3.1. Velocity of kinesin Kimers
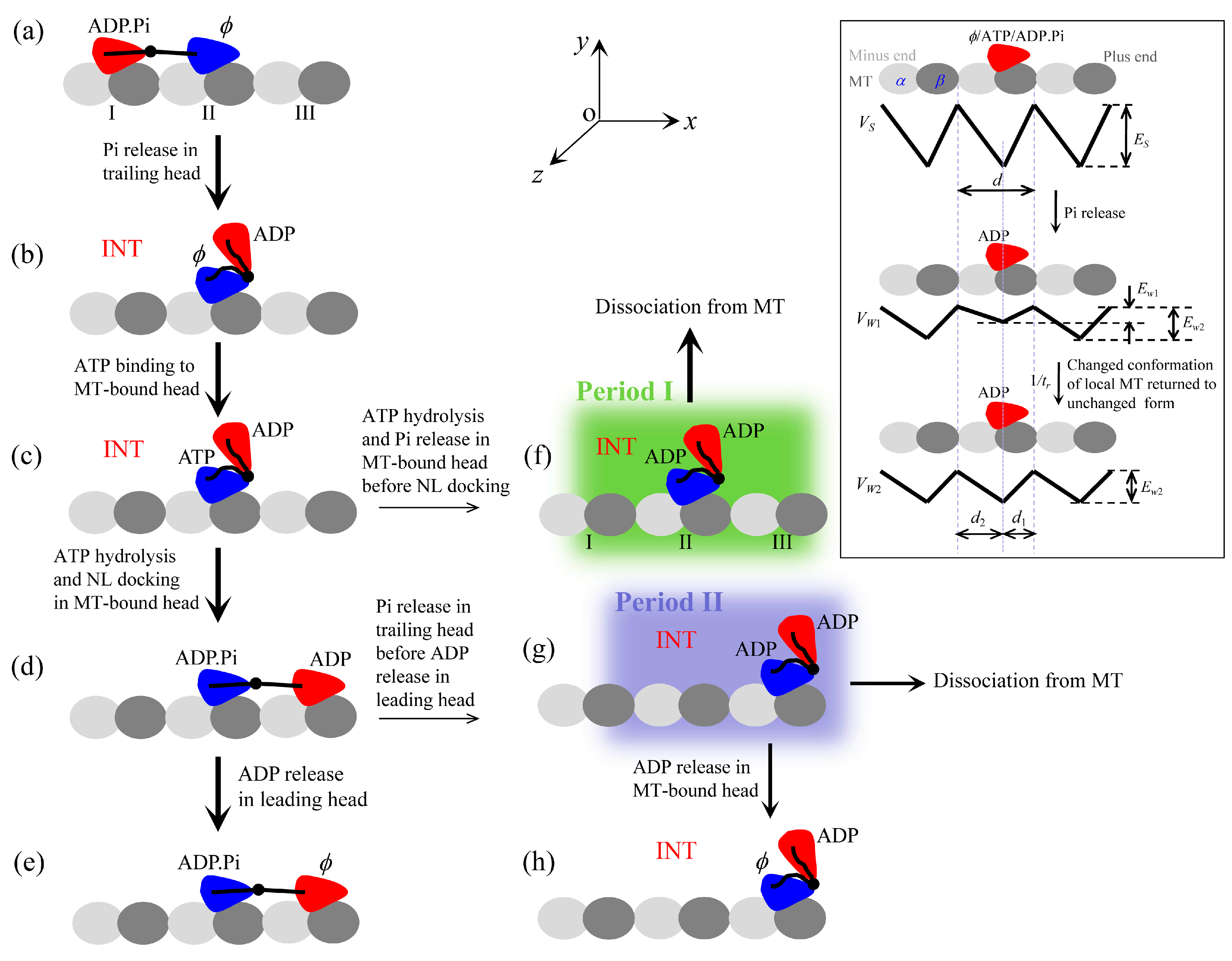
First, we study kinesin-5.
Figure 2a,b shows simulated results of the velocity
versus [ATP] at different values of the applied load and
versus the applied load at different values of [ATP], respectively. For comparison, the corresponding single-molecule data of Valentine et al. [
14] are also plotted in
Figure 2. It is seen that the simulated data show good agreement with the experimental data.
Second, we study kinesin-2.
Figure 3a (left panel) shows simulated results of the velocity
versus [ATP] under no applied load.
Figure 3b (left panel) shows simulated results of the velocity
versus the applied load at saturating [ATP] (2 mM). For comparisons, the single-molecule data of Andreasson et al. [
8] are shown in right panels of
Figure 3. It is seen that the simulated and experimental data are in good agreement with each other.
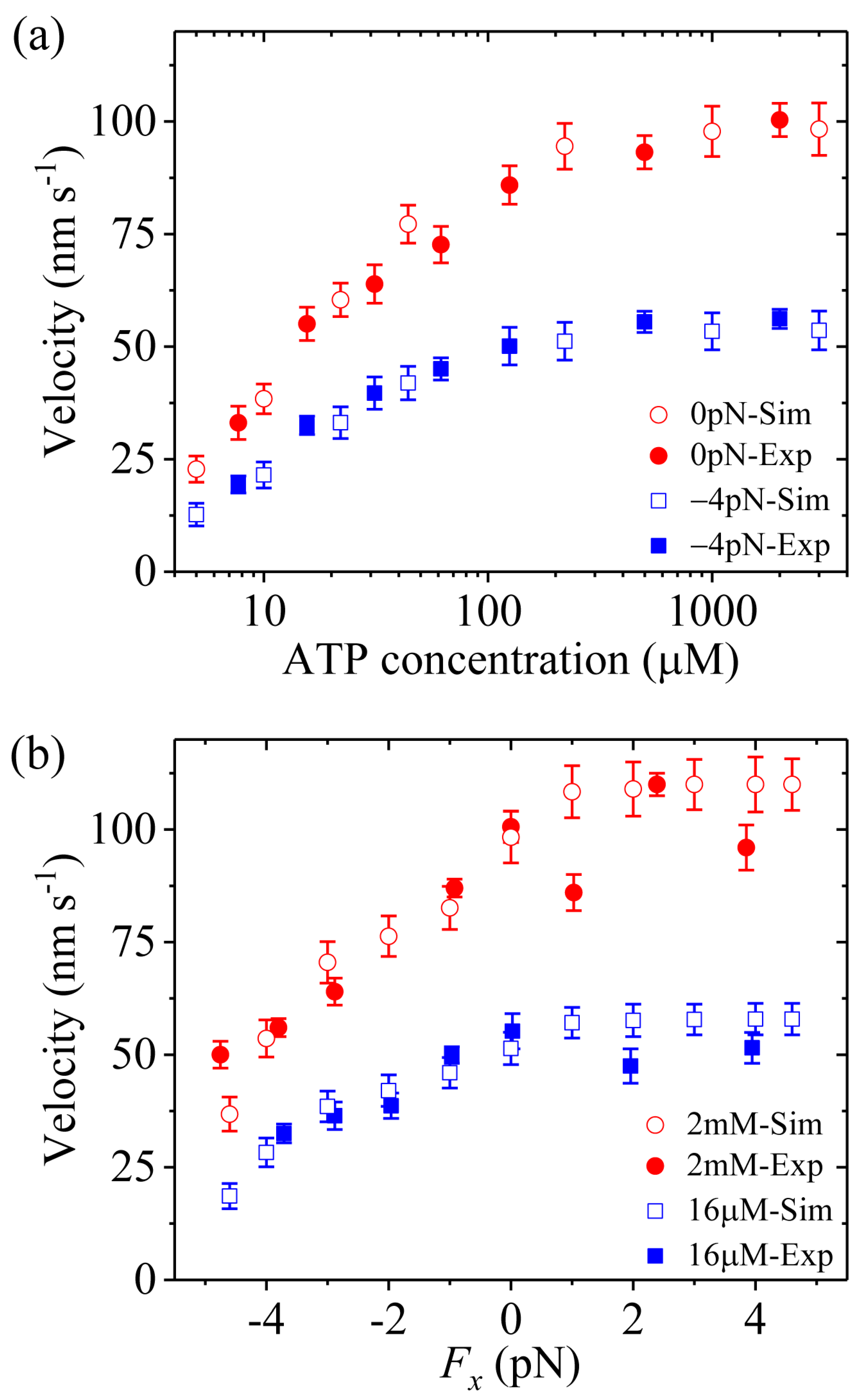
Third, we study kinesin-1. The results simulated with
= 1 were presented before [
21]. Here, we use
= 1.2 (see
Table 1) to make simulations.
