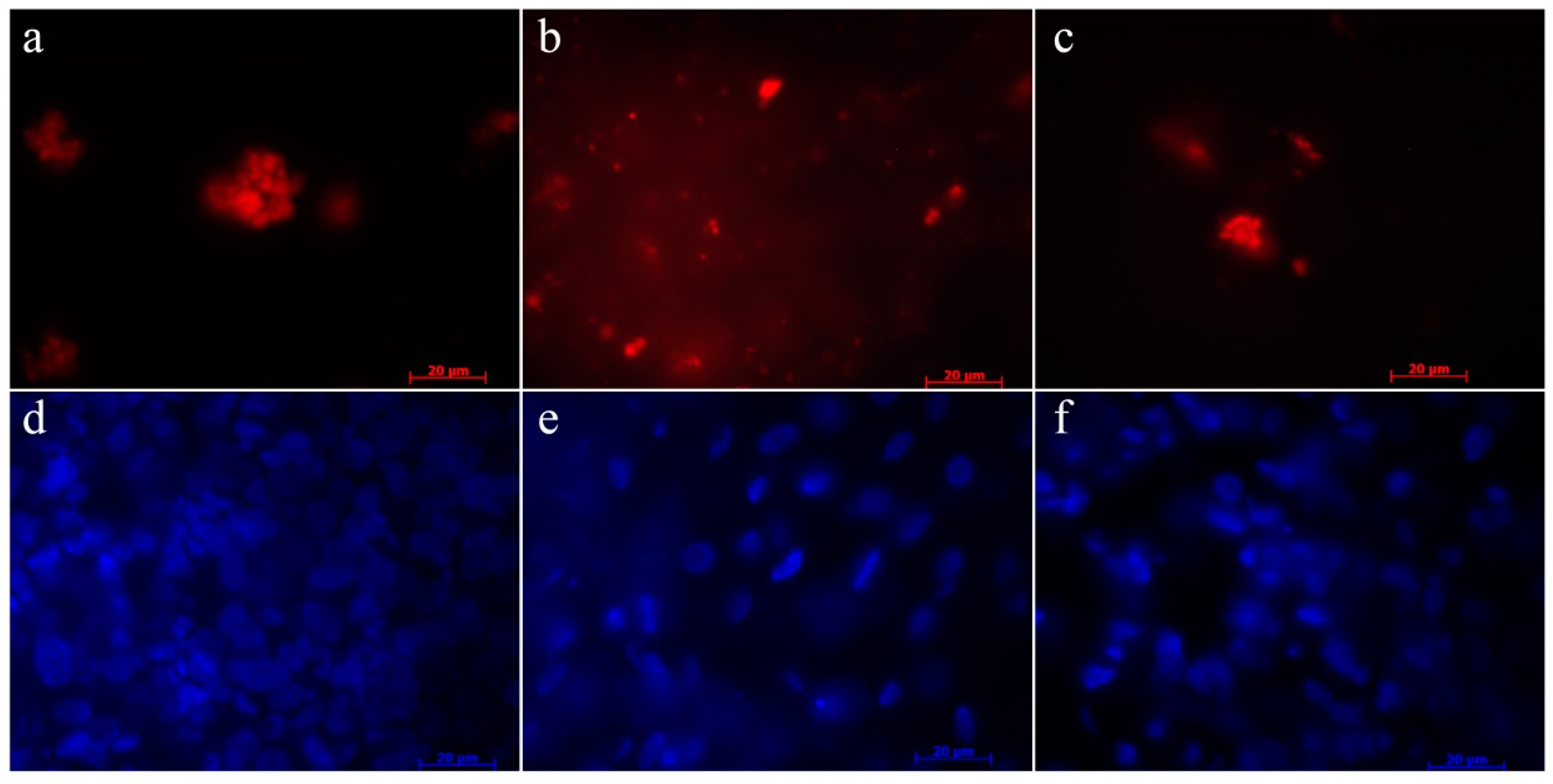
2.8. Homing of the USSC
3. Discussion
3.1. Subcritical Nature of the Defect
3.2. Less Bone Occurred in the Scaffold Groups
3.3. Fluorescent Labeling of USSC
3.4. External Fixation
3.5. Limitations of the Study
4. Materials and Methods
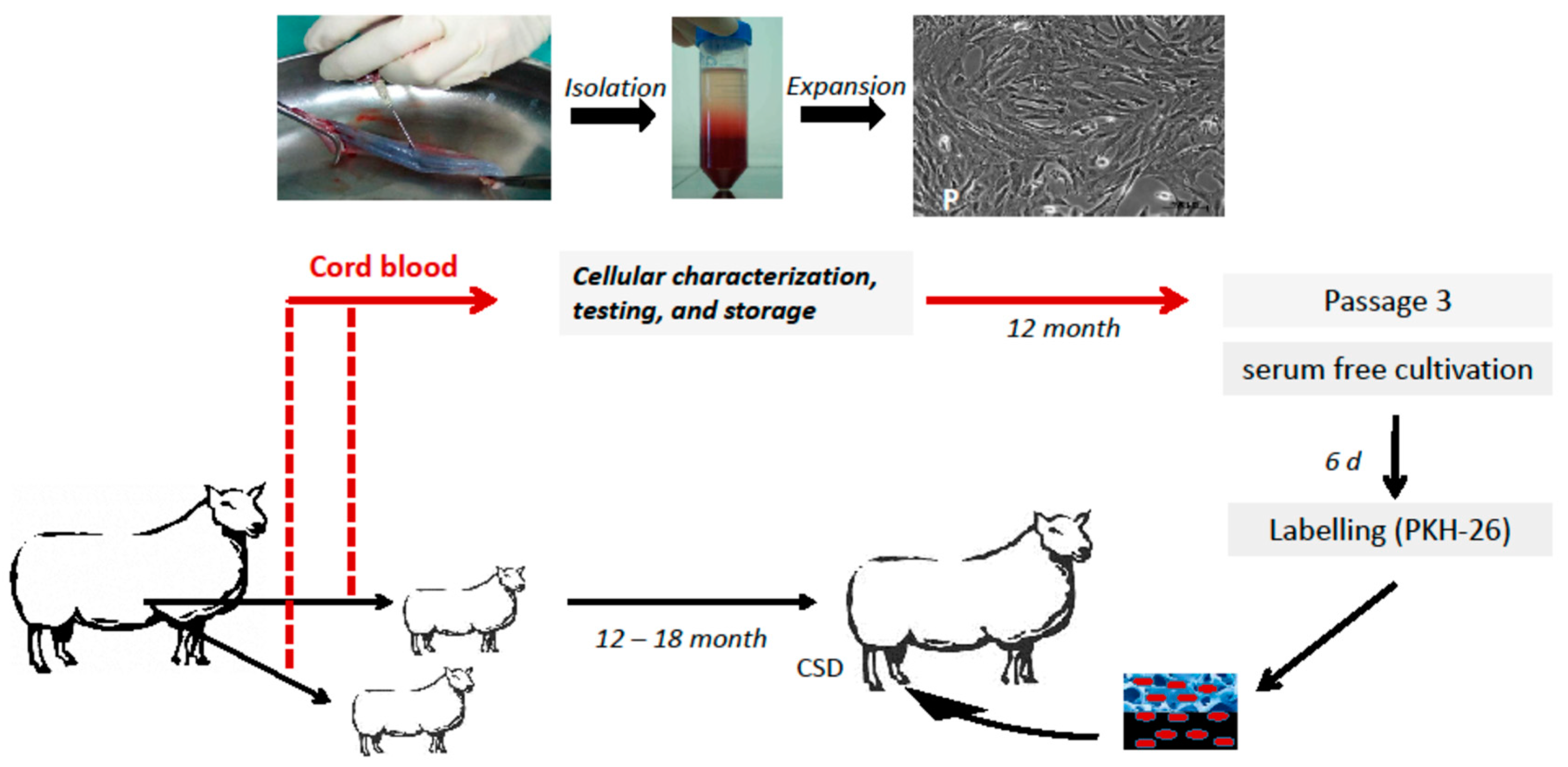
4.1. Animal Model and Study Design
4.2. Characterization of Autologous USSC
4.3. Labeling of USSC for Homing and Cell Tracking
4.4. Biomaterials
4.5. Biomaterial Insertion
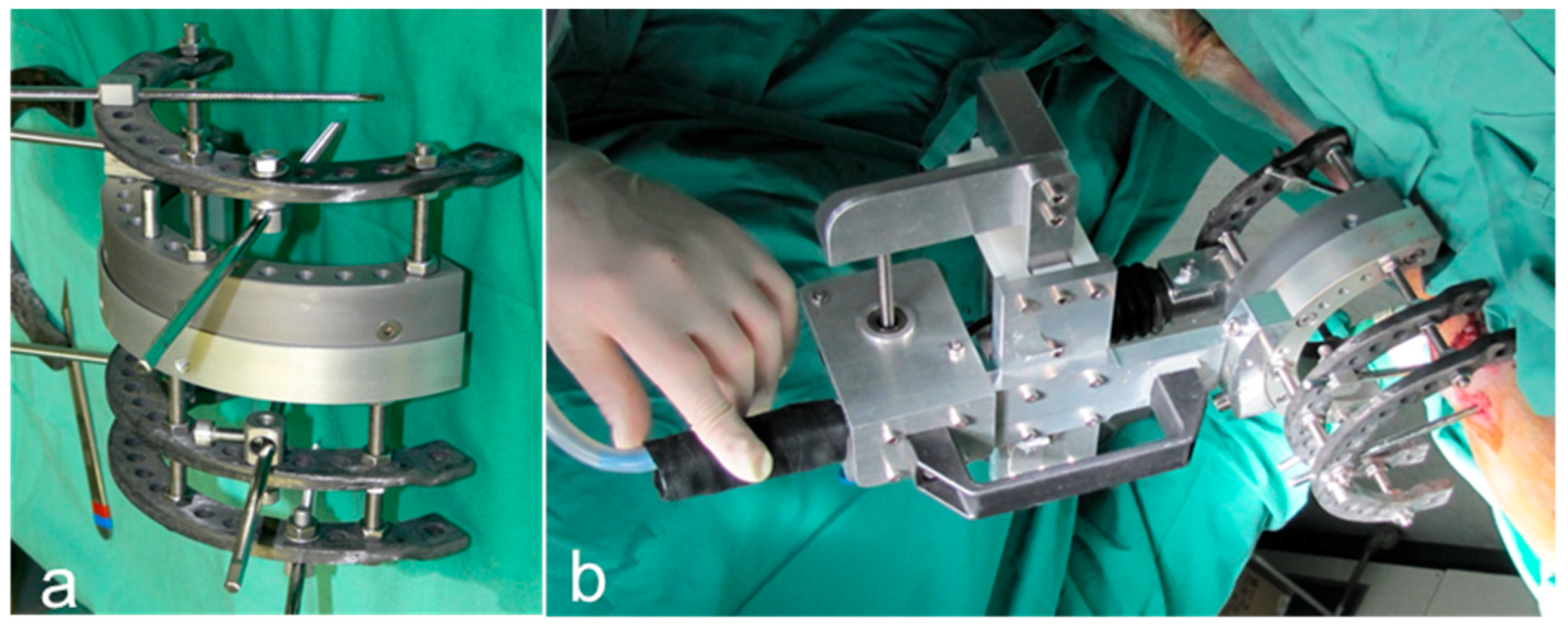
4.6. Animal Surgery
4.7. Biomechanical Testing
4.8. Radiographic Analysis and Analysis of Systemic Response
4.9. Histochemical Analysis
4.10. Detection of Labeled USSC
4.11. Data Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Petite, H.; Viateau, V.; Bensaid, W.; Meunier, A.; de Pollak, C.; Bourguignon, M.; Oudina, K.; Sedel, L.; Guillemin, G. Tissue-engineered bone regeneration. Nat. Biotechnol. 2000, 18, 959–963. [Google Scholar] [CrossRef] [PubMed]
- Dimitriou, R.; Jones, E.; McGonagle, D.; Giannoudis, P.V. Bone regeneration: Current concepts and future directions. BMC Med. 2011, 9, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Sen, M.K.; Miclau, T. Autologous iliac crest bone graft: Should it still be the gold standard for treating nonunions? Injury 2007, 38, S75–S80. [Google Scholar] [CrossRef]
- Field, J.R.; McGee, M.; Wildenauer, C.; Kurmis, A.; Margerrison, E. The utilization of a synthetic bone void filler (jax) in the repair of a femoral segmental defect. Vet. Comp. Orthop. Traumatol. 2009, 22, 87–95. [Google Scholar] [PubMed]
- Decambron, A.; Fournet, A.; Bensidhoum, M.; Manassero, M.; Sailhan, F.; Petite, H.; Logeart-Avramoglou, D.; Viateau, V. Low-dose bmp-2 and msc dual delivery onto coral scaffold for critical-size bone defect regeneration in sheep. J. Orthop. Res. 2017, 35, 2637–2645. [Google Scholar] [CrossRef]
