Castanopsis lamontii Water Extract Shows Potential in Suppressing Pathogens, Lipopolysaccharide-Induced Inflammation and Oxidative Stress-Induced Cell Injury
Abstract
:1. Introduction
2. Results and Discussion
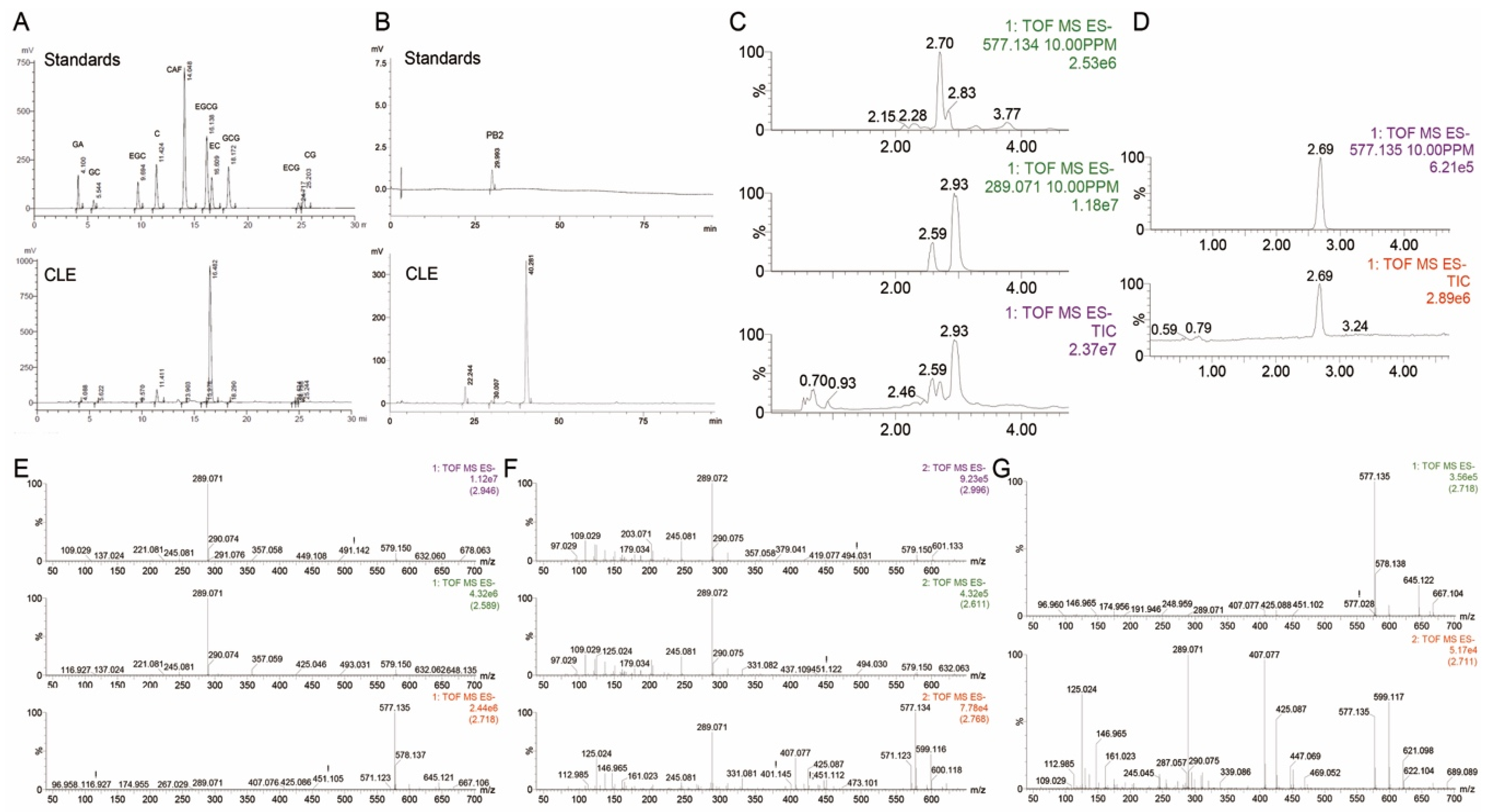
2.1. Main Chemical Composition of CLE
2.2. Antibacterial Activities of CLE
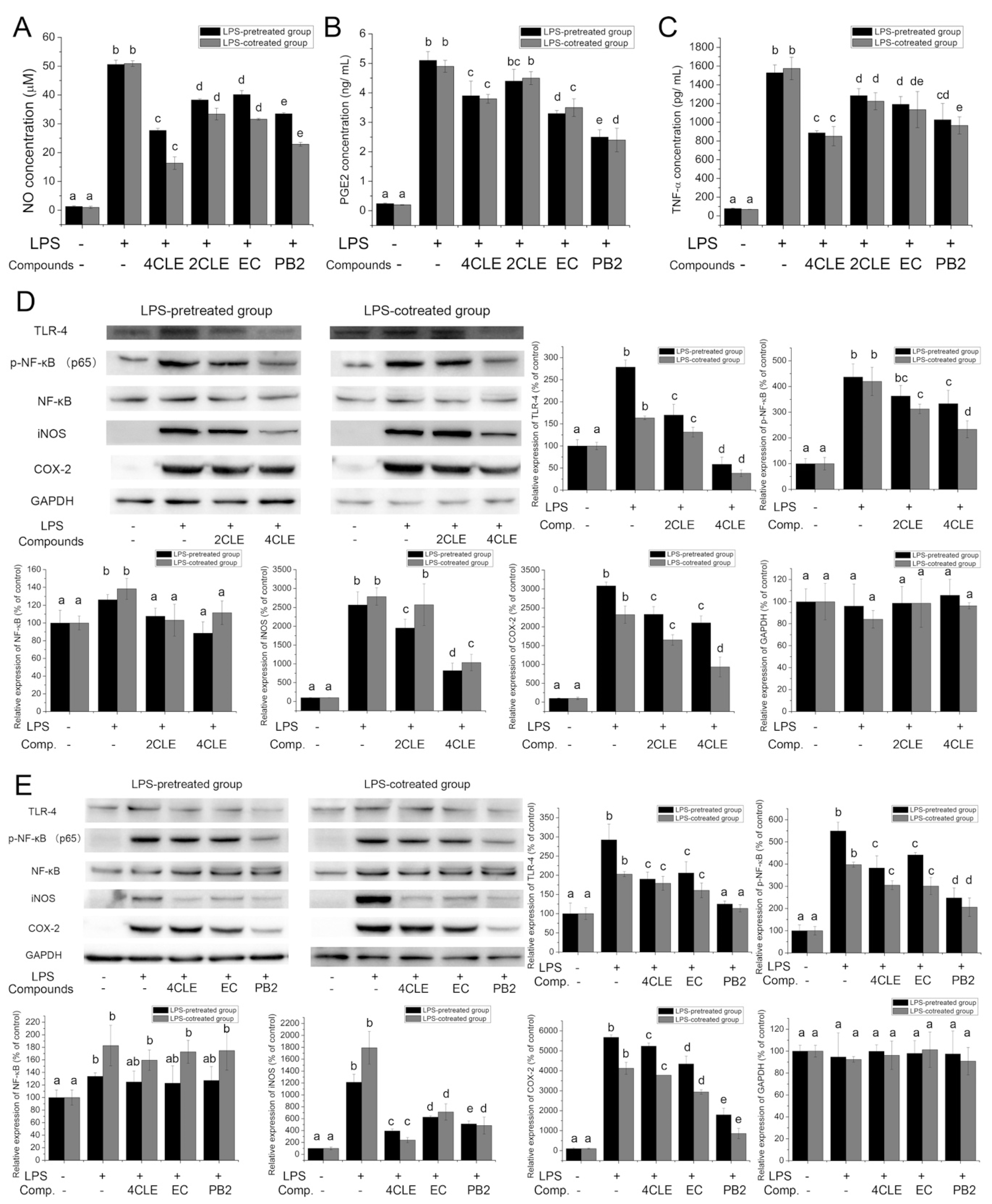
2.3. Anti-Inflammatory Activities of CLE
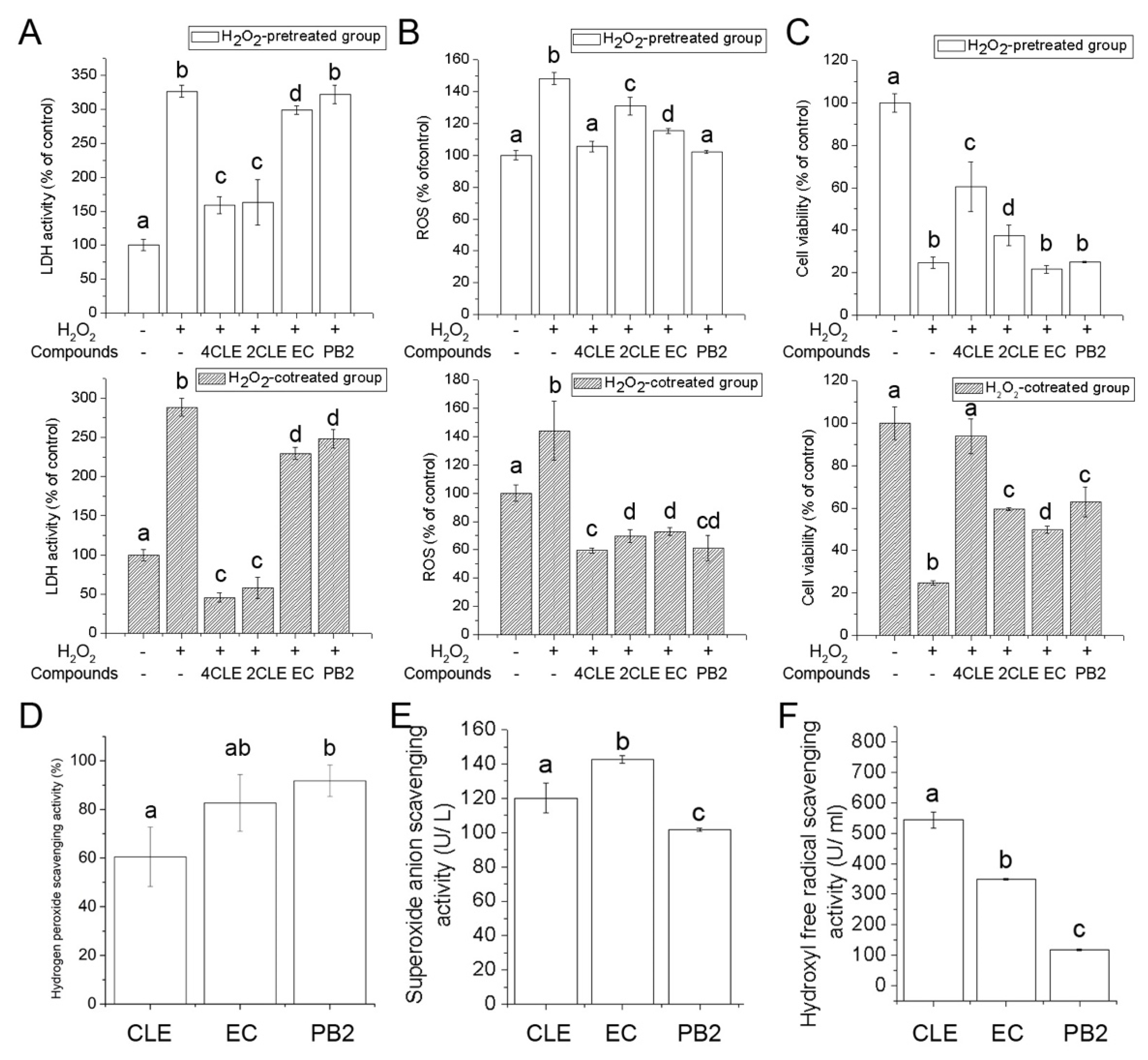
2.4. Antioxidant Activities of CLE
2.5. Acute Oral Toxicity of CLE
2.6. Effects of CLE, EC and PB2 on Normal RAW 264.7 Cells
3. Materials and Methods
3.1. Materials
3.2. Preparation of CLE
3.3. Determination of Chemical Composition
3.4. HPLC Analysis
3.5. UPLC-Q-TOF/MS Analysis
3.6. Determination of Minimum Inhibitory Concentrations (MIC) against Bacteria
3.7. Determination of Anti-Inflammatory Activities in LPS-Treated RAW264.7 Cells
3.8. Western Blot
3.9. Determination of Antioxidant Activities in Hydrogen Peroxide-Treated RAW264.7 Cells
3.10. Determination of Free Radical Scavenging Activities
3.11. Acute Oral Toxicity Tests
3.12. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, J.; Huang, Y.; Li, K.; Chen, Y.; Vanegas, D.; McLamore, E.S.; Shen, Y. Leaf extract from lithocarpus polystachyus rehd. Promote glycogen synthesis in t2dm mice. PLoS ONE 2016, 11, e0166557. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.K.; Tangpu, V. Antidiarrheal activity of lithocarpus dealbata and urena lobata extracts: Therapeutic implications. Pharm. Biol. 2007, 45, 223–229. [Google Scholar] [CrossRef]
- Khan, M.R.; Kihara, M.; Omoloso, A.D. Antimicrobial activity of lithocarpus celebicus. Fitoterapia 2001, 72, 703–705. [Google Scholar] [CrossRef]
