The Complete Plastid Genome of Magnolia zenii and Genetic Comparison to Magnoliaceae species
Abstract
1. Introduction
2. Results and Discussion
2.1. Genome Features and Guanine-Cytosine Content
2.2. Codon Usage Bias
2.3. Repeat Sequences
2.4. Comparative Analysis of Plastomes of Magnolia Species
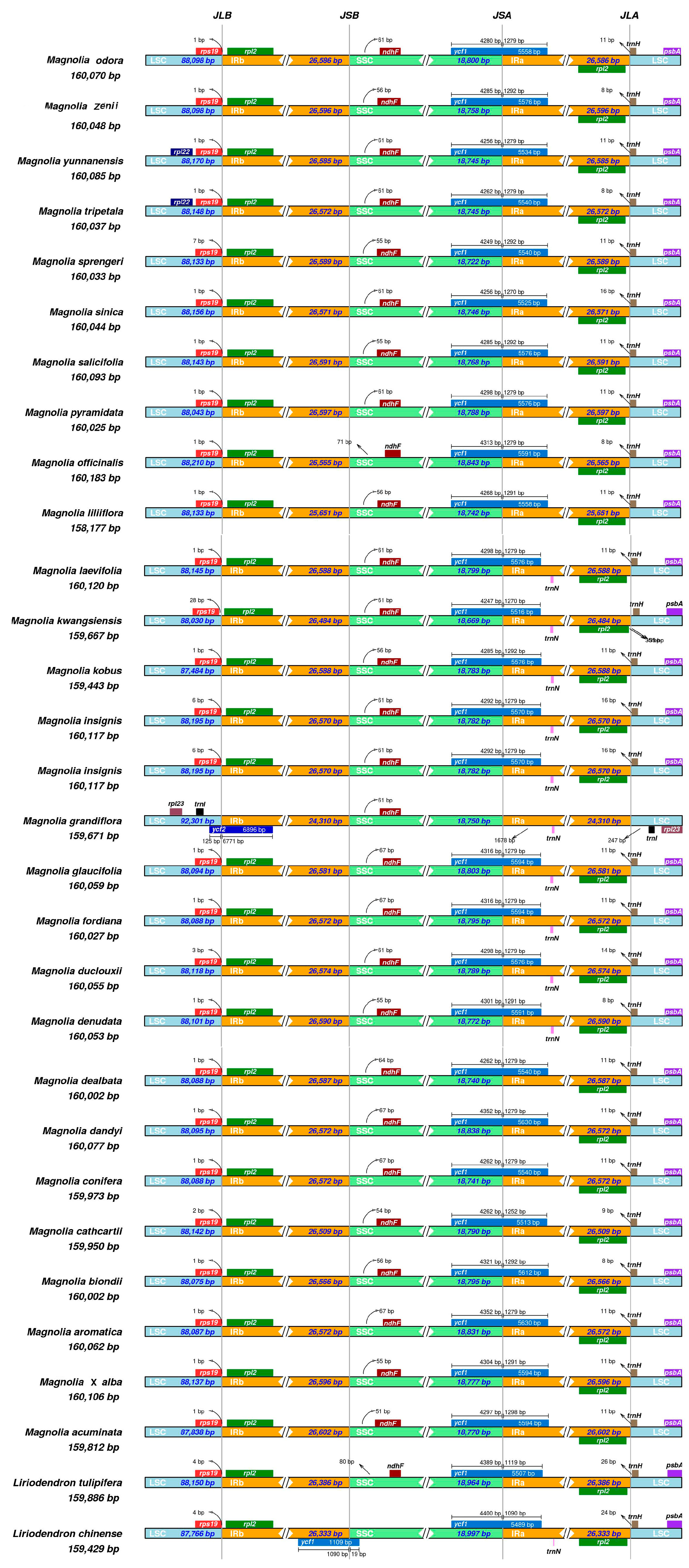
2.5. Junction Characteristics
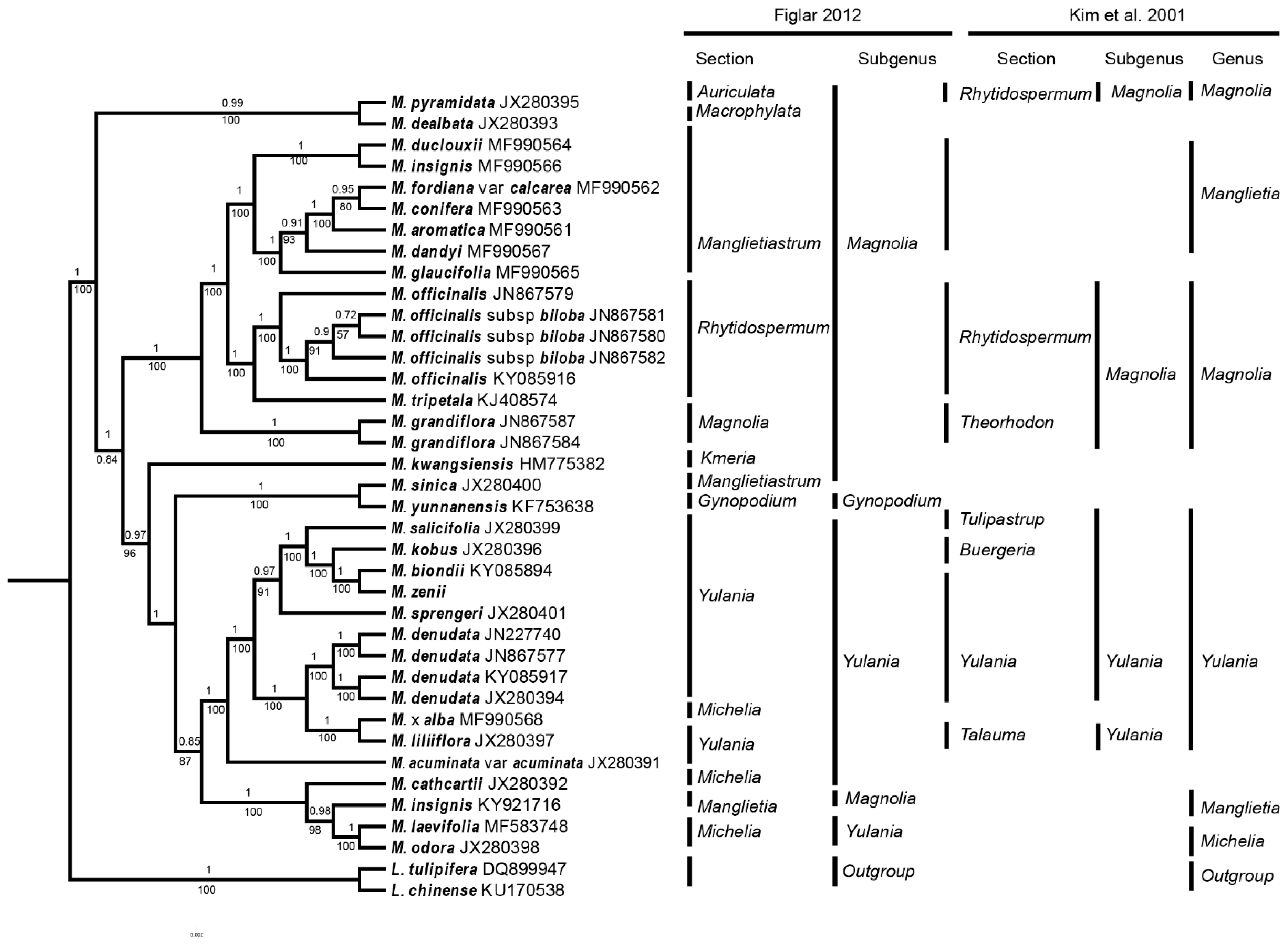
2.6. Phylogenetic Inference
3. Materials and Methods
3.1. DNA Extraction and Sequencing
3.2. Chloroplast Genome Assembling and Annotation
3.3. Whole Plastid Genome Comparison
3.4. Repeat Structure Identification
3.5. Phylogenetic Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, J.W.; Zhang, G.-F.; Chen, H.-Y. Population pattern and community characteristics of endemic and rare plant Magnolia zenii in Baohuashan National Forest Park. Guihaia 2008, 28, 489–494. [Google Scholar]
- Mei, J.G.; Guo, S.; Fu, Z.G.; Wei, W.J. Intra-and interspecific competition of endangered plant Magnolia zenii. Chinese J. Ecol. 2010, 29, 201–206. [Google Scholar]
- Shubin, Z. Study on Cuttage Propagation Technique of Magnolia zenii. J. Shandong For. Sci. Technol. 2008, 3. Available online: http://en.cnki.com.cn/Article_en/CJFDTotal-TREE200806014.htm (accessed on 9 November 2018).
- Song, X.; Li, J.; Li, Z. GC-MS analysis of volatile components from bark of Magnolia zenii. Pract. Pharm. Clin. Remedies 2012, 15, 651–652. [Google Scholar]
- Song, X.K.; Jing, L.I.; Li, Z.-H. GC-MS analysis of volatile components from seeds of Magnolia zenii Cheng. Pract. Pharm. Clin. Remedies 2012. Available online: http://en.cnki.com.cn/Article_en/CJFDTOTAL-LYLC201210018.htm (accessed on 9 November 2018).