Figure 4a,b shows simulated results of the velocity
versus [ATP] under a backward load (−2.5 pN) and
versus the applied load at saturating [ATP] (2 mM), respectively. For comparison, the single-molecule data of Andreasson et al. [
17] are also shown in
Figure 4b. From
Figure 4b we see that the simulated and experimental data are in good agreement with each other.
From
Figure 2a,
Figure 3a and
Figure 4a we see that for all of the three families of kinesin dimers the dependence of the velocity on [ATP] follows the Michaelis-Menten relation. From
Figure 2b,
Figure 3b and
Figure 4b we see that for all of the three families of kinesin dimers, the dependence of the velocity on force has a sigmoid form and the velocity decreases as the backward force increases. However, the velocities of kinesin-5 and kinesin-2 are less affected by the backward force than the velocity of kinesin-1, which arises mainly from the fact that kinesin-5 and kinesin-2 have larger NL-docking energy
ENL than kinesin-1 (see
Table 1).
Taken together, since the three families of kinesin dimers show much similar mechanism of the processive movement, they exhibit the similar feature on the dependence of the velocity upon [ATP] and upon the external force. However, since kinesin-5 and kinesin-2 have larger NL-docking energy than kinesin-1, the velocities of kinesin-5 and kinesin-2 are less affected by the backward force than the velocity of kinesin-1.
3.2. Processivity of Kinesin Dimers
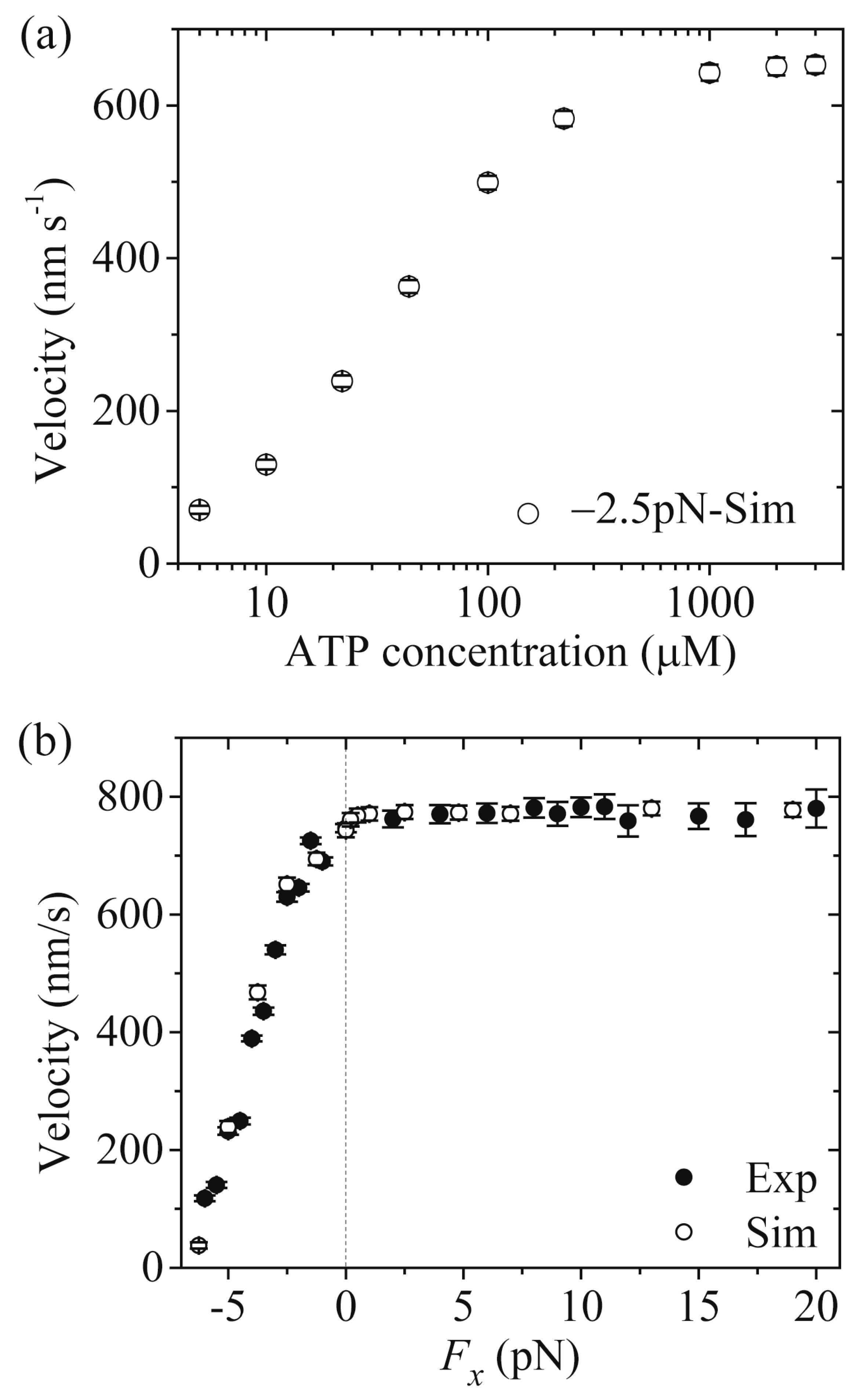
First, we study kinesin-5.