- Genco, C.A.; Van Dyke, T.; Amar, S. Animal models for porphyromonas gingivalis-mediated periodontal disease. Trends Microbiol. 1998, 6, 444–449. [Google Scholar] [CrossRef]
- Al-Charrakh, A.H.; Al-Khafaji, J.K.; Al-Rubaye, R.H. Prevalence of beta-hemolytic groups c and f streptococci in patients with acute pharyngitis. N. Am. J. Med. Sci. 2011, 3, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.B.; Shao, M.; Gao, H.Y.; Wu, B.; Wu, L.J. Study on bacteriostasis of fagopyrum cymosum meisn. Chin. J. Microecol. 2005, 17, 330–331. [Google Scholar]
- Ci, X.K.; Chen, L.P.; Ou, X.Y. Grape seed proanthocyanidin extracts inhibit lipopolysaccharide of porphyromonas gingivalis. Shanghai J. Stomatol. 2015, 24, 433–436. [Google Scholar]
- Sharma, A.; Sati, S.C.; Sati, O.P.; Sati, M.D.; Kothiyal, S.K.; Semwal, D.K.; Mehta, A. A new triterpenoid saponin and antimicrobial activity of ethanolic extract from sapindus mukorossi gaertn. J. Chem. 2013, 2013, 218510. [Google Scholar]
- Wang, W.; Chen, R.; Wang, J. Procyanidin b2 ameliorates carrageenan-induced chronic nonbacterial prostatitis in rats via anti-inflammatory and activation of the nrf2 pathway. Biochem. Biophys. Res. Commun. 2017, 493, 794–799. [Google Scholar] [CrossRef]
- Martinez-Micaelo, N.; Gonzalez-Abuin, N.; Pinent, M.; Ardevol, A.; Blay, M. Procyanidin b2 inhibits inflammasome-mediated il-1beta production in lipopolysaccharide-stimulated macrophages. Mol. Nutr. Food Res. 2015, 59, 262–269. [Google Scholar] [CrossRef]
- Yang, H.; Xiao, L.; Yuan, Y.; Luo, X.; Jiang, M.; Ni, J.; Wang, N. Procyanidin b2 inhibits nlrp3 inflammasome activation in human vascular endothelial cells. Biochem. Pharmacol. 