A Review on the Phytochemistry, Medicinal Properties and Pharmacological Activities of 15 Selected Myanmar Medicinal Plants
Abstract
:1. Introduction
2. Phytochemistry, Medicinal Properties and Ethnopharmacology of the Selected Myanmar Medicinal Plants
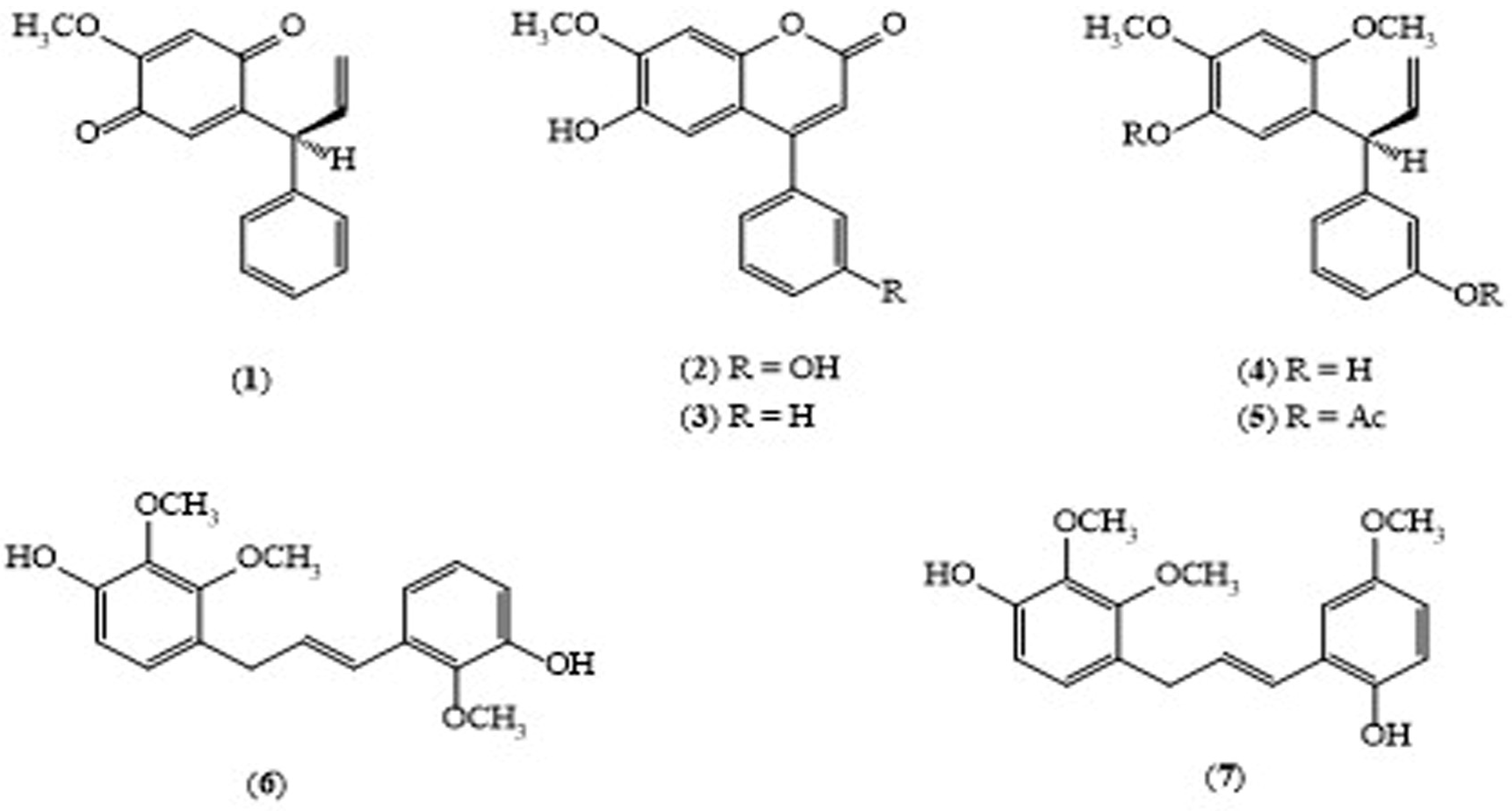
2.1. Dalbergia culrata Grah. (DC)
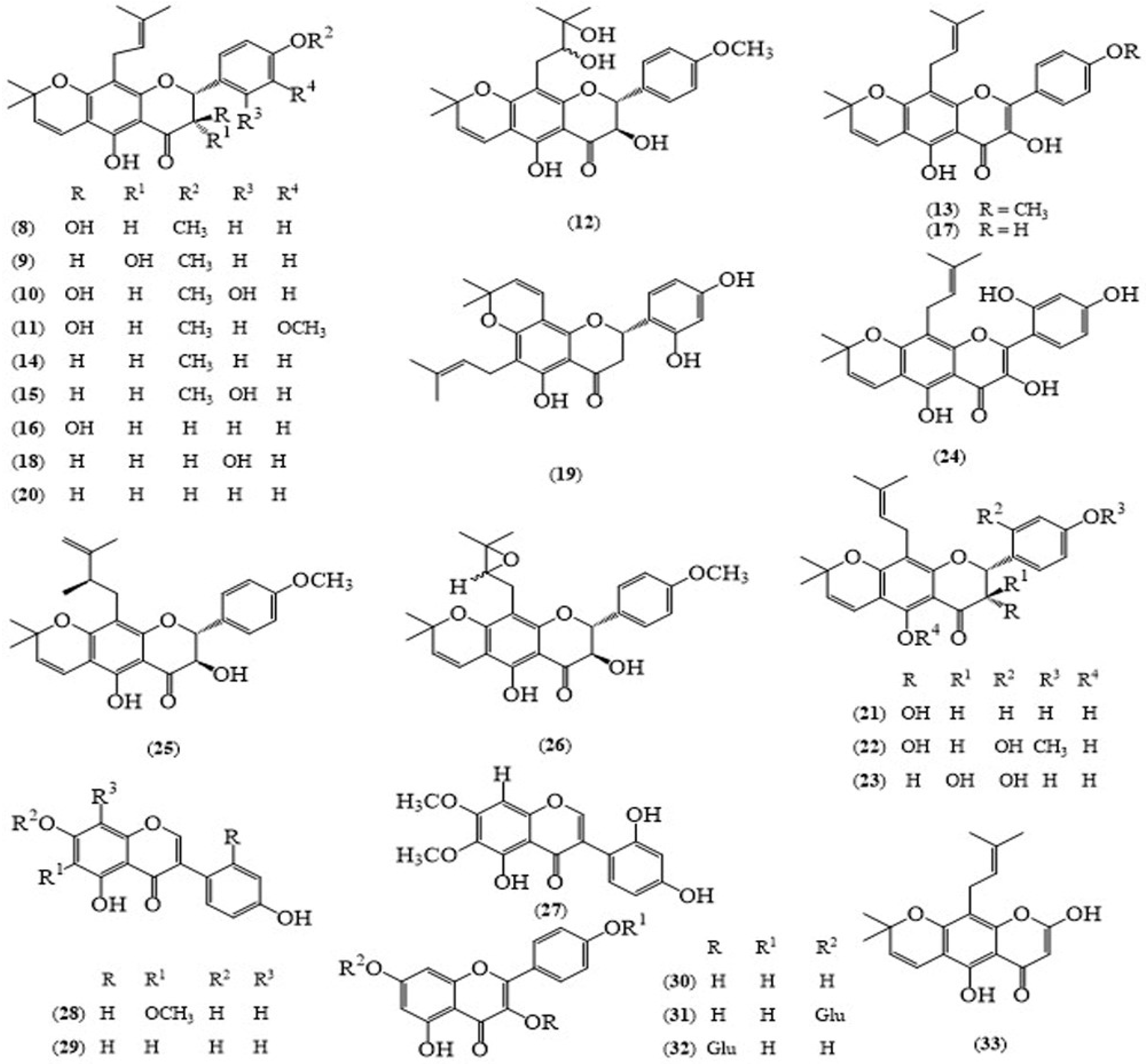
2.2. Eriosema chinense Vogel.
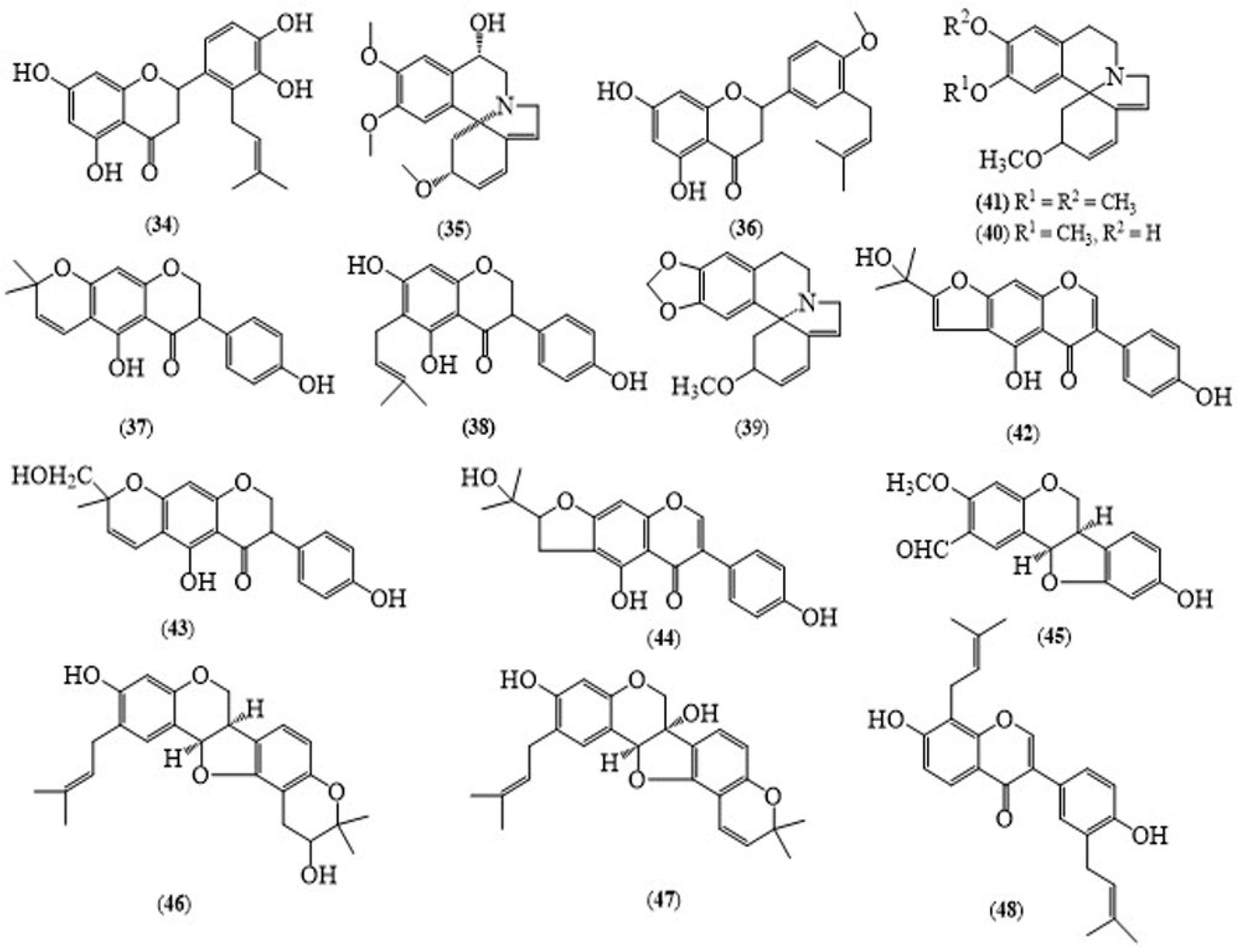
2.3. Erythrina suberosa Roxb.
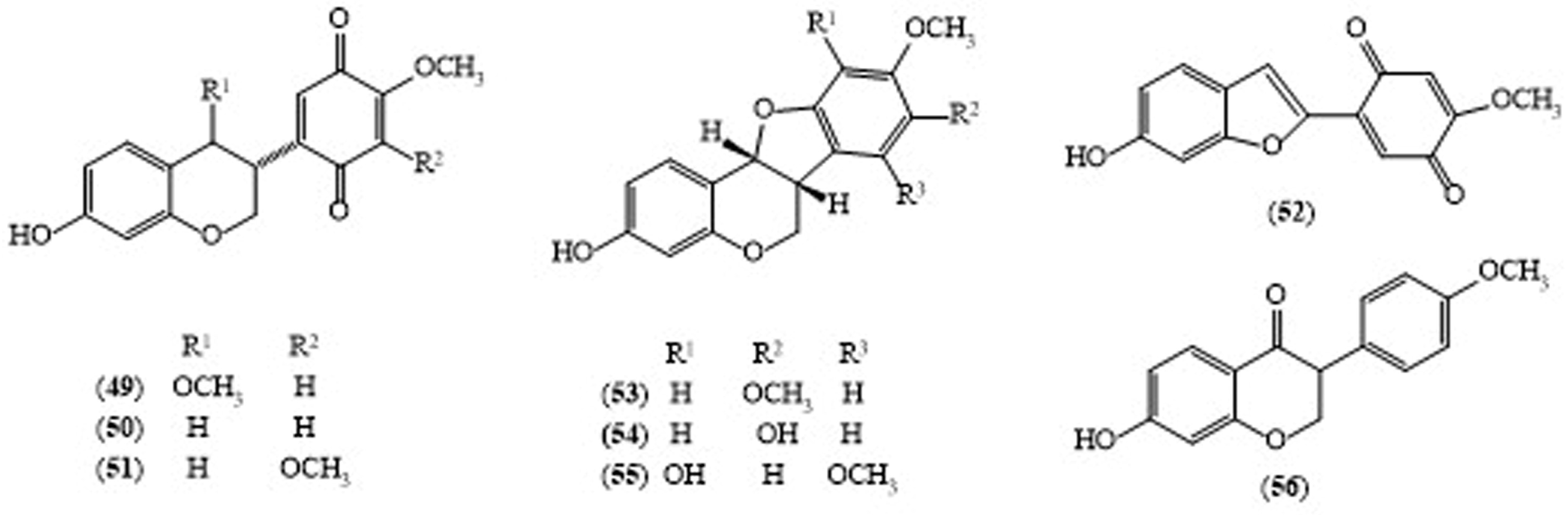
2.4. Millettia pendula BENTH.
2.5. Sesbania grandiflora (L.) Poir.
2.6. Tadehagi triquetrum (L.) H. Ohashi.
2.7. Andrographis echioides Nees.
2.8. Barleria cristata L.
2.9. Justicia gendarussa Burm F.
2.10. Premna integrifolia L.
2.11. Vitex trifolia L.
2.12. Acacia pennata (L.) Willd.
2.13. Cassia auriculata Linn.
2.14. Croton oblongifolius Roxb.
2.15. Glycosmis pentaphylla Correa
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fransworth, N.R.; Akerele, O.; Bingel, A.S.; Soejarto, D.D.; Guo, Z. Medicinal plants in therapy. Bull. WHO 1985, 63, 965–981. [Google Scholar] [CrossRef]
- Soe, K.; Ngwe, T.M. Medicinal Plants of Myanmar: Identification and Uses of Some 100 Commonly Used Species Series (1), 1st ed.; Shaung, T., Ed.; Forest Resource Environment Development and Conservation Association: Yangon, Myanmar, 2004; p. 255. [Google Scholar]
- Aung, H.T.; Sein, M.M.; Aye, M.M.; Thu, Z.M. A review of traditional medicinal plants from Kachin State, Northern Myanmar. Nat. Product Commun. 2016, 11, 353–364. [Google Scholar]
- Kress, W.J.; Defilipps, R.A.; Farr, E.; Kyi, Y.Y. A Checklist of the Trees, Shrubs, Herbs, and Climbers of Myanmar, Smithsonian Institution; The United States National Herbarium: Washington, DC, USA, 2003; Volume 45, pp. 1–590. [Google Scholar]
- Gamble, J.S. A Manual of Indian Timbers; Sampson Low Marston & Company: Marston, London, UK, 1922; p. 253. [Google Scholar]
- Takahashi, M.; Fuchino, H.; Satake, M.; Agatsuma, Y.; Sekita, S. In vitro screening of leishmanicidal activity in Myanmar timber extracts. Biol. Pharm. Bull. 2004, 27, 921–925. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Shilpi, J.A.; Mondal, H.; Hossain, F.; Anisuzzman, M.; Hasan, M.M.; Cordell, G.A. Ethnomedicinal, phytochemical, and pharmacological profile of the genus Dalbergia L. (Fabaceae). Phytopharmacology 2013, 4, 291–346. [Google Scholar]
- Eyton, W.B.; Ollis, W.D.; Sutherland, I.O.; Gottlieb, O.R.; Magalhaes, T.; Jackman, L.M. The neoflavonoid group of natural products-I: Dalbergions- a new class of quinones. Tetrahedron 1965, 21, 2683–2696. [Google Scholar] [CrossRef]
- Donnelly, D.M.X.; O’reilly, J.; Thompson, J. Neoflavonoids of Dalbergia cultrata. Phytochemistry 1972, 11, 823–826. [Google Scholar] [CrossRef]
- Ito, C.; Itoigawa, M.; Kanematsu, T.; Runagrungsi, N.; Higashihara, H.; Tokuda, H.; Nishino, H.; Furukawa, H. New cinnamylphenols from Dalbergia species with cancer chemopreventive activity. J. Nat. Prod. 2003, 66, 1574–1577. [Google Scholar] [CrossRef]
- Sutthiyaiyakit, S.; Thongnak, O.; Lhinhatrakool, T.; Yodchun, O.; Srimark, R.; Dowtaisong, P.; Chuankamnerdkarn, M. Cytotoxic and antimycobacterial prenylated flavonoids from the roots of Eriosema chinense. J. Nat. Prod. 2009, 72, 1092–1096. [Google Scholar] [CrossRef]
- Prasad, S.K.; Laloo, D.; Kumar, M.; Hemalatha, S. Antidiarrhoeal evaluation of root extract, its bioactive fraction, and lupinifolin isolated from Eriosema chinense. Planta Med. 2013, 79, 1620–1627. [Google Scholar] [CrossRef]
- Prasad, S.K.; Parmar, K.M.; Danta, C.C.; Laloo, D.; Hemalatha, S. Antidiarrhoeal activity of eriosematin E isolated from the roots of Eriosema chinense Vogel. Phytomedicine 2017, 24, 127–133. [Google Scholar] [CrossRef]
- Thongnest, S.; Lhinhatrakool, T.; Wetprasit, N.; Sutthivaiyakit, S. Eriosema chinense: A rich source of antimicrobial and antioxidant flavonoids. Phytochemistry 2013, 96, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Serrano, M.A.R.; Batista, A.N.-D.-L.; Bolzani, V.-D.-S.; Santos, L.-D.-A. Anxiolytic-like effects of erythrinian alkaloids from Erythrina suberosa. Quim. Nova. 2011, 34, 808–811. [Google Scholar]
- Chopra, R.N.; Nayar, S.L.; Chopra, I.C. Glossary of Indian Medicinal Plants; Council of scientific & Industrial Research: New Delhi, India, 1956. [Google Scholar]
- Gitte, T.A.; Kare, M.A.; Deshmukh, A.M. Ethno-medicinal studies on barks of some medicinal plants in Marathwada (M.S.), India-I. Recent Res. Sci. Technol. 2012, 4, 8–10. [Google Scholar]
- Chauha, P.; Saxena, V.K. A new prenylated flavone from Erythrina suberosa roots. Planta Med. 1987, 53, 221–222. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Pathania, A.S.; Saxena, A.K.; Vishwakarma, R.A.; Ali, A.; Bhushan, S. The anticancer potential of flavonoids isolated from the stem bark of Erythrina suberosa through induction of apoptosis and inhibition of STAT signalling pathway in human leukemia HL-60 cells. Chem.-Biol. Int. 2013, 205, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Chawla, A.S. Erythrina sp. 111: Chemical constituents of Erythrina suberosa Roxb. Seeds. J. Pharm. Sci. 1970, 59, 1179–1182. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Tanaka, T.; Etoh, H.; Watanabe, N.; Ahmad, M.; Qurashi, I.; Khand, M.R. Two new isoflavones from Erythrina suberosa var. glabrescences. Heterocycles 1998, 48, 2661–2666. [Google Scholar] [CrossRef]
- Tanaka, H.; Etoh, H.; Watanabae, N.; Shimizu, H.; Ahmad, M.; Rizwani, G.H. Erysubins, C-F, four isoflavonoids from Erythrina suberosa var. glabrescencens. Phytochemistry 2001, 56, 769–773. [Google Scholar] [CrossRef]
- Dhar, M.L.; Dhar, M.M.; Dhawan, B.N.; Mehrotra, B.N.; Ray, C. Screening of Indian plants for biological activity: Part-I. Indian J. Exp. Biol. 1968, 6, 232–247. [Google Scholar] [PubMed]
- Agrawal, S.K.; Agrawal, M.; Sharma, P.R.; Gupta, B.D.; Arora, S.; Saxena, A.K. Induction of Apoptosis in Human Promyekicytic Leukemia HL60 cells by an extract from Erythrina suberosa stem bark. Nutr. Cancer 2011, 63, 802–813. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Fuchino, H.; Sekita, S.; Satake, M.; Kiuchi, F. In vitro leishmanicidal constituents of Millettia pendula. Chem. Pharm. Bull. 2006, 54, 915–917. [Google Scholar] [CrossRef]
- Hayashi, Y.; Shirato, T.; Sakurai, K.; Takahashi, T. Isoflavonoids from the heartwood of Millettia pendula. Mojuzaki Gakkaishi 1978, 24, 898–901. [Google Scholar]
- Pari, L.; Uma, A. Protective effect of Sesbania grandiflora against erythromycin estolate induced hepatotoxicity. Therapie 2003, 58, 439–443. [Google Scholar] [CrossRef]