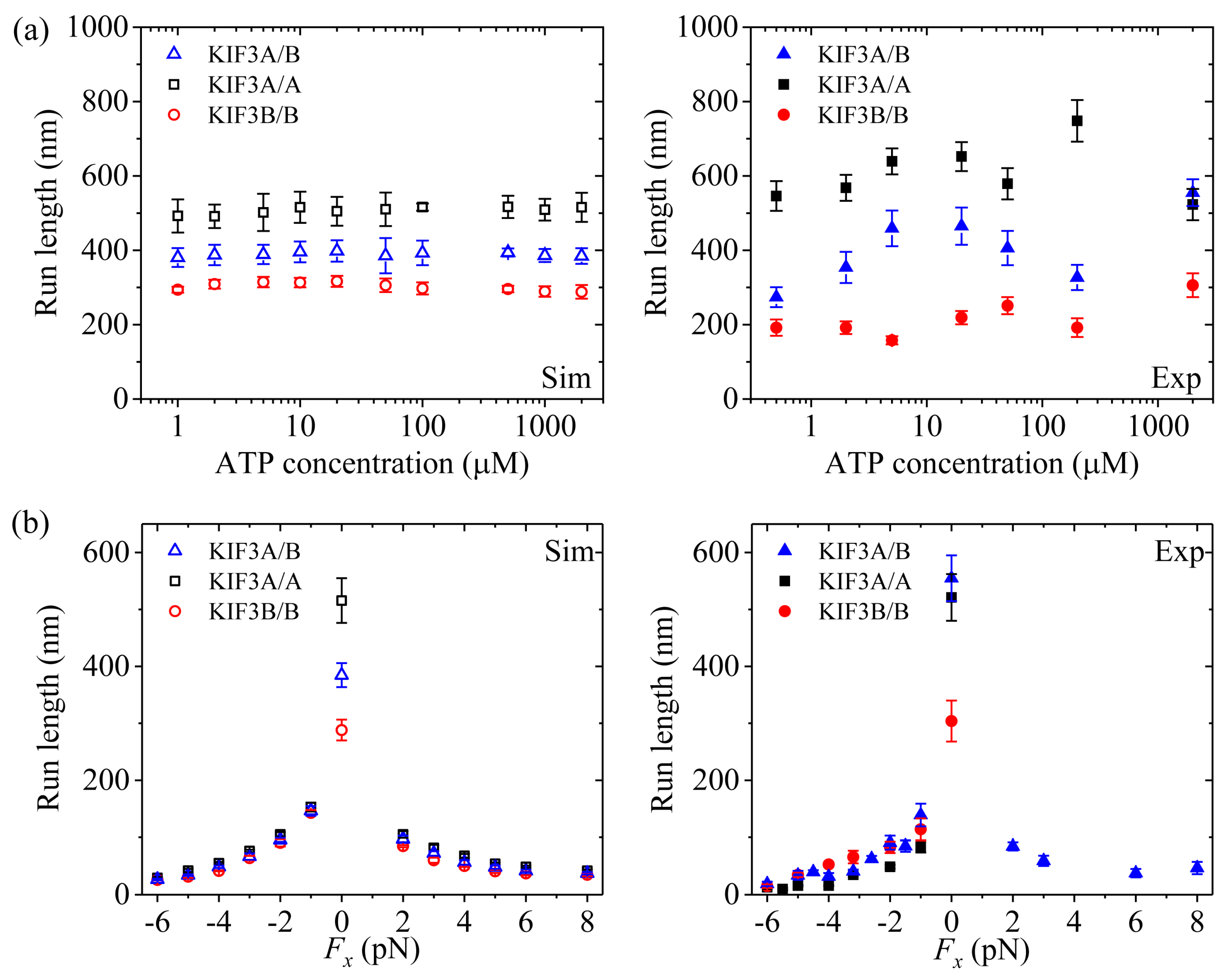
Figure 5a,b shows simulated results of the run length
versus [ATP] at different values of the applied load and
versus the applied load at different values of [ATP], respectively. For comparisons, the corresponding single-molecule data of Valentine and Block [
15] are also plotted in
Figure 5. It is seen that the simulated and experimental data are in quantitative agreement with each other.
Second, we study kinesin-2.
Figure 6a (left panel) shows simulated results of the run length
versus [ATP] under no applied load.
Figure 6b (left panel) shows simulated results of the run length
versus the applied load at saturating [ATP] (2 mM). For comparisons, the corresponding single-molecule data of Andreasson et al. [
8,
18] are shown in right panels of
Figure 6. It is seen that the simulated and experimental data show consistent with each other.
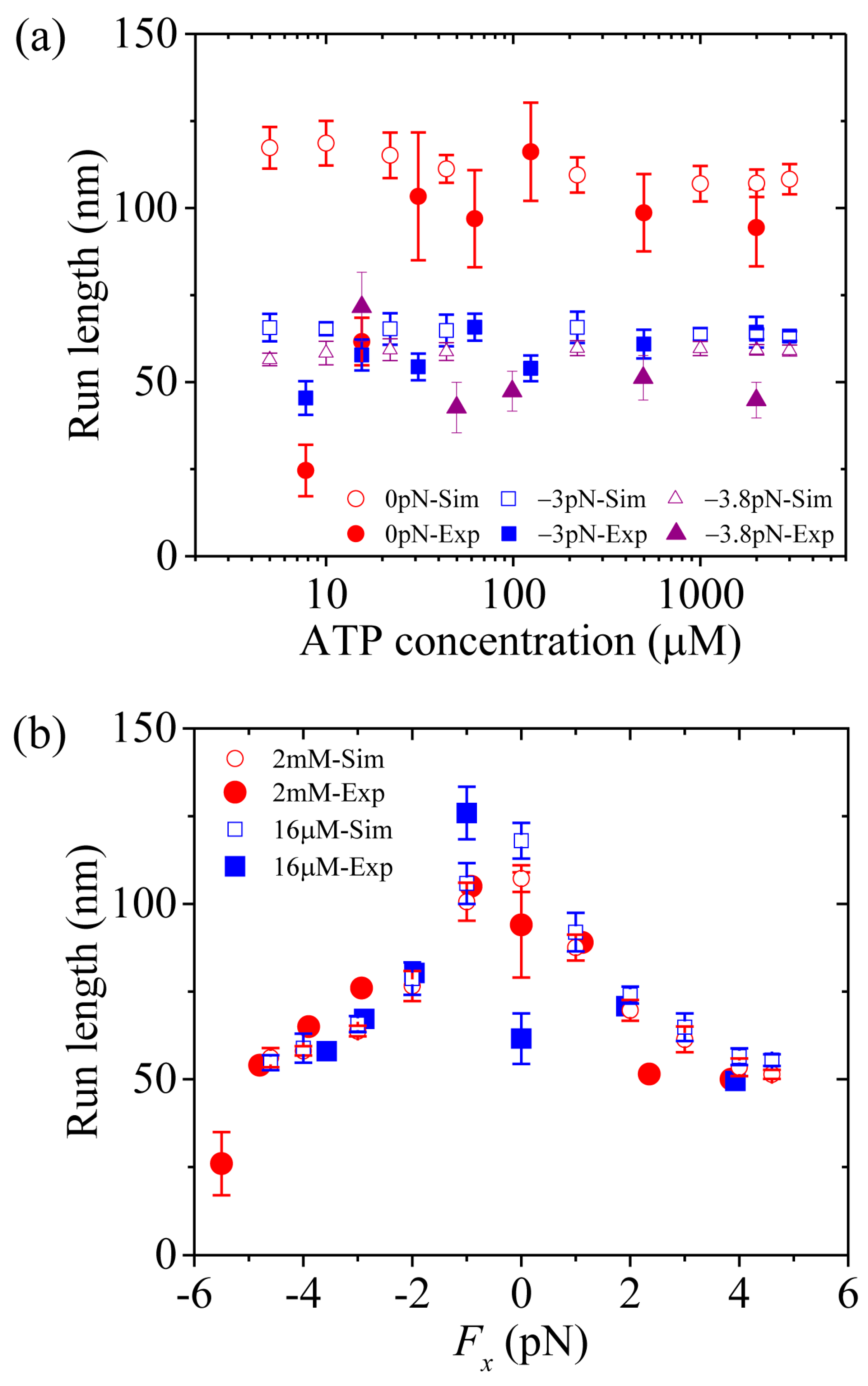
Third, we study kinesin-1. The results simulated using
= 1 were presented before [
21]. Here, we use
= 1.2 (see
Table 1) to make simulations.