2014, 92, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Sung, N.Y.; Yang, M.S.; Song, D.S.; Kim, J.K.; Park, J.H.; Song, B.S.; Park, S.H.; Lee, J.W.; Park, H.J.; Kim, J.H.; et al. Procyanidin dimer b2-mediated irak-m induction negatively regulates tlr4 signaling in macrophages. Biochem. Biophys. Res. Commun. 2013, 438, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.Y.; Liu, H.Q.; Xie, K.Q.; Yin, L.L.; Li, Y.; Kwik-Uribe, C.L.; Zhu, X.Z. Procyanidin dimer b2 [epicatechin-(4beta-8)-epicatechin] suppresses the expression of cyclooxygenase-2 in endotoxin-treated monocytic cells. Biochem. Biophys. Res. Commun. 2006, 345, 508–515. [Google Scholar] [CrossRef]
- Yang, D.J.; Liu, S.C.; Chen, Y.C.; Hsu, S.H.; Chang, Y.P.; Lin, J.T. Three pathways assess anti-inflammatory response of epicatechin with lipopolysaccharide-mediated macrophage raw264.7 cells. J. Food Biochem. 2015, 39, 334–343. [Google Scholar] [CrossRef]
- Hakim, J. Reactive oxygen species and inflammation. Comptes Rendus des Seances de la Societe de Biologie et de ses Filiales 1993, 187, 286–295. [Google Scholar] [PubMed]
- O’Brien, J.; Kla, K.M.; Hopkins, I.B.; Malecki, E.A.; McKenna, M.C. Kinetic parameters and lactate dehydrogenase isozyme activities support possible lactate utilization by neurons. Neurochem. Res. 2007, 32, 597–607. [Google Scholar] [CrossRef] [PubMed]
- Simos, Y.V.; Verginadis, I.I.; Toliopoulos, I.K.; Velalopoulou, A.P.; Karagounis, I.V.; Karkabounas, S.C.; Evangelou, A.M. Effects of catechin and epicatechin on superoxide dismutase and glutathione peroxidase activity, in vivo. Redox Rep. 2012, 17, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.Y.; Zhang, X.Y.; Guan, S.W.; Hua, Z.C. Protective effect of procyanidin b2 against ccl4-induced acute liver injury in mice. Molecules 2015, 20, 12250–12265. [Google Scholar] [CrossRef]
