The chemical conversion follows a sigmoidal trend characterized by a significant increase of the reaction rate approximately between 28 and 32 min of mechanical processing. The kinetic curve is quite different from the one observed for the same reaction carried out in solution, which corresponds to a simple first-order kinetics.
We show that such observation, in combination with a simplified kinetic modelling, can satisfactorily explain the experimental findings.
2.1. The Phenomenological Kinetic Model
Discussed in detail elsewhere [
16,
17,
18], the model takes into account the fundamental features of BM. Accordingly, it assumes that:
- (a)
Only a small amount of powder is processed during each collision;
- (b)
The powder is trapped between the impacting milling tools during each collision with approximately stochastic dynamics;
- (c)
For any component of the powder mixture, the amount involved in each collision corresponds to the average composition of the powder charge;
- (d)
The chemical composition of the processed powder remains uniform during the entire mechanical treatment.
Additionally, it is reasonable to assume that (i) the mechanical stresses generated during each collision result in critical loading conditions (CLCs) only in a subvolume of the trapped powder, and that (ii) such subvolume remains approximately constant during the entire mechanical treatment. CLCs can be regarded as the mechanical loading conditions that generate mechanical stresses intense enough to cause the mechanical deformation required to activate a given transformation process. Thus, represents the volume of effectively processed powder. Both CLCs and can vary from process to process.
For a given transformation, the volume fraction of effectively processed powders is , where is the total volume of powder inside the vial. Ideally, the powder charge can be divided into equal volume elements with the same probability of being processed in a given collision.
Once the first collision has occurred, the powder charge can be divided into two volume fractions, and , of powders processed respectively zero and one times. These two fractions are equal to and . As the second collision takes place, another volume fraction gets processed. Based on the model assumption that the powder charge remains always uniform, the volume fractions effectively processed zero, one, and two times become equal to , , and .
The process continues as the number of collisions,
, increases. The volume fraction
of powder not yet processed after
n collisions can be expressed as
This simply indicates that, during each collision,
decreases by the fraction
. The corresponding expression for the fraction
of the powder processed
times after
collisions is more complicated, as its variation is governed by two different contributions. During the
-th collision, a fraction of the powder that has been processed
times, namely
, is processed for the
-th time. This fraction contributes to
, thus it must be subtracted from
. On the other hand, the
n-th collision will process a fraction of
, equal to
, for the
i-th time, thus this amount must be added to
. Therefore, after the
-th collision, the volume fraction of powder effectively processed
i times is equal to
As long as the volume fraction
is small, i.e.,
<< 1, which is the case in typical BM experiments, the discrete Equations (1) and (2) can be written in the continuous forms
whereas Equation (4) is solved by
Equations (5) and (6), which satisfy the condition , can be regarded as the fundamental equations that describe the kinetics of ball mill-activated transformations.
A kinetic curve with a simple exponential character can be obtained by assuming that the final product forms when the powder is processed above CLCs for the first time. Under this assumption, the volume fraction
of the product phase can be expressed as
A sigmoid curve is obtained if the formation of the product requires that the powder undergoes CLCs twice. In this case,
If
CLCs are required, the resulting equation is more complicated, but retains the sigmoid shape and the volume fraction of the product can be expressed as
Similarly, if
CLCs are required to form an intermediate that evolves after
additional collisions, into a final product, the volume fraction of the intermediate can be expressed as
In all cases, the volume fraction of powder processed effectively during a collision, , can be regarded as a measure of the rate of the gradual mechanochemical transformation.
All of the model equations can be referred to mass fractions if density of reactants, intermediates if present, and products are taken into due account. In addition, under the assumption that the number of collisions, , is proportional to time, , all of the model equations reported above can be expressed as a function of time.
2.2. Analysis of Kinetic Data
The capability of mechanical processing by BM of inducing the Knoevenagel reaction raises a crucial question for its kinetic analysis. Is the reaction activated by the very first impact? Or, perhaps, a given fraction of powder must be hit twice to undergo the chemical transformation? At present, we do not have direct evidence concerning the point. Under these circumstances we make the simplest possible assumption that allows kinetic modelling. Accordingly, we assume that the Knoevenagel condensation takes place, in a small fraction of the powder trapped between ball and vial, when the powder has undergone CLCs once. If the rate coefficient, , accounting for the transformation rate remained constant, we could equal it to and try to use the equivalent of Equation (7) referred to time to describe the reaction kinetics. However, a constant does not seem to be the case for the observed transformation.
The rate coefficient measures the volume fraction processed effectively during a single collision. Therefore, it is proportional to the volume of material subjected to CLCs. In turn, the volume of material subjected to CLCs can be expected to scale with the amount of material that can be effectively trapped between the surfaces of milling tools during the collision.
The efficiency of trapping can vary greatly depending on the nature of the processed material. Trapping loose powder is quite difficult because of the intrinsic dynamics of the granular body inside the reactor. Thus, it can be expected, and it is also generally observed [
16,
17,
18], that only a very small fraction of the powder charge is effectively processed in individual collisions. In contrast, a greater trapping efficiency can be expected when the processed material coats the milling tools. Indeed, a significant fraction of the whole powder charge inside the reactor will be certainly loaded by milling tools under such circumstances.
