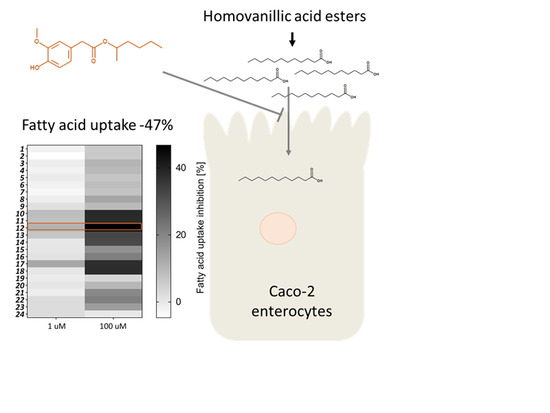
Biological Evaluation of Natural and Synthesized Homovanillic Acid Esters as Inhibitors of Intestinal Fatty Acid Uptake in Differentiated Caco-2 Cells
Abstract
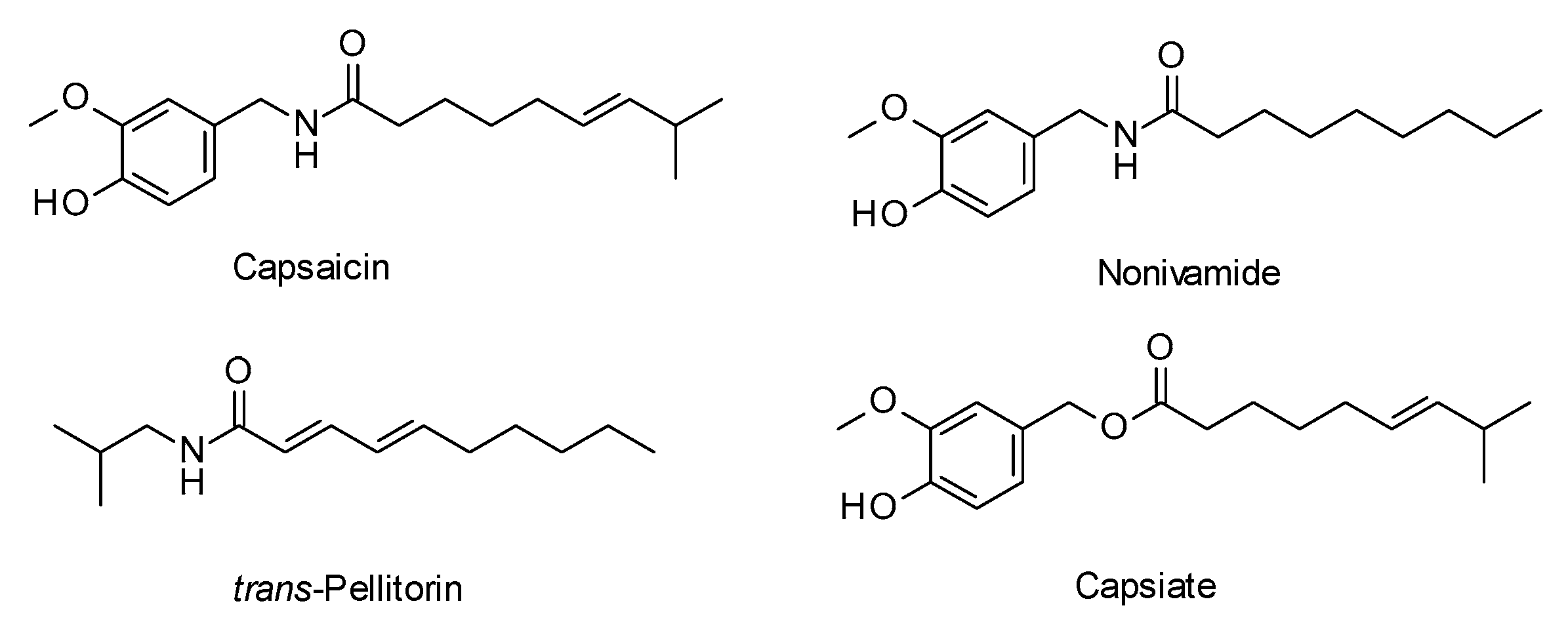
1. Introduction
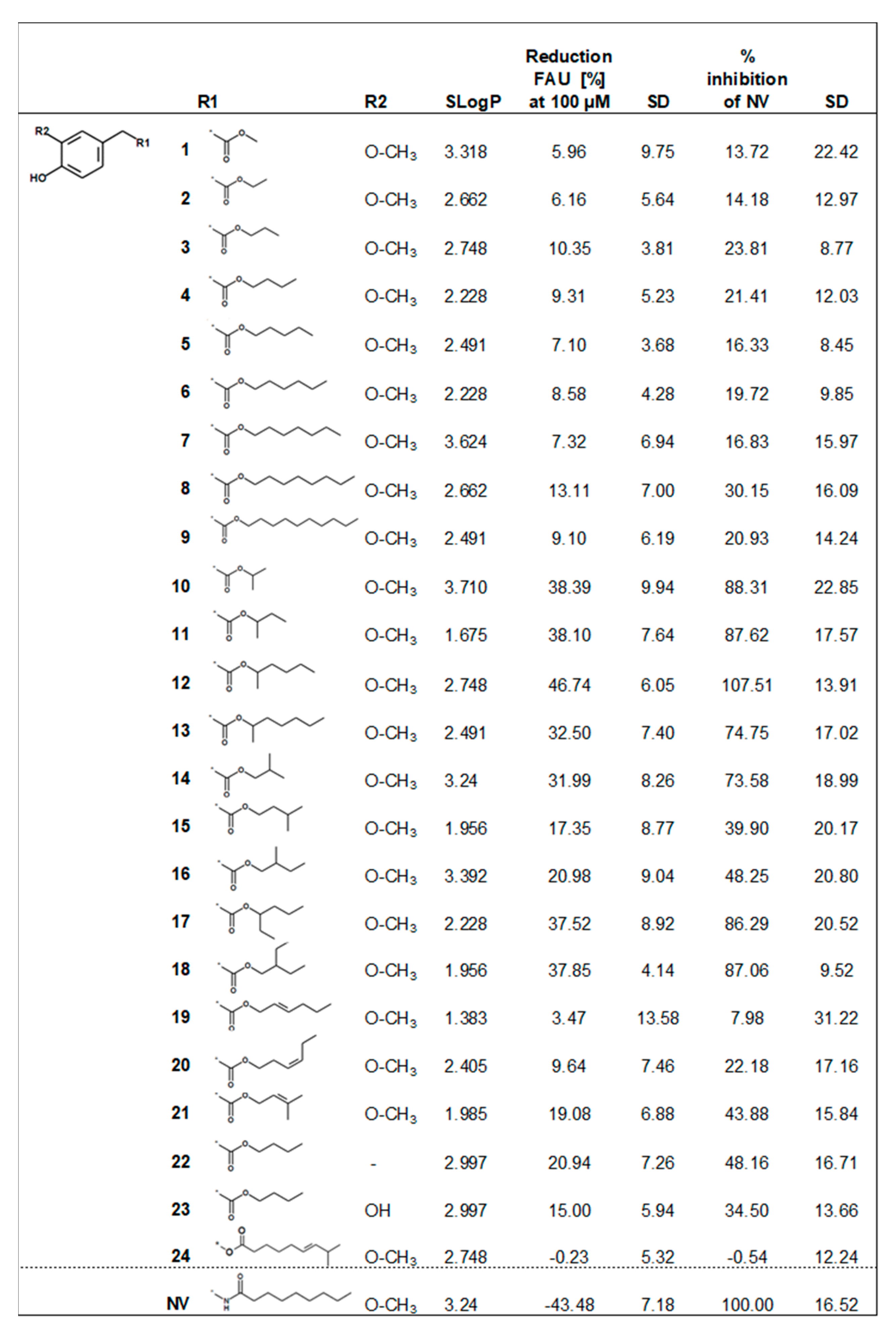
2. Results and Discussion
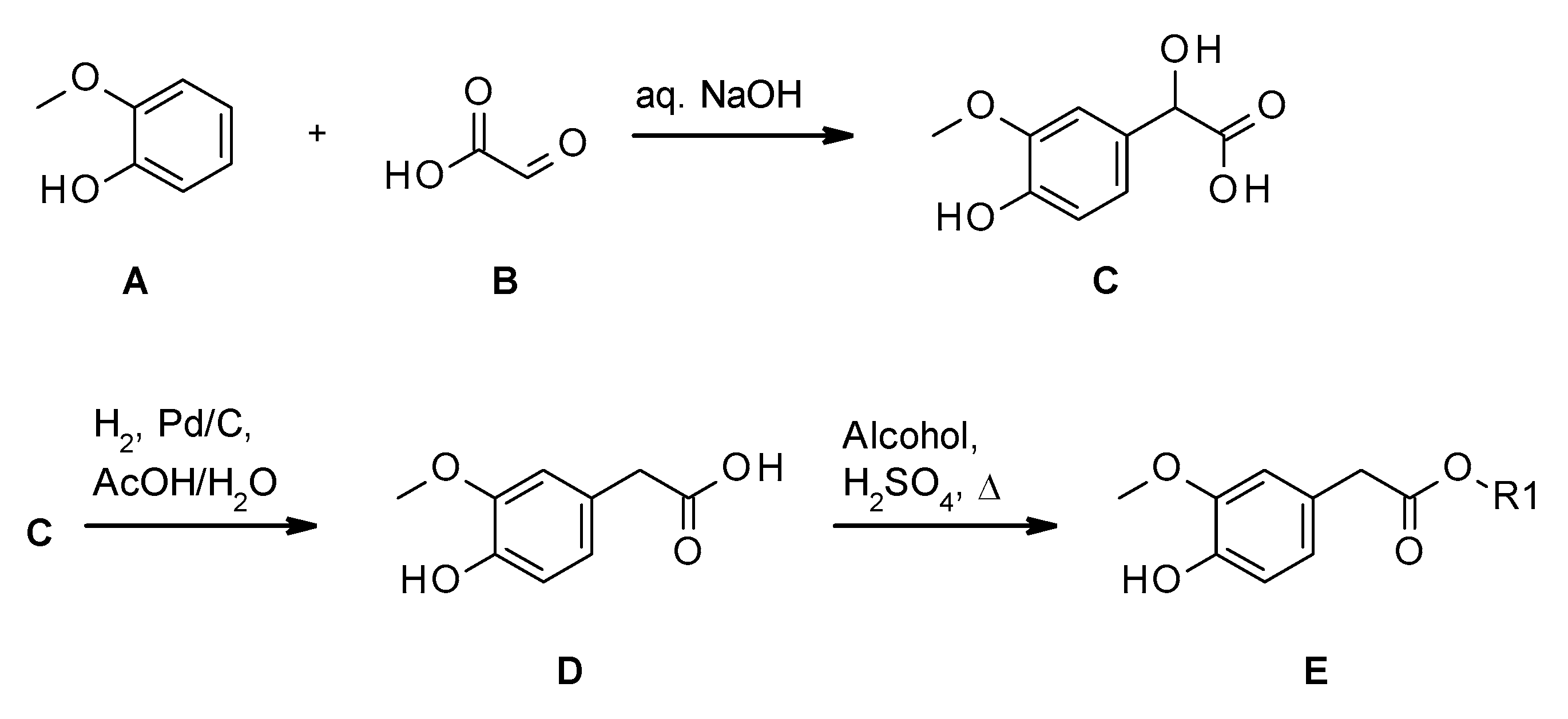
2.1. Synthesis of Homovanillate Esters
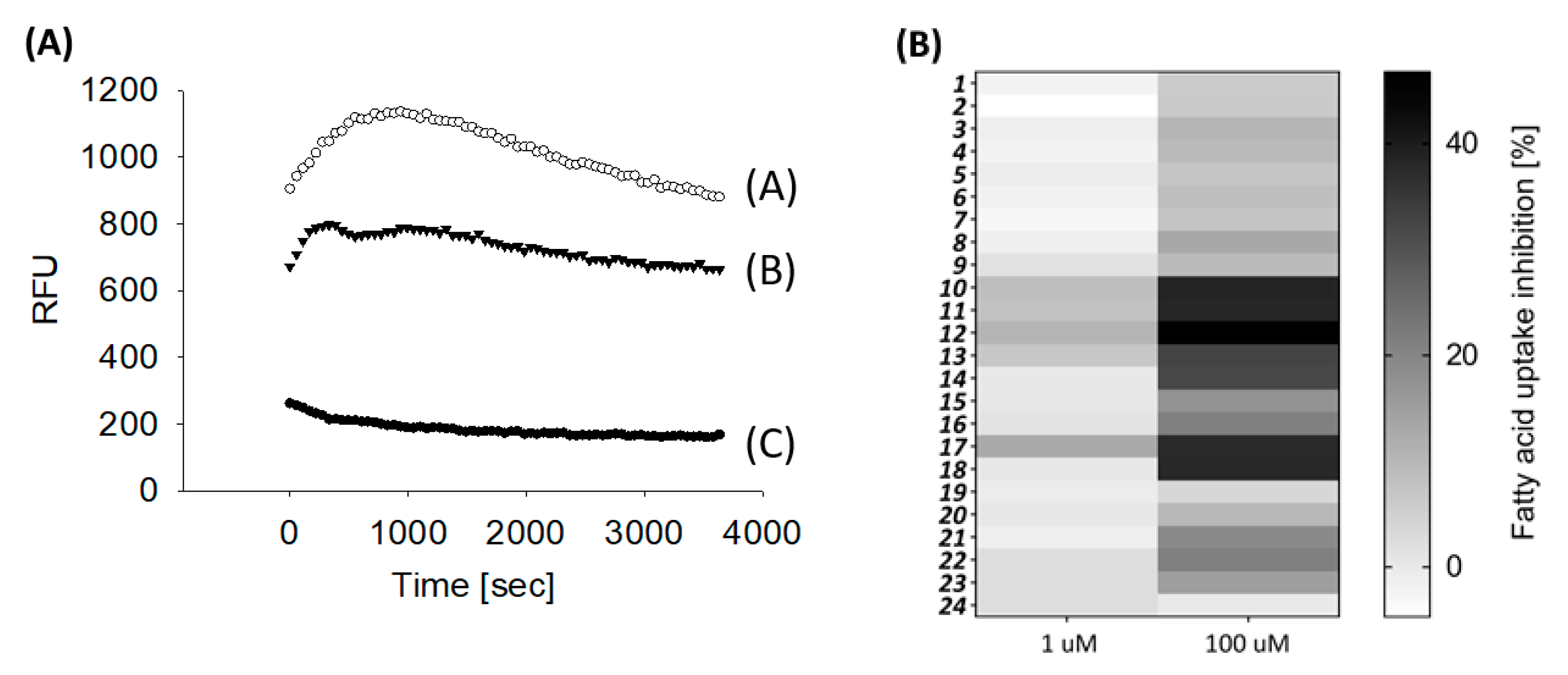
2.2. Biological Evaluation
3. Experimental Procedures
3.1. Chemistry Section
3.2. Synthesis
3.2.1. Method A
3.2.2. Method B
3.2.3. Method C
3.3. Biological Evaluation
3.3.1. Cell Culture
3.3.2. In Vitro Fatty Acid Uptake Studies
3.3.3. Physicochemcial Descriptors
3.3.4. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kumar, R.B.; Aronne, L.J. Efficacy comparison of medications approved for chronic weight management. Obesity 2015, 23, S4–S7. [Google Scholar] [CrossRef] [PubMed]