- Dilogo, I.H.; Kamal, A.F.; Gunawan, B.; Rawung, R.V. Autologous mesenchymal stem cell (mscs) transplantation for critical-sized bone defect following a wide excision of osteofibrous dysplasia. Int. J. Surg. Case Rep. 2015, 17, 106–111. [Google Scholar] [CrossRef] [PubMed]
- McDaniel, J.S.; Pilia, M.; Raut, V.; Ledford, J.; Shiels, S.M.; Wenke, J.C.; Barnes, B.; Rathbone, C.R. Alternatives to autograft evaluated in a rabbit segmental bone defect. Int. Orthop. 2016, 40, 197–203. [Google Scholar] [CrossRef]
- Fernandes, M.B.; Guimaraes, J.A.; Casado, P.L.; Cavalcanti Ados, S.; Goncalves, N.N.; Ambrosio, C.E.; Rodrigues, F.; Pinto, A.C.; Miglino, M.A.; Duarte, M.E. The effect of bone allografts combined with bone marrow stromal cells on the healing of segmental bone defects in a sheep model. BMC Vet. Res. 2014, 10, 36–47. [Google Scholar] [CrossRef]
- Giannini, S.; Vannini, F.; Lisignoli, G.; Facchini, A. Histological and immunohistochemical analysis of an allogenic bone graft engineered with autologous bone marrow mononuclear cells in the treatment of a large segmental defect of the ulna. A case report. Chir. Organi. Mov. 2008, 91, 171–175. [Google Scholar] [CrossRef]
- Weitao, Y.; Kangmei, K.; Xinjia, W.; Weili, Q. Bone regeneration using an injectable calcium phosphate/autologous iliac crest bone composites for segmental ulnar defects in rabbits. J. Mater. Sci. Mater. Med. 2008, 19, 2485–2492. [Google Scholar] [CrossRef]
- Kode, J.A.; Mukherjee, S.; Joglekar, M.V.; Hardikar, A.A. Mesenchymal stem cells: Immunobiology and role in immunomodulation and tissue regeneration. Cytotherapy 2009, 11, 377–391. [Google Scholar] [CrossRef] [PubMed]
- Le Blanc, K. Immunomodulatory effects of fetal and adult mesenchymal stem cells. Cytotherapy 2003, 5, 485–489. [Google Scholar] [CrossRef] [PubMed]