According to the experimental findings regarding the Knoevenagel condensation, the material inside the reactor forms a coating around the milling ball. Such coating forms gradually as a consequence of the ball impacting and rolling inside the reactor and we can suppose that it makes
increase with time. In particular, we can expect that the coating forms at a rate proportional to the total surface that can be coated. It follows that the coating rate can be expressed as
where
is the volume of material forming the coating layer around the milling ball and
is the radius of the coated ball. Now, we can suppose that the rate of radius increase remains constant. This means that
Thus,
where
is the radius of the uncoated ball. Once we substitute Equation (13) in Equation (11) and integrate Equation (11), the result is
It follows that the volume of the coated ball is
If we assume that the rate coefficient
is proportional to the volume
of the coated ball, then
where
is the total volume of powder inside the reactor and
is a proportionality constant. Thus, to a first, rough approximation, we can expect that
exhibits a cubic dependence on time.
It is also worth noting that experimental findings suggest that increases definitely only after about 20 min of mechanical processing. Therefore, it seems reasonable to assume that the material starts coating the ball only after an induction period .
The above-mentioned observations can be summarized as follows:
Here, is the apparent rate constant of the transformation for times shorter than the induction period , when the material is still unable to stick to the ball surface and form a stable coating. For times longer than , the apparent rate constant is affected by the additional contribution related to coating.
The variation of
with time does not allow any analytical description of the kinetic curve. However, it is still possible to write the equations as a function of time and solve them numerically.
Equivalent to Equations (3) and (4), Equations (19) and (20) provide a general description of the transformation kinetics. A best-fitting procedure allows us to find the expression of the kinetic curve and the spectrum of . values associated.
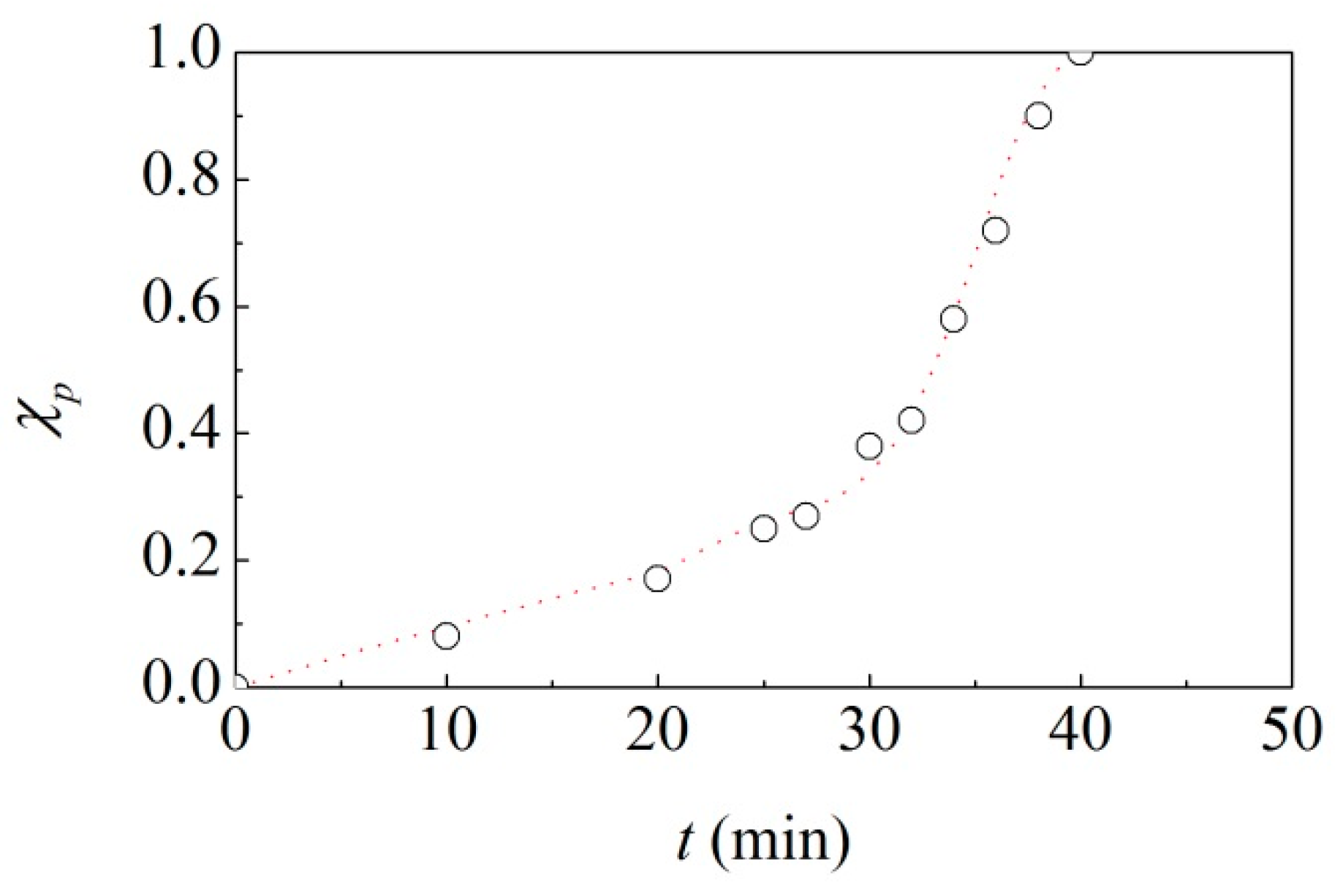
The hypothesis that the reaction activation requires that powder undergoes CLCs only once allows a very good fitting of the experimental data. As shown in
Figure 1, the resulting kinetic curve perfectly interpolates the experimental points. Such kinetic curve is obtained allowing
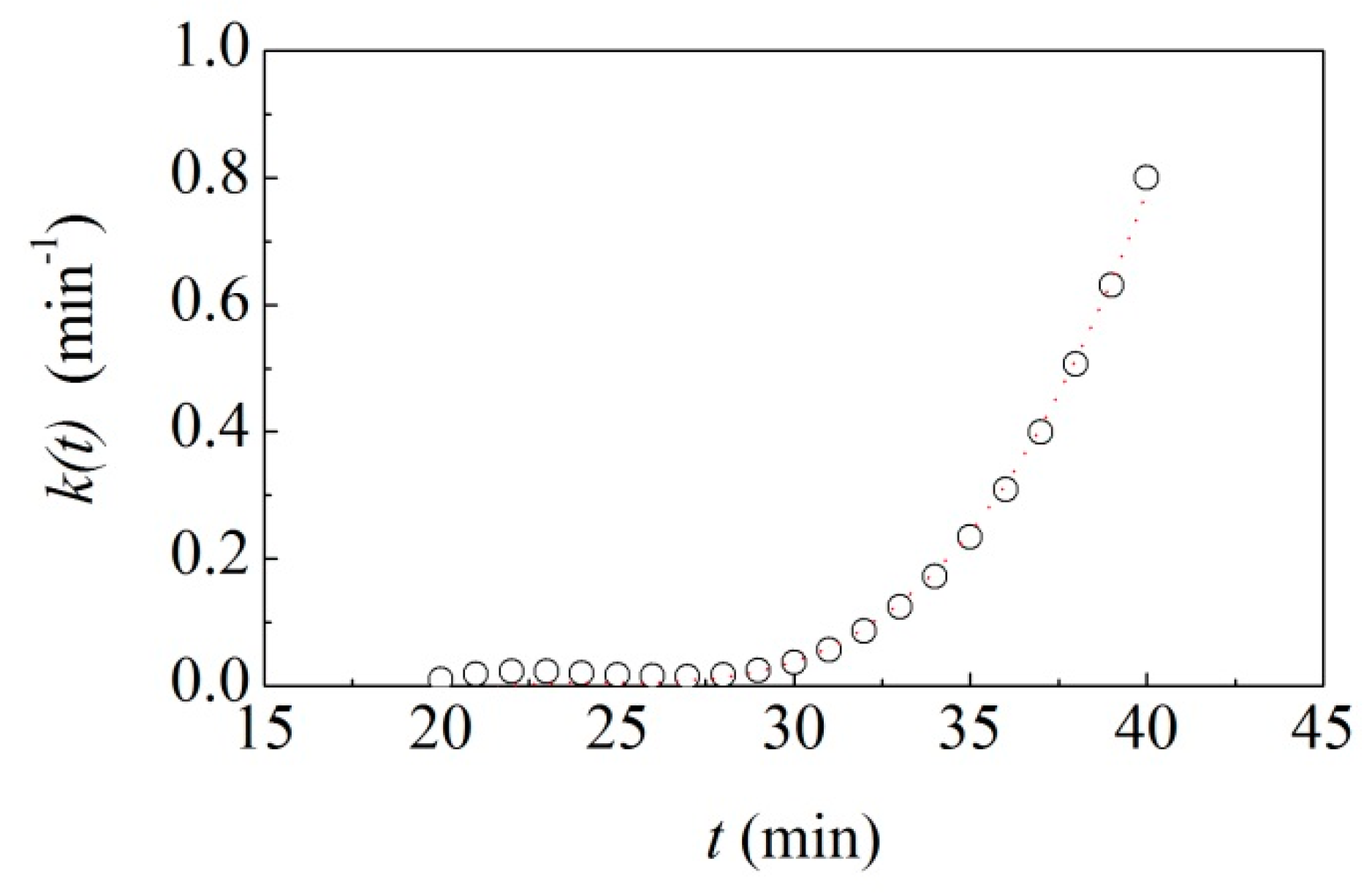
to vary in time as shown in
Figure 3. It can be seen that, starting from a relatively low value,
progressively increases up to about 0.8 min
−1.
