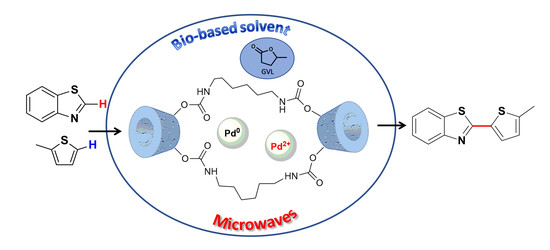
Microwave-Assisted Dehydrogenative Cross Coupling Reactions in γ-valerolactone with a Reusable Pd/β-cyclodextrin Crosslinked Catalyst
Abstract
1. Introduction
2. Results and Discussion
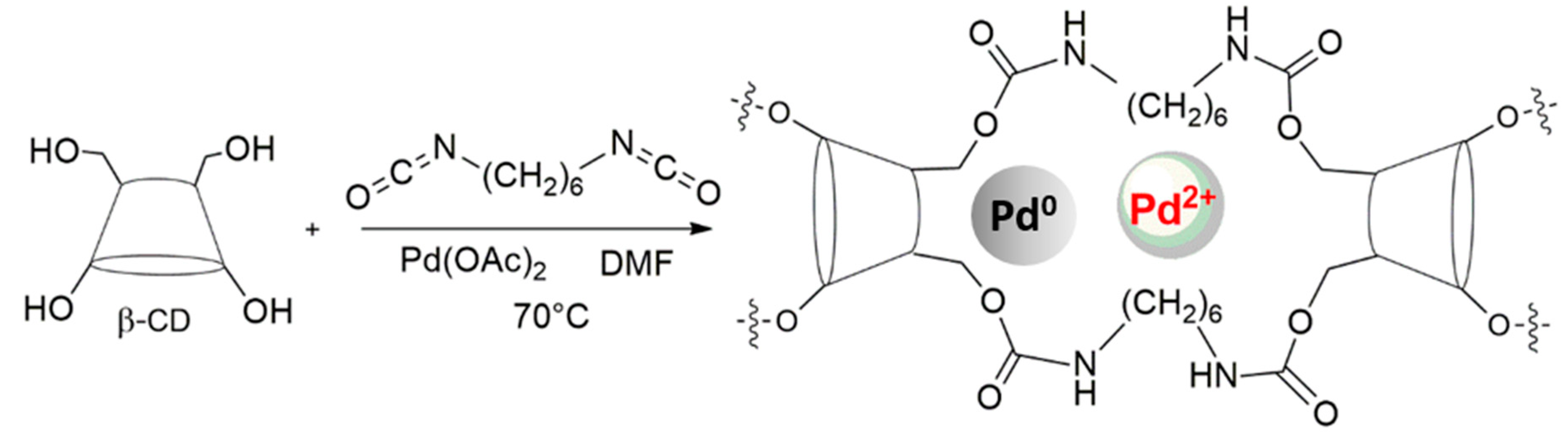
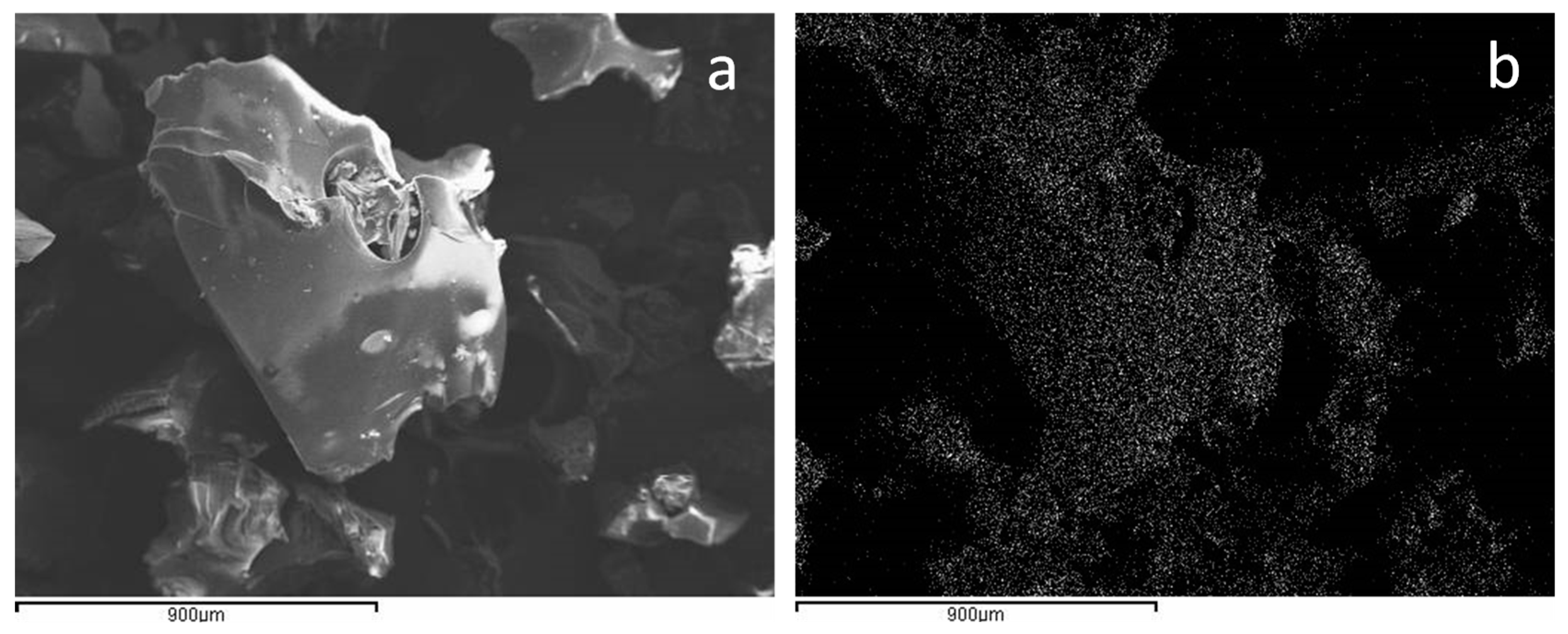
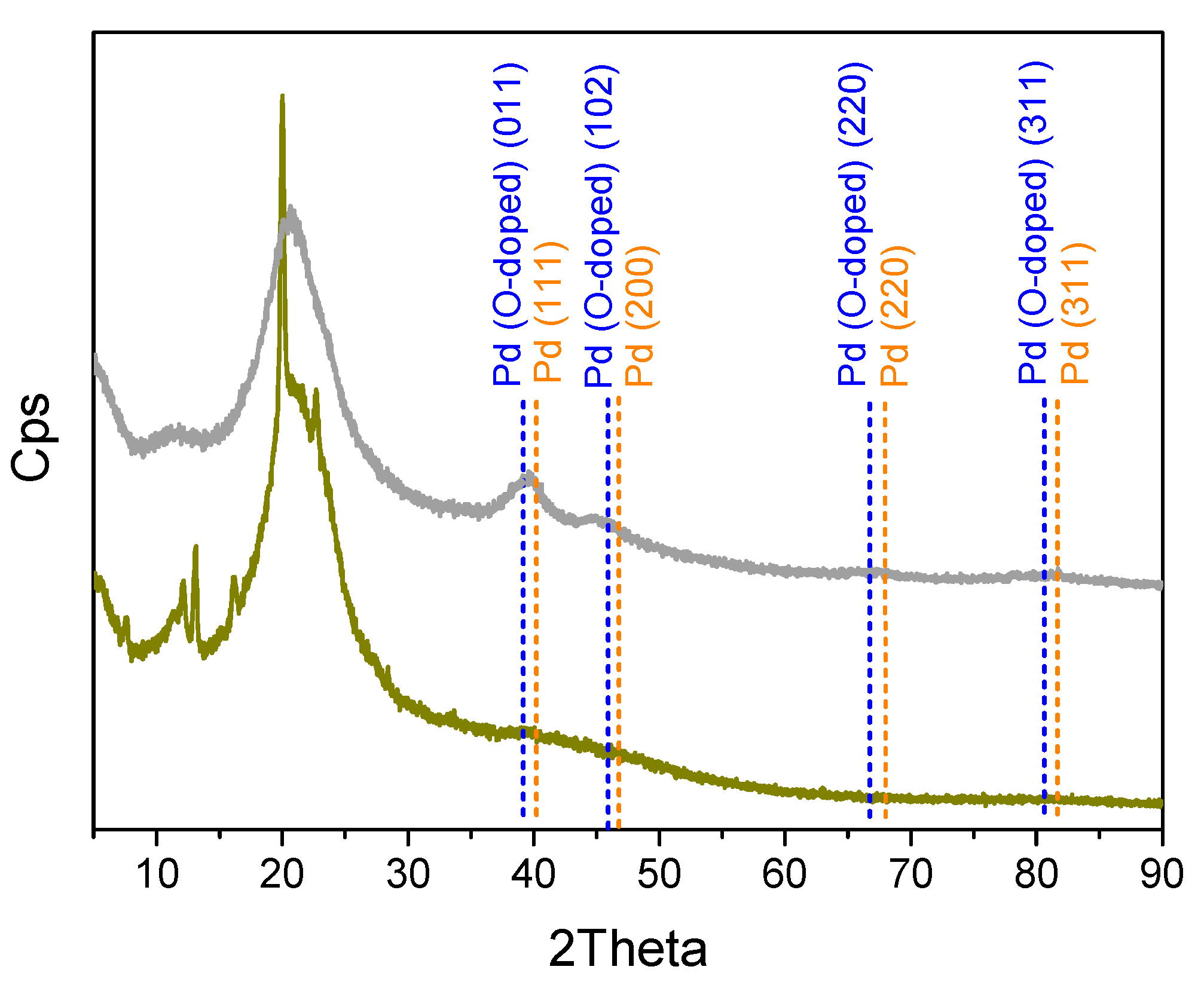
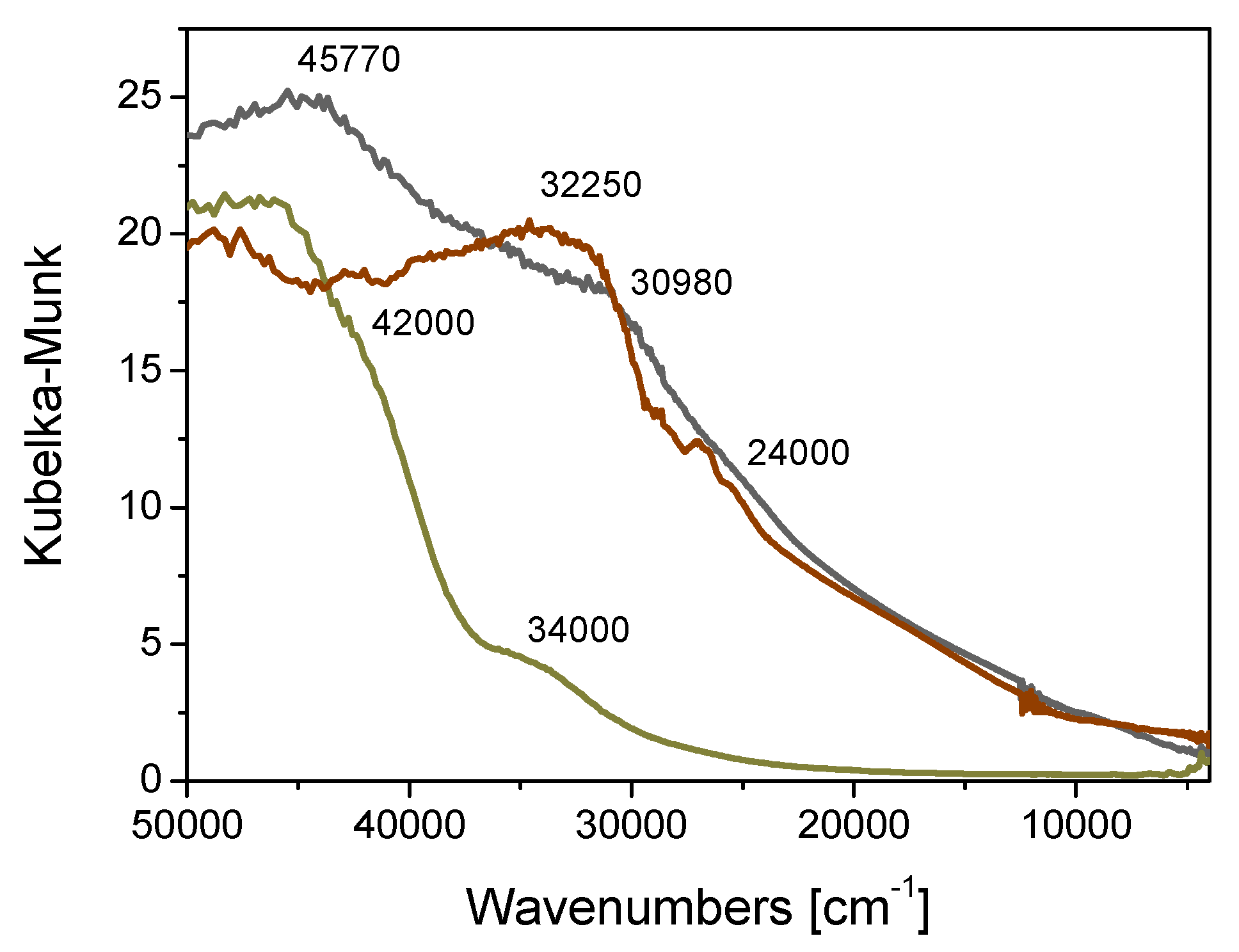
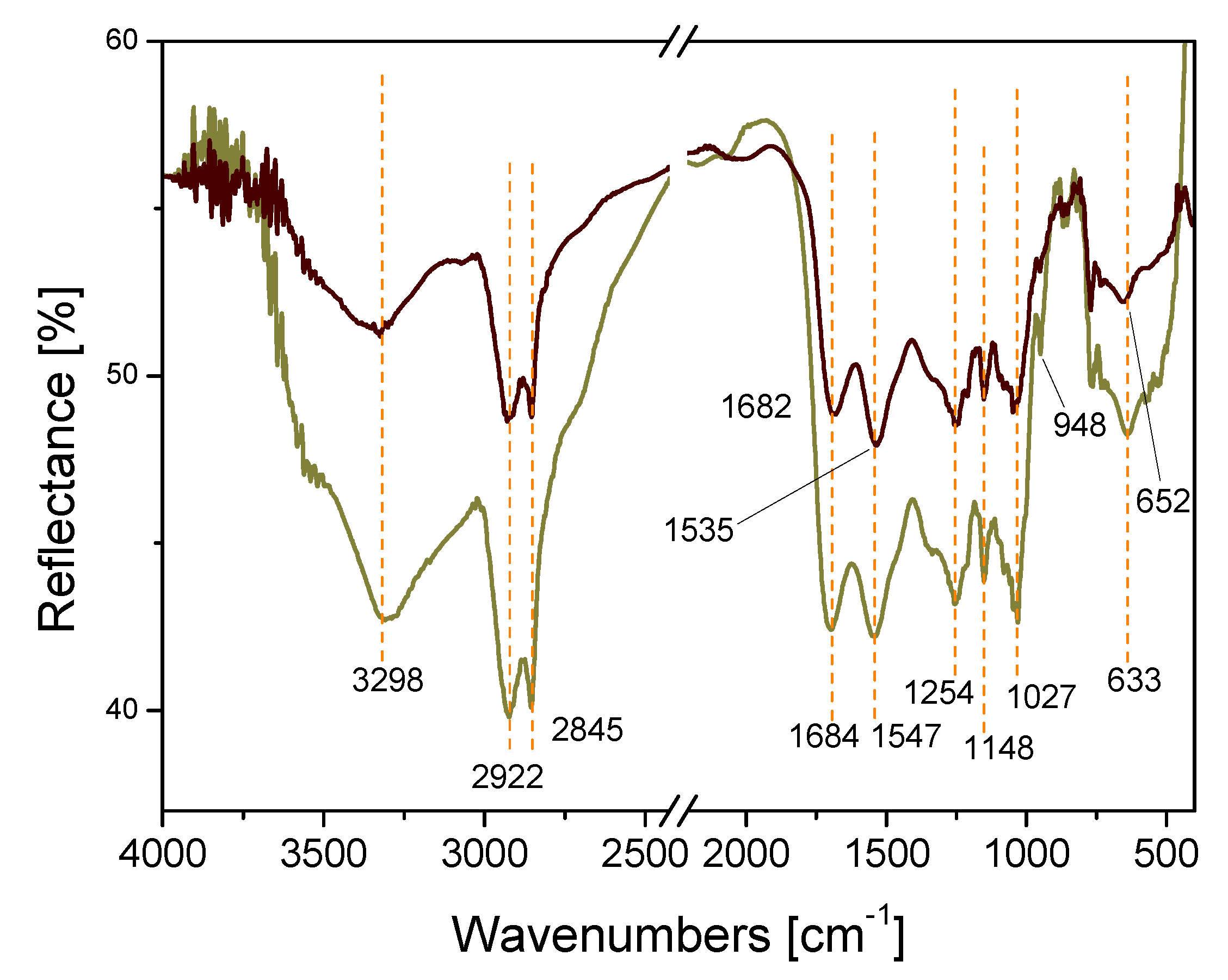
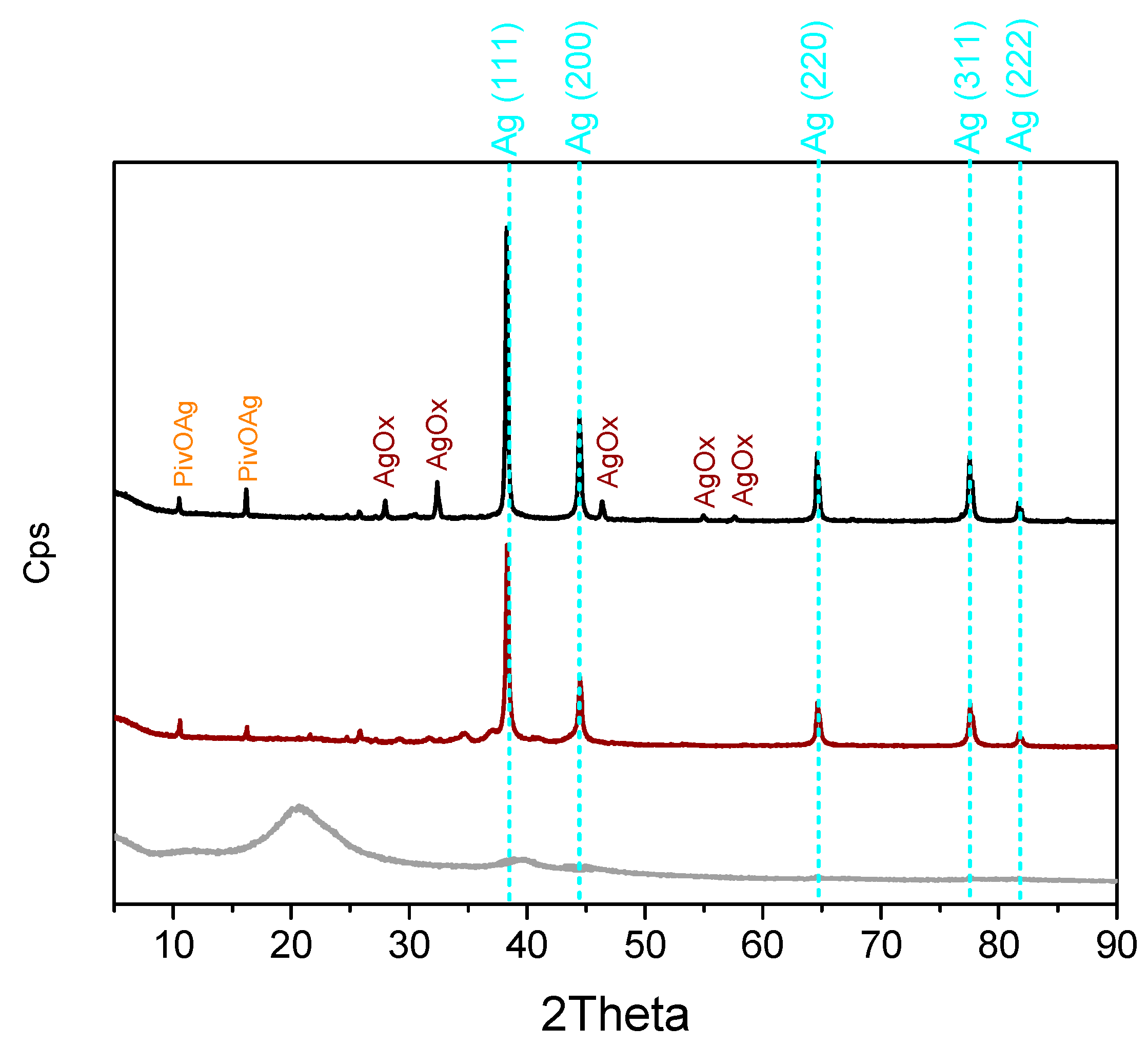
2.1. Catalyst Preparation and Characterization
2.2. Microwave-Assisted Dehydrogenative Cross Coupling Reactions
2.3. Catalyst Reusability
3. Materials and Methods
3.1. Catalyst Preparation
3.2. General Procedure for the MW-assisted Dehydrogenative Cross Coupling Reaction
3.3. General Procedure for Catalyst Recycling
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Watson, W.J.W. How do the fine chemical, pharmaceutical, and related Industries approach green chemistry and sustainability? Green Chem. 2012, 14, 251–259. [Google Scholar] [CrossRef]
- Beach, E.S.; Cui, Z.; Anastas, P.T. Green chemistry: A design framework for sustainability. Energy Environ. Sci. 2009, 2, 1038–1049. [Google Scholar] [CrossRef]
- Zhao, D.; You, J.; Hu, C. Recent Progress in Coupling of Two Heteroarenes. Chem. Eur. J. 2011, 17, 5466–5492. [Google Scholar] [CrossRef] [PubMed]
- Shan, Y.; Lai, M.; Li, R.; Wu, Z.; Zhao, M. Synthesis of Pyrazine–Thiazole Biheteroaryl Compounds through Base-Promoted Oxidative Cross-Dehydrogenative Coupling Reactions. Asian J. Org. Chem. 2017, 6, 1715–1718. [Google Scholar] [CrossRef]
- Hassan, J.; Sévignon, M.; Gozzi, C.; Schulz, E.; Lemaire, M. Aryl−Aryl Bond Formation One Century after the Discovery of the Ullmann Reaction. Chem. Rev. 2002, 102, 1359–1469. [Google Scholar] [CrossRef]
- Bringmann, G.; Mortimer, A.J.P.; Keller, P.A.; Gresser, M.J.; Garner, J.; Breuning, M. Atroposelective Synthesis of Axially Chiral Biaryl Compounds. Angew. Chem. Int. Ed. 2005, 44, 5384–5427. [Google Scholar] [CrossRef]