- Meesuk, L.; Tantrawatpan, C.; Kheolamai, P.; Manochantr, S. The immunosuppressive capacity of human mesenchymal stromal cells derived from amnion and bone marrow. Biochem. Biophys. Rep. 2016, 8, 34–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takizawa, N.; Okubo, N.; Kamo, M.; Chosa, N.; Mikami, T.; Suzuki, K.; Yokota, S.; Ibi, M.; Ohtsuka, M.; Taira, M.; et al. Bone marrow-derived mesenchymal stem cells propagate immunosuppressive/anti-inflammatory macrophages in cell-to-cell contact-independent and -dependent manners under hypoxic culture. Exp. Cell Res. 2017, 358, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Du, W.J.; Reppel, L.; Leger, L.; Schenowitz, C.; Huselstein, C.; Bensoussan, D.; Carosella, E.D.; Han, Z.C.; Rouas-Freiss, N. Mesenchymal stem cells derived from human bone marrow and adipose tissue maintain their immunosuppressive properties after chondrogenic differentiation: Role of hla-g. Stem. Cells Dev. 2016, 25, 1454–1469. [Google Scholar] [CrossRef] [PubMed]
- Schira, J.; Falkenberg, H.; Hendricks, M.; Waldera-Lupa, D.M.; Kogler, G.; Meyer, H.E.; Muller, H.W.; Stuhler, K. Characterization of regenerative phenotype of unrestricted somatic stem cells (ussc) from human umbilical cord blood (hucb) by functional secretome analysis. Mol. Cell Proteom. 2015, 14, 2630–2643. [Google Scholar] [CrossRef]
- Santourlidis, S.; Wernet, P.; Ghanjati, F.; Graffmann, N.; Springer, J.; Kriegs, C.; Zhao, X.; Brands, J.; Arauzo-Bravo, M.J.; Neves, R.; et al. Unrestricted somatic stem cells (ussc) from human umbilical cord blood display uncommitted epigenetic signatures of the major stem cell pluripotency genes. Stem. Cell Res. 2011, 6, 60–69. [Google Scholar] [CrossRef]
- Sensken, S.; Waclawczyk, S.; Knaupp, A.S.; Trapp, T.; Enczmann, J.; Wernet, P.; Kogler, G. In vitro differentiation of human cord blood-derived unrestricted somatic stem cells towards an endodermal pathway. Cytotherapy 2007, 9, 362–378. [Google Scholar] [CrossRef]