- Attorre, F.; Abeli, T.; Bacchetta, G.; Farcomeni, A.; Fenu, G.; De Sanctis, M.; Gargano, D.; Peruzzi, L.; Montagnani, C.; Rossi, G.; et al. How to include the impact of climate change in the extinction risk assessment of policy plant species? J. Nat. Conserv. 2018, 44, 43–49. [Google Scholar] [CrossRef]
- Cafarchia, C.; De, L.N.; Milillo, M.A.; Losacco, V.; Puccini, V. Fungistatic activity of a sesquiterpene lactone (tomentosin) isolated from fresh Inula viscosa (Asteraceae) flowers from the Puglia region. Parassitologia 2001, 43, 117–121. [Google Scholar] [PubMed]
- Park, H.-H.; Kim, S.-G.; Kim, M.J.; Lee, J.; Choi, B.-K.; Jin, M.-H.; Lee, E. Suppressive Effect of Tomentosin on the Production of Inflammatory Mediators in RAW264.7 Cells. Biol. Pharm. Bull. 2014, 37, 1177–1183. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.L.; Li, Y.Q.; Bai, M.; Wang, Y.L.; Wu, H. Variations in volatile oil yields and compositions of Magnolia zenii Cheng flower buds at different growth stages. Trees 2015, 29, 1649–1660. [Google Scholar] [CrossRef]
- Azuma, H.; Thien, L.B.; Kawano, S. Molecular Phylogeny of Magnolia (Magnoliaceae) Inferred from cpDNA Sequences and Evolutionary Divergence of the Floral Scents. J. Plant. Res. 1999, 112, 291–306. [Google Scholar] [CrossRef]
- Azuma, H.; García-Franco, J.G.; Rico-Gray, V.; Thien, L.B. Molecular phylogeny of the Magnoliaceae: The biogeography of tropical and temperate disjunctions. Am. J. Bot. 2001, 88, 2275–2285. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Chen, K.; Cuihua Gu Shaoyu Zheng, L.M. Comparative and phylogenetic analyses of 26 Magnoliaceae species based on complete chloroplast genome sequences. Can. J. For. Res. 2018, 48, 1456–1469. [Google Scholar] [CrossRef]
- Wu, W.; Chen, F.; Jing, D.; Liu, Z.; Ma, L. Isolation and Characterization of an AGAMOUS-Like Gene from Magnolia wufengensis (Magnoliaceae). Plant. Mol. Biol. Report. 2012, 30, 690–698. [Google Scholar] [CrossRef]
- Fang, H.; Yuan, Z. Studies on the Medicinal Plants of Chinese Magnoliaceae Analysis of the Constituents of the Volatile Oil from Flower Buds of Malznolia sargentiana. Chinese J. Pharm. Analysis 1988, 8, 266–269. [Google Scholar]
- Lee, J.-Y.; Ko, S.-H.; Mun, S.-J.; You, J.-H.; Kim, S.-W. Investigation of Forest Therapeutic Function According to the Antioxidant Activity and Total Phenolics in Magnoliaceae Flower. J. Korean Inst. For. Recreat. 2013, 17, 81–86. [Google Scholar] [CrossRef]
- Nooteboom, P.H.; Xia, N.; Yuhu, L. Magnoliaceae in Flora of China. Available online: http://www.efloras.org/florataxon.aspx?flora_id=2&taxon_id=10530 (accessed on 9 November 2018).
- Ma, F.; Liang, G. The Research Situation of Magnolia Linn. J. Guiyang College TCM 2015, 37, 92–96. [Google Scholar]
- Sui-Chao, H.E. Phylogenetic Relationship Between Magnolia wufengensis and Several Related Species in Subgenus Yulania Based on AFLP Marker Analysis. Bull. Bot. Res. 2008, 28, 288–292. [Google Scholar]
- Figlar, R.; Figlar, R.B. Proleptic branch initiation in Michelia and Magnolia subgenus Yulania provides basis for combinations in subfamily Magnolioideae. In The International Symposium on the Family Magnoliaceae; Science Press: Beijing, China, 1998; pp. 14–25. [Google Scholar]
- Azuma, H.; Chalermglin, P.; Nooteboom, H.P. Molecular phylogeny of Magnoliaceae based on plastid DNA sequences with special emphasis on some species from continental Southeast Asia. Thai For. Bull. 2011, 39, 148–165. [Google Scholar]
- Kim, S.; Park, C.W.; Kim, Y.D.; Suh, Y. Phylogenetic relationships in family Magnoliaceae inferred from ndhF sequences. Am. J. Bot. 2001, 88, 717–728. [Google Scholar] [CrossRef]
