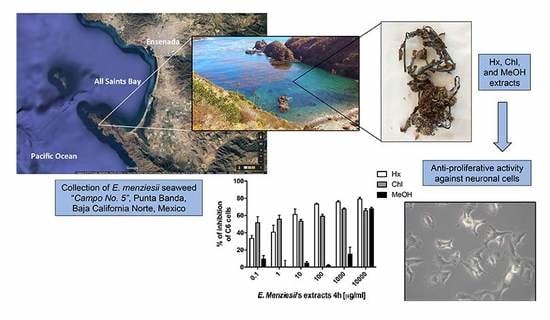
Brown Seaweed Egregia menziesii’s Cytotoxic Activity against Brain Cancer Cell Lines
Abstract
1. Introduction
2. Results
2.1. Cytotoxic Activity of E. menziesii’s Extracts over Artemia Salina Brine Shrimp
2.2. Determination of Cytotoxic Activity of E. menziesii’s Extracts against Nervous System Cell Lines
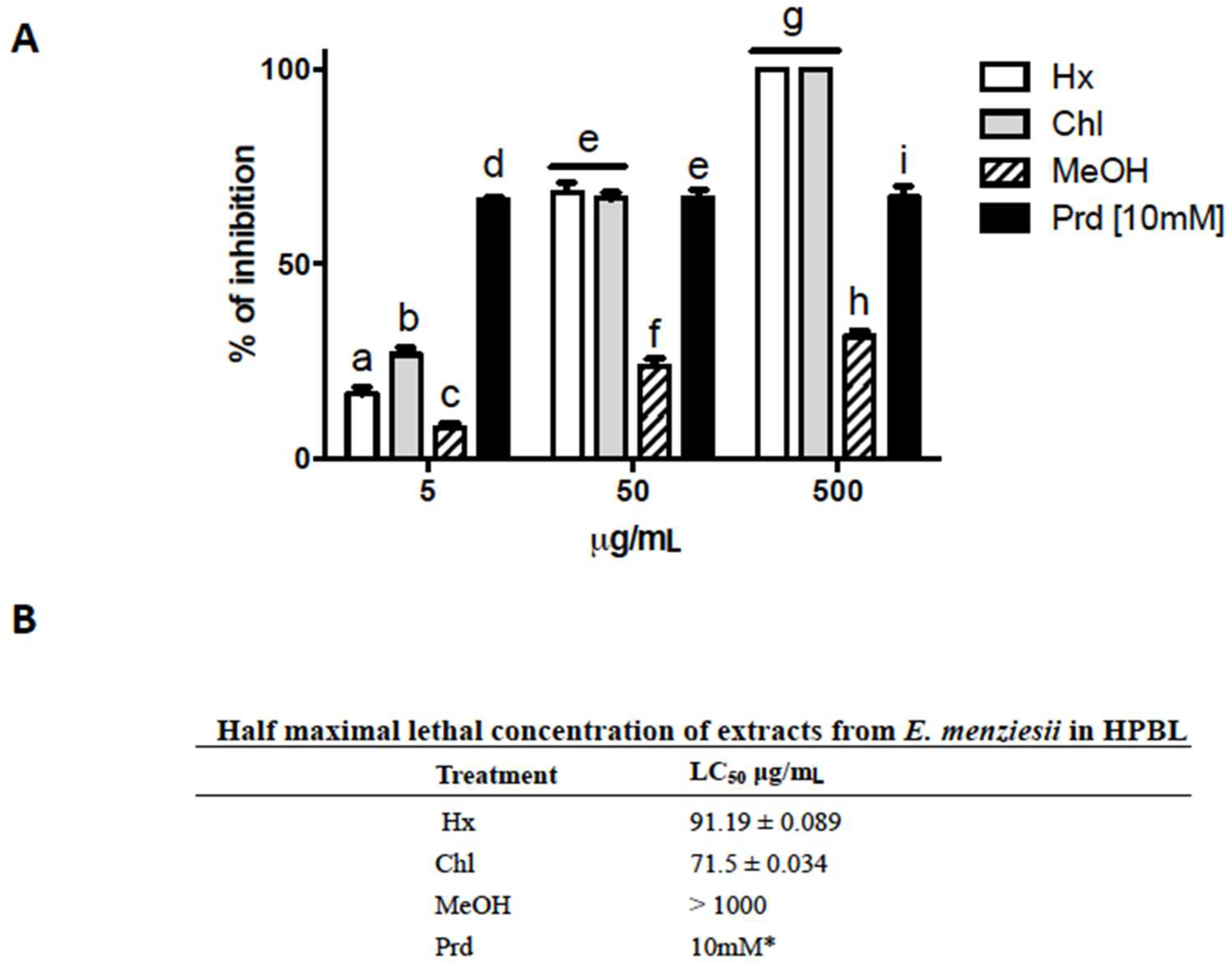
2.3. Cytotoxic Activity of E. menziesii Extracts over Human Peripheral Blood Lymphocytes (HPBL)
2.4. Cytotoxic Activity of E. menziesii Extracts against Differentiated and Non-Differentiated 3T3-L1 Cell Lines
3. Discussion
4. Materials and Methods
4.1. Drugs and Chemicals
4.2. Animals
4.3. Seaweed Samples and Preparation of Extracts
4.4. Cytotoxic Activity by Brine Shrimp Lethality Test
4.5. Lymphocyte Toxicity Test
4.6. Cell Culture
4.6.1. Bergmann Glia Primary Cultures
4.6.2. Cell Lines
4.6.3. In Vitro 3T3-L Cell Line Differentiation
4.7. In Vitro Viability Assays
4.8. Half Maximal Inhibitory Concentration (IC50) Determination
4.9. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- De Oliveira, M.N.; Freitas, A.L.P.; Carvalho, A.F.U.; Sarnpaio, T.M.T.; Farias, D.F.; Teixeira, D.I.A.; Gouveia, S.T.; Pereira, J.G.; de Sena, M.M.D.C. Nutritive and non-nutritive attributes of washed-up seaweeds from the coast of Ceara, Brazil. Food Chem. 2009, 115, 254–259. [Google Scholar] [CrossRef]
- Laurienzo, P. Marine polysaccharides in pharmaceutical applications: An overview. Mar. Drugs 2010, 8, 2435–2465. [Google Scholar] [CrossRef] [PubMed]
- Kilinç, B.; Cilik, S.; Turan, G.; Tekogul, H.; Koru, E. Seaweeds for Food and Industrial Applications. In Food Industry; Muzzalupo, I., Ed.; Intech Open: Izmir, Turkey, 2013. [Google Scholar]
- Lopes, G.; Pinto, E.; Andrade, P.B.; Valentao, P. Antifungal activity of phlorotannins against dermatophytes and yeasts: Approaches to the mechanism of action and influence on Candida albicans virulence factor. PLoS ONE 2013, 8, e72203. [Google Scholar] [CrossRef] [PubMed]
- Patra, J.K.; Das, G.; Baek, K.H. Chemical Composition and Antioxidant and Antibacterial Activities of an Essential Oil Extracted from an Edible Seaweed, Laminaria japonica L. Molecules 2015, 20, 12093–12113. [Google Scholar] [CrossRef] [PubMed]
- Galle, J.B.; Attioua, B.; Kaiser, M.; Rusig, A.M.; Lobstein, A.; Vonthron-Senecheau, C. Eleganolone, a diterpene from the French marine alga Bifurcaria bifurcata inhibits growth of the human pathogens Trypanosoma brucei and Plasmodium falciparum. Mar. Drugs 2013, 11, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Konig, G.M.; Wright, A.D.; Franzblau, S.G. Assessment of antimycobacterial activity of a series of mainly marine derived natural products. Planta. Med. 2000, 66, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Wang, A.; Lu, Z.; Qin, C.; Hu, J.; Yin, J. Overview on the antiviral activities and mechanisms of marine polysaccharides from seaweeds. Carbohydr. Res. 2017, 453, 1–9. [Google Scholar] [CrossRef]