- Sueblinvong, V.; Loi, R.; Eisenhauer, P.L.; Bernstein, I.M.; Suratt, B.T.; Spees, J.L.; Weiss, D.J. Derivation of lung epithelium from human cord blood-derived mesenchymal stem cells. Am. J. Respir. Crit. Care Med. 2008, 177, 701–711. [Google Scholar] [CrossRef]
- Kögler, G.; Sensken, S.; Airey, J.A.; Trapp, T.; Muschen, M.; Feldhahn, N.; Liedtke, S.; Sorg, R.V.; Fischer, J.; Rosenbaum, C.; et al. A new human somatic stem cell from placental cord blood with intrinsic pluripotent differentiation potential. J. Exp. Med. 2004, 200, 123–135. [Google Scholar] [CrossRef]
- Kögler, G.; Sensken, S.; Wernet, P. Comparative generation and characterization of pluripotent unrestricted somatic stem cells with mesenchymal stem cells from human cord blood. Exp. Hematol. 2006, 34, 1589–1595. [Google Scholar] [CrossRef] [PubMed]
- Jäger, M.; Degistirici, O.; Knipper, A.; Fischer, J.; Sager, M.; Krauspe, R. Bone healing and migration of cord blood-derived stem cells into a critical size femoral defect after xenotransplantation. J. Bone Miner. Res. 2007, 22, 1224–1233. [Google Scholar] [CrossRef] [PubMed]
- Degistirici, O.; Jager, M.; Knipper, A. Applicability of cord blood-derived unrestricted somatic stem cells in tissue engineering concepts. Cell. Prolif. 2008, 41, 421–440. [Google Scholar] [CrossRef] [PubMed]
- Rosada, C.; Justesen, J.; Melsvik, D.; Ebbesen, P.; Kassem, M. The human umbilical cord blood: A potential source for osteoblast progenitor cells. Calcif. Tissue Int. 2003, 72, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.T.; Maeng, H.; Chwae, Y.J.; Oh, D.J.; Kim, Y.M.; Yang, W.I. Effect of mesenchymal stem cell transplantation on the engraftment of human hematopoietic stem cells and leukemic cells in mice model. Int. J. Hematol. 