- Su, H.; Li, Y.; Hu, D.; Xie, L.; Ke, H.; Zheng, X.; Chen, W. Procyanidin b2 ameliorates free fatty acids-induced hepatic steatosis through regulating tfeb-mediated lysosomal pathway and redox state. Free Radic. Biol. Med. 2018, 126, 269–286. [Google Scholar] [CrossRef]
- Fu, W.; Sui, D.; Yu, X.; Gou, D.; Zhou, Y.; Xu, H. Protective effects of ginsenoside rg2 against h2o2-induced injury and apoptosis in h9c2 cells. Int. J. Clin. Exp. Med. 2015, 8, 19938–19947. [Google Scholar]
- Wang, Z.J.; Xie, J.H.; Kan, L.J.; Wang, J.Q.; Shen, M.Y.; Li, W.J.; Nie, S.P.; Xie, M.Y. Sulfated polysaccharides from cyclocarya paliurus reduce h2o2-induced oxidative stress in raw264.7 cells. Int. J. Biol. Macromol. 2015, 80, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Kruger, N.J. The bradford method for protein quantitation. Methods Mol. Biol. 1994, 32, 9–15. [Google Scholar] [PubMed]
- Zhao, Q.D.; Wang, Y.; Shu, L.X.; Song, Z.G.; Li, J.M. Determination of total saponins in anti-inflammatory and antasthmatic extractum. J. Tianjin Univ. Tradit. Chin. Med. 2009, 28, 201–203. [Google Scholar]
- Xu, Y.Q.; Hu, X.F.; Tang, P.; Jiang, Y.W.; Yuan, H.B.; Du, Q.Z.; Yin, J.F. The major factors influencing the formation of sediments in reconstituted green tea infusion. Food Chem. 2015, 172, 831–835. [Google Scholar] [CrossRef] [PubMed]
- de Freitas, V.A.; Glories, Y.; Laguerre, M. Incidence of molecular structure in oxidation of grape seed procyanidins. J. Agric. Food Chem. 1998, 46, 376–382. [Google Scholar] [CrossRef]
- Eloff, J.N. A sensitive and quick microplate method to determine the minimal inhibitory concentration of plant extracts for bacteria. Planta Medica 1998, 64, 711–713. [Google Scholar] [CrossRef]