- Gutierrez-Rodriguez, A.G.; Juarez-Portilla, C.; Olivares-Banuelos, T.; Zepeda, R.C. Anticancer activity of seaweeds. Drug Discov. Today 2018, 23, 434–447. [Google Scholar] [CrossRef]
- Ermakova, S.; Sokolova, R.; Kim, S.M.; Um, B.H.; Isakov, V.; Zvyagintseva, T. Fucoidans from Brown Seaweeds Sargassum hornery, Eclonia cava, Costaria costata: Structural Characteristics and Anticancer Activity. Appl. Biochem. Biotechnol. 2011, 164, 841–850. [Google Scholar] [CrossRef]
- Ahn, G.N.; Kim, K.N.; Cha, S.H.; Song, C.B.; Lee, J.; Heo, M.S.; Yeo, I.K.; Lee, N.H.; Jee, Y.H.; Kim, J.S.; et al. Antioxidant activities of phlorotannins purified from Ecklonia cava on free radical scavenging using ESR and H2O2-mediated DNA damage. Eur. Food Res. Technol. 2007, 226, 71–79. [Google Scholar] [CrossRef]
- Lee, S.H.; Han, J.S.; Heo, S.J.; Hwang, J.Y.; Jeon, Y.J. Protective effects of dieckol isolated from Ecklonia cava against high glucose-induced oxidative stress in human umbilical vein endothelial cells. Toxicol. In Vitro 2010, 24, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Wijesekara, I.; Yoon, N.Y.; Kim, S.K. Phlorotannins from Ecklonia cava (Phaeophyceae): Biological activities and potential health benefits. Biofactors 2010, 36, 408–414. [Google Scholar] [CrossRef]
- Lee, S.H.; Ko, C.I.; Jee, Y.; Jeong, Y.; Kim, M.; Kim, J.S.; Jeon, Y.J. Anti-inflammatory effect of fucoidan extracted from Ecklonia cava in zebrafish model. Carbohydr. Polym. 2013, 92, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Kang, K.A.; Piao, M.J.; Ko, D.O.; Wang, Z.H.; Lee, I.K.; Kim, B.J.; Jeong, I.Y.; Shin, T.; Park, J.W.; et al. Eckol protects V79-4 lung fibroblast cells against gamma-ray radiation-induced apoptosis via the scavenging of reactive oxygen species and inhibiting of the c-Jun NH2-terminal kinase pathway. Eur. J. Pharmacol. 2008, 591, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.S.; Kim, J.A.; Yoon, N.Y.; Kim, S.K. Induction of apoptosis by phloroglucinol derivative from Ecklonia Cava in MCF-7 human breast cancer cells. Food Chem. Toxicol. 2009, 47, 1653–1658. [Google Scholar] [CrossRef] [PubMed]
- IARC. World Cancer Report 2014; International Agency for Reaearch on Cancer: Lyon, France, 2014. [Google Scholar]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, 359–386. [Google Scholar] [CrossRef] [PubMed]
- Ohgaki, H.; Kleihues, P. Epidemiology and etiology of gliomas. Acta Neuropathol. 2005, 109, 93–108. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, R.W.; Ruefli, A.A.; Lowe, S.W. Apoptosis: A link between cancer genetics and chemotherapy. Cell 2002, 108, 153–164. [Google Scholar] [CrossRef]