2008, 87, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Tisato, V.; Naresh, K.; Girdlestone, J.; Navarrete, C.; Dazzi, F. Mesenchymal stem cells of cord blood origin are effective at preventing but not treating graft-versus-host disease. Leukemia 2007, 21, 1992–1999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, P.S.; Messina, D.J.; Hirsh, E.L.; Chi, N.; Goldman, S.N.; Lo, D.P.; Harris, I.R.; Popma, S.H.; Sachs, D.H.; Huang, C.A. Immunogenicity of umbilical cord tissue derived cells. Blood 2008, 111, 430–438. [Google Scholar] [CrossRef]
- Li, C.; Zhang, W.; Jiang, X.; Mao, N. Human-placenta-derived mesenchymal stem cells inhibit proliferation and function of allogeneic immune cells. Cell Tissue Res. 2007, 330, 437–446. [Google Scholar] [CrossRef]
- Maissen, O.; Eckhardt, C.; Gogolewski, S.; Glatt, M.; Arvinte, T.; Steiner, A.; Rahn, B.; Schlegel, U. Mechanical and radiological assessment of the influence of rhtgfbeta-3 on bone regeneration in a segmental defect in the ovine tibia: Pilot study. J. Orthop. Res. 2006, 24, 1670–1678. [Google Scholar] [CrossRef]
- Reichert, J.C.; Saifzadeh, S.; Wullschleger, M.E.; Epari, D.R.; Schutz, M.A.; Duda, G.N.; Schell, H.; van Griensven, M.; Redl, H.; Hutmacher, D.W. The challenge of establishing preclinical models for segmental bone defect research. Biomaterials 2009, 30, 2149–2163. [Google Scholar] [CrossRef] [Green Version]
- den Boer, F.C.; Wippermann, B.W.; Blokhuis, T.J.; Patka, P.; Bakker, F.C.; Haarman, H.J. Healing of segmental bone defects with granular porous hydroxyapatite augmented with recombinant human osteogenic protein-1 or autologous bone marrow. J. Orthop. Res. 2003, 21, 521–528. [Google Scholar] [CrossRef]
- Smith, J.O.; Tayton, E.R.; Khan, F.; Aarvold, A.; Cook, R.B.; Goodship, A.; Bradley, M.; Oreffo, R.O. Large animal in vivo evaluation of a binary blend polymer scaffold for skeletal tissue-engineering strategies; translational issues. J. Tissue Eng. Regen. Med. 2017, 11, 1065–1076. [Google Scholar] [CrossRef] [PubMed]
- Christou, C.; Oliver, R.A.; Pelletier, M.H.; Walsh, W.R. Ovine model for critical-size tibial segmental defects. Comp. Med. 2014, 64, 377–385. [Google Scholar] [PubMed]