- Padwal, R.S.; Rucker, D.; Li, S.K.; Curioni, C.; Lau, D.C. Long-term pharmacotherapy for obesity and overweight. Cochrane Database Syst. Rev. 2003. [Google Scholar] [CrossRef] [PubMed]
- Bray, G.A.; Tartaglia, L.A. Medicinal strategies in the treatment of obesity. Nature 2000, 404, 672–677. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.Y.; Liu, M.; Portincasa, P.; Wang, D.Q.-H. New insights into the molecular mechanism of intestinal fatty acid absorption. Eur. J. Clin. Invest. 2013, 43, 1203–1223. [Google Scholar] [CrossRef] [PubMed]
- Meunier, V.; Bourrie, M.; Berger, Y.; Fabre, G. The human intestinal epithelial cell line Caco-2; pharmacological and pharmacokinetic applications. Cell Biol. Toxicol. 1995, 11, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Grasset, E.; Pinto, M.; Dussaulx, E.; Zweibaum, A.; Desjeux, J.F. Epithelial properties of human colonic carcinoma cell line Caco-2: Electrical parameters. Am. J. Physiol. 1984, 247, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, I.J.; Raub, T.J.; Borchardt, R.T. Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability. Gastroenterology 1989, 96, 736–749. [Google Scholar] [CrossRef]
- Ho, S.Y.; Storch, J. Common mechanisms of monoacylglycerol and fatty acid uptake by human intestinal Caco-2 cells. Am. J. Physiol. Cell Physiol. 2001, 281, C1106–C1117. [Google Scholar] [CrossRef] [PubMed]
- Riedel, A.; Lang, R.; Rohm, B.; Rubach, M.; Hofmann, T.; Somoza, V. Structure-dependent effects of pyridine derivatives on mechanisms of intestinal fatty acid uptake: Regulation of nicotinic acid receptor and fatty acid transporter expression. J. Nutr. Biochem. 2014, 25, 750–757. [Google Scholar] [CrossRef] [PubMed]
- Constant, H.L.; Cordell, G.A.; West, D.P. Nonivamide, a constituent of Capsicum oleoresin. J. Nat. Prod. 1996, 59, 425–426. [Google Scholar] [CrossRef]