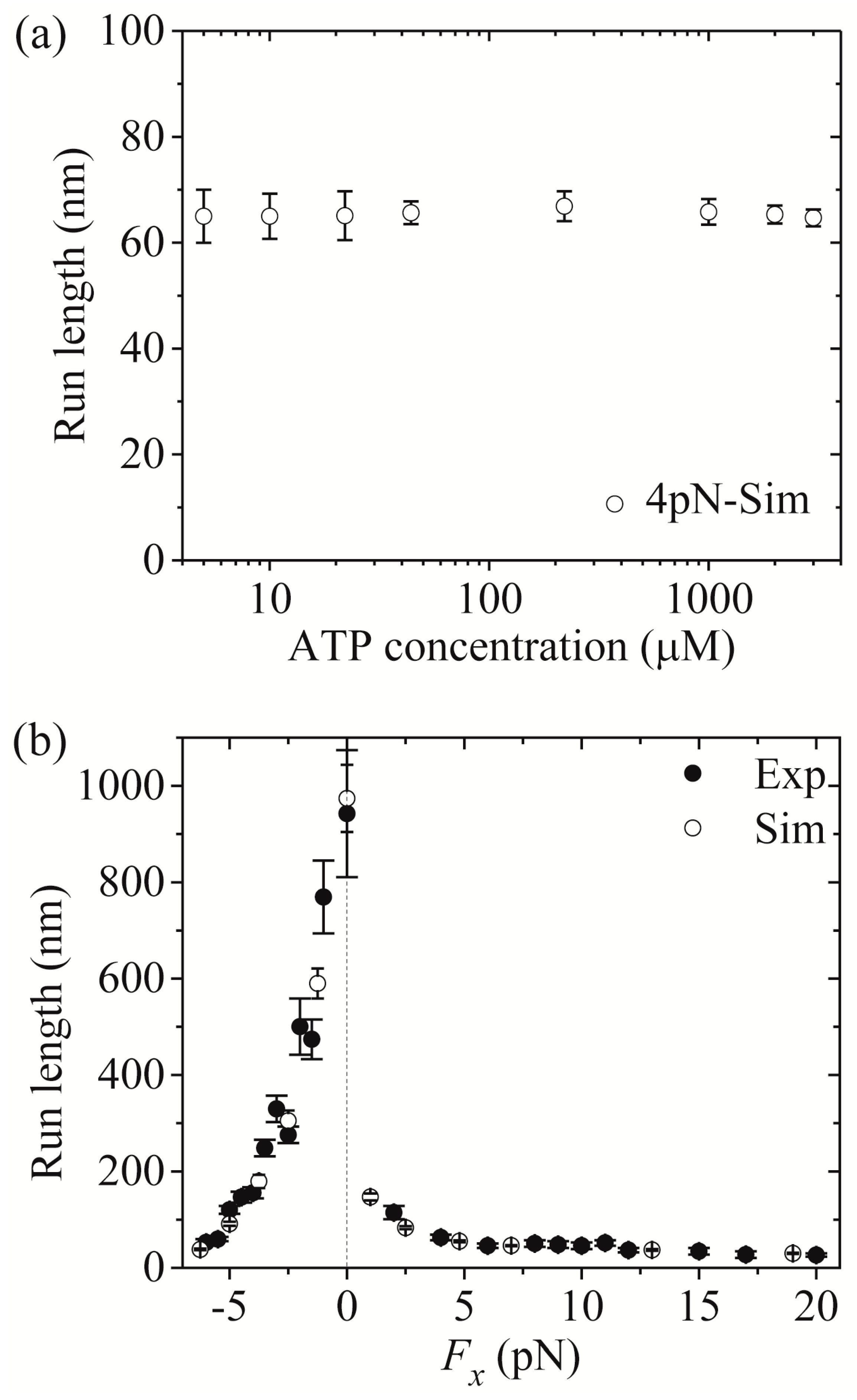
Figure 7a,b shows simulated results of the run length
versus [ATP] under a forward load (4 pN) and
versus the applied load at saturating [ATP] (2 mM), respectively, where the corresponding single-molecule data of Milic et al. [
16] and Andreasson et al. [
17] are also plotted for comparisons. The simulated data are in quantitative agreement with the experimental data.
Now, we make comparisons of the results among the three kinesin dimers. First, we focus on the dependence of the run length upon [ATP]. From
Figure 5a,
Figure 6a and
Figure 7a we see that the simulated run lengths for the three kinesin dimers are nearly independent of [ATP]. However, it is noted here that for simplicity of treatment, we have not taken into account the dissociation in the period of the
- head binding strongly to MT. Taking into account the small dissociation probability in the
long period when the MT-bound head in INT state is in
state (
Figure 1b) at low [ATP], the run length at low [ATP] should be slightly shorter than that at high [ATP], which is in better agreement with the single-molecule data [
8,
15,
16,
17].
Then, we turn to the run length
versus the applied load. By comparing
Figure 5b (for kinesin-5) with
Figure 7b (for kinesin-1), it is interestingly seen that for kinesin-5 the run length is nearly symmetric with respect to the loading direction, and by contrast, for kinesin-1 the run length is dramatically asymmetric with respect to the loading direction, with the run length under a moderate backward load being much larger than that under a moderate forward load. The rather different features of the effect of loading direction on the run length between the two kinesin dimers can be explained as follows.
Since the moving time of the detached ADP-head between the MT-binding site and INT position is very short (in the order of 1–10 μs), the dissociation of the dimer from MT takes place mainly in INT state, where one head with undocked NL is bound to MT and the other ADP-head is bound strongly to the MT-bound head. Moreover, since the MT-bound head in
, ATP or ADP.Pi state binds strongly to MT, its dissociation from MT is not considered. Hence, the dissociation of the dimer takes place mainly during two periods in INT state. One is the period after Pi is released from the MT-bound head and before the binding energy of the MT-bound ADP-head to MT changes from
Ew1 to
Ew2 (called Period I, see
Figure 1f). In Period I, since
Ew1 is very small, the MT-bound head can easily dissociate from MT by overcoming
Ew1 within time
tr. The other period is that the MT-bound head in INT state is in ADP state, with its binding energy to MT being
Ew2 (called Period II, see
Figure 1g). In Period II, the MT-bound head can dissociate from MT with a large probability by overcoming
Ew2 before ADP is released within a time (1/
kD) that is much longer than
tr.
For kinesin-1, in INT state the NL-docking rate of the MT-bound head in ADP.Pi state,
kNL = 800 s
−1 (see
Table 1), is only 5-fold larger than its Pi-release rate,
kPi = 160 s
−1 (see
Table 1), when the NL points forward. On the other hand, in INT state before the NL docking of the MT-bound head the NL is driven to point forward under the forward load. Thus, it has a large probability in each step for the Pi release to take place in INT state before the NL docking takes place under a forward load, giving a large probability for the occurrence of Period I. By contrast, under no or backward load with the NL of the MT-bound head being not pointed forward in INT state, since
kNL = 800 s
−1 or
= 667 s
−1 (
= 1.2, see
Table 1) is much (about 400-fold or 333-fold) larger than the Pi-release rate,
= 2 s
−1 (
kPi = 160 s
−1 and
= 80, see
Table 1), there is a very small probability for the occurrence of Period I. Thus, under a forward load the kinesin-1 dimer in INT state can dissociate from MT with a much larger probability than under no or the moderate backward load, giving the run length under a moderate forward load to be much smaller than that under no or the intermediate backward load, with the run length being dramatically asymmetric with respect to the loading direction.
For kinesin-5, in INT state the NL-docking rate of the MT-bound head in ADP.Pi state,
kNL = 300 s
−1 (see
Table 1), is 20-fold larger than its Pi-release rate,
kPi = 15 s
−1 (see
Table 1), when the NL points forward. Thus, under a forward load there is a noticeable probability in each step for the Pi release to take place in INT state before the NL docking takes place, giving a noticeable probability for the occurrence of Period I. By contrast, under no or a backward load, with the NL of the MT-bound head in INT state being not pointed in the forward direction,
kNL = 300 s
−1 or
kNL/
λ = 75 s
−1 (
λ = 4, see
Table 1) is much larger than the Pi-release rate
kPi/
ρ = 0.19 s
−1 (
kPi = 15 s
−1 and
ρ = 80, see
Table 1), giving a negligible probability for the occurrence of Period I. On the other hand, since the affinity of ADP-head to MT in Period II,
Ew2 = 36.5
kBT, is relatively small, a large dissociation rate is expected in Period II. Thus, under no or the backward load the dissociation of the motor from MT arises almost solely from the dissociation taking place in Period II, while under the forward load the dissociation arises from both the dissociation taking place in Period II and the dissociation taking place in Period I. Due to the large dissociation rate in Period II, under the forward load the dissociation taking place in Period II would make a larger contribution to the overall dissociation than the dissociation taking place in Period I. It is noted that in Period II the dissociation rate is symmetric with respect to the direction of the applied load. On the other hand, the backward load decreases the velocity whereas the forward load has nearly no effect on the velocity (
Figure 2b). By contrast, the forward load has an effect whereas the backward load has nearly no effect on the dissociation taking place in Period I. Consequently, a near symmetry of the run length with respect to the loading direction is expected.