- Yang, H.C.; Zeng, M.Y.; Dong, S.Y.; Liu, Z.Y.; Li, R.X. Anti-proliferative activity of phlorotannin extracts from brown algae Laminaria japonica Aresch. Chin. J. Oceanol. Limnol. 2010, 28, 122–130. [Google Scholar] [CrossRef]
- Ferreira, J.; Ramos, A.A.; Almeida, T.; Azqueta, A.; Rocha, E. Drug resistance in glioblastoma and cytotoxicity of seaweed compounds, alone and in combination with anticancer drugs: A mini review. Phytomedicine 2018, 48, 84–93. [Google Scholar] [CrossRef]
- Xue, M.L.; Ge, Y.L.; Zhang, J.Y.; Wang, Q.; Hou, L.; Liu, Y.C.; Sun, L.L.; Li, Q. Anticancer Properties and Mechanisms of Fucoidan on Mouse Breast Cancer In Vitro and In Vivo. PLoS ONE 2012, 7, e43483. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Ezeogu, L.; Zellmer, L.; Yu, B.; Xu, N.; Joshua Liao, D. Protecting the normal in order to better kill the cancer. Cancer Med. 2015, 4, 1394–1403. [Google Scholar] [CrossRef] [PubMed]
- Al-Fatlawi, A.A.; Al-Fatlawi, A.A.; Irshad, M.; Rahisuddin; Ahmad, A. Effect of parthenolide on growth and apoptosis regulatory genes of human cancer cell lines. Pharm. Biol. 2015, 53, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Green, H.; Meuth, M. An established pre-adipose cell line and its differentiation in culture. Cell 1974, 3, 127–133. [Google Scholar] [CrossRef]
- Vinayak, R.C.; Sabu, A.S.; Chatterji, A. Bio-Prospecting of a Few Brown Seaweeds for Their Cytotoxic and Antioxidant Activities. Evid.-Based Complement. Altern. 2011, 2011. [Google Scholar] [CrossRef] [PubMed]
- Asaduzzaman, M.; Rana, S.; Hasan Raqil, S.M.; Hossain, M.; Das, N. Cytotoxic (Brine Shrimp Lethality Bioassay) and antioxidant investigation of Barringtonia acutangula (L.). Int. J. Pharm. Sci. Res. 2015, 6, 1179–1185. [Google Scholar]
- Rajabi, S.; Ramazani, A.; Hamidi, M.; Naji, T. Artemia salina as a model organism in toxicity assessment of nanoparticles. Daru 2015, 23, 20. [Google Scholar] [CrossRef]
- Parra, A.L.; Yhebra, R.S.; Sardinas, I.G.; Buela, L.I. Comparative study of the assay of Artemia salina L. and the estimate of the medium lethal dose (LD50 value) in mice, to determine oral acute toxicity of plant extracts. Phytomedicine 2001, 8, 395–400. [Google Scholar]
- Kokkali, V.; Katramados, I.; Newman, J.D. Monitoring the Effect of Metal Ions on the Mobility of Artemia salina Nauplii. Biosensors 2011, 1, 36–45. [Google Scholar] [CrossRef]
- Gagliano, N.; Aldini, G.; Colombo, G.; Rossi, R.; Colombo, R.; Gioia, M.; Milzani, A.; Dalle-Donne, I. The potential of resveratrol against human gliomas. Anticancer Drugs 2010, 21, 140–150. [Google Scholar] [CrossRef]