Now, it is worth noting that Equation (18) satisfactorily describes the variation of . The best-fitted curve almost overlaps the estimates, providing significant support to the theoretical model proposed. In particular, it seems definitely reasonable to expect that cohesive states develop gradually under the effect of adhesive forces between the coated ball and the material present in the vial. The mechanism is analogous to that governing the formation of snowballs. Accordingly, the rate of adhesion to the coating depends on the amount of material involved in the coating itself and the adhesion process proceeds autocatalytically until all the material inside the vial has been collected or other competing disaggregation processes intervene to limit the growth of the coating layer.
The satisfactory best-fitting allowed obtaining reliable estimates for the different unknown quantities in Equation (18). In particular, it suggests an induction period,
, about 27 min long and, for times shorter than 27 min (not shown for clarity in
Figure 3), an initial value,
, of about 0.01 min
−1 for the rate coefficient. The rate of radius increase,
, is approximately equal to 0.1 mm min
−1, a value that properly accounts for the rapid formation of the coating layer.
The results discussed heretofore suggest that the kinetic modelling can suitably explain the experimental findings concerning the Knoevenagel condensation between vanillin and barbituric acid carried out under mechanical activation conditions [
8]. Specifically, it seems that the simplest possible kinetic assumption that the reaction occurs in a fraction of the processed material already at the first impact, combined with the hypothesis that the rate of coating formation is proportional to the total surface that can be coated, leads to kinetic equations able to reproduce the experimental data quite satisfactorily.