- Nie, Z.L.; Wen, J.; Azuma, H.; Qiu, Y.L.; Sun, H.; Meng, Y.; Sun, W.B.; Zimmer, E.A. Phylogenetic and biogeographic complexity of Magnoliaceae in the Northern Hemisphere inferred from three nuclear data sets. Mol. Phylogenet. Evol. 2008, 48, 1027–1040. [Google Scholar] [CrossRef]
- Daniell, H.; Lin, C.-S.; Yu, M.; Chang, W.-J. Chloroplast genomes: Diversity, evolution, and applications in genetic engineering. Genome Biol. 2016, 17. [Google Scholar] [CrossRef] [PubMed]
- Raman, G.; Park, V.; Kwak, M.; Lee, B.; Park, S.J. Characterization of the complete chloroplast genome of Arabis stellari and comparisons with related species. PLoS ONE 2017, 12, 1–18. [Google Scholar] [CrossRef]
- Kress, W.J.; Wurdack, K.J.; Zimmer, E.A.; Weigt, L.A.; Janzen, D.H. Use of DNA barcodes to identify flowering plants. Proc. Natl. Acad. Sci. USA 2005, 102, 8369–8374. [Google Scholar] [CrossRef]
- Ng, P.K.; Lin, S.M.; Lim, P.E.; Liu, L.C.; Chen, C.M.; Pai, T.W. Complete chloroplast genome of Gracilaria firma (Gracilariaceae, Rhodophyta), with discussion on the use of chloroplast phylogenomics in the subclass Rhodymeniophycidae. BMC Genomics 2017, 18, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Zhou, T.; Han, K.; Yang, Y.; Zhao, J.; Liu, Z.L. The complete chloroplast genome sequences of six rehmannia species. Genes 2017, 8. [Google Scholar] [CrossRef]
- Zhao, J.; Xu, Y.; Xi, L.; Yang, J.; Chen, H.; Zhang, J. Characterization of the Chloroplast Genome Sequence of Acer miaotaiense: Comparative and Phylogenetic Analyses. Molecules 2018, 23. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-W.; Hu, Z.-G.; Lin, X.-H.; Li, Q.; Gao, H.-H.; Luo, G.-A.; Chen, S.-L. High-throughput pyrosequencing of the complete chloroplast genome of Magnolia officinalis and its application in species identification. Acta Pharm. Sin. 2012, 47, 124–130. [Google Scholar]
- Li, X.; Gao, H.; Wang, Y.; Song, J.; Henry, R.; Wu, H.; Hu, Z.; Yao, H.; Luo, H.; Luo, K.; et al. Complete chloroplast genome sequence of Magnolia grandiflora and comparative analysis with related species. Sci. China Life Sci. 2013, 56, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Saina, J.K.; Gichira, A.W.; Li, Z.Z.; Hu, G.W.; Wang, Q.F.; Liao, K. The complete chloroplast genome sequence of Dodonaea viscosa: Comparative and phylogenetic analyses. Genetica 2018, 146, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Schmid, P.; Flegel, W.A. Codon usage in vertebrates is associated with a low risk of acquiring nonsense mutations. J. Transl. Med. 2011, 9, 87. [Google Scholar] [CrossRef]
- De Abreu, N.L.; Alves, R.J.V.; Cardoso, S.R.S.; Bertrand, Y.J.K.; Sousa, F.; Hall, C.F.; Pfeil, B.E.; Antonelli, A. The use of chloroplast genome sequences to solve phylogenetic incongruences in Polystachya Hook (Orchidaceae Juss). PeerJ 2018, 6. [Google Scholar] [CrossRef] [PubMed]
- Niu, D.K.; Yang, Y.F. Why eukaryotic cells use introns to enhance gene expression: Splicing reduces transcription-associated mutagenesis by inhibiting topoisomerase I cutting activity. Biol. Direct 2011, 6, 24. [Google Scholar] [CrossRef]
- Le Hir, H.; Nott, A.; Moore, M.J. How introns influence and enhance eukaryotic gene expression. Trends Biochem. Sci. 2003, 28, 215–220. [Google Scholar] [CrossRef]