- Mills, L.A.; Simpson, A.H. In vivo models of bone repair. J. Bone Joint Surg. Br. 2012, 94, 865–874. [Google Scholar] [CrossRef] [PubMed]
- Falavigna, A.; Righesso, O.; Volquind, D.; Teles, A.R. Anterior cervical interbody fusion with hydroxyapatite graft: Clinical and radiological analysis of graft breakage. Spine (Phila Pa 1976) 2009, 34, 2769–2774. [Google Scholar] [CrossRef] [PubMed]
- Pesenti, S.; Ghailane, S.; Varghese, J.J.; Ollivier, M.; Peltier, E.; Choufani, E.; Bollini, G.; Blondel, B.; Jouve, J.L. Bone substitutes in adolescent idiopathic scoliosis surgery using sublaminar bands: Is it useful? A case-control study. Int. Orthop. 2017, 41, 2083–2090. [Google Scholar] [CrossRef] [PubMed]
- Heimbach, B.; Grassie, K.; Shaw, M.T.; Olson, J.R.; Wei, M. Effect of hydroxyapatite concentration on high-modulus composite for biodegradable bone-fixation devices. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 1963–1971. [Google Scholar] [CrossRef]
- Currey, J.D. The relationship between the stiffness and the mineral content of bone. J. Biomech. 1969, 2, 477–480. [Google Scholar] [CrossRef]
- Currey, J.D. The mechanical consequences of variation in the mineral content of bone. J. Biomech. 1969, 2, 1–11. [Google Scholar] [CrossRef]
- Wang, W.; Yeung, K.W.K. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact. Mater. 2017, 2, 224–247. [Google Scholar] [CrossRef]
- Dell’Accio, F.; Vanlauwe, J.; Bellemans, J.; Neys, J.; De Bari, C.; Luyten, F.P. Expanded phenotypically stable chondrocytes persist in the repair tissue and contribute to cartilage matrix formation and structural integration in a goat model of autologous chondrocyte implantation. J. Orthop. Res. 2003, 21, 123–131. [Google Scholar] [CrossRef] [Green Version]