From
Figure 6b, it is seen that for kinesin-2, the run length under a forward load is also much smaller than that under no load, which is similar to the case of kinesin-1. As in the case of kinesin-1 (see above), this feature for kinesin-2 can also be explained as follows. In INT state, when the NL points forward under the forward load the NL-docking rate of the MT-bound head in ADP.Pi state,
kNL = 1200 s
−1 (see
Table 1), is only about 8-fold (KIF3A/A) and 6.7-fold (KIF3B/B) larger than the Pi-release rate,
kPi = 150 s
−1 for KIF3A/A and
kPi = 170 s
−1 for KIF3B/B (see
Table 1). Thus, the dissociation taking place in Period I under the forward load has a large effect on the run length.
By comparing
Figure 6b with
Figure 5b and
Figure 7b, it is interestingly seen that unlike kinesin-1 and kinesin-5, where run lengths are dependent only moderately on the backward load, there exist two regimes of processivity for kinesin-2: unloaded and loaded. Unloaded run lengths are long for kinesin-2, but even a small backward load can cause the run lengths to decrease greatly. The peculiar feature for kinesin-2 compared to kinesin-1 and kinesin-5 can be understood as follows.
For kinesin-1 and kinesin-5, under a backward load the NL-docking rates of the MT-bound heads in ADP.Pi state,
kNL/
λ = 667 s
−1 (
kNL = 800 s
−1 and
λ = 1.2, see
Table 1) and
kNL/
λ = 75 s
−1 (
kNL = 300 s
−1 and
λ = 4, see
Table 1), are much larger than the Pi-release rates,
kPi/
ρ = 2 s
−1 (
kPi = 160 s
−1 and
ρ = 80, see
Table 1) and
kPi/
ρ = 0.19 s
−1 (
kPi = 15 s
−1 and
ρ = 80, see
Table 1), respectively. Thus, the dissociation probabilities of the two motors from MT which arise from the dissociations taking place in period I are much smaller than those which arise from the dissociations taking place in period II, as in the case under no load. As a result, their run lengths are expected to depend only moderately upon the backward load. By contrast, for kinesin-2 KIF3A/A, under a backward load the NL-docking rate of the MT-bound head in ADP.Pi state,
kNL/
λ = 300 s
−1 (
kNL = 1200 s
−1 and
λ = 4, see
Table 1), is about 34-fold larger than the Pi-release rate,
kPi/
ρ = 8.8 s
−1 (
kPi = 150 s
−1 and
ρ = 17, see
Table 1). On the other hand, since the affinity of ADP-head to MT in Period II,
Ew2 = 40.8
kBT, is large, a small dissociation rate is expected in Period II. Thus, the dissociation probability of kinesin-2 KIF3A/A from MT which arises from the dissociation taking place in period I has a large effect on the run length. By comparison, under no load the NL-docking rate of the MT-bound head in ADP.Pi state,
kNL = 1200 s
−1 (see
Table 1), is about 136-fold larger than the Pi-release rate,
kPi/
ρ = 8.8 s
−1 (
kPi = 150 s
−1 and
= 17, see
Table 1). Hence, a backward load can cause a large reduction of the run length compared to that under no load.
It should be mentioned here that apart from the above explanation of the unloaded and loaded effects on the run length of kinesin-2, an additional possible explanation is stated as follows. For the unloaded case, in INT state an interaction could be present between the MT-bound head and coiled-coil segment, inhibiting Pi release (i.e., increasing
) in INT state and thus reducing the occurrence probability of Period I. By contrast, the load on the coiled-coil could disrupt this interaction between the MT-bound head and coiled-coil segment, with the Pi-release rate (i.e., with
) in INT state as given in
Table 1. In this work, we have not considered this effect. The inclusion of the effect will further enlarge the difference between the unloaded and loaded run lengths but have little effect on the velocity because
is very large.
Taken together, although the three families of kinesin dimers show much similar mechanism of processive movement they show rather different features of the run length versus the external load. The rather different features arise mainly from the different values of the rate constants of the ATPase activity and NL docking.
3.3. Dynamics of Kinesin Dimers with Changed NL Lengths
First, we study kinesin-5 with the NL of each head being shortened to 14 AAs via deletion of the last four AAs (ΔKKAL to make Kin5
14), as done in Shastry and Hancock [
20]. Since for Kin5
14 the internally stretched force on the NLs has a large value of about 33 pN in 2HB state (see
Figure S4, at extension of 4.1 nm), the rates of ADP release from the leading and trailing heads are taken as
and
(
kD = 50 s
−1, see
Table 1), with
= 7. In INT state, the rate of ADP release from the MT-bound head is also equal to
kD. Since the four deleted AAs are not involved in the NL docking and are distant away from the motor domain, the deletion should have no effect on the interaction between the head and MT, interaction between the two heads, NL docking and ATPase activity except ADP release. Hence, we take the same values for all parameters for Kin5
14 as those for the wild-type case (called Kin5
18) except
kD and the force-extension relation of the NL (see
Figure S4). In
Figure 8a,b (left panels) we show simulated results of the velocity and run length, respectively, under no load at saturating [ATP] (2 mM) for Kin5
14, and compare with the corresponding results for Kin5
18. Interestingly, from
Figure 8 we see that while the velocity for Kin5
14 is almost unchanged relative to Kin5
18, the run length for Kin5
14 is increased significantly. These results on the dependence of run length and velocity upon length of NL are in good agreement with the experimental data of Shastry and Hancock [
20] (right panels of
Figure 8). The results can be explained as follows. Since for both Kin5
18 and Kin5
14 the rates of ADP release from the leading head are much larger than the rates of Pi release in the trailing head, their velocities are mainly determined by the rates of Pi release in the trailing head. Since the rates of Pi release in the trailing head for Kin5
18 and Kin5
14 are the same, their velocities are nearly the same. Since for Kin5
14 the rate of ADP release from the leading head is much larger than that for Kin5
18, the probability of Pi release taking place in the trailing head before ADP release taking place in the leading head for Kin5
14 is much smaller than that for Kin5
18, i.e., the occurrence probability of Period II for Kin5
14 is much smaller than that for Kin5
18. Thus, the run length for Kin5
14 is much longer than that for Kin5
18.
Second, we study kinesin-2 (KIF3A/B) with the NL of each head being shortened to 14 AAs by deleting the last three AAs (ΔDAL in KIF3A and KIF3B heads to make Kin2
14), as done in Mickolajczyk and Hancock [
19]. As in the case of Kin5
14 (see above), for Kin2
14 the rates of ADP release from the leading and trailing heads are taken as
and
(
kD = 90 s
−1, see
Table 1), with
= 3. In INT state, the rate of ADP release from the MT-bound head is also equal to
kD. In addition, we take the same values for all parameters for Kin2
14 as those for the wild-type case (called Kin2
17) except
kD and the force-extension relation of the NL (see
Figure S5). In
Figure 8a,b (left panels) we show simulated results of the velocity and run length, respectively, under no load at saturating [ATP] (2 mM) for Kin2
14, and compare with the corresponding results for Kin2
17. For comparison, the available single-molecule data [
19] are shown in right panels of
Figure 8. We see that the simulated and experimental data are in agreement with each other. The velocity for Kin2
14 has a small increase relative to Kin2
17 and the run length for Kin2
14 has a large increase relative to Kin2
17, which can be understood as follows. For Kin2
17 the rate of ADP release from the leading head is smaller than the rate of Pi release in the trailing head whereas for Kin2
14 the rate of ADP release from the leading head is larger than the rate of Pi release in the trailing head, resulting in the velocity for Kin2
17 to be smaller than that for Kin2
14. Since for Kin2
17 the rate of ADP release from the leading head is smaller than that for Kin2
14, the probability of Pi release in the trailing head taking place before ADP release taking place in the leading head for Kin2
17 is larger than that for Kin2
14, i.e., the occurrence probability of Period II for Kin2
17 is larger than that that for Kin2
14. Thus, the run length for Kin2
17 is shorter than that for Kin2
14. In addition, the simulated mean duration of one-head-bound (1HB) or INT state for Kin2
14 (about 5.4 ms) is shorter than that for Kin2
17 (about 8.6 ms) and the mean duration of 2HB state for Kin2
14 (about 8.4 ms) is equal to that for Kin2
17 (about 8.4 ms), which are also consistent with the single-molecule data [
19].