- Kuang, D.-Y.; Wu, H.; Wang, Y.-L.; Gao, L.-M.; Zhang, S.-Z.; Lu, L.; Bonen, L. Complete chloroplast genome sequence of Magnolia kwangsiensis (Magnoliaceae): Implication for DNA barcoding and population genetics. Genome 2011, 54, 663–673. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.-Y.; Yu, Y.; Deng, Y.-Q.; Li, J.; Huang, Z.-X.; Zhou, S.-D. The Chloroplast Genome of Lilium henrici: Genome Structure and Comparative Analysis. Molecules 2018, 23. [Google Scholar] [CrossRef] [PubMed]
- Jian, H.-Y.; Zhang, Y.-H.; Yan, H.-J.; Qiu, X.-Q.; Wang, Q.-G.; Li, S.-B.; Zhang, S.-D. The Complete Chloroplast Genome of a Key Ancestor of Modern Roses, Rosa chinensis var. spontanea, and a Comparison with Congeneric Species. Molecules 2018, 23. [Google Scholar] [CrossRef]
- Zhou, J.; Cui, Y.; Chen, X.; Li, Y.; Xu, Z.; Duan, B.; Li, Y.; Song, J.; Yao, H. Complete Chloroplast Genomes of Papaver rhoeas and Papaver orientale: Molecular Structures, Comparative Analysis, and Phylogenetic Analysis. Molecules 2018, 23. [Google Scholar] [CrossRef] [PubMed]
- Provan, J.; Powell, W.; Hollingsworth, P.M. Chloroplast microsatellites: New tools for studies in plant ecology and evolution. Trends Ecol. Evol. 2001, 16, 142–147. [Google Scholar] [CrossRef]
- McCauley, D.E. The use of chloroplast DNA polymorphism in studies of gene flow in plants. Trends Ecol. Evol. 1995, 10, 198–202. [Google Scholar] [CrossRef]
- Deguilloux, M.-F.; Pemonge, M.-H.; Petit, R.J. Use of chloroplast microsatellites to differentiate oak populations. Ann. For. Sci. 2004, 61, 825–830. [Google Scholar] [CrossRef]
- Shen, X.; Wu, M.; Liao, B.; Liu, Z.; Bai, R.; Xiao, S.; Li, X.; Zhang, B.; Xu, J.; Chen, S. Complete Chloroplast Genome Sequence and Phylogenetic Analysis of the Medicinal Plant Artemisia annua. Molecules 2017, 22. [Google Scholar] [CrossRef]
- Xie, D.F.; Yu, Y.; Deng, Y.Q.; Li, J.; Liu, H.Y.; Zhou, S.D.; He, X.J. Comparative Analysis of the Chloroplast Genomes of the Chinese Endemic Genus Urophysa and Their Contribution to Chloroplast Phylogeny and Adaptive Evolution. Int. J. Mol. Sci. 2018, 19, 1847. [Google Scholar] [CrossRef] [PubMed]
- Borsch, T.; Quandt, D. Mutational dynamics and phylogenetic utility of noncoding chloroplast DNA. Plant. Syst. Evol. 2009, 282, 169–199. [Google Scholar] [CrossRef]
- F Costa, J.; Lin, S.M.; MacAya, E.C.; Fernández-Garciá, C.; Verbruggen, H. Chloroplast genomes as a tool to resolve red algal phylogenies: A case study in the Nemaliales. BMC Evol. Biol. 2016, 16, 1–13. [Google Scholar] [CrossRef]
- Darling, A.C.E. Mauve: Multiple Alignment of Conserved Genomic Sequence With Rearrangements. Genome Res. 2004, 14, 1394–1403. [Google Scholar] [CrossRef] [PubMed]
- Brudno, M.; Do, C.B.; Cooper, G.M.; Kim, M.F.; Davydov, E.; Green, E.D.; Sidow, A.; Batzoglou, S. LAGAN and Multi-LAGAN: Efficient tools for large-scale multiple alignment of genomic DNA. Genome Res. 2003, 13, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.; Guo, W.; Gupta, S.; Fan, W.; Mower, J.P. Evolutionary dynamics of the plastid inverted repeat: The effects of expansion, contraction, and loss on substitution rates. New Phytol. 2016, 209, 1747–1756. [Google Scholar] [CrossRef]
- Satjarak, A.; Graham, L.E. Comparative DNA sequence analyses of Pyramimonas parkeae (Prasinophyceae) chloroplast genomes. J. Phycol. 2017, 53, 415–424. [Google Scholar] [CrossRef]
- Saina, J.K.; Li, Z.Z.; Gichira, A.W.; Liao, Y.Y. The Complete Chloroplast Genome Sequence of Tree of Heaven (Ailanthus altissima (Mill.) (Sapindales: Simaroubaceae), an Important Pantropical Tree. Int. J. Mol. Sci. 2018, 19. [Google Scholar] [CrossRef]
- Neubig, K.; Whitten, W.; Carlsward, B.; Blanco, M.; Endara, L.; Williams, N.; Moore, M. Phylogenetic utility of ycf1 in orchids: A plastid gene more variable than matK. Plant. Syst. Evol. 2009, 277, 75–84. [Google Scholar] [CrossRef]