- Mellinghoff, I.K.; Gilbertson, R.J. Brain Tumors: Challenges and Opportunities to Cure. J. Clin. Oncol. 2017, 35, 2343–2345. [Google Scholar] [CrossRef]
- Butowski, N.A.; Sneed, P.K.; Chang, S.M. Diagnosis and treatment of recurrent high-grade astrocytoma. J. Clin. Oncol. 2006, 24, 1273–1280. [Google Scholar] [CrossRef]
- Furnari, F.B.; Fenton, T.; Bachoo, R.M.; Mukasa, A.; Stommel, J.M.; Stegh, A.; Hahn, W.C.; Ligon, K.L.; Louis, D.N.; Brennan, C.; et al. Malignant astrocytic glioma: Genetics, biology, and paths to treatment. Genes Dev. 2007, 21, 2683–2710. [Google Scholar] [CrossRef]
- Stupp, R.; Pavlidis, N.; Jelic, S.; Force, E.G.T. ESMO Minimum Clinical Recommendations for diagnosis, treatment and follow-up of malignant glioma. Ann. Oncol. 2005, 16 (Suppl. 1), i64–i65. [Google Scholar] [CrossRef] [PubMed]
- Santandreu, F.M.; Brell, M.; Gene, A.H.; Guevara, R.; Oliver, J.; Couce, M.E.; Roca, P. Differences in mitochondrial function and antioxidant systems between regions of human glioma. Cell Physiol. Biochem. 2008, 22, 757–768. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Wang, D.C.; Cheng, Y.; Qian, M.; Zhang, M.; Shen, Q.; Wang, X. Roles of tumor heterogeneity in the development of drug resistance: A call for precision therapy. Semin. Cancer Biol. 2017, 42, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Dagogo-Jack, I.; Shaw, A.T. Tumour heterogeneity and resistance to cancer therapies. Nat. Rev. Clin. Oncol. 2018, 15, 81–94. [Google Scholar] [CrossRef]
- Athukorala, Y.; Kim, K.N.; Jeon, Y.J. Antiproliferative and antioxidant properties of an enzymatic hydrolysate from brown alga, Ecklonia cava. Food Chem. Toxicol. 2006, 44, 1065–1074. [Google Scholar] [CrossRef]
- Moghadamtousi, S.Z.; Karimian, H.; Khanabdali, R.; Razavi, M.; Firoozinia, M.; Zandi, K.; Kadir, H.A. Anticancer and Antitumor Potential of Fucoidan and Fucoxanthin, Two Main Metabolites Isolated from Brown Algae. Sci. World J. 2014, 2014. [Google Scholar]
- Liu, Y.; Zheng, J.; Zhang, Y.; Wang, Z.; Yang, Y.; Bai, M.; Dai, Y. Fucoxanthin Activates Apoptosis via Inhibition of PI3K/Akt/mTOR Pathway and Suppresses Invasion and Migration by Restriction of p38-MMP-2/9 Pathway in Human Glioblastoma Cells. Neurochem. Res. 2016, 41, 2728–2751. [Google Scholar] [CrossRef]
- Gong, A.; Guo, P.; Gong, L.; Liang, H. Aplysin suppresses the invasion of glioma cells by targeting Akt pathway. Int. J. Clin. Exp. Med. 2016, 9, 8062–8068. [Google Scholar]
- Ermakova, S.; Men’shova, R.; Vishchuk, O.; Kim, S.M.; Um, B.H.; Isakov, V.; Zvyagintseva, T. Water-soluble polysaccharides from the brown alga Eisenia bicyclis: Structural characteristics and antitumor activity. Algal Res. 2013, 2, 51–58. [Google Scholar] [CrossRef]