- Ye Li, Y.C., S. K.; Li, L.; Qin, L.; Wang, X.L.; Lai, Y.X. Bone defect animal models for testing efficacy of bone substitute biomaterials. J. Orthop. Transl. 2015, 3, 95–104. [Google Scholar]
- Jäger, M.; Bachmann, R.; Scharfstadt, A.; Krauspe, R. Ovine cord blood accommodates multipotent mesenchymal progenitor cells. In Vivo 2006, 20, 205–214. [Google Scholar]
- Herten, M.; Grassmann, J.P.; Sager, M.; Benga, L.; Fischer, J.C.; Jager, M.; Betsch, M.; Wild, M.; Hakimi, M.; Jungbluth, P. Bone marrow concentrate for autologous transplantation in minipigs. Characterization and osteogenic potential of mesenchymal stem cells. Vet. Comp. Orthop. Traumatol. 2013, 26, 34–41. [Google Scholar] [CrossRef]
- Jäger, M.; Krauspe, R. Antigen expression of cord blood derived stem cells under osteogenic stimulation in vitro. Cell Biol. Int. 2007, 31, 950–957. [Google Scholar] [CrossRef] [PubMed]
- Herten, M.; Rothamel, D.; Schwarz, F.; Friesen, K.; Koegler, G.; Becker, J. Surface- and nonsurface-dependent in vitro effects of bone substitutes on cell viability. Clin. Oral. Investig. 2009, 13, 149–155. [Google Scholar] [CrossRef]
- Herten, M.; Bisdas, T.; Knaack, D.; Becker, K.; Osada, N.; Torsello, G.B.; Idelevich, E.A. Rapid in vitro quantification of s. Aureus biofilms on vascular graft surfaces. Front. Microbiol. 2017, 8, 2333–2340. [Google Scholar] [CrossRef]
- Jäger, M.; Feser, T.; Denck, H.; Krauspe, R. Proliferation and osteogenic differentiation of mesenchymal stem cells cultured onto three different polymers in vitro. Ann. Biomed. Eng. 2005, 33, 1319–1332. [Google Scholar] [CrossRef]
- Jäger, M.; Herten, M.; Fochtmann, U.; Fischer, J.; Hernigou, P.; Zilkens, C.; Hendrich, C.; Krauspe, R. Bridging the gap: Bone marrow aspiration concentrate reduces autologous bone grafting in osseous defects. J. Orthop. Res. 2011, 29, 173–180. [Google Scholar] [CrossRef]
- Available online: https://www.geistlich.de/de/orthopaedie/products/orthoss/kundennutzen/ (accessed on 18 December 2018).