- Gao, Y.; Rankin, G.O.; Tu, Y.; Chen, Y.C. Theaflavin-3, 3′-digallate decreases human ovarian carcinoma ovcar-3 cell-induced angiogenesis via akt and notch-1 pathways, not via mapk pathways. Int. J. Oncol. 2016, 48, 281–292. [Google Scholar] [CrossRef]
Sample Availability: Samples of Castanopsis lamontii water extract, epicatechin and procyanidin B2 are available from the authors. |

| Components | Contents (%) |
|---|---|
| Polyphenols | 48.38 ± 0.192 |
| Soluble sugars | 24.43 ± 1.944 |
| Saponins | 12.19 ± 0.312 |
| Amino acids | 2.520 ± 0.168 |
| Polysaccharides | 2.184 ± 0.048 |
| Proteins | 1.440 ± 0.240 |
| Flavonoids | 1.056 ± 0.000 |
| Caffeine | Not detected |
| Components | Concentrations (μg/mL) |
|---|---|
| Epicatechin (EC) | 120.1 ± 1.1 |
| Procyanidin B2 (PB2) (including isomers) | 34.4 ± 3.2 |
| Catechin (C) | 9.6 ± 0.5 |
| Gallic acid (GA) | Not detected |
| Gallocatechin (GC) | 0.1 ± 0.0 |
| Epigallocatechin (EGC) | 0.5 ± 0.0 |
| Epigallocatechin gallate (EGCG) | 0.3 ± 0.0 |
| Gallocatechin gallate (GCG) | 0.1 ± 0.0 |
| Epicatechin gallate (ECG) | 0.1 ± 0.0 |
| Catechin gallate (CG) | 0.2 ± 0.0 |
| Concentrations of CLE (mg/mL) | 0 | 0.156 | 0.313 | 0.625 | 1.25 | 2.50 | 5.00 | 10.0 |
|---|---|---|---|---|---|---|---|---|
| Porphyromonas gingivalis | + | + | + | - | - | - | - | - |
| β-hemolytic Streptococcus | + | + | + | + | - | - | - | - |
| Staphylococcus aureus | + | + | + | + | + | - | - | - |
| Escherichia coli | + | + | + | + | - | - | - | - |
| Concentrations of EC (mg/mL) | 0 | 0.078 | 0.156 | 0.313 | 0.625 | 1.25 | 2.50 | 5.00 |
| Porphyromonas gingivalis | + | + | + | + | + | - | - | - |
| β-hemolytic Streptococcus | + | + | + | + | + | - | - | - |
| Staphylococcus aureus | + | + | + | + | + | - | - | - |
| Escherichia coli | + | + | + | + | + | - | - | - |