- Teas, J.; Vena, S.; Cone, D.L.; Irhimeh, M. The consumption of seaweed as a protective factor in the etiology of breast cancer: Proof of principle. J. Appl. Phycol. 2013, 25, 771–779. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.; Park, G.M.; Kim, S.N.; Amna, T.; Lee, S.; Shin, W.S. Glioblastoma-specific anticancer activity of pheophorbide a from the edible red seaweed Grateloupia elliptica. J. Microbiol. Biotechnol. 2014, 24, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Villarreal-Gomez, L.J.; Soria-Mercado, I.E.; Guerra-Rivas, G.; Ayala-Sanchez, N.E. Antibacterial and anticancer activity of seaweeds and bacteria associated with their surface. Rev. Biol. Mar. Oceanog. 2010, 45, 267–275. [Google Scholar] [CrossRef]
- Zhang, J.; Lou, X.; Jin, L.; Zhou, R.; Liu, S.; Xu, N.; Liao, D.J. Necrosis, and then stress induced necrosis-like cell death, but not apoptosis, should be the preferred cell death mode for chemotherapy: Clearance of a few misconceptions. Oncoscience 2014, 1, 407–422. [Google Scholar] [CrossRef] [PubMed]
- Das, S.K.; Hashimoto, T.; Shimizu, K.; Yoshida, T.; Sakai, T.; Sowa, Y.; Komoto, A.; Kanazawa, K. Fucoxanthin induces cell cycle arrest at G0/G1 phase in human colon carcinoma cells through up-regulation of p21WAF1/Cip1. Biochim. Biophys. Acta 2005, 1726, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Hyun, K.H.; Yoon, C.H.; Kim, R.K.; Lim, E.J.; An, S.; Park, M.J.; Hyun, J.W.; Suh, Y.; Kim, M.J.; Lee, S.J. Eckol suppresses maintenance of stemness and malignancies in glioma stem-like cells. Toxicol. Appl. Pharmacol. 2011, 254, 32–40. [Google Scholar] [CrossRef]
- Kang, C.W.; Park, M.S.; Kim, N.H.; Lee, J.H.; Oh, C.W.; Kim, H.R.; Kim, G.D. Hexane extract from Sargassum serratifolium inhibits the cell proliferation and metastatic ability of human glioblastoma U87MG cells. Oncol. Rep. 2015, 34, 2602–2608. [Google Scholar] [CrossRef]
- Do, H.; Pyo, S.; Sohn, E.H. Suppression of iNOS expression by fucoidan is mediated by regulation of p38 MAPK, JAK/STAT, AP-1 and IRF-1, and depends on up-regulation of scavenger receptor B1 expression in TNF-α- and IFN-γ-stimulated C6 glioma cells. J. Nutr. Biochem. 2010, 21, 671–679. [Google Scholar] [CrossRef]
- Lv, Y.; Song, Q.; Shao, Q.; Gao, W.; Mao, H.; Lou, H.; Qu, X.; Li, X. Comparison of the effects of marchantin C and fucoidan on sFlt-1 and angiogenesis in glioma microenvironment. J. Pharm. Pharmacol. 2012, 64, 604–609. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.Y.; Chang, C.S.; Yeh, W.L.; Tang, C.H.; Cheung, C.W.; Leung, Y.M.; Liu, J.F.; Wong, K.L. The novel phloroglucinol derivative BFP induces apoptosis of glioma cancer through reactive oxygen species and endoplasmic reticulum stress pathways. Phytomedicine 2012, 19, 1093–1100. [Google Scholar] [CrossRef] [PubMed]