- Bringmann, G.; Gulder, T.; Gulder, T.A.M.; Breuning, M. Atroposelective Total Synthesis of Axially Chiral Biaryl Natural Products. Chem. Rev. 2011, 111, 563–639. [Google Scholar] [CrossRef] [PubMed]
- Miyaura, N. Cross-Coupling Reactions: A Practical Guide; Springer: Berlin, Germany, 2002. [Google Scholar]
- Diederich, F.; Stang, P.J. Metal-Catalyzed Cross-Coupling Reactions; Wiley-VCH: Weinheim, Germany, 1998. [Google Scholar]
- Miyaura, N.; Suzuki, A. Palladium-Catalyzed Cross-Coupling Reactions of Organoboron Compounds. Chem. Rev. 1995, 95, 2457–2483. [Google Scholar] [CrossRef]
- Suzuki, A. Cross-Coupling Reactions of Organoboranes: An Easy Way to Construct C−C Bonds (Nobel Lecture). Angew. Chem. Int. Ed. 2011, 50, 6722–6737. [Google Scholar] [CrossRef] [PubMed]
- Dwight, T.A.; Rue, N.R.; Charyk, D.; Josselyn, R.; DeBoef, B. C–C Bond Formation via Double C–H Functionalization: Aerobic Oxidative Coupling as a Method for Synthesizing Heterocoupled Biaryls. Org. Lett. 2007, 9, 3137–3139. [Google Scholar] [CrossRef] [PubMed]
- Stuart, D.R.; Fagnou, K. The Catalytic Cross-Coupling of Unactivated Arenes. Science 2007, 316, 1172–1775. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lan, J.; You, J. Oxidative C–H/C–H Coupling Reactions between Two (Hetero)arenes. Chem. Rev. 2017, 117, 8787–8863. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, L.; Sheldon, R.A. Fundamentals of green chemistry: Efficiency in reaction design. Chem. Soc. Rev. 2012, 41, 1437–1451. [Google Scholar]
- Liu, G.; Romo, D. Cover Picture: Total Synthesis of (+)-Omphadiol. Angew. Chem. Int. Ed. 2011, 50, 7537–7540. [Google Scholar] [CrossRef] [PubMed]
- Xi, P.; Yang, F.; Qin, S.; Zhao, D.; Lan, J.; Gao, G.; Hu, C.; You, J. Palladium(II)-Catalyzed Oxidative C–H/C–H Cross-Coupling of Heteroarenes. J. Am. Chem. Soc. 2010, 132, 1822–1824. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Song, G.; Zhang, H.; Li, X. Palladium-Catalyzed Oxidative Cross-Coupling between Pyridine N-Oxides and Indoles. Org. Lett. 2011, 13, 1766–1769. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, A.D.; Mandal, D.; Yamaguchi, J.; Itami, K. Oxidative C–H/C–H Coupling of Azine and Indole/Pyrrole Nuclei: Palladium Catalysis and Synthesis of Eudistomin U. Chem. Lett. 2011, 40, 555–557. [Google Scholar] [CrossRef]
- Han, W.; Mayer, P.; Ofial, A.R. Palladium-katalysierte dehydrierende Kreuzkupplungen von Benzazolen mit Azolen. Angew. Chem. 2011, 123, 2226–2230. [Google Scholar] [CrossRef]
- Bugaut, X.; Glorius, F. Palladium-Catalyzed Selective Dehydrogenative Cross-Couplings of Heteroarenes. Angew. Chem. Int. Ed. 2011, 50, 7479–7481. [Google Scholar] [CrossRef]
- Shinde, V.N.; Dhiman, S.; Krishnan, R.; Kumar, D.; Kumar, A. Synthesis of imidazopyridine-fused indoles via one-pot sequential Knoevenagel condensation and cross dehydrogenative coupling. Org. Biomol. Chem. 2018, 16, 6123–6132. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, Z.; Cheng, Y.; Lan, J.; She, Z.; You, J. Oxygen as an Oxidant in Palladium/Copper-Cocatalyzed Oxidative C–H/C–H Cross-Coupling between Two Heteroarenes. Sci. China Chem. 2015, 58, 1292–1296. [Google Scholar] [CrossRef]
- Andrade, C.; Yanez, C.O.; Ahn, H.-Y.; Urakami, T.; Bondar, M.V.; Komatsu, M.; Belfield, K.D. Two-Photon Fluorescence Vascular Bioimaging with New Bioconjugate Probes Selective toward the Vascular Endothelial Growth Factor Receptor 2. Bioconjugate Chem. 2011, 22, 2060–2071. [Google Scholar] [CrossRef] [PubMed]
- Rai, G.; Vyjayanti, V.N.; Dorjsuren, D.; Simeonov, A.; Jadhav, A.; Wilson, D.M.; Maloney, D.J. Synthesis, Biological Evaluation, and Structure–Activity Relationships of a Novel Class of Apurinic/Apyrimidinic Endonuclease 1 Inhibitors. J. Med. Chem. 2012, 55, 3101–3112. [Google Scholar] [CrossRef]