- Murota, K.; Storch, J. Uptake of Micellar Long-Chain Fatty Acid and sn-2-Monoacylglycerol into Human Intestinal Caco-2 Cells Exhibits Characteristics of Protein-Mediated Transport. J. Nutr. 2005, 135, 1626–1630. [Google Scholar] [CrossRef] [PubMed]
- Rohm, B.; Riedel, A.; Ley, J.P.; Widder, S.; Krammer, G.E.; Somoza, V. Capsaicin, nonivamide and trans-pellitorine decrease free fatty acid uptake without TRPV1 activation and increase acetyl-coenzyme A synthetase activity in Caco-2 cells. Food Funct. 2015, 6, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Ley, J.P.; Krammer, G.; Looft, J.; Reinders, G.; Bertram, H.-J. Structure-activity relationships of trigeminal effects for artificial and naturally occurring alkamides related to spilanthol. In Developments in Food Science; Elsevier: Amsterdam, The Netherlands, 2006; Volume 43, pp. 21–24. [Google Scholar]
- Baskaran, P.; Covington, K.; Bennis, J.; Mohandass, A.; Lehmann, T.; Thyagarajan, B. Binding Efficacy and Thermogenic Efficiency of Pungent and Nonpungent Analogs of Capsaicin. Molecules 2018, 23, 3198. [Google Scholar] [CrossRef] [PubMed]
- Baskaran, P.; Krishnan, V.; Ren, J.; Thyagarajan, B. Capsaicin induces browning of white adipose tissue and counters obesity by activating TRPV1 channel-dependent mechanisms. Br. J. Pharmacol. 2016, 173, 2369–2389. [Google Scholar] [CrossRef] [PubMed]
- Hong, Q.; Xia, C.; Xiangying, H.; Quan, Y. Capsinoids suppress fat accumulation via lipid metabolism. Mol. Med. Rep. 2015, 11, 1669–1674. [Google Scholar] [CrossRef] [PubMed]
- Backes, M.; Ley, J.P.; Degenhardt, A.; Paetz, S.; Reichelt, K.; Riess, T.; Klose, B.; Hentschel, F. Homovanillic Ester, More Particularly for Achieving an Impression of Heat and/or Spiciness. Available online: http://www.freepatentsonline.com/y2019/0276386.html (accessed on 23 June 2019).