Third, we study kinesin-1 with the NL of each head being extended to 17 AAs by inserting three AAs (DAL) into the C-terminal portion of the linker region (called Kin1
17), as done in Mickolajczyk and Hancock [
19]. Since for Kin1
17 the internally stretched force on the NLs is small in 2HB state (see
Figure S6, at extension of 4.1 nm), the rates of ADP release from both heads are taken to be the same, with
(
kD = 350 s
−1 and
= 2.3), as done for kinesin-5 and kinesin-2 (see above). In INT state, the rate of ADP release from the MT-bound head is also equal to
. Since the added AAs in the NL are distant away from the motor domain, the addition should have no effect on the interaction between the head with MT, interaction between the two heads, NL docking and ATPase activity except ADP release. Thus, we take the same values for all parameters for Kin1
17 as those for wild-type kinesin-1 (Kin1
14) except
kD and the force-extension relation of the NLs (see
Figure S6).
In
Figure 8a,b (left panels) we show simulated results of the velocity and run length, respectively, under no load at saturating [ATP] (2 mM) for Kin1
17, where for comparison the corresponding simulated results for Kin1
14 are re-shown. The available single-molecule data [
19] are shown in right panels of
Figure 8. We see that the simulated and experimental data are in agreement with each other. Both the velocity and run length for Kin1
17 are reduced relative to those for Kin1
14, with the velocity having a small reduction and the run length having a large reduction, which can be explained as follows. For Kin1
17 with the nearly zero internally stretched force of the NLs, upon Pi release the trailing head cannot escape the potential well of depth
Ew1 = 19.8
kBT (see
Table 1) within time
tr with 100% probability, and thus more than one ATP molecules are hydrolyzed in the trailing head to make a forward step (noting that for kinesin-2 and kinesin-5, since
Ew1 18
kBT, even with nearly zero tension on the NLs, upon Pi release the trailing head can escape the potential well of depth
Ew1 within time
tr with nearly 100% probability). By contrast, for Kin1
14, upon Pi release the large internally stretched force (about 30 pN) of the NLs can easily drive the trailing head to escape the potential well of depth
Ew1 within time
tr with 100% probability, and thus one ATP hydrolysis gives one forward step. Consequently, the velocity for Kin1
17 is smaller than that for Kin1
14. Since for Kin1
17 the rate of ADP release from the leading head is smaller than that for Kin1
14, the occurrence probability of Period II for Kin1
17 is larger than for Kin1
14. Thus, the run length for Kin1
17 is shorter than that for Kin1
14. In addition, the simulated mean duration of 1HB state for Kin1
14 (about 3.2 ms) is shorter than that for Kin1
17 (about 5.3 ms) and the mean duration of 2HB state for Kin1
14 (about 7.8 ms) is close to that for Kin1
17 (about 8.4 ms), which are also consistent with the experimental data [
19].
Taken together, for the three families of kinesin dimers with longer NL lengths (17 or 18 AAs) the run lengths are reduced largely relative to those with shorter NL length (14 AAs). The reductions arise from the fact that the occurrence probability of INT state with the ADP-head bound to MT with the weak binding energy Ew2 (i.e., the occurrence probability of Period II) is increased greatly, which is in turn due to the fact that the ADP-release rate in the leading head is decreased greatly because of the much reduced internally stretched force on the NLs.
3.4. Limping Effect of Kinesin Homodimers
Up to now, for simplicity of treatment, we have implicitly considered that the distance between the C-terminal end (i.e., the end connecting to the coiled-coil stalk) of one NL and that of another NL along the
x direction (the movement direction) is zero. By considering a very small but non-zero distance along the
x direction, which is called biased distance and is denoted by
<<
d, and using model of
Figure 1 but with the NL-docking rate
kNL in ATP and ATP.Pi state, we showed that the asymmetric or limping movement dynamics of kinesin-1 homodimer can be explained well in our previous work [
24].
In this section, we also consider a non-zero
but finite values of
kNL to study the limping effect of homodimeric kinesin-1, kinesin-2 KIF3B/B and kinesin-5 with and without changes of NL lengths by using model of
Figure 1 (considering a non-zero
, the schematic of two successive forward steps of the homodimer at saturating [ATP] is shown in
Figure S7). As discussed in detail before [
24], the available evidence indicated that during the processive stepping of the kinesin dimer, the structure of the coiled-coil stalk is kept very stable, without unwinding [
46], and moreover the stalk is kept in the fixed orientation [
47,
48]. Thus, to study limping we take the following into consideration. For odd steps (from
Figure S7a to
Figure S7c), before stepping the NL of each head in 2HB state is stretched to a length
(
Figure S7a) and after stepping the NL is stretched to a length
(
Figure S7c). For even steps (from
Figure S7c to
Figure S7e), before stepping the NL is stretched to a length
(
Figure S7c) and after stepping the NL is stretched to a length
(
Figure S7e). In this section, we focus on saturating [ATP] and take
= 0.4 nm, which is much smaller than
d = 8.2 nm, and values of all other parameter as given in
Table 1.
First, we consider homodimeric kinesin-1. We fix longitudinal force
Fx = −5 pN and vary vertical force
Fy, as done in the single-molecule optical trapping experiments [
49,
50].