- Munde-Wagh, K.B.; Wagh, V.D.; Toshniwal, S.S.; Sonawane, B.R. Phytochemical, antimicrobial evaluation and determination of total phenolic and flavonoid contents of Sesbania grandiflora flower extract. Int. J. Pharm. Sci. 2012, 4, 229–232. [Google Scholar]
- Hussain, A.Z.; Kumaresan, S. GC-MS studies and phytochemical screening of Sesbania grandiflora L. J. Chem. Pharm. Res. 2014, 6, 43–47. [Google Scholar]
- Husan, N.; Osman, H.; Mohamad, S.; Chong, W.K.; Awang, K.; Zahariluddin, A.S.M. The chemical components of Sesbania grandiflora root and their antituberculosis activity. Pharmaceuticals 2012, 5, 882–889. [Google Scholar] [CrossRef]
- Mustafa, R.A.; Hamid, A.A.; Mohamed, S.; Bakar, F.A. Total phenolic compounds, flavonoids, and radical scavenging activity of 21 selected tropical plants. J. Food Sci. 2010, 75, C28–C35. [Google Scholar] [CrossRef]
- Laladhas, K.P.; Cheriyan, V.T.; Puliappadamba, V.T.; Bava, S.V.; Unnithan, R.G.; Vijayammal, P.L.; Anto, R.J. A novel protein fraction from Sesbania grandiflora shows potential anticancer and chemopreventive efficacy, in vitro and in vivo. J. Cell. Mol. Med. 2010, 14, 636–646. [Google Scholar]
- Ramesh, T.; Mahesh, R.; Begum, V.H. Effect of Sesbania grandiflora on lung antioxidant defense system in cigarette smoke exposed rats. Int. J. Biol. Chem. 2007, 1, 141–148. [Google Scholar]
- Ramesh, T.; Sureka, C.; Bhuvana, S.; Begum, V.H. Sesbania grandiflora diminishes oxidative stress and ameliorates antioxidant capacity in liver and kidney or rats exposed to cigarette smoke. J. Physiol. Pharmacol. 2010, 61, 467–476. [Google Scholar]
- Doddola, S.; Pasupulati, H.; Koganti, B.; Prasad, K.V. Evaluation of Sesbania grandiflora for antiurolithiatic and antioxidant properties. J. Nat. Med. 2008, 62, 300–307. [Google Scholar] [CrossRef]
- Sreelatha, S.; Padma, P.R.; Umasankari, E. Evaluation of anticancer activity of ethanol extract of Sesbania grandiflora (Agati Sesban) against Ehrlich ascited carcinoma in Swiss albino mice. J. Ethnopharmacol. 2011, 134, 984–987. [Google Scholar] [CrossRef]
- Kumar, N.S.; Dhanyaraj, F.S. Phytochemical analysis and antimicrobial activities of Sesbania grandiflora (L) leaf extracts. Int. J. Pharm. Sci. Rev. Res. 2016, 36, 144–148. [Google Scholar]
- Kasture, V.S.; Deshmukh, V.K.; Chopde, C.T. Anxiolytic and anticonvulsive activity of Sesbania grandiflora leaves in experimental animals. Phytother. Res. 2002, 16, 455–460. [Google Scholar] [CrossRef]
- Kumar, R.; Janadri, S.; Kumar, S.; Dhanajaya, D.R.; Swamy, S. Evaluation of antidiabetic activity of alcoholic extract of Sesbania grandiflora flower in alloxan induced diabetic rats. Asian J. Pharm. Pharmacol. 2015, 1, 21–26. [Google Scholar]
- Kale, I.; Khan, M.A.; Irfan, Y.; Goud, A.V. Hepatoprotective potential of ethanolic and aqueous extract of flowers of Sesbania grandiflora (Linn) induced by CCl4. Asian Pac. J. Trop. Biomed. 2012, 2, S670–S679. [Google Scholar] [CrossRef]
- Das, J.; Das, M.P.; Velusamy, P. Sesbania grandiflora leaf extract mediated green synthesis of antibacterial silver nanoparticles against selected human pathogens. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 104, 265–270. [Google Scholar] [CrossRef]
- Ajitha, B.; Reddy, Y.A.K.; Rajesh, K.M.; Reddy, P.S. Sesbania grandiflora leaf extract assisted green synthesis of silver nanoparticles: Antimicrobial activity. Mater. Today Proc. 2013, 3, 1977–1984. [Google Scholar] [CrossRef]
- Xiang, W.; Li, R.-T.; Mao, Y.-L.; Zhang, H.-J.; Li, S.-H.; Song, Q.-S.; Sun, H.-D. Four new prenylated isoflanonoids in Tagehagi triquetrum. J. Agric. Food. Chem. 2005, 53, 267–271. [Google Scholar] [CrossRef]
- Zhang, R.-T.; Cheng, G.-G.; Feng, T.; Cai, X.-H.; Luo, X.-D. Four new isoflavanones from Tadehagi triquetrum. Nat. Prod. Bioprospect. 2011, 1, 121–123. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, C.Y.; Zhang, T.; Zhao, D.; An, N.; Li, Y.; Zhu, N.; Wang, S.; Chen, F.; Zhang, X. Four new prenylated isoflavonoids in Tadehagi triquetrum. Nat. Prod. Res. 2015, 29, 1–5. [Google Scholar]
- Zhang, X.; Chen, C.; Li, Y.; Chen, D.; Dong, L.; Na, W.; Wu, C.; Zhang, J.; Li, Y. Tadehaginosides, A-J, phenylpropanoid glucosides from Tadehagi triquetrum, enhance glucose uptake via the upregulation of PPARy and GLUT-4 in C2C12 myotubes. J. Nat. Prod. 2016, 79, 1249–1258. [Google Scholar] [CrossRef]
- Lwin, H.S.; Tu, M. Effect of Desmodium triquetrum extract on some pathogenic bacteria. Union Burma J. Life Sci. 1968, 1, 66–70. [Google Scholar]
- Moe, S.; Naing, K.S.; Htay, M.N.N. Herbal medicines used by tuberculosis patients in Myanmar. Euro. J. Med. Plants 2018, 22, 1–10. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Feng, B.M.; Tang, L.; Shi, L.Y.; Shi, H.F. Research of Tadehagi triquetrum (L.) DC. J. Dalian Univ. 2007, 28, 49–51. [Google Scholar]
- Zhou, X.D.; Shi, L.Y.; Yu, D.Y.; Shi, H.B.; Miao, Y.B.; Tang, L.; Feng, B.M.; Wang, Y.Q. Effect of active part extracted from Tadehagi triquetrum (Linn.) Ohashi on type I allergy induced by immunoglobulin E. Central South. Pharm. 2011, 9, 35–38. [Google Scholar]
- Tang, A.; Chen, X.; Lu, Q.; Zheng, N.; Wei, Y.; Wu, X. Antihepatotoxic effect of tadehaginoside, extracted from Tadehagi triquetrum (L.), against CCl4-lesioned rats through activating the Nrf2 signaling pathway and attenuating the inflammatory response. Inflammation 2014, 37, 1006–1014. [Google Scholar] [CrossRef]
- Gamble, J.S. Flora of the Presidency of Madras; Botanical Survey of India: Calcutta, India, 1956; Volume 2, p. 1051. [Google Scholar]
- Kirtikar, K.R.; Basu, B.D. Indian Medicinal Plants; Bishen Singh Mahendra Pal Singh: New Delhi, India, 1975; Volume 3, pp. 1884–1886. [Google Scholar]
- Kanchana, G.; Nirubama, K.; Rubalakshmi, G. Phytochemical screening and antimicrobial activity of Andrographis echioides (L.) Nees-an indigenous medicinal plant. World J. Pharm. Pharm. Sci. 2014, 3, 702–710. [Google Scholar]
- Govindachar, T.R.; Parthasarathy, P.C.; Pai, B.R.; Subramania, P.S. Chemical examination of Andrographis echioides-I: Structure and synthesis of echioidinin. Tetrahedron 1965, 21, 2633–2640. [Google Scholar] [CrossRef]
- Govindachar, T.R.; Parthasarathy, P.C.; Pai, B.R.; Subramania, P.S. Chemical examination of Andrographis echioides-II: Structure and synthesis of echioidin. Tetrahedron 1965, 21, 3715–3720. [Google Scholar] [CrossRef]
- Jayaprakasam, B.; Damu, A.G.; Gunasekar, D.; Blond, A.; Bodo, B. Dihydroechioidinin, a flavanone from Andrographis echioides. Phytochemistry 1999, 52, 935–937. [Google Scholar] [CrossRef]