- Appendino, G.; Cravotto, G.; Palmisano, G.; Annunziata, R.; Szallasi, A. Synthesis and Evaluation of Phorboid 20-Homovanillates: Discovery of a Class of Ligands Binding to the Vanilloid (Capsaicin) Receptor with Different Degrees of Cooperativity. J. Med.Chem. 1996, 39, 3123–3131. [Google Scholar] [CrossRef] [PubMed]
- Umumura, S.; Iwata, F.; Ataka, K.; Shiraishi, H. Process for Preparation of 3-Alkoxy-4-hydroxyphenylacetic Acid. U.S. Patent 4,401,830, 30 August 1983. [Google Scholar]
- Silverman, R.B.; Holladay, M.W. The Organic Chemistry of Drug Design and Drug Action; Academic Press: San Diego, CA, USA, 2014. [Google Scholar]
- Ohnuki, K.; Haramizu, S.; Watanabe, T.; Yazawa, S.; Fushiki, T. CH-19 sweet, nonpungent cultivar of red pepper, increased body temperature in mice with vanilloid receptors stimulation by capsiate. J. Nutr. Sci. Vitaminol. 2001, 47, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Yoneshiro, T. Capsinoids and related food ingredients activating brown fat thermogenesis and reducing body fat in humans. Curr. Opin. Lipidol. 2013, 24, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Shirai, Y.; Ueno, S.; Nakayama, A.; Ikeuchi, K.; Ubukata, K.; Mihara, R.; Bernard, B.K. Studies of the toxicological potential of capsinoids, XII: Pharmacokinetic study of capsinoid-containing CH-19 Sweet extract in rats. Int. J. Toxicol. 2010, 29, 15S–21S. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Traber, M.G.; Kayden, H.J.; Rindler, M.J. Polarized secretion of newly synthesized lipoproteins by the Caco-2 human intestinal cell line. J. Lipid Res. 1987, 28, 1350–1363. [Google Scholar] [PubMed]
Sample Availability: Samples of the compounds 1–24 are available from the authors. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lieder, B.; Hans, J.; Hentschel, F.; Geissler, K.; Ley, J. Biological Evaluation of Natural and Synthesized Homovanillic Acid Esters as Inhibitors of Intestinal Fatty Acid Uptake in Differentiated Caco-2 Cells. Molecules 2019, 24, 3599. https://doi.org/10.3390/molecules24193599
Lieder B, Hans J, Hentschel F, Geissler K, Ley J. Biological Evaluation of Natural and Synthesized Homovanillic Acid Esters as Inhibitors of Intestinal Fatty Acid Uptake in Differentiated Caco-2 Cells. Molecules. 2019; 24(19):3599. https://doi.org/10.3390/molecules24193599
Chicago/Turabian StyleLieder, Barbara, Joachim Hans, Fabia Hentschel, Katrin Geissler, and Jakob Ley. 2019. "Biological Evaluation of Natural and Synthesized Homovanillic Acid Esters as Inhibitors of Intestinal Fatty Acid Uptake in Differentiated Caco-2 Cells" Molecules 24, no. 19: 3599. https://doi.org/10.3390/molecules24193599
APA StyleLieder, B., Hans, J., Hentschel, F., Geissler, K., & Ley, J. (2019). Biological Evaluation of Natural and Synthesized Homovanillic Acid Esters as Inhibitors of Intestinal Fatty Acid Uptake in Differentiated Caco-2 Cells. Molecules, 24(19), 3599. https://doi.org/10.3390/molecules24193599