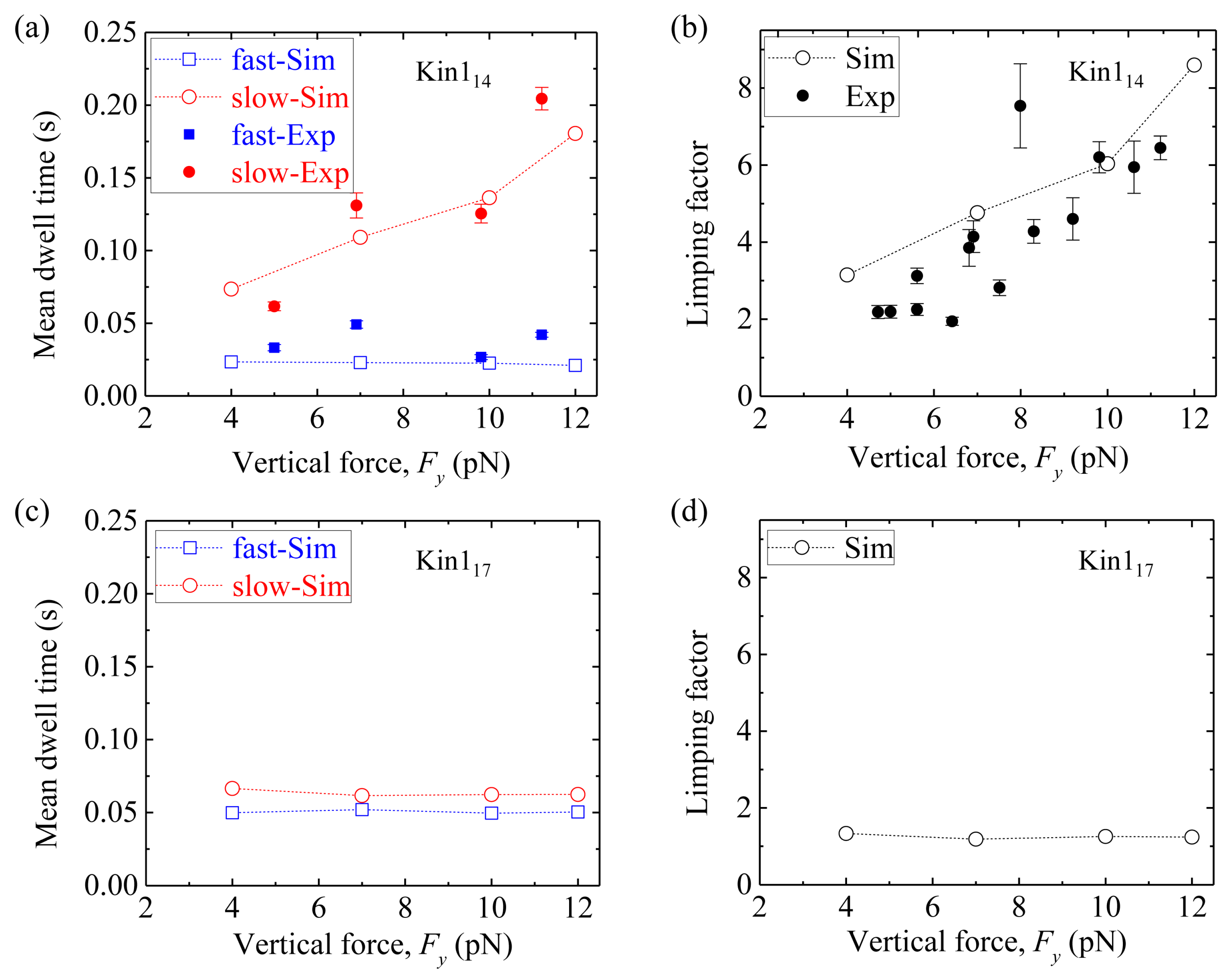
Figure 9a shows simulated results of the mean dwell time of the odd steps and that of the even steps
versus vertical force
Fy for Kin1
14, where for comparison the available single-molecule data [
49,
50] are also shown. We see that the mean dwell time of the odd steps are longer than the even steps, and the mean dwell time of the odd steps (also called slow steps) increases with
Fy whereas the mean dwell time of the even steps (also called fast steps) remains invariant.
Figure 9b shows the results of the limping factor (defined as the ratio of the mean dwell time of the slow steps to that of the fast steps) calculated using data of
Figure 9a, and compares with the experimental data [
49,
50].
We see that the limping factor for Kin1
14 increases with
Fy. All the simulated results are consistent with the experimental data [
49,
50].
Figure 9c shows simulated results of the mean dwell time of the odd steps and that of the even steps
versus Fy for Kin1
17. We see that both the mean dwell time of the odd steps and that of even steps are nearly independent of
Fy and both have the nearly same values, giving nearly no limping effect, with the limping factor nearly equal to one (
Figure 9d).
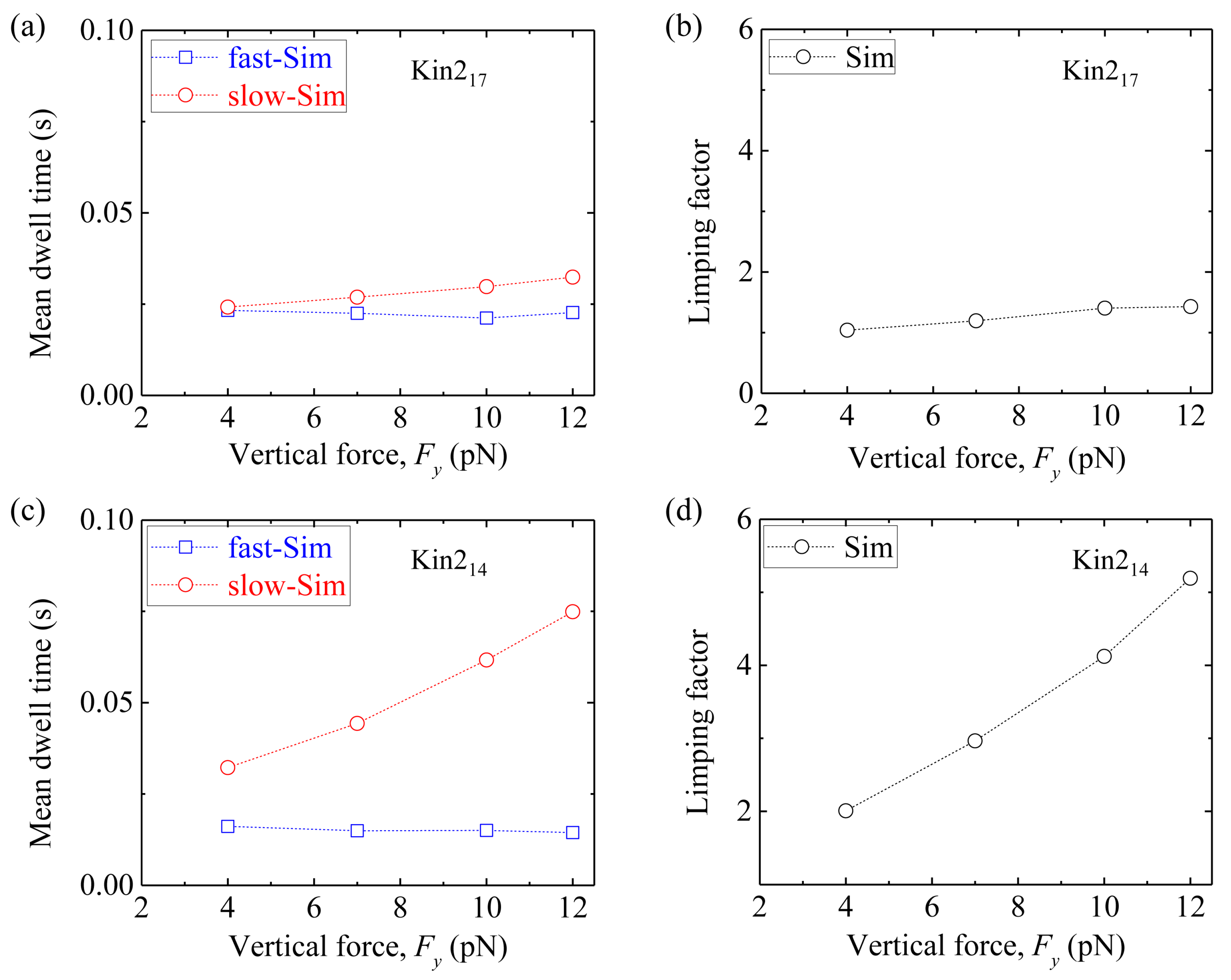
Second, we consider homodimeric KIF3B/B kinesin-2. We fix longitudinal force
Fx = −5.7 pN and vary vertical force
Fy.
Figure 10a shows simulated results of the mean dwell time of the odd steps and that of the even steps
versus Fy for Kin2
17. The corresponding results of the limping factor
versus Fy are shown in
Figure 10b. It is seen that Kin2
17 shows nearly no limping effect, as in the case of Kin1
17.
Figure 10c shows simulated results of the mean dwell time of the odd steps and that of the even steps
versus Fy for Kin2
14. The corresponding results of the limping factor
versus Fy are shown in
Figure 10d. As expected, Kin2
14 shows similar limping effect to Kin1
14.