- Shen, D.-Y.; Juang, S.-H.; Kuo, P.-C.; Huang, G.-J.; Chan, Y.-Y.; Damu, A.G.; Wu, T.-S. Chemical constituents from Andrographis echioides and their anti-inflammatory activity. Int. J. Mol. Sci. 2013, 14, 496–514. [Google Scholar] [CrossRef]
- Jayaprakasam, B.; Gunasekar, D.; Rao, K.V.; Blond, A.; Bodo, B. Androechin, a new chalcone glucoside from Andrographis echioides. J. Asian Nat. Prod. Res. 2001, 3, 43–48. [Google Scholar] [CrossRef]
- Mathivanan, D.; Suseem, S.R. Identified bioactive constituents on leaf of Andrographis echioides grown on Vellore District, Tamil Nadu. Der Pharm. Lett. 2015, 7, 138–147. [Google Scholar]
- Basu, S.K.; Rupeshkumar, M.; Kavitha, K. Hepatoprotective and antioxidant effect of Andrographis echioides N. against acetaminophen induced hepatotoxicity in rats. J. Biol. Sci. 2009, 9, 351–356. [Google Scholar]
- Basu, S.K.; Rupeshkumar, M.; Kavitha, K. Studies on the anti-inflammatory, analgestic and antipyretic properties of Andrographic echioides Nees. Int. J. Pharmacol. 2009, 5, 251–256. [Google Scholar]
- Murthy, J.R.; Rajaraman, M.; Meera, R. Phytochemical constituents and diuretic activity of leaf extracts of Androgarphis echioides-l-Nees. Int. J. Pharm. Chem. Sci. 2012, 1, 1659–1665. [Google Scholar]
- Raja, R.R.; Jeevanreddy, K. Pharmacognostical phytochemical and anti-ulcer activity of Andrographis echioides (Acanthaceae). J. Pharm. Phytochem. 2014, 3, 39–49. [Google Scholar]
- Punnagai, K.; Chellathai, D.D.; Karthik, V.P.; Josephine, I.G. Evaluation of antifungal activity of ethanolic extract of Andrographis echioides—An in vitro study. Int. J. Pharm. Bio. Sci. 2016, 7, 6–10. [Google Scholar]
- Rajkumar, S.; Jebanesan, A.; Nagarajan, R. Synergistic effect of Andrographis echioides and Cadaba trifoliata leaf extracts against larvae of dengue mosquito Aedes aegypti L. Asian Pac. J. Trop. Biomed. 2012, 2, S1588–S1591. [Google Scholar] [CrossRef]
- Punnagai, K.; Chellathai, D.; Latharani, M.; Radhika, R. Evaluation of antibacterial activity of ethanolic extract of Andrographic echioides—An in vitro study. Int. J. Phytopharmacol. 2016, 7, 214–217. [Google Scholar]
- Mathivanan, D.; Suseem, S.R. Chemical and biological evaluation of Andrographis echioides leaf extracts collected from the Vellore district in Tamil Nadu, India. Pac. Sci. Rev. A Nat. Sci. Eng. 2016, 18, 138–144. [Google Scholar] [CrossRef]
- Gambhire, M.N.; Wnakhede, S.S.; Juvekar, A.R. Antiinflammatory activity of aqueous extract of Barleria cristata leaves. J. Young Pharm. 2009, 1, 220–224. [Google Scholar]
- Khare, C.P. Indian Medicinal Plants: An Illustrated Dictionary, 1st ed.; Springer: Berlin, Germany, 2009. [Google Scholar]
- Narmadha, R.; Devaki, K. Barleria cristata Linn.: Phytochemical screening and HPTLC analysis. Int. Res. J. Pharm. 2012, 3, 301–307. [Google Scholar]
- Abd El-Mawla, A.M.A.; Ahmed, A.S.; Ibraheim, Z.Z.; Ernst, L. Phenylethanoid glycosides from Barleria cristata L. Callus cultures. Bull. Pharm. Sci. Assiut. Univ. 2005, 28, 199–204. [Google Scholar]
- Hemalatha, K.; Hareeka, N.; Sunitha, D. Chemical constituents isolated from leaves of Barleria cristata Linn. Int. J. Pharm. Bio. Sci. 2012, 3, 609–615. [Google Scholar]
- Chowdhury, N.; Hasan, A.A.; Tareq, F.S.; Ahsan, M.; Zafrul Azam, A.T.M. 4-Hydroxy-trans-cinnamate Derivatives and Triterpene from Barleria cristata. Dhaka Univ. J. Pharm. Sci. 2013, 12, 143–145. [Google Scholar] [CrossRef]
- Gambhire, M.; Juvekar, A.; Wankhede, S. Evaluation of anti-inflammatory activity of methanol extract of Barleria cristata leaves by in vivo and in vitro methods. Int. J. Pharmcol. 2008, 7, 1–4. [Google Scholar] [CrossRef]
- Amutha, K.; Victor Arokia, D. In vitro antioxidant activity of ethanolic extract of Barleria cristata L. leaves. Res. J. Pharm. Phytochem. 2009, 1, 209–212. [Google Scholar]
- Charoenchai, P.; Vajrodava, S.; Somprasong, W.; Mahidol, C.; Ruchirawat, S.; Kittakoop, P. Part 1: Antiplasmodial, cytotoxic, radical scavenging and antioxidant activities of Thai plants in the family Acanthaceae. Planta Med. 2010, 76, 1940–1943. [Google Scholar] [CrossRef]
- Sastri, B.N. Wealth of India: Raw Materials; Council of Scientific and Industrial Research: New Delhi, India, 1959; Volume 5, pp. 312–313. [Google Scholar]
- Kirtikar, K.R.; Basu, B.D. Indian Medicinal Plants, 2nd ed.; International Book Publisher: Dehradun, India, 1994; Volume 3, pp. 1896–1898. [Google Scholar]
- Zhang, H.-J.; Rumschlag-Booms, E.; Guan, Y.-F.; Liu, K.-L.; Wang, D.-Y.; Li, W.-F.; Nguyen, V.H.; Cuong, N.M.; Soejarto, D.D.; Fong, H.H.S.; et al. Anti-HIV diphyllin glycosides from Justicia gendarussa. Phytochemistry 2017, 136, 94–100. [Google Scholar] [CrossRef]
- Souza, L.G.S.; Almeida, M.C.S.; Lemos, T.L.G.; Ribeiro, P.R.V.; Canuto, K.M.; Braz-Filho, R.; Cistia, C.N.D.; Sant’Anna, C.M.R.; Barreto, F.S.; Moraes, M.O.D. Brazoides AD new alkaloids from Justicia gendarussa Burm. F. species. J. Braz. Chem. Soc. 2017, 28, 1281–1287. [Google Scholar]
- Zhang, H.-J.; Rumschlag-Booms, E.; Guan, Y.-F.; Wang, D.-Y.; Liu, K.-L.; Li, W.-F.; Nguyen, V.H.; Cuong, N.M.; Soejarto, D.D.; Fong, H.H.S.; Rong, L. Potent inhibitor of drug-resistant HIV-1 strains identified from the medicinal plant Justicia gendarussa. J. Nat. Prod. 2017, 80, 1798–1807. [Google Scholar] [CrossRef]
- Krishna, K.L.; Mruthunjaya, K.; Patel, J.A. Antioxidant and hepatoprotective potential of stem methanolic extract of Justicia gendarussa Burm. Int. J. Pharmacol. 2010, 6, 72–80. [Google Scholar] [CrossRef]
- Bhagya, N.; Chandrashekar, K.R. Evaluation of plant and callus extracts of Justicia gendarussa Burm. F. for phytochemicals and antioxidant activity. Int J. Pharm. Pharm. Sci. 2013, 5, 82–85. [Google Scholar]
- Jothimanivannan, C.; Kumar, R.S.; Subramanian, N. Anti-inflammatory and analgestic activities of ethanol extract of aerial parts of Justicia gendarussa Burm. Int. J. Pharmacol. 2010, 6, 278–283. [Google Scholar]
- Periyanayagam, K.; Umamaheswari, B.; Suseela, L.; Padmini, M.; Ismail, M. Evaluation of antiangiogenic effect of the leaves of Justicia gendarussa (Burm. f) (Acanthaceae) by Chrio Allontoic Membrane Method. Am. J. Infect. Dis. 2009, 5, 180–182. [Google Scholar] [CrossRef]
- Subramanian, N.; Jothimanivannan, C.; Kumar, R.S.; Kameshwaran, S. Evaluation of anti-anxiety activity of Justicia gendarussa Burm. Pharmacologia 2013, 4, 405–407. [Google Scholar]