- Elia, U.; Flescher, E. Combined chemotherapy or biotherapy with jasmonates: Targeting energy metabolism for cancer treatment. Curr. Pharm. Biotechnol. 2013, 14, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Taki-Nakano, N.; Ohzeki, H.; Kotera, J.; Ohta, H. Cytoprotective effects of 12-oxo phytodienoic acid, a plant-derived oxylipin jasmonate, on oxidative stress-induced toxicity in human neuroblastoma SH-SY5Y cells. Biochim. Biophys. Acta 2014, 1840, 3413–3422. [Google Scholar] [CrossRef] [PubMed]
- Todd, J.S.; Proteau, P.J.; Gerwick, W.H. The Absolute Configuration of Ecklonialactones A, B, and E, Novel Oxylipins from Brown Algae of the Genera Ecklonia and Egregia. J. Nat. Prod. 1994, 57, 171–174. [Google Scholar] [CrossRef]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly)phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.J.; Nam, S.J.; Kong, G.; Kim, M.K. A case-control study on seaweed consumption and the risk of breast cancer. Brit. J. Nutr. 2010, 103, 1345–1353. [Google Scholar] [CrossRef] [PubMed]
- Nanri, A.; Mizoue, T.; Shimazu, T.; Ishihara, J.; Takachi, R.; Noda, M.; Iso, H.; Sasazuki, S.; Sawada, N.; Tsugane, S.; et al. Dietary patterns and all-cause, cancer, and cardiovascular disease mortality in Japanese men and women: The Japan public health center-based prospective study. PLoS ONE 2017, 12, e0174848. [Google Scholar] [CrossRef]
- DOF. Carta Nacional Pesquera. Secretaría de Agricultura, Ganadería; Desarrollo Rural, Pesca y Alimentación, Ed.; Diario Oficial de la Federación: Mexico City, Mexico, 2012. [Google Scholar]
- Lincoln, R.A.; Strupinski, K.; Walker, J.M. The use of Artemia nauplii (Brine shrimp larvae) to detect toxic compounds from microalgal cultures. Int. J. Pharmacogn. 1996, 34, 384–389. [Google Scholar] [CrossRef]
- Ortega, A.; Eshhar, N.; Teichberg, V.I. Properties of Kainate Receptor Channels on Cultured Bergmann Glia. Neuroscience 1991, 41, 335–349. [Google Scholar] [CrossRef]
- Limb, G.A.; Salt, T.E.; Munro, P.M.; Moss, S.E.; Khaw, P.T. In vitro characterization of a spontaneously immortalized human Muller cell line (MIO-M1). Investig. Ophthalmol. Vis. Sci. 2002, 43, 864–869. [Google Scholar] [PubMed]
- Ramirez-Zacarias, J.L.; Castro-Munozledo, F.; Kuri-Harcuch, W. Quantitation of adipose conversion and triglycerides by staining intracytoplasmic lipids with Oil red O. Histochemistry 1992, 97, 493–497. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
Sample Availability: Samples of the MeOH, Chl and Hx extracts are available from the authors. |

| Cell Line | Hx | Chl | MeOH | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 4 h | 24 h | 48 h | 4 h | 24 h | 48 h | 4 h | 24 h | 48 h | |