- Rodrigues, J.R.; Charris, J.; Camacho, J.; Barazarte, A.; Gamboa, N.; Nitzsche, B.; Hoepfner, M.; Lein, M.; Jung, K.; Abramjuk, C. N′-Formyl-2-(5-nitrothiophen-2-yl)benzothiazole-6-carbohydrazide as a potential anti-tumour agent for prostate cancer in experimental studies. J. Pharm. Pharmacol. 2013, 65, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Huang, X.; He, Q.; Xie, Y.; Yang, C. Palladium-catalyzed oxidative C–H/C–H cross-coupling of benzothiazoles with thiophenes and thiazoles. Chem. Commun. 2014, 50, 3996–3999. [Google Scholar] [CrossRef] [PubMed]
- Cravotto, G.; Carnaroglio, D. (Eds.) Microwave Chemistry; De Gruyter GmbH: Berlin, Germany, 2017. [Google Scholar]
- Kokel, A.; Schäfer, C.; Török, B. Application of microwave-assisted heterogeneous catalysis in sustainable synthesis design. Green Chem. 2017, 19, 3729–3751. [Google Scholar] [CrossRef]
- Polshettiwar, V.; Varma, R.S. Microwave-Assisted Organic Synthesis and Transformations using Benign Reaction Media. Acc. Chem. Res. 2008, 41, 629–639. [Google Scholar] [CrossRef] [PubMed]
- Calcio Gaudino, E.; Rinaldi, L.; Rotolo, L.; Carnaroglio, D.; Pirola, C.; Cravotto, G. Heterogeneous phase microwave-assisted reactions under CO2 or CO pressure. Molecules 2016, 21, 253. [Google Scholar] [CrossRef] [PubMed]
- Calcio Gaudino, E.; Manzoli, M.; Carnaroglio, D.; Wu, Z.; Grillo, G.; Rotolo, L.; Medlock, J.; Bonrath, W.; Cravotto, G. Sonochemical preparation of alumina-sphere loaded Pd nanoparticles for multi-litre alkyne semi-hydrogenation in a continuous flow microwave reactor. RCS Adv. 2018, 8, 7029–7039. [Google Scholar]
- Tuck, C.O.; Pérez, E.; Horváth, I.T.; Sheldon, R.A.; Poliakoff, M. Valorization of biomass: Deriving more value from waste. Science 2012, 337, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Tabasso, S.; Carnaroglio, D.; Calcio Gaudino, E.; Cravotto, G. Microwave, ultrasound and ball mill procedures for bio-waste valorisation. Green Chem. 2015, 17, 684–693. [Google Scholar] [CrossRef]
- Jérôme, F.; Luque, R. (Eds.) Bio-Based Solvents; Wiley: Hoboken, NJ, USA, 2017; ISBN 9781119065432. [Google Scholar]
- Clarke, C.J.; Tu, W.-C.; Levers, O.; Bröhl, A.; Hallett, J.P. Green and Sustainable Solvents in Chemical Processes. Chem. Rev. 2018, 118, 747–800. [Google Scholar] [CrossRef] [PubMed]