- Paval, J.; Kaitheri, S.K.; Potu, B.K.; Govindan, S.; Kumar, R.S.; Narayanan, S.N.; Moorkoth, S. Anti-arthritic potential of the plant Justicia gendarussa Burm. F. Clinics 2009, 64, 357–360. [Google Scholar] [CrossRef]
- Ayob, Z.; Samad, A.A.; Bohari, S.P.M. Cytotoxicity activities in local Justicia gendarussa crude extracts against human cancer cell lines. J. Teknol. Sci. Eng. 2013, 64, 45–52. [Google Scholar] [CrossRef]
- Ayob, Z.; Bohari, S.P.M.; Samad, A.A.; Jamil, S. Cytotoxic activities against breast cancer cells of local Justicia gendarussa crude extract. Evid.-Based Complement. Altern. Med. 2014, 2014, 1–12. [Google Scholar] [CrossRef]
- Sivasakthi, A.; Vijayalakshmi, M. Antibacterial activities of phytochemical extracts from the leaves of Justicia gendarussa Burm. F. Int. J. Pharm. Biol. Sci. 2014, 5, 433–438. [Google Scholar]
- Sharma, K.K.; Saikia, R.; Kotoky, J.; Kalita, J.C.; Devi, R. Antifungal activity of Solanum melogena L., Lawsonia inermis L. and Justicia gendarussa B. against Dermatophytes. Int. J. Pharm. Tech. Res. 2011, 3, 1636–1640. [Google Scholar]
- Saha, M.R.; Debnath, P.C.; Rahman, A.; Islam, A.U. Evaluation of in vitro anthelmintic activities of leaf and stem extracts of Justicia gendarussa. Bangladesh J. Pharmacol. 2012, 7, 50–53. [Google Scholar] [CrossRef]
- Kumar, K.S.; Vijayan, V.; Bhaskar, S.; Krishnan, K.; Shalini, V.; Helen, A. Anti-inflammatory potential of an ethyl acetate isolated from Justicia gendarussa roots through inhibition of iNOS and COX-2 expression via NF-jB pathway. Cell. Immunol. 2012, 272, 283–289. [Google Scholar] [CrossRef]
- Loi, D.T. The Glossary of Vietnamese Medicinal Plants and Items; Hanoi Medicine Publishing House: Hanoi, Vietnam, 2000; pp. 209–210. [Google Scholar]
- Gokhani, R.H.; Lahiri, S.K.; Santani, D.D.; Shah, M.B. Evaluation of immunomodulatory activity of Clerodendrum phlomidis and Premna integrifolia root. Int. J. Pharmacol. 2005, 3, 352–356. [Google Scholar]
- Basu, N.K.; Dandiya, P.C. Chemical Investigation of Premna integrifolia Linn. J. Amer. Pharm. Assoc. 1947, 36, 389–391. [Google Scholar] [CrossRef]
- Vadivu, R.; Suresh, J.A.; Girinath, K.; Kannan, B.P.; Vimala, R.; Kumar, N.M.S. Evaluation of Hepatoprotective and in-vitro cytotoxic activity of leaves of P. serratifolia Linn. J. Sci. Res. 2009, 1, 145–152. [Google Scholar] [CrossRef]
- Hang, N.T.B.; Ky, P.T.; Minh, C.V.; Cuong, N.X.; Thao, N.P.; Kiem, P.V. Study on the chemical constituents of Premna integrifolia L. Nat. Prod. Commun. 2008, 3, 1449–1452. [Google Scholar]
- Yadav, D.; Tiwari, N.; Gupta, M.M. Diterpenoids from Premna integrifolia. Phytochem. Lett. 2010, 3, 143–147. [Google Scholar] [CrossRef]
- Yadav, D.; Gupta, M.M. Isolation and HPTLC analysis of Iridoids in Premna integrifolia, an important ingredient of Ayurvedic drug dashmool. J. Planar Chromatogr. 2013, 26, 260–266. [Google Scholar] [CrossRef]
- Yadav, D.; Masood, N.; Luqman, S.; Brindha, P.; Gupta, M.M. Antioxidant furofuran lignans from Premna integrifolia. Ind. Crops Prod. 2013, 41, 397–402. [Google Scholar] [CrossRef]
- Rahman, A.; Shanta, Z.S.; Rashid, M.A.; Parvin, T.; Afrin, S.; Khatun, M.K.; Sattar, M.A. In vitro antibacterial properties of essential oil and organic extracts of Premna integrifolia Linn. Arabian J. Chem. 2011, 9, S475–S479. [Google Scholar] [CrossRef]
- Majumder, R.; Akter, S.; Naim, Z.; Al-Amin, M.; Alam, M.B. Antioxidant and anti-diabetic activities of the methanolic extract of Premna integrifolia bark. Advan. Biol. Res. 2014, 8, 29–36. [Google Scholar]
- Khatun, H.; Majumder, R.; Mamun, A.; Alam, E.K.; Jami, S.I.; Alam, B. Preliminary pharmacological activity of the methanolic extract of Premna integrifolia barks in rats. Avicenna J. Phytomed. 2014, 4, 215–224. [Google Scholar]
- Kimura, T.; Kimura, T. Medicinal Plants of Japan in Color; Hoikusha Publishing: Osaka, Japan, 1981; p. 183. [Google Scholar]
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China; Beijing Chemical Industry Press: Beijing, China, 2010; Volume 1, pp. 340–341. [Google Scholar]
- Ono, M.; Sawamura, H.; Ito, Y.; Mizuki, K.; Nohara, T. Diterpenoids from the fruits of Vitex trifolia. Phytochemistry 2000, 55, 873–877. [Google Scholar] [CrossRef]
- Ono, M.; Ito, Y.; Nohara, T. Four new halimane-type diterpenes, Vitetrifolins D-G from the fruit of Vitex trifolia. Chem. Pharm. Bull. 2001, 49, 1220–1222. [Google Scholar] [CrossRef]
- Li, W.-X.; Cui, C.-B.; Cai, B.; Yao, X.-S. Labdane-type diterpenes as new cell cycle inhibitors and apoptosis inducers from Vitex trifolia L. J. Asian Nat. Prod. Res. 2005, 7, 95–105. [Google Scholar] [CrossRef]
- Li, W.-X.; Cui, C.-B.; Cai, B.; Wang, H.-Y.; Yao, X.-S. Flavonoids from Vitex trifolia L. inhibit cell cycle progression at G2/M phase and induce apoptosis in mammalian cancer cells. J. Asian Nat. Prod. Res. 2005, 7, 615–626. [Google Scholar] [CrossRef]
- Zheng, C.-J.; Zhu, J.-Y.; Yu, W.; Ma, X.-Q.; Rahman, K.; Qin, L.-P. Labdane-type diterpenoids from the fruits of Vitex trifolia. J. Nat. Prod. 2013, 76, 287–291. [Google Scholar] [CrossRef]
- Jangwan, J.S.; Aquino, R.P.; Mencherini, T.; Picerno, P.; Singh, R. Chemical constituents of ethanol extract and free radical scavenging activity of Vitex trifolia Linn. Acta Chim. Pharm. Indica. 2015, 5, 1–7. [Google Scholar]
- Tiwari, N.; Luqman, S.; Masood, N.; Gupta, M.M. Validated high performance thin layer chromatographic method for simultaneous quantification of major iridoids in Vitex trifolia and their antioxidant studies. J. Pharm. Biomed. Anal. 2012, 61, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Kannathasan, K.; Senthilkumar, A.; Venkatesalu, V. Mosquito larvicidal activity of methyl-p-hydroxybenzoate isolated from the leaves of Vitex trifolia Linn. Acta Trop. 2011, 120, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Tandon, S.; Mittal, A.K.; Pant, A.K. Insect growth regulatory activity of Vitex trifolia and Vitex agnus-castus essential oils against Spilosoma obliqua. Fitoterapia 2008, 79, 283–286. [Google Scholar] [CrossRef] [PubMed]
- Matsui, M.; Kumar-Roine, S.; Darius, H.T.; Chinain, M.; Laurent, D.; Pauillac, S. Characterisation of the anti-inflammatory potential of Vitex trifolia L. (Labiatae), a multipurpose plant of the Pacific traditional medicine. J. Ethnopharmacol. 2009, 126, 427–433. [Google Scholar] [CrossRef]
- Matsui, M.; Conquy, M.A.; Coste, A.; Kumar-Roine, S.; Pipy, B.; Laurent, D.; Pauillac, S. Aqueous extract of Vitex trifolia L. (Labiatae) inhibits LPS-dependent regulation of inflammatory mediators in RAW 264.7 macrophages through inhibition of Nuclear Factor kappa B translocation and expression. J. Ethnopharmacol. 2012, 143, 24–32. [Google Scholar] [CrossRef]