However, there are at least two issues that deserve further investigation in the attempt of gaining a deeper insight into the behaviour of the processed material and validating, as far as possible, the kinetic model proposed. On the one hand, it is worth investigating whether or not cohesive, rubber-like states can form under the effects of a mechanical action different from the one imparted to milling balls by the Retsch MM400 shaker-type ball mill used in previous work [
8]. On the other, it is highly desirable to obtain direct evidence concerning the formation kinetics of the coating layer around the milling ball in order to support the hypothesis underlying Equations (14)–(16).
To this aim, we expressly designed and carried out experiments involving a different ball mill and a different adhesive material. Specifically, in view of the number of preliminary runs to be done under different conditions, we used polyvinyl acetate glue. In addition, we equipped the ball mill with the sensors needed to monitor the milling conditions and obtain in situ indirect information on the rheological behaviour of the material inside the vial. Details are given in the following.
2.3. Supporting Experiments
Experiments were performed using a SPEX Mixer/Mill 8000. It was chosen because of the different mechanical action with respect to the Retsch MM400 ball mill. While the latter makes the vials oscillate on the horizontal plane with a simple harmonic motion, the SPEX Mixer/Mill 8000 swings the vial along a three-dimensional trajectory that combines a vertical harmonic motion with synchronous oscillations on the horizontal plane. It follows that the vial movement results in a more efficient stirring of powder and a wider volume spanned by the milling ball. In addition, we also used a SPEX vial with a flat base, which makes ball trajectories inside the vial more complicated and impacts on the vial walls more energetically.