- Horváth, I.T.; Mehdi, H.; Fábos, V.; Boda, L.; Mika, L.T. γ-Valerolactone—A sustainable liquid for energy and carbon-based chemicals. Green Chem. 2008, 10, 238–242. [Google Scholar] [CrossRef]
- Ismalaj, E.; Strappaveccia, G.; Ballerini, E.; Elisei, F.; Piermatti, O.; Gelman, D.; Vaccaro, L. γ-Valerolactone as a Renewable Dipolar Aprotic Solvent Deriving from Biomass Degradation for the Hiyama Reaction. ACS Sustain. Chem. Eng. 2014, 2, 2461–2464. [Google Scholar] [CrossRef]
- Tabasso, S.; Calcio Gaudino, E.; Rinaldi, L.; Ledoux, A.; Larini, P.; Cravotto, G. Microwave-assisted, ligand-free, direct C–H arylation of thiophenes in biomass-derived γ-valerolactone. New J. Chem. 2017, 41, 9210–9215. [Google Scholar] [CrossRef]
- Santoro, S.; Marrocchi, A.; Lanari, D.; Ackermann, L.; Vaccaro, L. Towards Sustainable C–H Functionalization Reactions: The Emerging Role of Bio-Based Reaction Media. Chem. Eur. J. 2018, 24, 13383–13390. [Google Scholar] [CrossRef]
- Ferlin, F.; Santoro, S.; Ackermann, L.; Luigi Vaccaro, L. Heterogeneous C–H alkenylations in continuousflow: Oxidative palladium-catalysis in a biomass derived reaction medium. Green Chem. 2017, 19, 2510–2514. [Google Scholar] [CrossRef]
- Strappaveccia, G.; Ismalaj, E.; Petrucci, C.; Lanari, D.; Marrocchi, A.; Drees, M.; Facchetti, A.; Vaccaro, L. A biomass-derived safe medium to replace toxic dipolar solvents and access cleaner Heck coupling reactions. Green Chem. 2015, 17, 365–372. [Google Scholar] [CrossRef]
- Strappaveccia, G.; Luciani, L.; Bartollini, E.; Marrocchi, A.; Pizzo, F.; Vaccaro, L. γ-Valerolactone as an alternative biomass-derived medium for the Sonogashira reaction. Green Chem. 2015, 17, 1071–1076. [Google Scholar] [CrossRef]
- Cravotto, G.; Calcio Gaudino, E.; Tagliapietra, S.; Carnaroglio, D.; Procopio, A. A Green Approach to Heterogeneous Catalysis Using Ligand-Free, Metal-Loaded Cross-Linked Cyclodextrins. Green Process. Synth. 2012, 1, 269–273. [Google Scholar] [CrossRef]