Third, we consider homodimeric kinesin-5. We fix longitudinal force
Fx = −3 pN and vary vertical force
Fy.
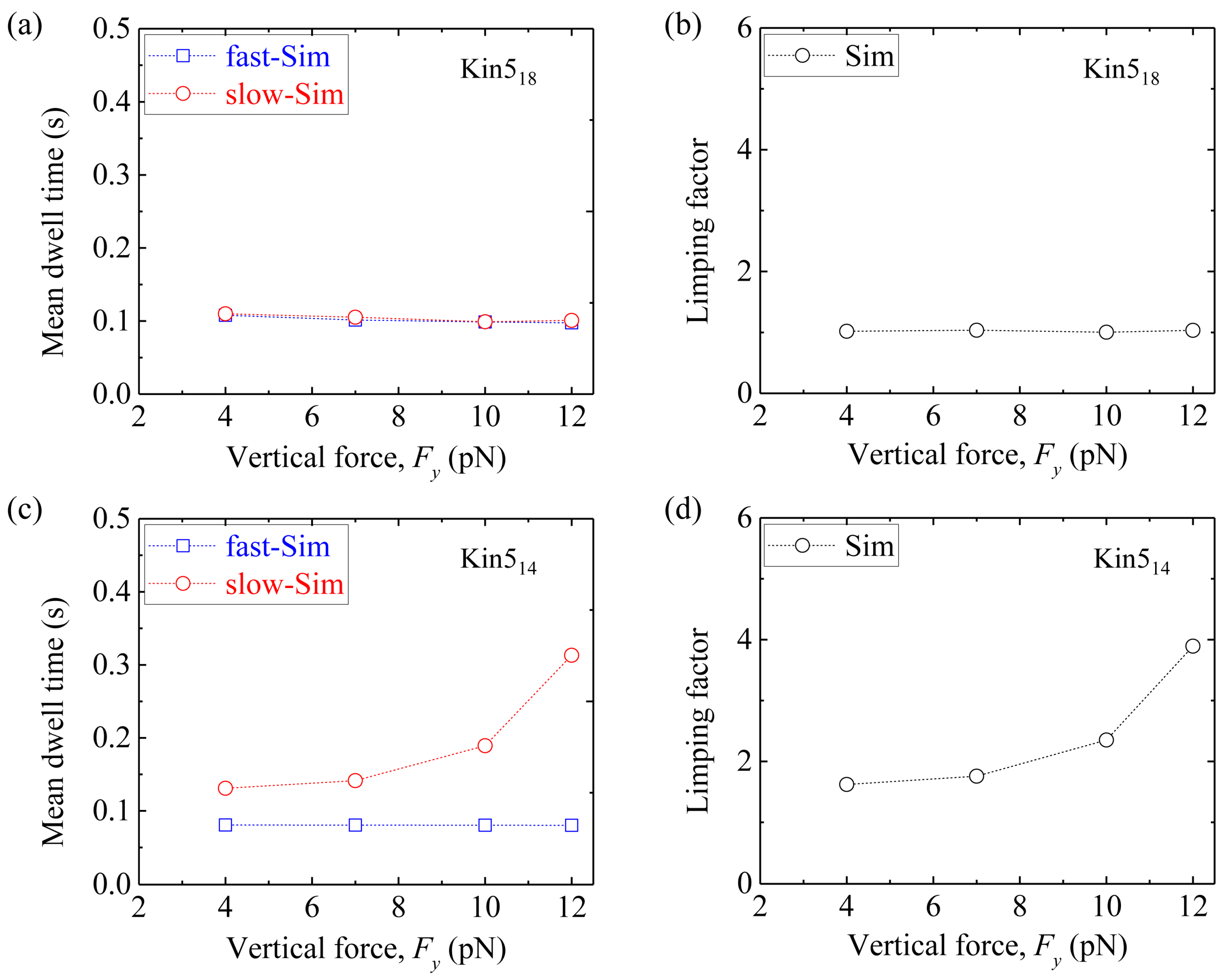
Figure 11a shows simulated results of the mean dwell time of the odd steps and that of the even steps
versus Fy for Kin5
18. The corresponding results of the limping factor
versus Fy are shown in
Figure 11b. As expected, Kin5
18 shows no limping effect, as in the case of Kin1
17 and Kin2
17.
Figure 11c shows simulated results of the mean dwell time of the odd steps and that of the even steps
versus Fy for Kin5
14. The corresponding results of the limping factor
versus Fy are shown in
Figure 11d. As expected, Kin5
14 shows similar limping effect to Kin1
14 and Kin2
14.
Taken together, our simulated results show that while the wild-type kinesin-1 homodimer (Kin1
14) shows the limping behavior, the kinesin-1 homodimer with extended NLs (Kin1
17) does not show the limping behavior. By contrast, while the homodimeric kinesin-2 (Kin2
17) and kinesin-5 (Kin5
18) without change of the NLs do not show the limping behavior, the homodimeric kinesin-2 and kinesin-5 with shortened NLs (Kin2
14 and Kin5
14) show the limping behavior. While the simulated results on the limping dynamics of Kin1
14 are consistent with the available single-molecule data [
49,
50], the predictions on the limping effect of Kin1
17, Kin2
17, Kin5
18, Kin2
14 and Kin5
14 can be tested easily by using single-molecule optical trappings.
It is noted here that the consideration that during the processive stepping of the kinesin dimer its stalk is kept in the fixed orientation is not inconsistent with the recent single-molecule data of Ramaiya et al. [
51]. This consideration implies that an intrinsic torque is present to keep the stalk in its equilibrium angle, where the line connecting the two C-terminus ends of NLs is parallel to the
x axis. From the force-extension relation of the NL of the wild-type kinesin-1 head (
Figure S1), it is seen that when the NL of the head is stretched to a length
= 4.3 nm the internally elastic force has a large value of about 53 pN, whereas when the NL is stretched to a length
= 3.9 nm, the internally elastic force is only about 15 pN. Thus, if the line connecting the two C-terminus ends of NLs is deviated slightly from that along the
x axis, on average, a torque is present to make the coiled-coil stalk rotate in one direction during kinesin-1 processive movement at high [ATP], because a rotation angle relaxes to the equilibrium angle slowly with a timescale of 1 s [
51]. Thus, accompanying the forward processive movement at high [ATP], the stalk would rotate gradually in one direction by overcoming the intrinsic torque until the torque caused by the stretching of the NL becomes equal to the intrinsic torque. However, at very low [ATP], a long time is present in INT state, during which the rotation induced by the torque caused by the stretching of the NL can be relaxed to its equilibrium angle. Thus, at very low [ATP], no rotation of the stalk would be observed, whereas at high [ATP] a rotation of small angle in one direction would be observed. Moreover, since the line connecting the two C-terminus ends of NLs can be deviated from the
x axis in both directions with equal probability, both right- and left-handed rotations would be observed with equal probability. All these are in agreement with the single-molecule data of Ramaiya et al. [
51]. It is noted that since the rotation angle is small (< 10°) [
51], the rotation has little effect on the value of
.
In addition, from the force-extension relation of the NL of the Kin1
17 head (
Figure S6), it is seen that when the NL of each head is stretched to a length
= 4.3 nm the internally elastic force is only about 11 pN, and when the NL is stretched to a length
= 3.9 nm the internally elastic force is only about 5 pN. Thus, it is predicted that for Kin1
17, nearly no rotation of the stalk would be observed even at saturating [ATP]. Similarly, it is predicted that for a kinesin homodimer possessing no limping effect such as homodimeric kinesin-2 (Kin2
17) and kinesin-5 (Kin5
18), nearly no rotation of the stalk would be observed even at saturating [ATP]. By contrast, for a kinesin homodimer possessing pronounced limping effect, the rotation of small angle would be observed at high [ATP].