The use of a SPEX Mixer/Mill 8000 has another advantage. The milling dynamics have been thoroughly studied under the most diverse experimental conditions [
19,
20,
21], which provided significant help in the planning of experiments and the interpretation of their results. Following previous work [
19], a single milling ball was used. Furthermore, the ball mill was equipped with a piezoelectric transducer in order to monitor the occurrence of impacts between the ball and the vial walls. The sensor was placed on the bottom end of the vial and connected to a computer to record the sequence of electric signals generated by impacts. The polymer, initially in the liquid phase, was added as a partially dried powder.
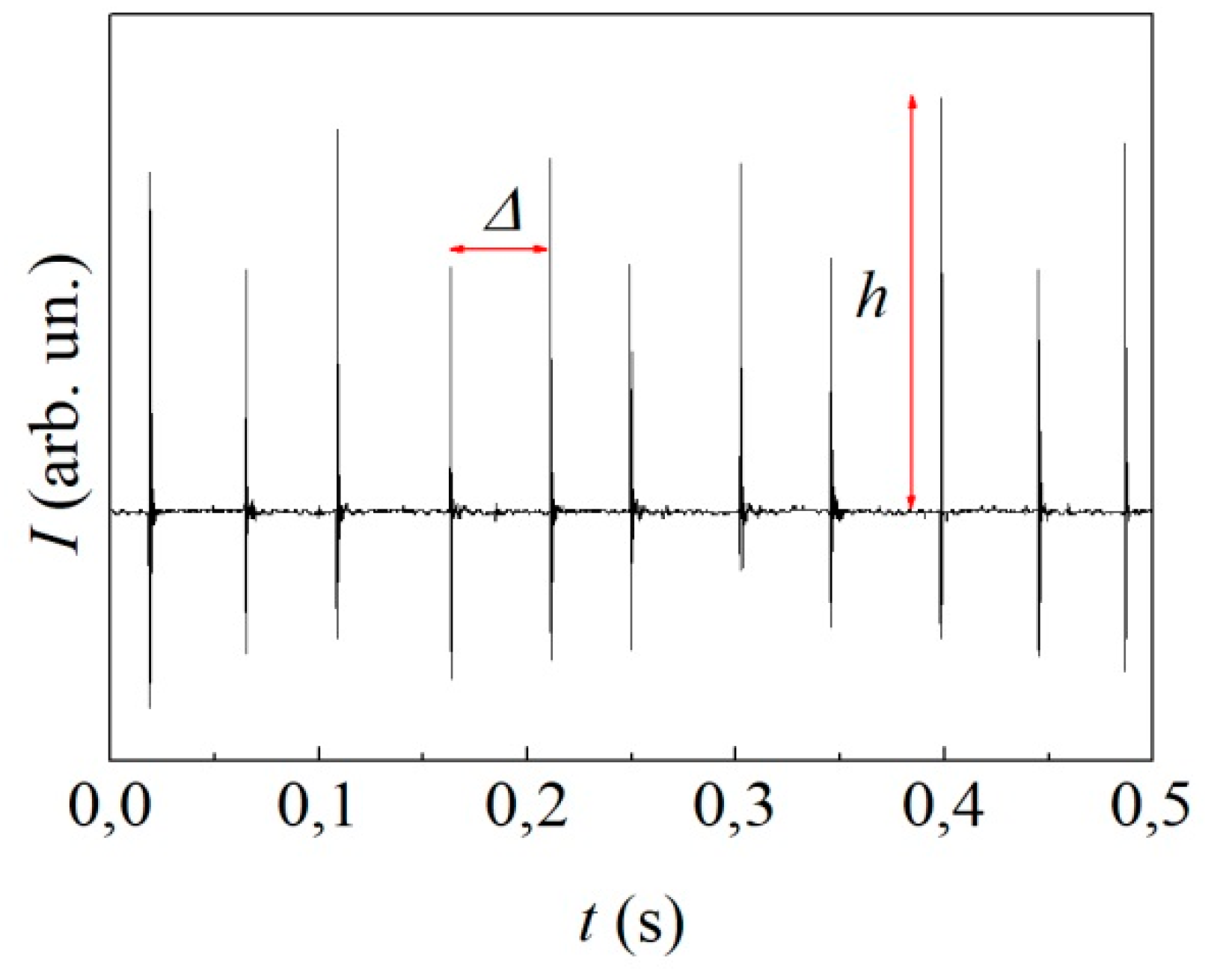
A short sequence of signals generated by the piezoelectric sensor is shown in
Figure 4. Signals are regularly spaced, thus indicating that the ball underwent regular dynamics. Two impacts per vial cycle were detected, which corresponds to an impact frequency of about 23.2 Hz.
We recorded long sequences of signals to gain information on the ball dynamics on long time-scales. Although more complex analyses can be performed, the distance between two consecutive signals and the intensity of signals allow sufficient insight in this respect. The former gives information on the periodicity of the ball motion, with relatively short values pointing out the occurrence of irregular trajectories with multiple rebounds on the vial base and cylindrical wall. The latter measures the energy dissipation at collisions, with a decrease of signal intensity for less energetic collisions.
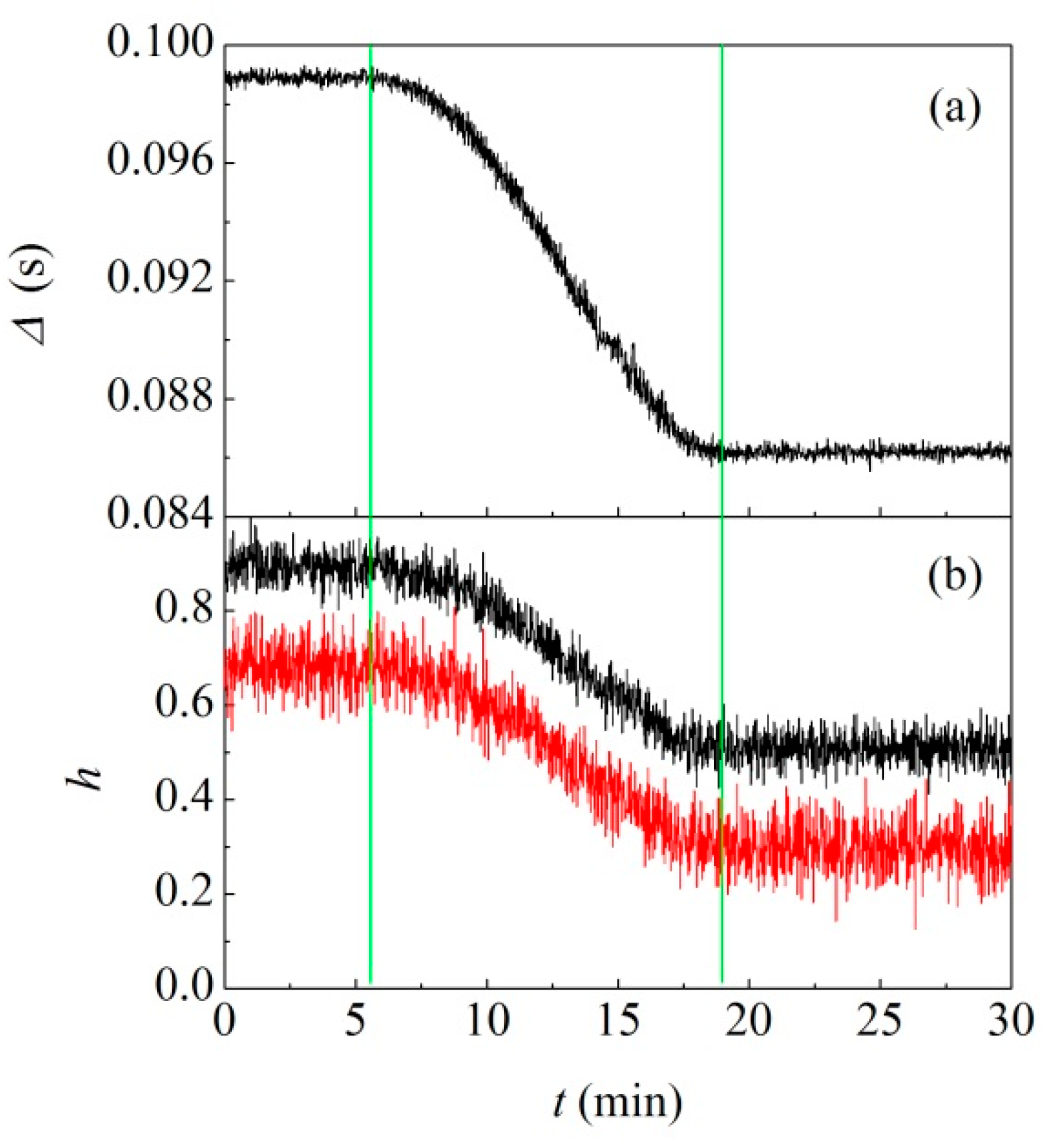
The distance between two consecutive signals,
, is shown in
Figure 5a as a function of time,
.
It can be seen that