- Calcio Gaudino, E.; Tagliapietra, S.; Palmisano, G.; Martina, K.; Carnaroglio, D.; Cravotto, G. Microwave-assisted, green synthesis of 4(3H)-quinazolinones under CO pressure in -valerolactone and reusable Pd/-cyclodextrin cross-linked catalyst. ACS Sustain. Chem. Eng. 2017, 5, 9233–9243. [Google Scholar] [CrossRef]
- Cintas, P.; Cravotto, G.; Calcio Gaudino, E.; Orio, L.; Boffa, L. Reticulated Pd(II)/Cu(I) cyclodextrin complexes as recyclable green catalyst for Sonogashira alkynylation. Catal. Sci. Technol. 2012, 2, 85–87. [Google Scholar] [CrossRef]
- Calcio Gaudino, E.; Carnaroglio, D.; Martina, K.; Palmisano, G.; Penoni, A.; Cravotto, G. Highly efficient microwave-assisted CO Aminocarbonylation with a recyclable Pd(II)/TPP-β-Cyclodextrin cross-linked catalyst. Org. Process Res. Dev. 2015, 19, 499–505. [Google Scholar] [CrossRef]
- Gao, Y.; Zhao, X.; Dong, B.; Zheng, L.; Li, N.; Zhang, S. Inclusion Complexes of β-Cyclodextrin with Ionic Liquid Surfactants. J. Phys. Chem. B 2006, 110, 8576–8581. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Shao, W.; Sun, W.; Kou, Y.; Li, X.; Yang, H. One-step fabrication of β-cyclodextrin modified magnetic graphene oxide nanohybrids for adsorption of Pb(II), Cu(II) and methylene blue in aqueous solutions. Appl. Surf. Sci. 2018, 459, 544–553. [Google Scholar] [CrossRef]
- Osorio, G.P.; Moyado, S.F.; Petranovskii, V.; Simakov, A. PdO/Al2O3-(Ce1−X ZrX)O2 catalysts: Effect of the sol-gel support composition. Catal. Lett. 2006, 110, 53–60. [Google Scholar] [CrossRef]
- Mullassery, M.D.; Fernandez, N.B.; Thomas, D. Microwave- assisted green synthesis of acrylamide cyclodextrin-grafted silylated bentonite for the controlled delivery of tetracycline hydrochloride. Sustain. Chem. Pharm. 2018, 10, 103–111. [Google Scholar] [CrossRef]
- Feiz, A.; Loni, M.; Naderi, S.; Bazgir, A. The β-cyclodextrin decorated with palladium nanoparticles without pretreatment: An efficient heterogeneous catalyst for biaryls synthesis. Appl. Organometal. Chem. 2018, 32, e4608. [Google Scholar] [CrossRef]
