Solvent-Free C-3 Coupling of Azaindoles with Cyclic Imines
Abstract
1. Introduction
2. Results
3. Materials and Methods
3.1. General Methods
Procedures
3.2. Synthesis of New Compounds
3.2.1. Synthesis of 3-(1,2,3,4-Tetrahydroisoquinolin-1-yl)-7-azaindole
3.2.2. Synthesis of 3-(1,2,3,4-Tetrahydro-β-carboline-1-yl)-7-azaindole
3.2.3. Synthesis of 3-(4,5,6,7-Tetrahydrothieno[3,2-c]pyridin-4-yl)-7-azaindole
3.2.4. Synthesis of 3-(2,3,4,5-Tetrahydro-1H-benz[c]azepin-1-yl)-7-azaindole
3.2.5. Synthesis of 3-(1,2,3,4-Tetrahydroisoquinolin-1-yl)-4-azaindole
3.2.6. Synthesis of 3-(1,2,3,4-Tetrahydroisoquinolin-1-yl)-6-azaindole
3.2.7. Synthesis of 3-(1,2,3,4-Tetrahydro-β-carboline-1-yl)-4-azaindole
3.2.8. Synthesis of 3-(1,2,3,4-Tetrahydro-β-carboline-1-yl)-6-azaindole
3.2.9. Synthesis of 3-(4,5,6,7-Tetrahydrothieno[3,2-c]pyridin-4-yl)-4-azaindole
3.2.10. Synthesis of 3-(4,5,6,7-Tetrahydrothieno[3,2-c]pyridin-4-yl)-6-azaindole
3.2.11. Synthesis of 3-(2,3,4,5-Tetrahydro-1H-benzo[c]azepin-1-yl)-4-azaindole
3.2.12. Synthesis of 3-(2,3,4,5-Tetrahydro-1H-benzo[c]azepin-1-yl)-6-azaindole
3.2.13. Synthesis of 3-(1,2,3,4-Tetrahydroisoquinolin-1-yl)-5-azaindole
3.2.14. Synthesis of 3-(1,2,3,4-Tetrahydro-β-carboline-1-yl)-5-azaindole
3.2.15. Synthesis of 3-(4,5,6,7-Tetrahydrothieno[3,2-c]pyridin-4-yl)-5-azaindole
3.2.16. Synthesis of 3-(2,3,4,5-Tetrahydro-1H-benzo[c]azepin-1-yl)-5-azaindole
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ghose, A. K.; Herbertz, T.; Pippin, D. A.; Salvino, J. M.; Mallamo, J. P. Knowledge Based Prediction of Ligand Binding Modes and Rational Inhibitor Design for Kinase Grug Discovery. J. Med. Chem. 2008, 51, 5149–5155. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Hamajima, T.; Nakano, M.; Sato, H.; Dickerson, S.H.; Lackey, K.E. Knowledge-Based Design of 7-azaindoles as Selective B-Raf Inhibitors. Bioorg. Med. Chem. Lett. 2008, 18, 4610–4614. [Google Scholar] [CrossRef] [PubMed]
- Mérour, J.Y.; Buron, F.; Plé, K.; Bonnet, P.; Routier, S. The Azaindole Framework in the Design of Kinase Inhibitors. Molecules 2014, 19, 19935–19979. [Google Scholar] [CrossRef] [PubMed]
- Pires, M.J.; Poeira, D.L.; Purificacao, S.I.; Marques, M.M.B. Synthesis of Substituted 4-, 5-, 6-, and 7-Azaindoles from Aminopyridines via a Cascade C–N Cross-Coupling/Heck Reaction. Org. Lett. 2016, 18, 3250–3253. [Google Scholar] [CrossRef] [PubMed]
- Irie, T.; Sawa, M. 7-Azaindole: A Versatile Scaffold for Developing Kinase Inhibitors. Chem. Pharm. Bull. 2018, 66, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Manning, G.; Whyte, D.B.; Martinez, R.; Hunter, T.; Sudarsanam, S. The Protein Kinase Complement of the Human Genome. Science 2002, 298, 1912–1934. [Google Scholar] [CrossRef]
- Bollag, G.; Tsai, J.; Zhang, J.; Zhang, C.; Ibrahim, P.; Nolop, K.; Hirth, P. Vemurafenib: The First Drug Approved for BRAF-Mutant Cancer. Nature 2012, 11, 873–886. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.; Lee, J.T.; Wang, W.; Zhang, J.; Cho, H.; Mamo, S.; Bremer, R.; Gillette, S.; Kong, J.; Haass, N.K.; et al. Discovery of a Selective Inhibitor of Oncogenic B-Raf Kinase with Potent Antimelanoma Activity. Proc. Natl. Acad. Sci. USA 2008, 105, 3041–3046. [Google Scholar] [CrossRef]
- Ito, M.; Yamazaki, S.; Yamagami, K.; Kuno, M.; Morita, Y.; Okuma, K.; Nakamura, K.; Chida, N.; Inami, M.; Inoue, T.; et al. A Novel JAK Inhibitor, Peficitinib, Demonstrates Potent Efficacy in a Rat Adjuvant-Induced Arthritis Model. J. Pharmacol. Sci. 2017, 133, 25–33. [Google Scholar] [CrossRef]