- Drescher, A.; Stephanie, R.; Calsa, T.; Carrer, H.; Bock, R. The two largest chloroplast genome-encoded open reading frames of higher plants are essential genes. Plant. J. 2000, 22, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.L.; Sun, G.L.; Zhang, D.M. Molecular evolution and phylogeny of the angiosperm ycf2 gene. J. Syst. Evol. 2010, 48, 240–248. [Google Scholar] [CrossRef]
- Dugas, D.V.; Hernandez, D.; Koenen, E.J.M.; Schwarz, E.; Straub, S.; Hughes, C.E.; Jansen, R.K.; Nageswara-Rao, M.; Staats, M.; Trujillo, J.T.; et al. Mimosoid legume plastome evolution: IR expansion, tandem repeat expansions, and accelerated rate of evolution in clpP. Sci. Rep. 2015, 5, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Resour, G.; Wang, G.; Hou, N.; Zhang, S.; Luo, Y. Characterization of the complete chloroplast genomes of seven Manglietia and one Michelia species (Magnoliales: Magnoliaceae). Conserv. Genet. Resour. 2017, 1. [Google Scholar] [CrossRef]
- Maier, U.G.; Karin, K.; Sabine, B.; Funk, H.T.; Kirsten, K. Complete DNA sequences of the plastid genomes of two parasitic flowering plant species, Cuscuta reflexa and Cuscuta gronovii. BMC Plant. Biol. 2007, 7, 1–12. [Google Scholar] [CrossRef][Green Version]
- Wicke, S.; Müller, K.F.; de Pamphilis, C.W.; Quandt, D.; Wickett, N.J.; Zhang, Y.; Renner, S.S.; Schneeweiss, G.M. Mechanisms of functional and physical genome reduction in photosynthetic and nonphotosynthetic parasitic plants of the broomrape family. Plant. Cell. 2013, 25, 3711–3725. [Google Scholar] [CrossRef] [PubMed]
- Saski, C.; Lee, S.B.; Daniell, H.; Wood, T.C.; Tomkins, J.; Kim, H.G.; Jansen, R.K. Complete Chloroplast Genome Sequence of Glycine max and Comparative Analyses with other Legume Genomes. Plant. Mol. Biol. 2005, 59, 309–322. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, E.N.; Ruhlman, T.A.; Sabir, J.S.M.; Hajrah, N.H.; Alharbi, N.S.; Al-Malki, A.L.; Bailey, C.D.; Jansen, R.K. Plastid genome sequences of legumes reveal parallel inversions and multiple losses of rps16 in papilionoids. J. Syst. Evol. 2015, 53, 458–468. [Google Scholar] [CrossRef]
- Chumley, T.W.; Palmer, J.D.; Mower, J.P.; Fourcade, H.M.; Calie, P.J.; Boore, J.L.; Jansen, R.K. The complete chloroplast genome sequence of Pelargonium x hortorum: Organization and evolution of the largest and most highly rearranged chloroplast genome of land plants. Mol. Biol. Evol. 2006, 23, 2175–2190. [Google Scholar] [CrossRef]
- Shaw, J.; Lickey, E.B.; Schilling, E.E.; Small, R.L. Comparison of whole chloroplast genome sequences to choose noncoding regions for phylogenetic studies in angiosperms: The Tortoise and the hare III. Am. J. Bot. 2007, 94, 275–288. [Google Scholar] [CrossRef]
- Niu, Z.; Xue, Q.; Zhu, S.; Sun, J.; Liu, W.; Ding, X. The Complete Plastome Sequences of Four Orchid Species: Insights into the Evolution of the Orchidaceae and the Utility of Plastomic Mutational Hotspots. Front. Plant. Sci. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Figlar, R.B. Magnolia Classification. Available online: https://www.magnoliasociety.org/Classification (accessed on 9 November 2018).
- Qiu, Y.-L.; Chase, M.W.; Parks, C.R. A Chloroplast Eastern Section Study Rytidospermum of Magnolia. Am. J. Bot. 1995, 82, 1582–1588. [Google Scholar] [CrossRef]
- Doyle, J.J.; Doyle, J.L. A Rapid DNA Isolation Procedure for Small Quantities of Fresh Leaf Tissue. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- Dierckxsens, N.; Mardulyn, P.; Smits, G. NOVOPlasty: De novo assembly of organelle genomes from whole genome data. Nucleic Acids Res. 2016, 45. [Google Scholar] [CrossRef]