- Santoro, S.; Ferlin, F.; Luciani, L.; Ackermann, L.; Vaccaro, L. Biomass-derived solvents as effective media for cross-coupling reactions and C–H functionalization processes. Green Chem. 2017, 19, 1601–1612. [Google Scholar] [CrossRef]
- Bechtoldt, A.; Baumert, M.E.; Vaccaro, L.; Ackermann, L. Ruthenium(II) oxidase catalysis for C–H alkenylations in biomass-derived γ-valerolactone. Green Chem. 2018, 20, 398–402. [Google Scholar] [CrossRef]
- Campbell, A.N.; Stahl, S.S. Overcoming the “Oxidant Problem”: Strategies to Use O2 as the Oxidant in Organometallic C–H Oxidation Reactions Catalyzed by Pd (and Cu). Acc. Chem. Res. 2012, 45, 851–863. [Google Scholar] [CrossRef] [PubMed]
- Lanci, M.P.; Remy, M.S.; Kaminsky, W.; Mayer, J.M.; Sanford, M.S. Oxidatively Induced Reductive Elimination from (tBu2bpy)Pd(Me)2: Palladium(IV) Intermediates in a One-Electron Oxidation Reaction. J. Am. Chem. Soc. 2009, 131, 15618–15620. [Google Scholar] [CrossRef] [PubMed]
- Khusnutdinova, J.R.; Rath, N.P.; Mirica, L.M. Stable Mononuclear Organometallic Pd(III) Complexes and Their C-C Bond Formation Reactivity. J. Am. Chem. Soc. 2010, 132, 7303–7306. [Google Scholar] [CrossRef] [PubMed]
- Seligson, A.L.; Trogler, W.C. One-electron oxidative cleavage of palladium(II) alkyl and phenoxo bonds. J. Am. Chem. Soc. 1992, 114, 7085–7089. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |
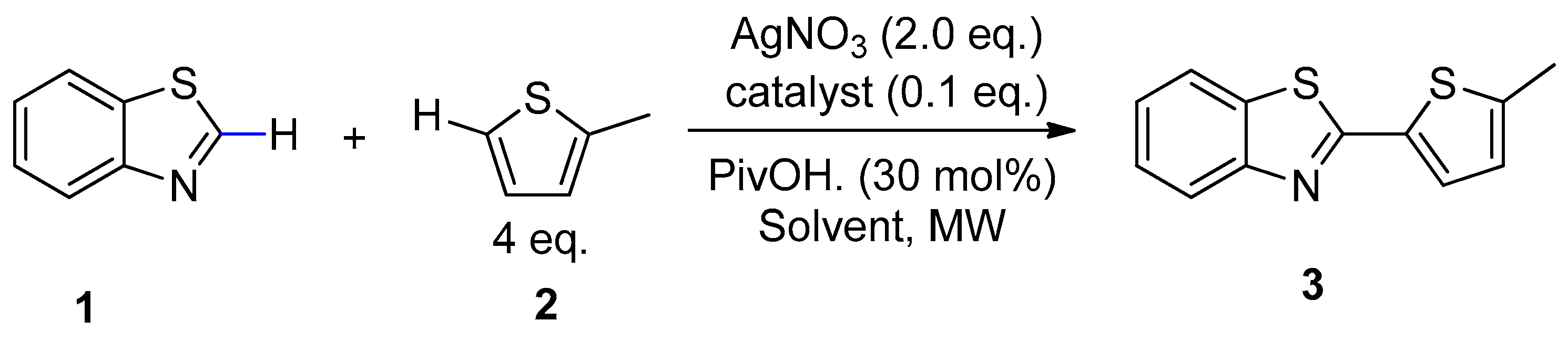

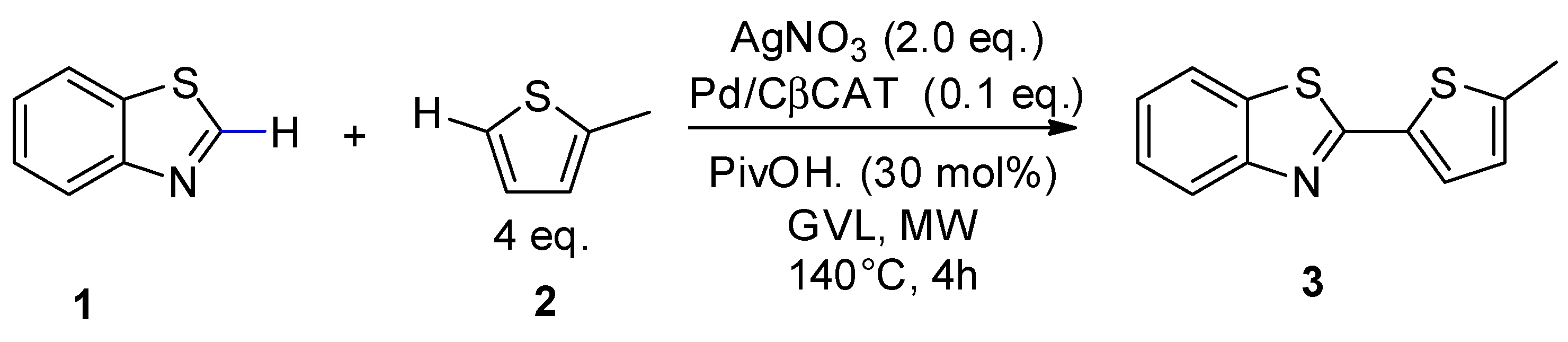
| Entry | Solvent | Yield (%) b | |
|---|---|---|---|
| Pd(OAc)2 | Pd/CβCAT | ||
| 1 | DMA | 100 (89) c | 95 (56) c |
| 2 | GVL | 76 (46) c | 95 (43) c |
| 3 | Ethyl lactate | 60 (40 homo) | 57 (42 homo) |
| 4 | Ethyl levulinate | 34 (65 homo) | 15 (58 homo) |
| Entry | Ligand b | Solvent | Yield (%) c | |
|---|---|---|---|---|
| Pd(OAc)2 | Pd/CβCAT | |||
| 1 | TFA | DMA | 46 (53 homo) | 16 (83 homo) |
| 2 | TFA | GVL | 33 (67 homo) | 29 (66 homo) |