- Hernandez, M.M.; Heraso, C.; Villarreal, M.L.; Vargas-Arispuro, I. Biological activities of crude plant extracts from Vitex trifolia L. (Verbenaceae). J. Ethnopharmacol. 1999, 67, 37–44. [Google Scholar] [CrossRef]
- Lalchhandama, K. Efficacy and structural effects of Acacia pennata root bark upon the avian parasitic helminth, Raillietina echinobothrida. Pharm. J. 2013, 5, 17–21. [Google Scholar] [CrossRef]
- Khare, C.P. Indian Medicinal Plants: An Illustrated Dictionary; Springer: New York, NY, USA, 2008; Volume 8. [Google Scholar]
- Rifai, Y.A.; Arai, M.; Kovano, T.; Kowithayakom, T.; Ishibashi, M. Terpenoids and a Flavonoid Glycoside from Acacia pennata Leaves as Hedgehog/GLI-Mediated Transcriptional Inhibitors. J. Nat. Prod. 2010, 73, 995–997. [Google Scholar] [CrossRef]
- Dongmo, A.B.; Myyamoto, T.; Yoshikawa, K.; Arihara, S.; Lacaille-Dubois, M.A. Flavonoids from Acacia pennata and their cyclooxygenase (COX-1 and COX-2) inhibitory activities. Planta Med. 2007, 73, 1202–1207. [Google Scholar] [CrossRef]
- Kim, A.; Choi, I.; Htwe, K.M.; Chin, Y.-W.; Kim, I.; Yoon, K.D. Flavonoid glycosides from the aerial parts of Acacia pennata in Myanmar. Phytochemistry 2015, 118, 17–22. [Google Scholar] [CrossRef]
- Dongmo, A.B.; Nguelefack, T.; Lacaille-Dubois, M.A. Antinociceptive and anti-inflammatory activities of Acacia pennata wild (Mimosaceae). J. Ethnophamacol. 2005, 98, 201–206. [Google Scholar] [CrossRef]
- Sowndhararajan, K.; Joseph, J.M.; Manian, S. Antioxidant and free radical scavenging activities of indan Acacias: Acacia leucophloea (Roxb.) Willd., Acacia ferruginea DC., Acacia dealbata Links., and Acacia pennata (L.) Willd. Int. J. Food Prop. 2013, 16, 1717–1729. [Google Scholar] [CrossRef]
- Annie, S.; Rajagopal, P.L.; Malini, S. Effect of Cassia auriculata Linn. Root extract on cisplatin and gentamicin-induced renal injury. Phytomedicine 2005, 12, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, K.N.; Patel, M.B.; Valera, H.R.; Patil, S.D.; Surana, S.J. Hepatoprotective activity of flowers of Cassia curiculata R. Br against paracetamol induced liver injury. J. Nat. Remed. 2009, 9, 85–90. [Google Scholar]
- Raj, J.Y.; John Peter, M.P.; Joy, V. Chemical compounds investigation of Cassia auriculata seeds: A potential folklore medicinal plant. Asian J. Plant. Sci. Res. 2012, 2, 187–192. [Google Scholar]
- Balakrishna, R.B.; Patel, J.R.; Prabhakaran, V. Cassia auriculata- A phytopharmacological review. J. Adv. Drug Res. 2011, 1, 46–57. [Google Scholar]
- Nakamura, S.; Xu, F.; Ninomiya, K.; Nakashima, S.; Oda, Y.; Morikawa, T.; Muraoka, O.; Yoshikawa, M.; Matsuda, H. Chemical Structures and hepatoprotective effects of constituents from Cassia auriculata leaves. Chem. Pharm. Bull. 2014, 62, 1026–1031. [Google Scholar] [CrossRef]
- Vijayaraj, P.S.; Muthukumar, K.; Sabarirajan, J.; Nachiappan, V. Evaluation of antihyperlipidemic activity of ethanolic extract of Cassia auriculata flowers. Ind. J. Biochem. Biophys. 2011, 48, 54–58. [Google Scholar]
- Gupta, S.; Sharma, S.B.; Bandal, S.K.; Prabhu, K.M. Antihyperglycemic and hypolipidemic activity of aqueous extract of Cassia auriculata L. leaves in experimental diabetes. J. Ethnopharmcol. 2009, 123, 499–503. [Google Scholar] [CrossRef]
- Jaydeokar, A.V.; Bandawane, D.D.; Bibave, K.H.; Patil, T.V. Hepatoprotective potential of Cassia auriculata roots on ethanol and natitubercular drug-induced hepatotoxicity in experimental models. Pharm. Biol. 2014, 52, 344–355. [Google Scholar] [CrossRef] [PubMed]
- Rajagopal, S.K.; Manickam, P.; Periyasamy, V.; Namasiyayam, N. Activity of Cassia auriculata leaf extract in rats with alcoholic liver injury. J. Nutr. Biochem. 2003, 14, 452–458. [Google Scholar] [CrossRef]
- John, C.M.; Sandrasaigaran, P.; Tong, C.K.; Adam, A.; Ramasamy, R. Immunomodulatory activity of polyphenols derived from Cassia auriculata flowers in aged rats. Cell. Immunol. 2011, 271, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Sommit, D.; Petsom, A.; Ishikawa, J.; Roengsumran, S. Cytotoxic activity of Natural Labdanes and their semi-synthetic modified derivatives from Croton oblongifolius. Planta Med. 2003, 69, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Ngamrojnavanich, N.; Sirimongkon, S.; Roengsumran, S.; Petsom, A.; Kamimura, H. Inhibition of Na+, K+-ATPase activity by (−)-ent-kaar-16-en-19-oic acid and its derivatives. Planta Med. 2003, 69, 555–556. [Google Scholar]
- Wijesekera, K. A bioactive diterpene; Nasimalum A from Croton oblongifolius Roxb. Prayog. Ras. 2017, 1, 41–44. [Google Scholar]
- Yongsa-ad, W.; Ngamrojanavanich, N.; Mahidol, C.; Ruchirawat, S.; Prawat, H.; Kittakoop, P. Diterpenoids from the roots of Croton oblongifolius. Planta Med. 2007, 73, 1491–1494. [Google Scholar] [CrossRef]
- Pudhom, K.; Vilaivan, T.; Ngamrojanayanich, N.; Dechangyipart, S.; Sommit, D.; Petsom, A.; Roengsumran, S. Furanocembranoids from the stem bark of Croton oblongifolius. J. Nat. Prod. 2007, 70, 659–661. [Google Scholar] [CrossRef]
- Roengsumran, S.; Petsom, A.; Kuptipyanuwat, N.; Vilaivan, T.; Ngamrojnavanich, N.; Chaichantipyuth, C.; Phuthong, S. Cytotoxic labdane diterpenoids from Croton oblongifolius. Phytochemistry 2001, 56, 103–107. [Google Scholar] [CrossRef]
- Roengsumran, S.; Petsom, A.; Sommit, D.; Vilaivan, T. Labdane diterpenoids from Croton oblongifolius. Phytochemistry 1999, 50, 449–453. [Google Scholar] [CrossRef]
- Roengsumran, S.; Singtothong, P.; Pudhom, K.; Ngamrochanayanich, N.; Petsom, A.; Chaichantipyuth, C. Neocrotocembranal from Croton oblongifolius. J. Nat. Prod. 1999, 62, 1163–1164. [Google Scholar] [CrossRef] [PubMed]
- Roengsumran, S.; Achayindee, S.; Petsom, A.; Pudhom, K.; Singtothong, P.; Surachetapan, C.; Vilaiyan, T. Two new cembranoids from Croton oblongifolius. J. Nat. Prod. 1998, 61, 652–654. [Google Scholar] [CrossRef] [PubMed]
- Aiyar, V.N.; Seshadri, T.R. Components of Croton oblongifolius-III constitution of oblongifolic acid. Tetrahedron 1970, 26, 5275–5279. [Google Scholar] [CrossRef]
- Rao, P.S.; Sachder, G.P.; Seshadri, T.R.; Singh, H.B. Isolation and constitution of oblongifoliol, a new diterpene of Croton oblongifolius L. Tetrahedron Lett. 1968, 45, 4685–4688. [Google Scholar] [CrossRef]
- Adiga, P.G.; Shridhar, N.B.; Sanganal, J.S.; Rao, S.; Shilpa, N.; Aparna, V.; Santhosh, I.M. In vivo anti-tumor efficary of Croton oblongifolius in DMBA induced mammary tumor in rats. Indian J. Anim. Res. 2016, 3301, 1–4. [Google Scholar]