remains approximately constant during the first 6 min of BM. Then, it decreases slowly for other 13 min, after which it keeps the constant value of 23.2 Hz, twice the milling frequency. Overall, data indicate that the ball never undergoes chaotic dynamics. However, after about 20 min, there is a clear transition to an extremely regular behaviour. Based on the impact frequency as well as on previous work on other systems [
19,
20,
21], it can be inferred that the ball has reached the most regular possible dynamics. Accordingly, it simply travels between the opposite bases undergoing almost perfectly inelastic collisions.
The relative intensity of signals,
, normalized to the maximum
value observed, is plotted in
Figure 5b. Two distinct sequences of
values were detected, simply due to the position of the piezoelectric sensor. Being placed externally on the bottom end of the vial, it recorded with higher intensity the impacts occurring on the bottom base. Those occurring on the vial cap were much less intense, also because of the O-ring that, while assuring a good sealing of the vial, dampened any vibration. Stronger and weaker signals exhibited a similar variation with time. They initially kept an approximately constant intensity. Then, the intensity decreased progressively, and significantly, until a new constant value was reached.
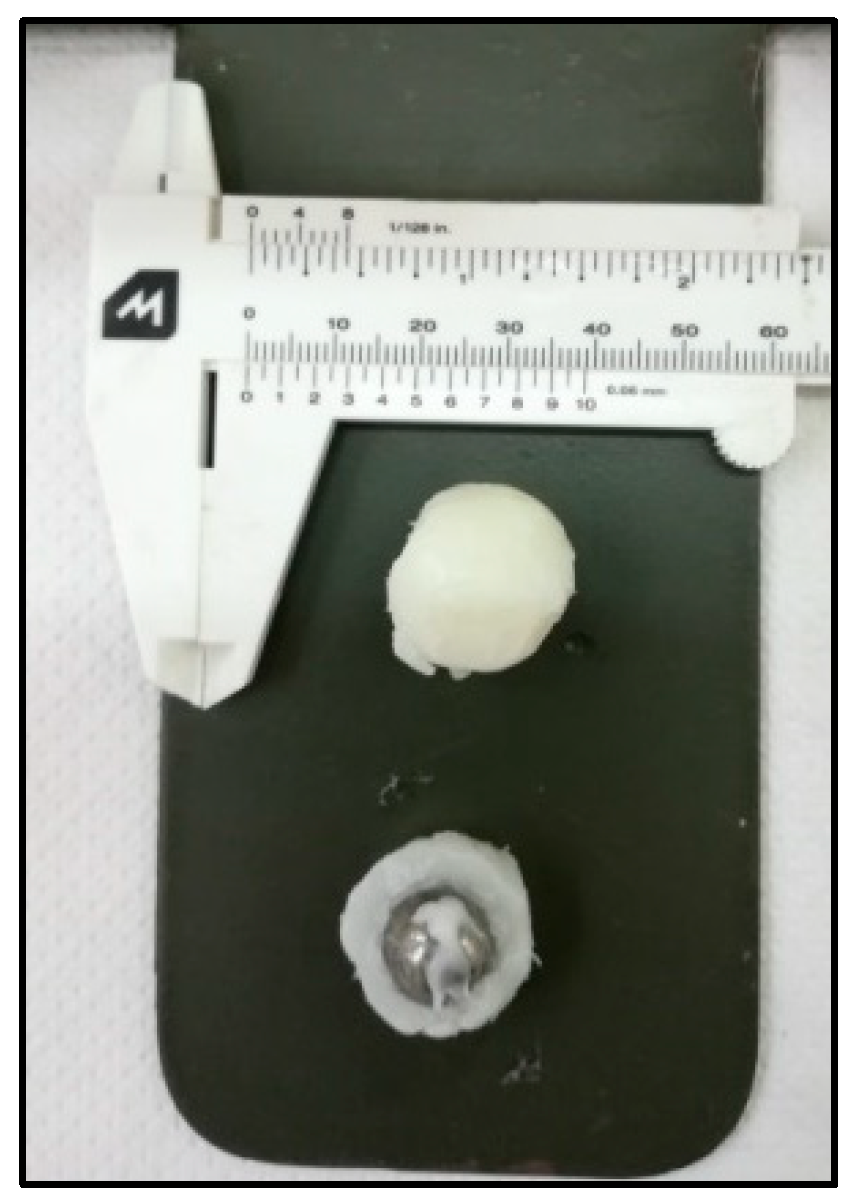
The variation of
is substantially synchronous with the variation of the distance
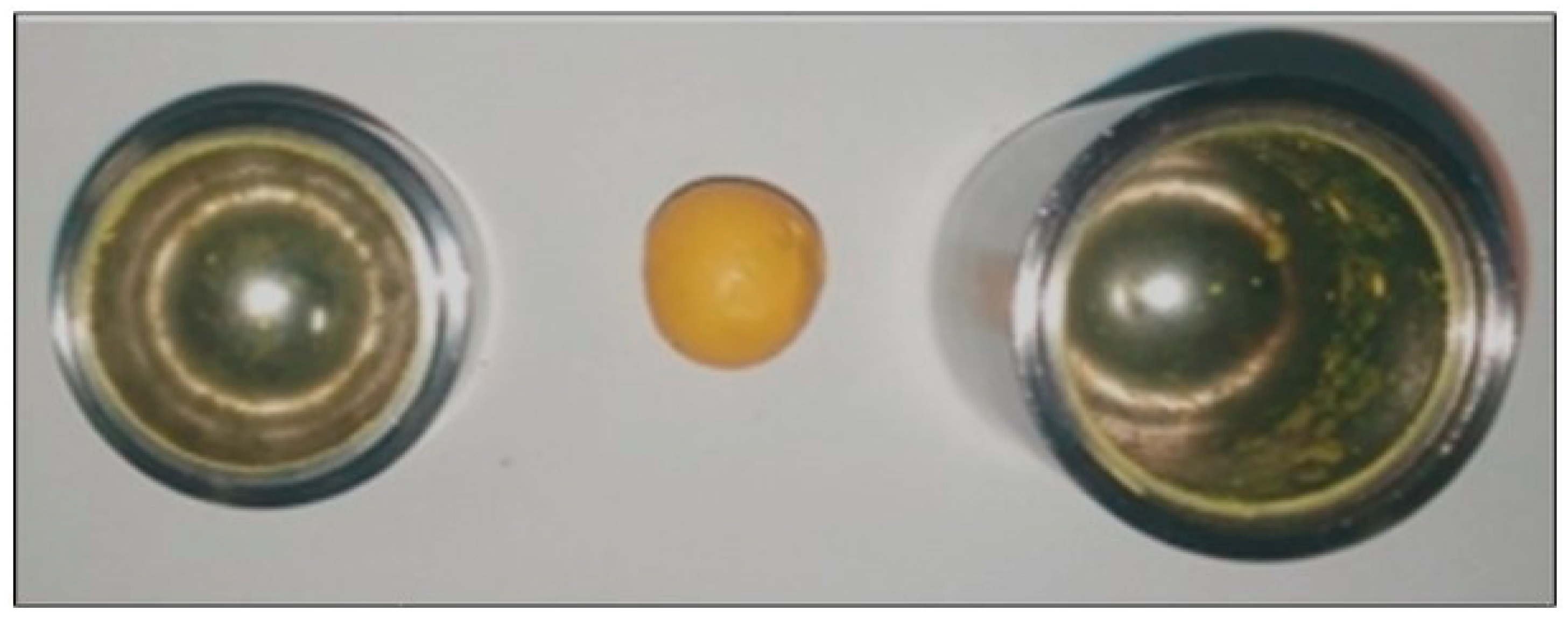
between consecutive impacts. This strongly suggests that something happened to the ball after about 6 min of mechanical processing, as evident from the direct inspection of the ball after 3 min and after 24 min of milling. Before the transition in the ball dynamics occurred, the ball was still quite clean. Conversely, once the ball had reached the new dynamics, the ball was completely coated with material as shown by the picture in
Figure 6.