| Concentrations of PB2 (mg/mL) | 0 | 0.020 | 0.039 | 0.078 | 0.156 | 0.313 | 0.625 | 1.25 |
| Porphyromonas gingivalis | + | + | + | + | + | + | + | + |
| β-hemolytic Streptococcus | + | + | + | + | + | + | + | + |
| Staphylococcus aureus | + | + | + | + | + | + | + | + |
| Escherichia coli | + | + | + | + | + | + | + | + |
| Gender | Initial Average Body Weight (g) | Final Average Body Weight (g) | Death | LD50 (g/kg) |
|---|---|---|---|---|
| Female | 20.6 ± 1.3 | 28.7 ± 1.5 | 0 | >20.0 |
| Male | 20.1 ± 1.2 | 30.4 ± 1.6 | 0 | >20.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.; Zhang, X.; Yin, J.; Du, Q.; Tu, Y.; Shi, J.; Xu, Y. Castanopsis lamontii Water Extract Shows Potential in Suppressing Pathogens, Lipopolysaccharide-Induced Inflammation and Oxidative Stress-Induced Cell Injury. Molecules 2019, 24, 273. https://doi.org/10.3390/molecules24020273
Gao Y, Zhang X, Yin J, Du Q, Tu Y, Shi J, Xu Y. Castanopsis lamontii Water Extract Shows Potential in Suppressing Pathogens, Lipopolysaccharide-Induced Inflammation and Oxidative Stress-Induced Cell Injury. Molecules. 2019; 24(2):273. https://doi.org/10.3390/molecules24020273
Chicago/Turabian StyleGao, Ying, Xinzhong Zhang, Junfeng Yin, Qizhen Du, Youying Tu, John Shi, and Yongquan Xu. 2019. "Castanopsis lamontii Water Extract Shows Potential in Suppressing Pathogens, Lipopolysaccharide-Induced Inflammation and Oxidative Stress-Induced Cell Injury" Molecules 24, no. 2: 273. https://doi.org/10.3390/molecules24020273
APA StyleGao, Y., Zhang, X., Yin, J., Du, Q., Tu, Y., Shi, J., & Xu, Y. (2019). Castanopsis lamontii Water Extract Shows Potential in Suppressing Pathogens, Lipopolysaccharide-Induced Inflammation and Oxidative Stress-Induced Cell Injury. Molecules, 24(2), 273. https://doi.org/10.3390/molecules24020273