- Ahmed, B.; Alam, T.; Varshney, M.; Khan, S.A. Hepatoprotective activity of two plants belonging to the Apiaceae and the Euphorbiaceae family. J. Ethnopharmacol. 2002, 79, 313–316. [Google Scholar] [CrossRef]
- Sastri, B.N. The wealth of India: Raw Materials; CSIR: New Delhi, India, 1956; Volume 4, p. 150. [Google Scholar]
- Sreejith, P.S.; Praseeja, R.J.; Asha, V.V. A review on the pharmacology and phytochemistry of traditional medicinal plant, Glycosmis pentaphylla (Retz.) Correa. J. Pharm. Res. 2012, 5, 2723–2728. [Google Scholar]
- Ito, C.; Kondo, Y.; Rao, K.S.; Tokuda, H.; Nishino, H.; Furukawa, H. Chemical constituents of Glycosmis pentaphylla. Isolation of a novel naphthoquinone and a new acridone alkaloid. Chem. Pharm. Bull. 1999, 47, 1579–1581. [Google Scholar] [CrossRef]
- Wang, J.; Di, Y.; Yang, X.; Li, S.; Wang, Y.; Hao, X. Hydroquinone diglycoside acyl esters from the stems of Glycosmis pentaphylla. Phytochemistry 2006, 67, 486–491. [Google Scholar] [CrossRef]
- Yang, G.Z.; Wu, Y.; Chen, Y. Alkaloids from the stems of Glycosmis pentaphylla. Helv. Chim. Acta. 2012, 95, 1449–1454. [Google Scholar] [CrossRef]
- Chen, Y.; Tian, E.L.; Hu, X.; Li, J.; Yang, G.-Z. Glycopentosides, D-F, three new phenolic glycosides from Glycosmis pentaphylla. Helv. Chim. Acta 2005, 98, 1160–1166. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, C.; Wu, Y.; Mo, S.; Wang, S.; Yang, G.; Mei, Z. Glycosmisines A and B: Isolation of two new carbazole-indole-type dimeric alkaloids from Glycosmis pentaphylla and an evaluation of their antiproliferative activities. Org. Biomol. Chem. 2005, 13, 6773–6781. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, M.; Chakraborty, D.P. Some minor constituents from Glycosmis pentaphylla. Phytochemistry 1977, 16, 2007–2008. [Google Scholar] [CrossRef]
- Mukherjee, S.; Mukherjee, M.; Ganglily, S.N. Glycozolinine, a carbazole derivative from Glycosmis pentaphylla. Phytochemistry 1983, 22, 1064–1065. [Google Scholar] [CrossRef]
- Govindachari, T.R.; Pai, B.R.; Subramaniam, P.S. Alkaloids of Glycosmis pentaphylla (Retz.) Correa. Tetrahedron 1966, 22, 3245–3252. [Google Scholar] [CrossRef]
- Chowdhury, B.K.; Mustapha, A.; Garba, M.; Bhattacharyya, P. Carbazole and 3-methylcarbazole from Glycosmis pentaphylla. Phytochemistry 1987, 26, 2138–2139. [Google Scholar]
- Bhattacharyya, P.; Chakrabarity, P.K.; Chowdhury, B.K. Glycozolidol, and antibacterial carbazole alkaloid from Glycosmis pentaphylla. Phytochemistry 1985, 24, 882–883. [Google Scholar] [CrossRef]
- Bhattachryya, P. Glycozolidal, a new carbazole alkaloid from Glycosmis pentaphylla. J. Nat. Prod. 1984, 48, 465–466. [Google Scholar] [CrossRef]
- Bhattacharyya, P.; Chowdhury, B.K. Glycolone, a quinolone alkaloid from Glycosmis pentaphylla. Phytochemistry 1985, 24, 634–635. [Google Scholar] [CrossRef]
- Zhang, Q.-B.; Ding, G.; Zhang, T.; Si, J.-G.; Song, B.; Wang, M.-H.; Chen, J.-H.; Yu, M.; Gu, Y.-C.; Zou, Z.-M. New furanopyridine alkaloids from the leaves of Glycosmis pentaphylla. Phytochem. Lett. 2006, 18, 51–54. [Google Scholar] [CrossRef]
- Sripisut, T.; Ritthiwigrom, T.; Promgool, T.; Yossathera, K.; Deachathai, S.; Phakhodee, W.; Cheenpracha, S.; Laphookhieo, S. Glycopentaphyllone: The first isolation of hydroperoxyquinolone from the fruits of Glycosmis pentaphylla. Phytochem. Lett. 2012, 5, 379–381. [Google Scholar] [CrossRef]
- Howlader, A.; Rizwan, F.; Sultana, S.; Rahman, M.R.; Shams-Ud-Doha, K.M.; Mowla, R.; Apu, A.S. Antimicrobial, antioxidant and cytotoxic effects of methanolic extracts of leaves and stems of Glycosmis pentaphylla (Retz.). J. Appl. Pharm. Sci. 2011, 1, 137–140. [Google Scholar]
- Quader, M.A.; Nutan, M.T.H.; Rashid, M.A. Antitumor alkaloid from Glycosmis pentaphylla. Fitoterapia 1999, 70, 305–307. [Google Scholar] [CrossRef]
- Nayak, S.S.; Jain, R.; Sahoo, A.K. Hepatoprotective activity of Glycosmis pentaphylla against paracetamol-induced hepatotoxicity in Swiss albino mice. Pharm. Biol. 2011, 49, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Agarwal, M.; Bhatia, V.; Jha, S.K.; Dinesh, J. In vitro antioxidant activity of crude extracts of the plant Glycosmis pentaphylla Correa. Int. J. Pharm. Sci. Rev. Res. 2011, 6, 159–162. [Google Scholar]

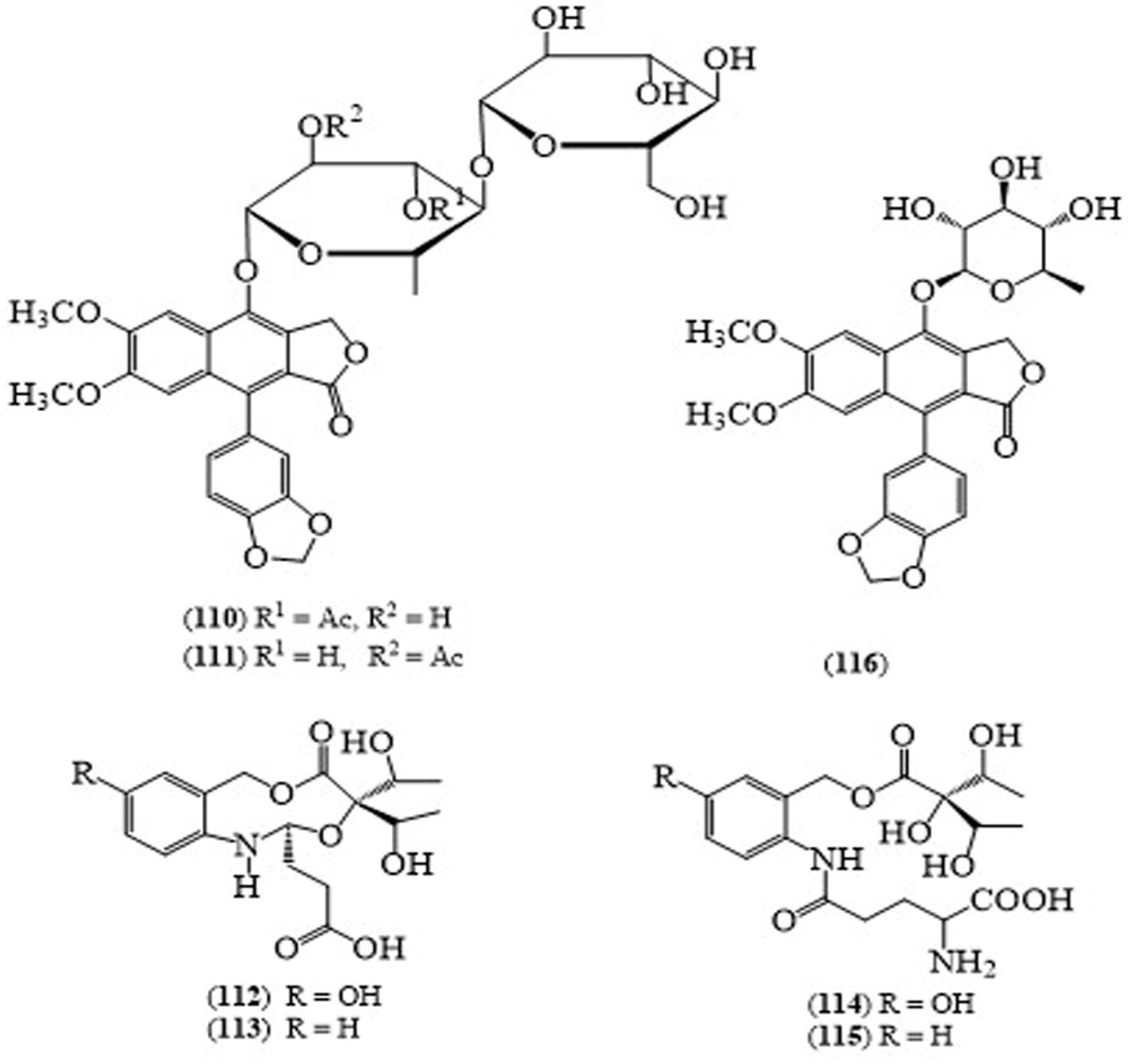
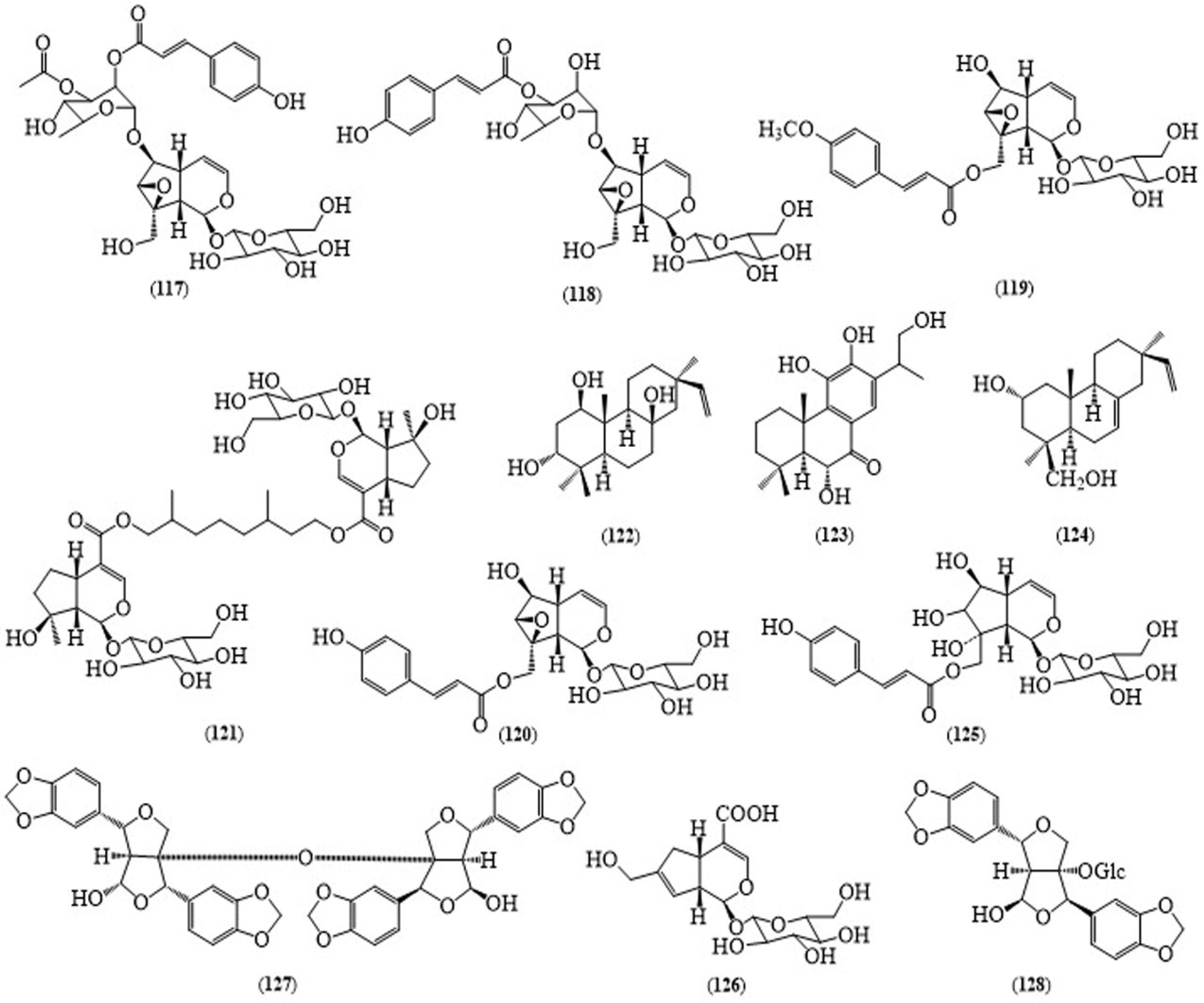


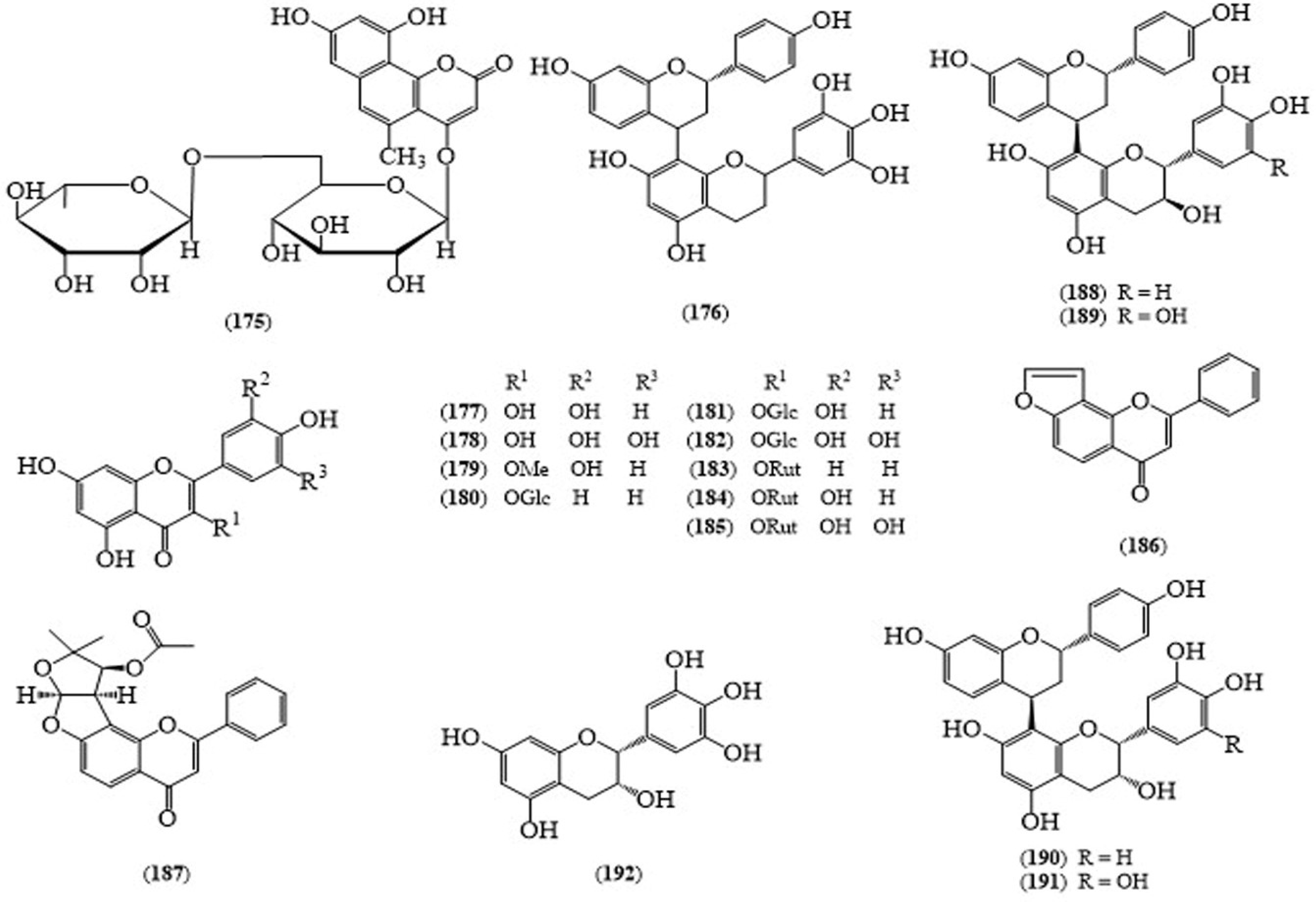

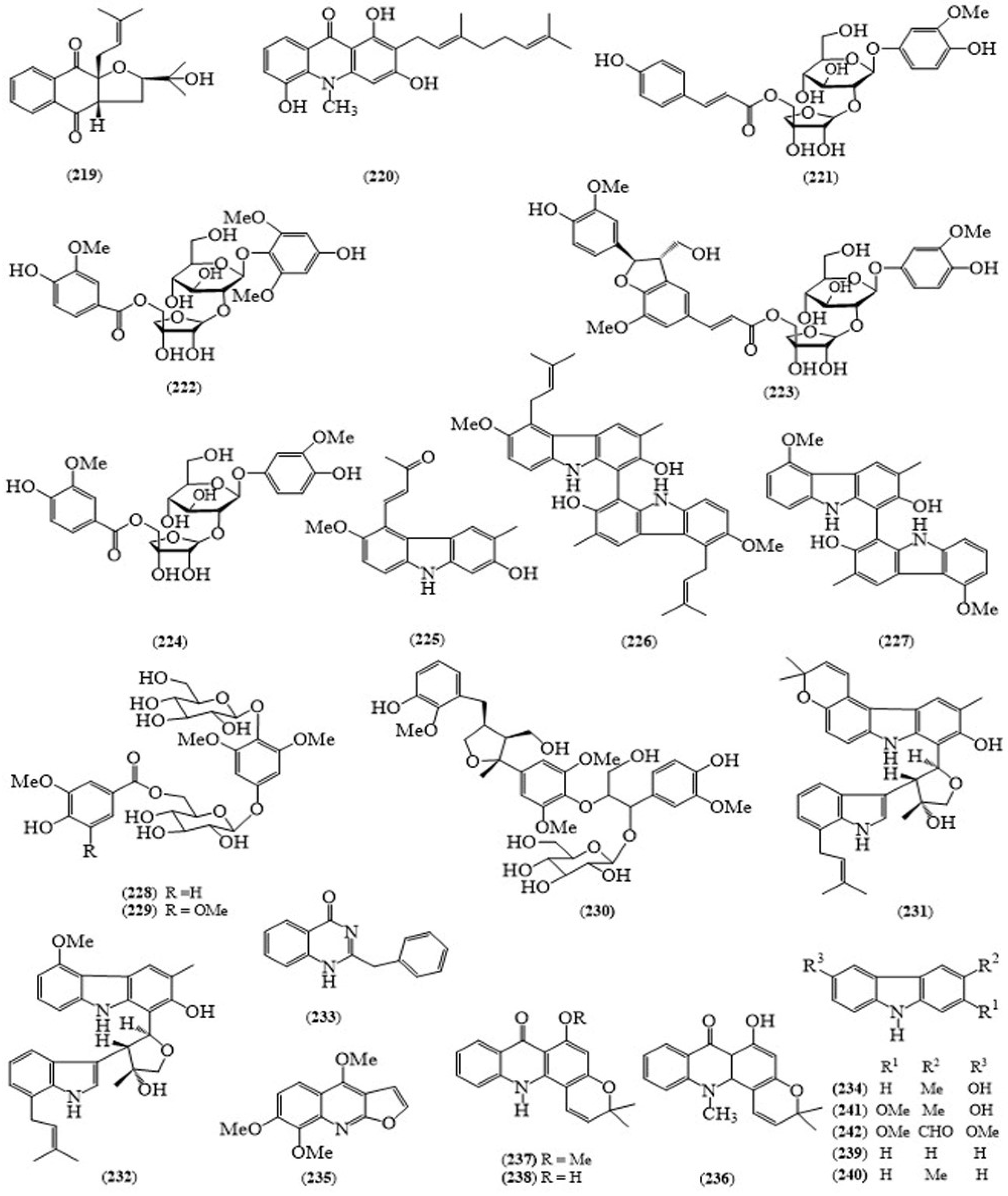
| No | Scientific Name | Family Name | Local Name | Ref(s) |
|---|---|---|---|---|
| 1 | Dalbergia cultrata | Fabaceae | Yin-daik | [4] |
| 2 | Eriosema chinense | Fabaceae | Peik-san-gale | [4] |
| 3 | Erythrina suberosa | Fabaceae | Ka-thit | [4] |
| 4 | Millettia pendula | Fabaceae | Thin-win | [4] |
| 5 | Sesbania grandiflora | Fabaceae | Paukpan-phyu | [4] |
| 6 | Tadehagi triquetrum | Fabaceae | Lauk-thay or shwe-gu-than-hlet | [4] |
| 7 | Andrographis echioides | Acanthaceae | Sega-gyi-hmwe-tu | [4] |
| 8 | Barleria cristata | Acanthaceae | Leik-tha-ywe-pya | [4] |
| 9 | Justicia gendarussa | Acanthaceae | Pha-wa-net | [4] |
| 10 | Premna integrifolia | Verbenaceae | Taungtan-gyi | [4] |
| 11 | Vitex trifolia | Verbenaceae | Kyanung-ban | [4] |
| 12 | Acacia pennata | Mimosaceae | Suyit or Suboke-gyi | [4] |
| 13 | Cassia auriculata | Caesalpiniaceae | Peik-thingat | [4] |
| 14 | Croton oblongifolius | Euphorbiaceae | Thetyin-gyi | [4] |
| 15 | Glycomis pentaphylla | Rutaceae | Taw-shauk | [4] |
| Source | Compound | Biological/Pharmacological Activities | Reference(s) |
|---|---|---|---|
| D. culrata (Stem bark) | Dalberatin A (6) | Cancer chemopreventive activity with IC50 of 212 (mol ratio/32 pmol TPA) | [10] |
| D. culrata (Stem bark) | Dalberatin B (7) | Cancer chemopreventive activity with IC50 of 303 (mol ratio/32 pmol TPA) | [10] |
| E. chinense (Roots) | Khonklonginol A (8) | Cytotoxicity against KB (IC50 3.1 μg/mL), NCI-H187 (IC50 3.0 μg/mL), Antimycobacterial activity against Mycobacterium tuberculosis H37Ra (MIC 25 μg/mL), Antimicrobial activity against Bacillus cereus (MIC 150 μg/mL), Staphylococcus agalactiae (MIC 2.3 μg/mL) and S. pyrogenes (MIC 2.3 μg/mL), Antioxidant activity (IC50 7.919 mM) | [11,12] |
| E. chinense (Roots) | Khonklonginol B (9) | Cytotoxicity against KB (IC50 3.8 μg/mL), NCI-H187 (IC50 4.3 μg/mL) | [11] |
| E. chinense (Roots) | Lupinifolinol (16) | Cytotoxicity against KB (IC50 1.73 μg/mL), NCI-H187 (IC50 3.5 μg/mL), Antimicrobial activity against Candia albicans (IC50 75 μg/mL), Bacillus cereus (IC50 4.7 μg/mL), Listeria monocytogenes (IC50 9.4 μg/mL), Staphylococcus aureus (IC50 75 μg/mL), S. aureusRASA (IC50 9.4 μg/mL), S. agalactiae (IC50 4.7 μg/mL), S. epidermidis (IC50 9.4 μg/mL), S. pyrogenes (IC50 2.3 μg/mL), Antioxidant activity (IC50 1.768 mM) | [11,12] |