| BC | >1000 a | >1000 c | >1000 g | >1000 a | >1000 e | >1000 i | >1000 a | >1000 b | >1000 c |
| C6 | 9.51 ± 1.45 b | 9.59 ± 1.34 d | 8.59 ± 0.93 h | 9.82 ± 0.83 b | 8.86 ± 1.23 f | 7.39 ± 1.43 j | >1000 a | >1000 b | >1000 c |
| MIO-M1 | >1000 a | 88.48 ± 1.65 e | 10.08 ± 1.98 i | 90.11 ± 1.23 c | 86.32 ± 1.39 g | 9.41 ± 1.93 k | >1000 a | >1000 b | >1000 c |
| N1-115 | 10.94 ± 1.93 b | >1000 c | >1000 g | >1000 a | >1000 e | >1000 i | >1000 a | >1000 b | 10.23 ± 1.23 d |
| U737 | >1000 a | 891.96 ± 1.23 f | 906.73 ± 1.73 j | 105.71 ± 1.83 d | 108.85 ± 1.93 h | 95.76 ± 1.35 l | >1000 a | >1000 b | >1000 c |
| Extract | Fibroblast State | Adipose State | ||||
|---|---|---|---|---|---|---|
| 4 h | 24 h | 48 h | 4 h | 24 h | 48 h | |
| Hx | >1000 a | >1000 a | 105.01 ± 2.12 b | >1000 a | 110.15 ± 4.15 b | 8.78 ± 3.12 c |
| Chl | >1000 c | >1000 c | 107.79 ± 1.98 d | 13.17 ± 3.13 d | 109.15 ± 3.11 e | 8.46 ± 4.16 d |
| MeOH | >1000 e | >1000 e | >1000 e | >1000 f | >1000 f | 107.79 ± 2.67 g |
| Extract | Obtained Amount (g) | Yield (%) |
|---|---|---|
| Hx | 1.48 | 0.25 |
| Chl | 2.96 | 0.49 |
| MeOH | 79.65 | 13.27 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olivares-Bañuelos, T.; Gutiérrez-Rodríguez, A.G.; Méndez-Bellido, R.; Tovar-Miranda, R.; Arroyo-Helguera, O.; Juárez-Portilla, C.; Meza-Menchaca, T.; Aguilar-Rosas, L.E.; Hernández-Kelly, L.C.R.; Ortega, A.; et al. Brown Seaweed Egregia menziesii’s Cytotoxic Activity against Brain Cancer Cell Lines. Molecules 2019, 24, 260. https://doi.org/10.3390/molecules24020260
Olivares-Bañuelos T, Gutiérrez-Rodríguez AG, Méndez-Bellido R, Tovar-Miranda R, Arroyo-Helguera O, Juárez-Portilla C, Meza-Menchaca T, Aguilar-Rosas LE, Hernández-Kelly LCR, Ortega A, et al. Brown Seaweed Egregia menziesii’s Cytotoxic Activity against Brain Cancer Cell Lines. Molecules. 2019; 24(2):260. https://doi.org/10.3390/molecules24020260
Chicago/Turabian StyleOlivares-Bañuelos, Tatiana, Anllely G. Gutiérrez-Rodríguez, Rodolfo Méndez-Bellido, Ricardo Tovar-Miranda, Omar Arroyo-Helguera, Claudia Juárez-Portilla, Thuluz Meza-Menchaca, Luis E. Aguilar-Rosas, Luisa C. R. Hernández-Kelly, Arturo Ortega, and et al. 2019. "Brown Seaweed Egregia menziesii’s Cytotoxic Activity against Brain Cancer Cell Lines" Molecules 24, no. 2: 260. https://doi.org/10.3390/molecules24020260
APA StyleOlivares-Bañuelos, T., Gutiérrez-Rodríguez, A. G., Méndez-Bellido, R., Tovar-Miranda, R., Arroyo-Helguera, O., Juárez-Portilla, C., Meza-Menchaca, T., Aguilar-Rosas, L. E., Hernández-Kelly, L. C. R., Ortega, A., & Zepeda, R. C. (2019). Brown Seaweed Egregia menziesii’s Cytotoxic Activity against Brain Cancer Cell Lines. Molecules, 24(2), 260. https://doi.org/10.3390/molecules24020260