- Leteve, M.; Passuti, N. Current Concepts in Bone Graft Substitutes. New J. Glass Ceram. 2018, 8, 39–54. [Google Scholar] [CrossRef]
- Windhagen, H.; Witte, F.; Hurschler, C.; Maciejewski, O.; Linnenberg, D.; Thorey, F. Bone turnover during distraction osteogenesis in an experimental sheep model. Arch. Orthop. Trauma. Surg. 2002, 122, 279–282. [Google Scholar] [PubMed]
- Windhagen, H.; Kolbeck, S.; Bail, H.; Schmeling, A.; Raschke, M. Quantitative assessment of in vivo bone regeneration consolidation in distraction osteogenesis. J. Orthop. Res. 2000, 18, 912–919. [Google Scholar] [CrossRef] [PubMed]
- Windhagen, H.; Bail, H.; Schmeling, A.; Kolbeck, S.; Weiler, A.; Raschke, M. A new device to quantify regenerate torsional stiffness in distraction osteogenesis. J. Biomech. 1999, 32, 857–860. [Google Scholar] [CrossRef]
- Thorey, F.; Windhagen, H.; Linnenberg, D.; Nolle, O.; Maciejewski, O.; Spies, C. Assessment of bone healing during callus distraction by an automatic torsional stiffness metering system. Biomed. Tech. (Berl.) 2000, 45, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, F.; Ferrari, D.; Herten, M.; Mihatovic, I.; Wieland, M.; Sager, M.; Becker, J. Effects of surface hydrophilicity and microtopography on early stages of soft and hard tissue integration at non-submerged titanium implants: An immunohistochemical study in dogs. J. Periodontol. 2007, 78, 2171–2184. [Google Scholar] [CrossRef]
- Donath, K. The diagnostic value of the new method for the study of undecalcified bones and teeth with attached soft tissue (sage-schliff (sawing and grinding) technique). Pathol. Res. Pract. 1985, 179, 631–633. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |



| Group | Distance between Original Bone Fragments (mm) | Measurement Area (mm2) | Bone Area (mm2) | Bone Area Percentage (%) |
|---|---|---|---|---|
| Test | 22.7 ± 2.3 | 452.1 ± 55.4 | 262.5 ± 40.2 | 59.2 ± 13.0 |
| HA | 21.9 | 457.0 ± 08.8 | 222.0 ± 15.5 | 48.6 ± 2.9 |
| Control | 16.8 ± 0.8 | 298.2 ± 63.4 | 246.3 ± 57.0 | 82.5 ± 5.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herten, M.; Zilkens, C.; Thorey, F.; Tassemeier, T.; Lensing-Höhn, S.; Fischer, J.C.; Sager, M.; Krauspe, R.; Jäger, M. Biomechanical Stability and Osteogenesis in a Tibial Bone Defect Treated by Autologous Ovine Cord Blood Cells—A Pilot Study. Molecules 2019, 24, 295. https://doi.org/10.3390/molecules24020295
Herten M, Zilkens C, Thorey F, Tassemeier T, Lensing-Höhn S, Fischer JC, Sager M, Krauspe R, Jäger M. Biomechanical Stability and Osteogenesis in a Tibial Bone Defect Treated by Autologous Ovine Cord Blood Cells—A Pilot Study. Molecules. 2019; 24(2):295. https://doi.org/10.3390/molecules24020295
Chicago/Turabian StyleHerten, Monika, Christoph Zilkens, Fritz Thorey, Tjark Tassemeier, Sabine Lensing-Höhn, Johannes C. Fischer, Martin Sager, Rüdiger Krauspe, and Marcus Jäger. 2019. "Biomechanical Stability and Osteogenesis in a Tibial Bone Defect Treated by Autologous Ovine Cord Blood Cells—A Pilot Study" Molecules 24, no. 2: 295. https://doi.org/10.3390/molecules24020295
APA StyleHerten, M., Zilkens, C., Thorey, F., Tassemeier, T., Lensing-Höhn, S., Fischer, J. C., Sager, M., Krauspe, R., & Jäger, M. (2019). Biomechanical Stability and Osteogenesis in a Tibial Bone Defect Treated by Autologous Ovine Cord Blood Cells—A Pilot Study. Molecules, 24(2), 295. https://doi.org/10.3390/molecules24020295