| E. chinense (Roots) | Dehydrolupinifolinol (17) | Antimycobacterial activity against Mycobacterium tuberculosis H37Ra (MIC 12.5 μg/mL) | [11] |
| E. chinense (Roots) | Flemichin D (18) | Antimycobacterial activity against Mycobacterium tuberculosis H37Ra (MIC 12.5 μg/mL), Antimicrobial activity against with the values of (IC50 ˃ 150 μg/mL) each for Candida albicans, Escherichia coli, Klebsiella pneumoniae and Pseudomonas aeruginosa and (IC50 4.7 μg/mL) for each Bacillus cereus, Enterococcus faecalis, Listeria monocytogenes, Staphylococcus aureus, S. aureus RASA, S. agalactiae, S. epidermidis and S. pyrogenes, Antioxidant activity (IC50 0.538 mM) | [11,12] |
| E. chinense (Roots) | Eriosemaone A (19) | Antimycobacterail activity against Mycobacterium tuberculosis H37Ra (MIC 12.5 μg/mL) | [11] |
| E. chinense (Roots) | Lupinifolin (20) | Antimycobacterial activity against Mycobacterium tuberculosis H37Ra (MIC 12.5 μg/mL) | [11] |
| E. chinense (Roots) | 3-epi-lupinifolinol (21) | Antioxidant activity (IC50 0.681 mM) | [12] |
| E. chinense (Roots) | 2′-dihydroxy lupinifolinol (23) | Antimicrobial activity against Candia albicans (IC50 37.5 μg/mL), Escherichia coli (IC50 75 μg/mL), Klebsiella pneumoniae (IC50 75 μg/mL), and Pseudomonas aeruginosa (IC50 75 μg/mL), Bacillus cereus (IC50 2.3 μg/mL), Enterococcus faecalis (IC50 9.4 μg/mL), Listeria monocytogenes (IC50 9.4 μg/mL), Staphylococcus aureus (IC50 4.7 μg/mL), S. aureusRASA (IC50 4.7 μg/mL), S. agalactiae (IC50 4.7 μg/mL), S. epidermidis (IC50 37.5 μg/mL), S. pyrogenes (IC50 2.3 μg/mL), Antioxidant activity (IC50 0.252 mM) | [12] |
| E. chinense (Roots) | 3,5,2′,4′-Tetrahydroxy-6″,6″ dimethylpyrano (2″,3″:7,6)-8-(3‴,3‴-dimethylallyl) flavone (24) | Antimicrobial activity against Candia albicans (IC50 75 μg/mL), Escherichia coli (IC50 ˃ 150 μg/mL), Klebsiella pneumoniae (IC50 150 μg/mL), and Pseudomonas aeruginosa (IC50 150 μg/mL), Bacillus cereus (IC50 9.4 μg/mL), Enterococcus faecalis (IC50 18.8 μg/mL), Listeria monocytogenes (IC50 18.8 μg/mL), Staphylococcus aureus (IC50 9.4 μg/mL), S. aureusRASA (IC50 9.4 μg/mL), S. agalactiae (IC50 9.4 μg/mL), S. epidermidis (IC50 9.4 μg/mL), S. pyrogenes (IC50 9.4 μg/mL), Antioxidant activity (IC50 0.035 mM) | [12] |
| E. chinense (Roots) | Tectorigenin (28) | Antimicrobial activity against with the values of (IC50 > 150 μg/mL) for each Candida albicans, Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Bacillus cereus, Enterococcus faecalis, Listeria monocytogenes, Staphylococcus aureus, S. aureusRASA, S. agalactiae, S. epidermidis and S. pyrogenes, Antioxidant activity (IC50 3.666 mM) | [12] |
| E. chinense (Roots) | Genistein (29) | Antimicrobial activity against Candida albicans (IC50 75 μg/mL) and (IC50 150 μg/mL) for each Klebsiella pneumoniae, Pseudomonas aeruginosa, Bacillus cereus, Staphylococcus aureus, S. agalactiae and S. pyrogenes | [12] |
| E. chinense (Roots) | Kaempferol (30) | Antimicrobial activity against with the values of (IC50 > 150 μg/mL) each for Candida albicans, Klebsiella pneumoniae, Pseudomonas aeruginosa, Bacillus cereus, Staphylococcus aureus, S. agalactiae and S. pyrogenes, Antioxidant activity (IC50 0.028 mM) | [12] |
| E. chinense (Roots) | Kaempferol-7-O-β-d-glucopyranoside (31) | Antimicrobial activity against with the values of (IC50 > 150 μg/mL) for each Candida albicans, Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Bacillus cereus, Listeria monocytogenes, Staphylococcus agalactiae and S. epidermidis, Antioxidant activity (IC50 0.651 mM) | [12] |
| E. chinense (Roots) | Astragalin (32) | Antimicrobial activity against with the values of (IC50 150 μg/mL) for each Candida albicans, Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Bacillus cereus, Enterococcus faecalis, Staphylococcus aureus, S. agalactiae, S. epidermidis and S. pyrogenes, Antioxidant activity (IC50 0.681 mM) | [12] |
| E. suberosa (Stem bark) | 4′-methoxy licoflavanone (36) | The cytotoxic effects on apoptosis in human leukemia HL-60 cells and their potency to induce cancer cell death | [19] |
| E. suberosa (Stem bark) | Alpinumisoflavone (37) | The cytotoxic effects on apoptosis in human leukemia HL-60 cells and their potency to induce cancer cell death | [19] |
| E. suberosa (Stem bark) | Erysodine (40) | The anxiolytic effects in the elevated plus-maze and the light-dark transition model | [15] |
| E. suberosa (Flowers) | Erysotrine (41) | The anxiolytic effects in the elevated plus-maze and the light-dark transition model | [15] |
| M. pendula (Timber) | Millettilone A (49) | Leishmanicidal activity (IC50 9.3 μg/mL) | [25] |
| M. pendula (Timber) | 3R-Claussequinone (50) | Leishmanicidal activity (IC50 1.2 μg/mL) | [25] |
| M. pendula (Timber) | Pendulone (51) | Leishmanicidal activity (IC50 0.07 μg/mL) | [25] |
| M. pendula (Timber) | Secundiflorol I (53) | Leishmanicidal activity (IC50 86 μg/mL) | [25] |
| M. pendula (Timber) | 3,8-Dihydroxy-9-methoxy pterocarpan (54) | Leishmanicidal activity (IC50 2.9 μg/mL) | [25] |
| M. pendula (Timber) | 3,10-Dihydroxy-7,9-dimethoxypterocarpan (55) | Leishmanicidal activity (IC50 77 μg/mL) | [25] |
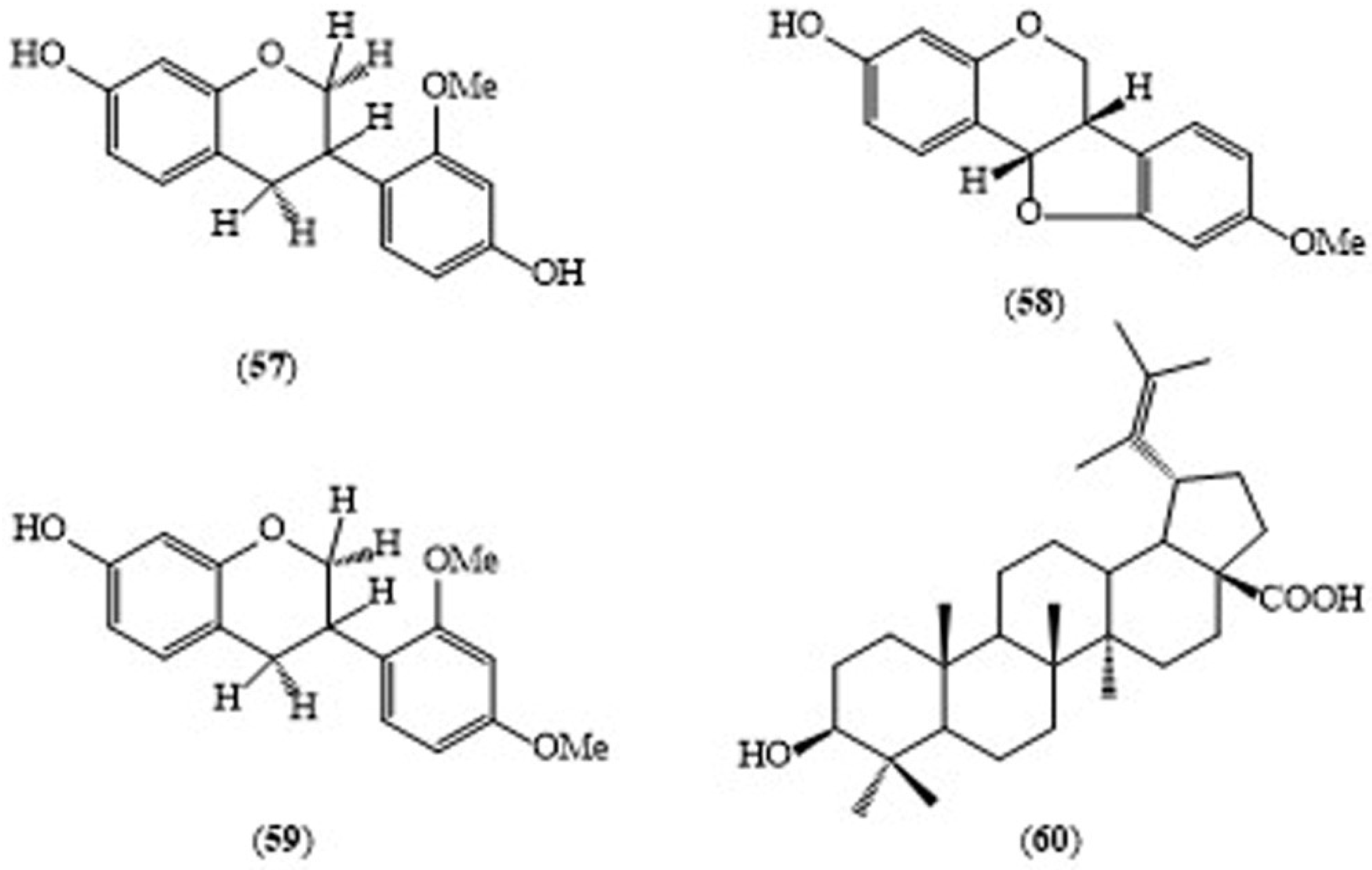
| S. grandiflora (Roots) | Isovestitol (57) | Antituberculosis activity against M. tuberculosis H37Rv (MIC 50 μg/mL) | [27] |
| S. grandiflora (Roots) | Medicarpin (58) | Antituberculosis activity against M. tuberculosis H37Rv (MIC 50 μg/mL) | [27] |
| S. grandiflora (Roots) | Sativan (59) | Antituberculosis activity against M. tuberculosis H37Rv (MIC 50 μg/mL) | [27] |
| S. grandiflora (Roots) | Betulinic acid (60) | Antituberculosis activity against M. tuberculosis H37Rv (MIC 100 μg/mL) | [27] |
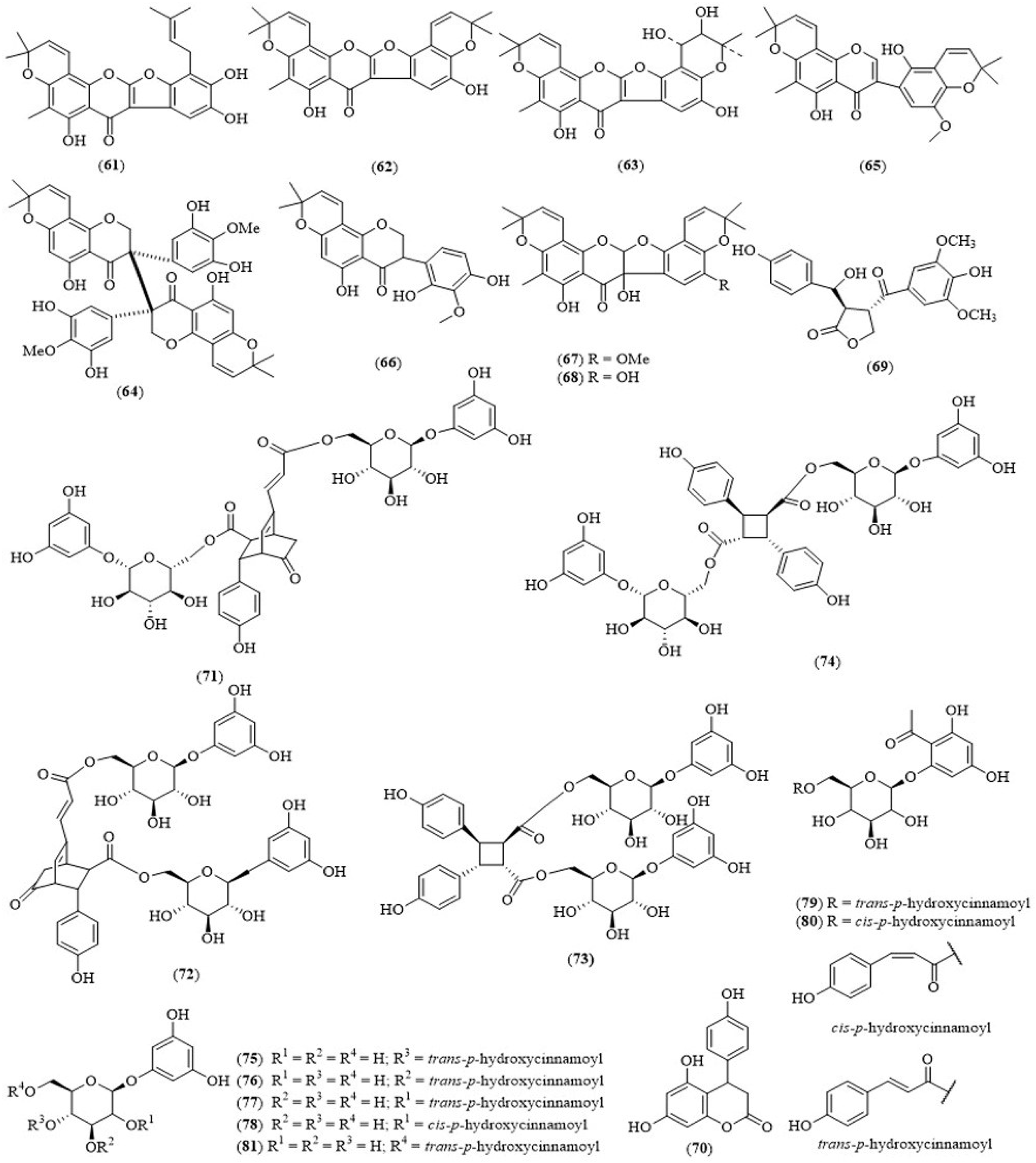
| T. treiquetrum (whole plant) | Tadehaginosin (69) | Hypoglycemic activity in vitro by HepG2 cells | [45] |
| T. treiquetrum (whole plant) | 3,4-Dihydro-4-(4′-hydroxyphenyl)-5,7-dihydroxycoumarin (70) | Hypoglycemic activity in vitro by HepG2 cells | [45] |
| T. treiquetrum (Aerial part) | Tadehaginosides C–J (73–80) | Antidiabetic activity | [46] |
| T. treiquetrum (Aerial part) | Tadehaginoside (81) | Antidiabetic activity | [46] |
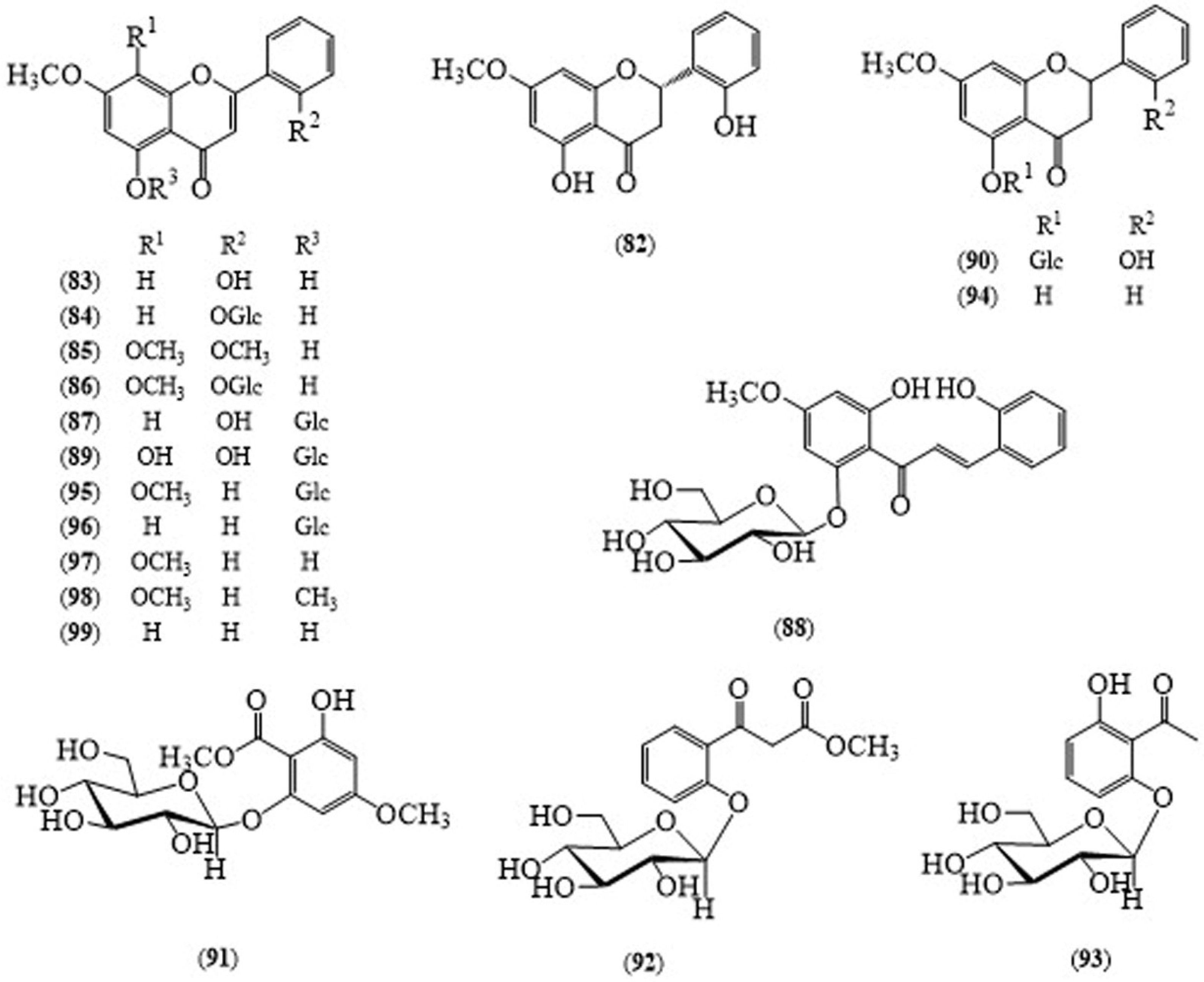
| A. echioides (whole plant) | Dihydroechioidinin (82) | Anti-inflammatory activity with the IC50 of 37.6 ± 1.2 μM | [58] |