- Liu, C.; Shi, L.; Zhu, Y.; Chen, H.; Zhang, J.; Lin, X.; Guan, X. CpGAVAS, an integrated web server for the annotation, visualization, analysis, and GenBank submission of completely sequenced chloroplast genome sequences. BMC Genomics 2012, 13. [Google Scholar] [CrossRef] [PubMed]
- Lohse, M.; Drechsel, O.; Bock, R. OrganellarGenomeDRAW (OGDRAW): A tool for the easy generation of high-quality custom graphical maps of plastid and mitochondrial genomes. Curr. Genet. 2007, 52, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Peden, J.F. Analysis of codon usage. Biosystems. 1999, 5, 45–50. [Google Scholar]
- Doose, D.; Grand, C.; Lesire, C. MAUVE runtime: A component-based middleware to reconfigure software architectures in real-time. Robotic Computing (IRC), IEEE International Conference, Taichung, Taiwan, 10–12 April 2017, IEEE: Taichung, Taiwan, 2017; pp. 208–211. [Google Scholar]
- Mayor, C.; Brudno, M.; Schwartz, J.R.; Poliakov, A.; Rubin, E.M.; Frazer, K.A.; Pachter, L.S.; Dubchak, I. Vista: Visualizing global DNA sequence alignments of arbitrary length. Bioinformatics 2000, 16, 1046–1047. [Google Scholar] [CrossRef]
- Frazer, K.A.; Pachter, L.; Poliakov, A.; Rubin, E.M.; Dubchak, I. VISTA: Computational tools for comparative genomics. Nucleic Acids Res. 2004, 32, 273–279. [Google Scholar] [CrossRef]
- Brudno, M.; Malde, S.; Poliakov, A.; Do, C.B.; Couronne, O.; Dubchak, I.; Batzoglou, S. Glocal alignment: Finding rearrangements during alignment. Bioinformatics 2003, 19. [Google Scholar] [CrossRef]
- Amiryousefi, A.; Hyvönen, J.; Poczai, P. IRscope: An online program to visualize the junction sites of chloroplast genomes. Bioinformatics 2018, 34, 3030–3031. [Google Scholar] [CrossRef] [PubMed]
- Librado, P.; Rozas, J. DnaSP v5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics 2009, 25, 1451–1452. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, S.; Schleiermacher, C. REPuter: Fast computation of maximal repeats in complete genomes. Bioinformatics 1999, 15, 426–427. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, S. REPuter: The manifold applications of repeat analysis on a genomic scale. Nucleic Acids Res. 2001, 29, 4633–4642. [Google Scholar] [CrossRef] [PubMed]
- Thiel, T.; Michalek, W.; Varshney, R.K.; Graner, A. Exploiting EST databases for the development and characterization of gene-derived SSR-markers in barley (Hordeum vulgare L.). Theor. Appl. Genet. 2003, 106, 411–422. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef]
- Akaike, H. A new look at the statistical model identification. IEEE Trans. Automat. Contr. 1974, 19, 716–723. [Google Scholar] [CrossRef]
- Pol, D. Empirical problems of the hierarchical likelihood tatio test for model selection. Syst. Biol. 2004, 53, 949–962. [Google Scholar] [CrossRef]
- Posada, D.; Buckley, T.R. Model selection and model averaging in phylogenetics: Advantages of akaike information criterion and bayesian approaches over likelihood ratio tests. Syst. Biol. 2004, 53, 793–808. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.A.; Pfeiffer, W.; Schwartz, T. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In 2010 Gateway Computing Environments Workshop (GCE), New Orleans, LA, USA, 14 November 2010; IEEE: New Orleans, LA, USA, 2010. [Google Scholar]
- Stamatakis, A.; Hoover, P.; Rougemont, J. A rapid bootstrap algorithm for the RAxML web servers. Syst. Biol. 2008, 57, 758–771. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef] [PubMed]