| 3 | Lutidine | DMA | 67 (32 homo) | 23 (72 homo) |
| Entry | Oxidant | Solvent | Yield(%) b | |
|---|---|---|---|---|
| Pd(OAc)2 | Pd/CβCAT | |||
| 1 | O2 15 bar | DMA | 31 | 41 |
| 2 | O2 15 bar | GVL | 21 | 9 |
| 3 | K2S2O8 + O2 15 bar | DMA | 0 | 0 |
| 4 | Ag2O (1eq.) + O2 15 bar | DMA | 0 | 0 |
| 5 | AgNO3 (1eq.) + O2 15 bar | DMA | 89 | 80 |
| 6 | AgNO3 (1eq.) + O2 15 bar | GVL | 53 | 54 |
| 7 | Ag2O (2eq.) | DMA | 18 | Traces |
| 8 | Ag2O (2eq.) | GVL | 15 | Traces |
| 9 | AgNO3 (2eq.) | DMA | 100 | 95 |
| 10 | AgNO3 (2eq.) | GVL | 76 | 95 |
| 11 c | AgNO3 (2eq.) | DMA | n.d. | 0 (98 homo) |
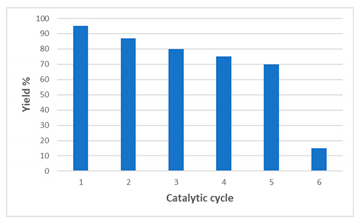
| Run | Yield a |
|---|---|
| 1 | 95 |
| 2 | 87 |
| 3 | 80 |
| 4 | 75 |
| 5 | 70 |
| 6 | 15 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tabasso, S.; Calcio Gaudino, E.; Acciardo, E.; Manzoli, M.; Giacomino, A.; Cravotto, G. Microwave-Assisted Dehydrogenative Cross Coupling Reactions in γ-valerolactone with a Reusable Pd/β-cyclodextrin Crosslinked Catalyst. Molecules 2019, 24, 288. https://doi.org/10.3390/molecules24020288
Tabasso S, Calcio Gaudino E, Acciardo E, Manzoli M, Giacomino A, Cravotto G. Microwave-Assisted Dehydrogenative Cross Coupling Reactions in γ-valerolactone with a Reusable Pd/β-cyclodextrin Crosslinked Catalyst. Molecules. 2019; 24(2):288. https://doi.org/10.3390/molecules24020288
Chicago/Turabian StyleTabasso, Silvia, Emanuela Calcio Gaudino, Elisa Acciardo, Maela Manzoli, Agnese Giacomino, and Giancarlo Cravotto. 2019. "Microwave-Assisted Dehydrogenative Cross Coupling Reactions in γ-valerolactone with a Reusable Pd/β-cyclodextrin Crosslinked Catalyst" Molecules 24, no. 2: 288. https://doi.org/10.3390/molecules24020288
APA StyleTabasso, S., Calcio Gaudino, E., Acciardo, E., Manzoli, M., Giacomino, A., & Cravotto, G. (2019). Microwave-Assisted Dehydrogenative Cross Coupling Reactions in γ-valerolactone with a Reusable Pd/β-cyclodextrin Crosslinked Catalyst. Molecules, 24(2), 288. https://doi.org/10.3390/molecules24020288