| A. echioides (whole plant) | 5,7,8-Trimethoxyflavone (98) | Anti-inflammatory activity with the IC50 of 39.1 ± 1.3 μM | [58] |
| J. gendarussa (Stem and Bark) | Justiprocumin B (111) | Anti-HIV activity against a broad spectrum of HIV strains with IC50 values in the range of 15–21 nM (AZT, IC50 77–95 nM), nevirapine resistant isolate HIV-1N119 with an IC50 value of 495 nM and AZT resistant isolate HIV-11617-1 with (IC50 185 nM) | [80] |
| J. gendarussa (Stem and Bark) | Patentiflorin A (116) | Anti-HIV activity against a broad spectrum of HIV strains with IC50 values in the range of 24-37 nM (AZT, IC50 77–95 nM), drug-resistant HIV-1 isolate of both the nucleotide analogue (AZT) and (nevirapine) | [82] |
| P. integrifolia (Stem bark) | 10-O-trans-p-Methoxycinnamoyl catalpol (119) | Antioxidant activity with the IC50 value of 0.37 μM/mL in DPPH free radical scavenging assay | [101] |
| P. integrifolia (Stem bark) | 4″-Hydroxy-E-globularinin (125) | Antioxidant activity with the IC50 value of 0.29 μM/mL in DPPH free radical scavenging assay | [101] |
| P. integrifolia (Stem bark) | Premnosidic acid (126) | Antioxidant activity | [101] |
| P. integrifolia (Stem bark) | Premnadimer (127) | Antioxidant activity | [101] |
| P. integrifolia (Stem bark) | 4β-Hydroxyasarinin-1-O-β-glucopyranoside (128) | Antioxidant activity | [101] |
| V. trifolia (Aerial part) | Agnuside (152) | Antioxidant activity (IC50 9.81 μg) in DPPH and (IC50 12.90 μg) NO radical scavenging assays | [114] |
| V. trifolia (Aerial part) | Negundoside (153) | Antioxidant activity (IC50 9.96 μg) in DPPH and (IC50 16.25 μg) NO radical scavenging assays | [114] |
| V. trifolia (Aerial part) | 6-p-Hydroxybenzoyl mussaenosidic acid (154) | Antioxidant activity (IC50 10.31 μg) in DPPH and (IC50 13.51 μg) and NO radical scavenging assays | [114] |
| V. trifolia (Leaves) | Methyl-p-hydroxybenzoate (155) | Mosquito larvicidal activity against LC50 values of methyl-p-hydroxybenzoate were 5.77 ppm against Culex quinquefasciatus and 4.74 ppm against Aedes aegypti | [115] |
| A. pennata (Leaves) | Taepeenin D (156) | Hedgehog/GLi-mediated transcriptional activity with IC50 value of 1.6 Μm, Cytotoxic against pancreatic (PANC1) cells (IC50 3.2 μM) and prostate (DU145) cells (IC50 3.4 μM) | [122] |
| A. pennata (Leaves) | (+)-Drim-8-ene (157) | Hedgehog/GLi-mediated transcriptional activity with IC50 value of 13.5 μM, Cytotoxic against pancratic (PANC1) cells (IC50 15.1 μM) and prostate (DU145) cells (IC50 23.2 μM) | [122] |
| A. pennata (Leaves) | Quercetin 3-O-β-d-glucopyranosyl-4-O-β-d-glucopyranoside (158) | Hedgehog/GLi-mediated transcriptional activity with IC50 value of 10.5 μM, Cytotoxic against pancreatic (PANC1) cells (IC50 26.6 μM) and prostate (DU145) cells (IC50 30.0 μM) | [122] |
| C.auriculata (Leaves) | Pseudosemiglabrin (187) | Hepatoprotective effects [inhibition % 37.0 ± 3.7 (p ˂ 0.01)] at 30 μM | [135] |
| C. auriculata (Leaves) | (2S)-7,4-Dihydroxy flavan (4β→8)-catechin (188) | Hepatoprotective effects [inhibition % 33.5 ± 2.9 (p < 0.01)] at 30 μM | [135] |
| C. auriculata (Leaves) | (2S)-7,4-Dihydroxy flavan (4β→8)-gallocatechin (189) | Hepatoprotective effects [inhibition % 28.2 ± 4.7 (p < 0.01)] at 30 μM | [135] |
| C. auriculata (Leaves) | (−)-Epigallocatechin (192) | Hepatoprotective effects [inhibition % 24.4 ± 3.1 (p < 0.01)] at 30 μM | [135] |
| C. oblongifolius (Leaves) | Nasimalun A (193) | Cytotoxicity toward MOLT-3 cell line with IC50 26.44 μg/mL, Antibacterial activity MIC value of 50 μg/mL for Bacilus cereus and 100 μg/mL for Staphylococcus aureus and Staphylococcus epidermidis | [139] |
| C. oblongifolius (Stem bark) | Furanocembranoid 1 (201) | Cytotoxic effects against human tumor cell lines BT474 (IC50 7.8 μg/mL), CHAGO (IC50 7.0 μg/mL), Hep-G2 (IC50 5.6 μg/mL), KATO-3 (IC50 5.9 μg/mL) and SW-620s (IC50 6.3 μg/mL) by MTT colorimetric method | [142] |
| C. oblongifolius (Stem bark) | Furanocembranoid 3 (203) | Cytotoxic effects against human tumor cell lines BT474 (IC50 9.6 μg/mL), CHAGO (IC50 7.1 μg/mL), Hep-G2 (IC50 5.7 μg/mL), KATO-3 (IC50 8.2 μg/mL) and SW-620s (IC50 5.6 μg/mL) by MTT colorimetric method | [142] |
| C. oblongifolius (Stem bark) | Furanocembranoid 4 (204) | Cytotoxic effects against human tumor cell lines BT474 (IC50 9.6 μg/mL), CHAGO (IC50 9.3 μg/mL), Hep-G2 (IC50 6.1 μg/mL), KATO-3 (IC50 8.1 μg/mL) and SW-620s (IC50 6.0 μg/mL) by MTT colorimetric method | [142] |
| C. oblongifolius (Stem bark) | (−)-ent-kuar-16-en-19-oic acid (205) | Inhibition of Na+, K+-ATPase activity with an IC50 of 2.2 × 10−5 M | [138] |
| C. oblongifolius (Stem bark) | Croblongifolin (206) | Cytotoxic effects against human tumor cell lines including HEP-G2 (IC50 0.35 μM), BT474 (IC50 0.12 μM), SW-620 (IC50 0.47 μM) CHAGO (IC50 0.24 μM) and KATO-3 (IC50 0.35 μM) | [143] |
| C. oblongifolius (Stem bark) | Neocrotocembranal (215) | Cytotoxicity against P-388 cell culture in vitro (IC50 6.48 μg/mL) | [145] |
| G. pentaphylla (Stem) | Biscarbalexine A (227) | Cytotoxicity against human cancer cell lines A549 (IC50 56.06 μM), HepG-2 (IC50 60.06 μM) and Huh-7 (IC50 73.16 μM) | [156] |
| G. pentaphylla (Stem) | Glycosmisine A (231) | Cytotoxicity against human cancer cell lines A549 (IC50 43.68 μM), HepG-2 (IC50 50.30 μM) and Huh-7 (IC50 30.60 μM) | [156] |
| G. pentaphylla (Stem) | Glycosmisine B (232) | Cytotoxicity against human cancer cell lines A549 (IC50 57.10 μM), HepG-2 (IC50 62.89 μM) and Huh-7 (IC50 62.87 μM) | [156] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aye, M.M.; Aung, H.T.; Sein, M.M.; Armijos, C. A Review on the Phytochemistry, Medicinal Properties and Pharmacological Activities of 15 Selected Myanmar Medicinal Plants. Molecules 2019, 24, 293. https://doi.org/10.3390/molecules24020293
Aye MM, Aung HT, Sein MM, Armijos C. A Review on the Phytochemistry, Medicinal Properties and Pharmacological Activities of 15 Selected Myanmar Medicinal Plants. Molecules. 2019; 24(2):293. https://doi.org/10.3390/molecules24020293
Chicago/Turabian StyleAye, Mya Mu, Hnin Thanda Aung, Myint Myint Sein, and Chabaco Armijos. 2019. "A Review on the Phytochemistry, Medicinal Properties and Pharmacological Activities of 15 Selected Myanmar Medicinal Plants" Molecules 24, no. 2: 293. https://doi.org/10.3390/molecules24020293
APA StyleAye, M. M., Aung, H. T., Sein, M. M., & Armijos, C. (2019). A Review on the Phytochemistry, Medicinal Properties and Pharmacological Activities of 15 Selected Myanmar Medicinal Plants. Molecules, 24(2), 293. https://doi.org/10.3390/molecules24020293