- Booy, G.; Hendriks, R.J.J.; Smulders, M.J.M.; Van Groenendael, J.M.; Vosman, B. Genetic diversity and the survival of populations. Plant. Biol. 2000, 2, 379–395. [Google Scholar] [CrossRef]
- Breed, M.F.; Stead, M.G.; Ottewell, K.M.; Gardner, M.G.; Lowe, A.J. Which provenance and where? Seed sourcing strategies for revegetation in a changing environment. Conserv. Genet. 2013, 14, 1–10. [Google Scholar] [CrossRef]
- Byrne, M.; Stone, L.; Millar, M.A. Assessing genetic risk in revegetation. J. Appl. Ecol. 2011, 48, 1365–1373. [Google Scholar] [CrossRef]
Sample Availability: The annotated plastid genome sequence of M. zenii was deposited in GenBank (Accession number MH607378). Samples of the compounds are available from the authors. |
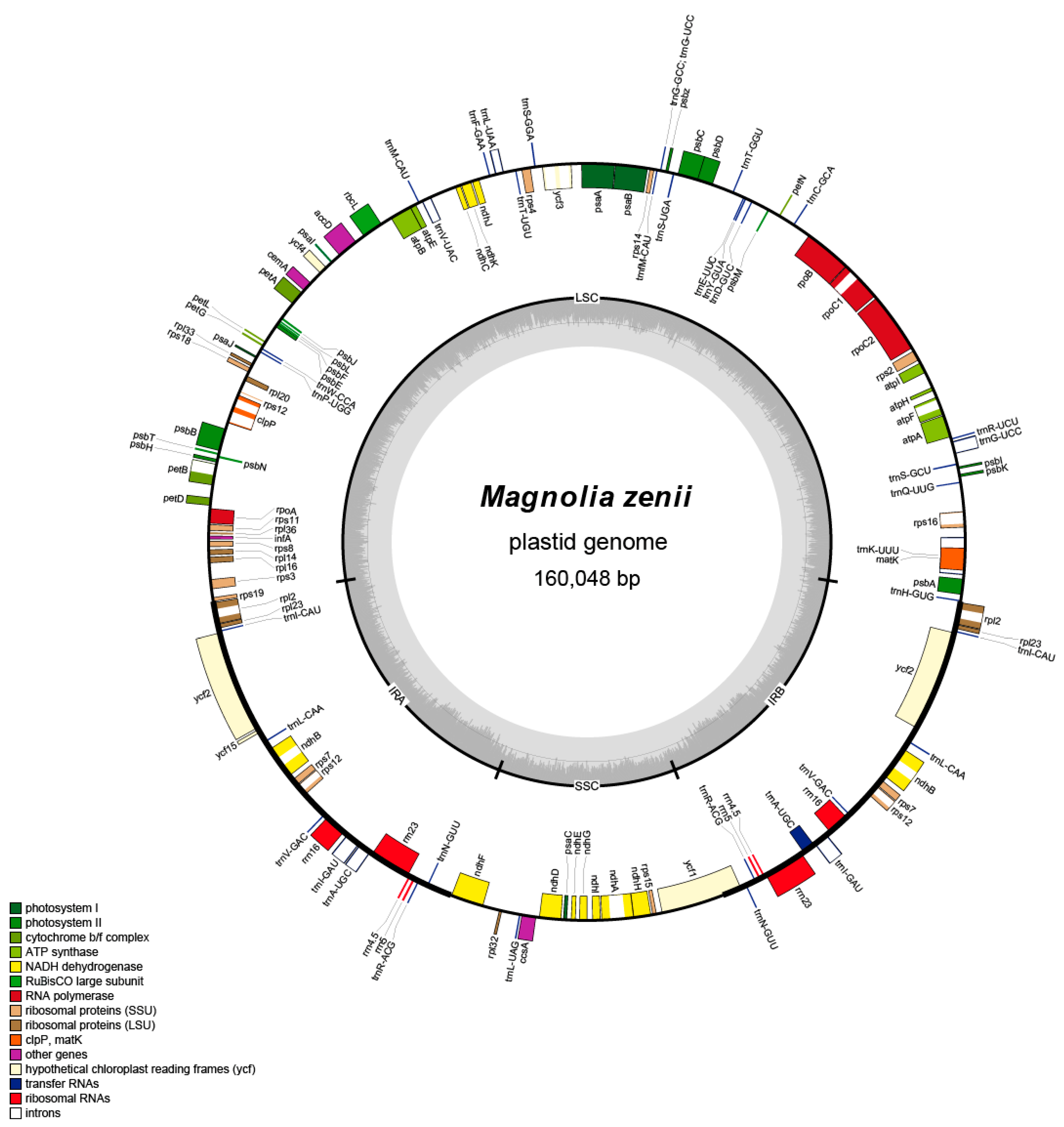

| Category | Name of Group | Name of Gene | ||||
|---|---|---|---|---|---|---|
| Self-replication | Ribosomal RNA | rrn16 | rrn23 | rrn4.5 | rrn5 | |
| Transfer RNA | trnA-UGC | trnC-GCA | trnD-GUC | trnE-UUC | trnF-GAA | |
| trnfM-CAU | trnG-GCC | trnG-UCC | trnH-GUG | trnI-CAU | ||
| trnI-GAU | trnK-UUU | trnL-CAA | trnL-UAA | trnL-UAG | ||
| trnM-CAU | trnN-GUU | trnP-UGG | trnQ-UUG | trnR-ACG | ||
| trnR-UCU | trnS-GCU | trnS-GGA | trnS-UGA | trnT-GGU | ||
| trnT-UGU | trnV-GAC | trnV-UAC | trnW-CCA | trnY-GUA | ||
| Small subunit of ribosome | rps11 | rps12 | rps14 | rps15 | rps16 | |
| rps18 | rps19 | rps2 | rps3 | rps4 | ||
| rps7 | rps8 | |||||
| Large subunit of ribosome | rpl12 | rpl14 | rpl16 | rpl2 | rpl20 | |
| rpl23 | rpl32 | rpl33 | rpl36 | |||
| RNA polymerase subunits | rpoA | rpoB | rpoC1 | rpoC2 | ||
| Photosynthesis | Subunits of photosystem I | psaA | psaB | psaC | psaI | psaJ |
| Subunits of photosystem II | psbA | psbB | psbC | psbD | psbE | |
| psbF | psbH | psbI | psbJ | psbK | ||
| psbL | psbM | psbN | psbT | psbz | ||
| Subunits of cytochrome | petA | petB | petD | petG | petL | |
| petN | ||||||
| Subunits of ATP synthase | atpA | atpB | atpE | atpF | atpH | |
| atpI | ||||||
| Large subunit of RuBisCO | rbcL | |||||
| Subunits of NADH | ndhA | ndhB | ndhC | ndhD | ndhE | |
| ndhF | ndhG | ndhH | ndhI | ndhJ | ||
| ndhK | ||||||
| Other gene | Translational initiation factor | infA | ||||
| Maturase | matK | |||||
| Envelope membrane protein | cemA | |||||
| Subunit of acetyl-CoA | accD | |||||
| C-type cytochrome synthesis gene | ccsA | |||||
| Protease | clpP | |||||
| Unknown function | Conserved open reading frames | ycf1 | ycf15 | ycf2 | ycf3 | ycf4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Sylvester, S.P.; Li, M.; Zhang, C.; Li, X.; Duan, Y.; Wang, X. The Complete Plastid Genome of Magnolia zenii and Genetic Comparison to Magnoliaceae species. Molecules 2019, 24, 261. https://doi.org/10.3390/molecules24020261
Li Y, Sylvester SP, Li M, Zhang C, Li X, Duan Y, Wang X. The Complete Plastid Genome of Magnolia zenii and Genetic Comparison to Magnoliaceae species. Molecules. 2019; 24(2):261. https://doi.org/10.3390/molecules24020261
Chicago/Turabian StyleLi, Yongfu, Steven Paul Sylvester, Meng Li, Cheng Zhang, Xuan Li, Yifan Duan, and Xianrong Wang. 2019. "The Complete Plastid Genome of Magnolia zenii and Genetic Comparison to Magnoliaceae species" Molecules 24, no. 2: 261. https://doi.org/10.3390/molecules24020261
APA StyleLi, Y., Sylvester, S. P., Li, M., Zhang, C., Li, X., Duan, Y., & Wang, X. (2019). The Complete Plastid Genome of Magnolia zenii and Genetic Comparison to Magnoliaceae species. Molecules, 24(2), 261. https://doi.org/10.3390/molecules24020261