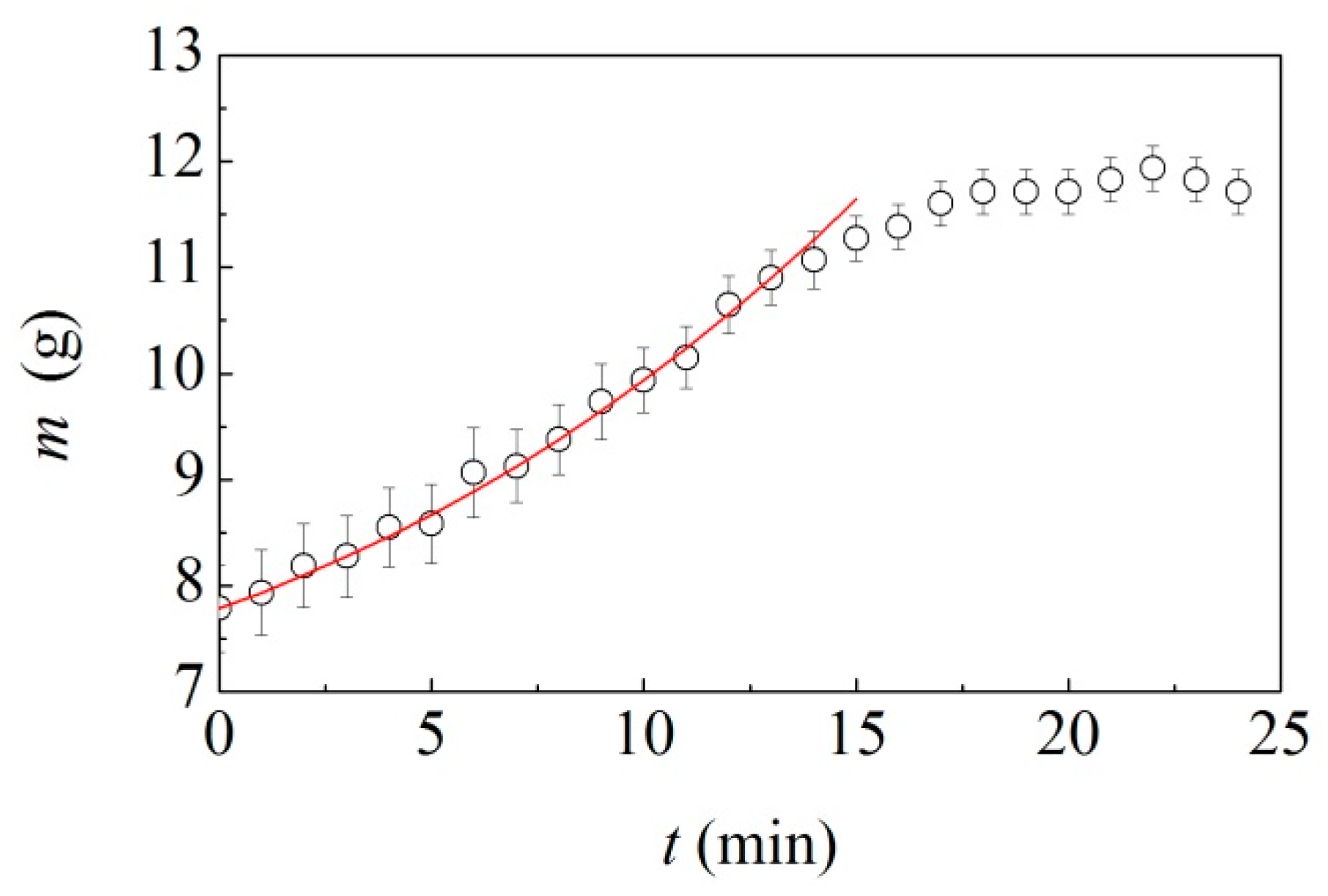
Based on the evidence mentioned above, we opened regularly the vial to weigh the ball during the transition period starting from 5 min of milling. We repeated the experiments three times to suitably account for experimental uncertainties. At the beginning of BM, the material inside the jar kept its powder form, although it showed tendency to aggregate. As the coating layer around the ball began to form, part of the powder adhered to the rubber-like cohesive state. Generally, it could be recognized and easily separated from the coated ball. In the later BM stages, the material not yet involved in the formation of the cohesive layer around the ball also formed irregular coatings on the vial. Eventually, all the material was consumed in the coating layer. The results are shown in
Figure 7, where the mass of the coated ball,
, is plotted as a function of time,
. Data arranged according to a concave up, increasing curve.
Assuming that the mechanical processing does not change the density of the adhesive material, so that it keeps constant throughout the milling, Equation (14) can be readily utilized for expressing the mass of the coated ball. It can be rewritten as
where
and
are the densities of adhesive and ball, equal to 1.1 and 7.85 g cm
−3 respectively. We used Equation (21) to interpolate the experimental data in
Figure 7. It can be seen that it works quite well. Thus, it can be reasonably deduced that the coating around the ball grows proportionally to the total surface of the coated layer and that the thickness of the coated layer varies linearly with time.