- Tap, W.D.; Wainberg, Z.A.; Anthony, S.P.; Ibrahim, P.N.; Zhang, C.; Healey, J.H.; Chmielowski, B.; Staddon, A.P.; Cohn, A.L.; Shapiro, G.I.; et al. Structure-Guided Blockade of CSF1R Kinase in Tenosynovial Giant-Cell Tumor. Engl. J. Med. 2015, 373, 428–437. [Google Scholar] [CrossRef]
- Adams, N.D.; Adams, J.L.; Burgess, J.L.; Chaudhari, A.M.; Copeland, R.A.; Donatelli, C.A.; Drewry, D.H.; Fisher, K.E.; Hamajima, T.; Hardwicke, M.A.; et al. Discovery of GSK1070916, A Potent and Selective Inhibitor of Aurora B/C Kinase. J. Med. Chem. 2010, 53, 3973–4001. [Google Scholar] [CrossRef] [PubMed]
- Tokushige, H.; Inatani, M.; Nemoto, S.; Sakaki, H.; Katayama, K.; Uehata, M.; Tanihara, H. Effects of Topical Administration of Y-39983, a Selective Rho-Associated Protein Kinase Inhibitor, on Ocular Tissues in Rabbits and Monkeys. Invest. Ophthalmol. Vis. Sci. 2007, 48, 3216–3222. [Google Scholar] [CrossRef] [PubMed]
- Szatmári, I.; Sas, J.; Fülöp, F. C-3 Functionalization of Indole Derivatives with Isoquinolines. Curr. Org. Chem. 2016, 20, 2036–2038. [Google Scholar] [CrossRef][Green Version]
- Santos, A.; Mortinho, A.; Marques, M. Metal-Catalyzed Cross-Coupling Reactions on Azaindole Synthesis and Functionalization. Molecules 2018, 23, 2673. [Google Scholar] [CrossRef] [PubMed]
- Ghobrial, M.; Harhammer, K.; Mihovilovic, M.D.; Schmürch, M. Facile, Solvent and Ligand Free Iron Catalyzed Direct Functionalization of N-Protected Tetrahydroisoquinolines and Isochroman. Chem. Commun. 2010, 46, 8836. [Google Scholar] [CrossRef] [PubMed]
- Ghobrial, M.; Schmürch, M.; Mihovilovic, M.D. Direct Functionalization of (Un)protected Tetrahydroisoquinoline and Isochroman under Iron and Copper Catalysis: Two Metals, Two Mechanisms. J. Org. Chem. 2011, 76, 8781–8793. [Google Scholar] [CrossRef] [PubMed]
- Szatmári, I.; Sas, J.; Fülöp, F. Catalyst-Free Coupling of Indole Derivatives with 3,4-dihydroisoquinoline and Related Compounds. Tetrahedron Lett. 2013, 54, 5069–5071. [Google Scholar] [CrossRef]
- Sas, J.; Szatmári, I.; Fülöp, F. One-Pot α-Arylation of β-Carboline with Indole and Naphthol Derivatives. Curr. Org. Synth. 2016, 13, 611–616. [Google Scholar] [CrossRef][Green Version]
- Chen, Z.; Hu, G.; Li, D.; Chen, J.; Li, Y.; Zhou, H.; Xie, Y. Synthesis and Vasodilator Effects of Rutaecarpine Analogues which Might be Involved Transient Receptor Potential Vanilloid Subfamily, Member 1 (TRPV1). Bioorg. Med. Chem. 2009, 17, 2351–2359. [Google Scholar] [CrossRef]
- Herz, W.; Tsai, L. Sulfur Analogs of Isoquinolines. IV. The Pictet-Gams Reaction and Attempts to Prepare Analogs of Papaverine1,2. J. Chem. Soc. 1955, 77, 3529–3533. [Google Scholar] [CrossRef]
- Meyers, A.I.; Hutchings, R.H. The Asymmetric Synthesis of 1-alkyl-2,3,4,5-tetrahydro-benzazepines and Benzo[β]-1-azabicyclo[5,3,1]decanes. Tetrahedron 1993, 49, 1807–1820. [Google Scholar] [CrossRef]
- Jakubec, P.; Helliwell, M.; Dixon, D.J. Cyclic Imine Nitro-Mannich/ Lactamization Cascades: A Direct Stereoselective Synthesis of Multicyclic Piperidinone Derivatives. D. J. Org. Lett. 2008, 10, 4267–4270. [Google Scholar] [CrossRef] [PubMed]
- Khodaei, M.M.; Khosropour, A.R.; Moghanian, H. A Simple and Efficient Procedure for the Synthesis of Amidoalkyl Naphthols by p-TSA in Solution or under Solvent-Free Conditions. Synlett 2006, 6, 916–920. [Google Scholar] [CrossRef]
- Khosropour, A.R.; Khodaei, M.M.; Moghanian, H. A Facile, Simple and Convenient Method for the Synthesis of 14-Alkyl or Aryl-14-H-Dibenzo[a,j]xanthenes Catalyzed by pTSA in Solution and Solvent-Free Conditions. Synlett 2005, 6, 955–958. [Google Scholar] [CrossRef]
- Csütörtöki, R.; Szatmári, I.; Mándi, A.; Kurtán, T.; Fülöp, F. Synthesis of Hydroxynaphthyl-Substituted α-Amino Acid Derivatives via a Modified Mannich Reaction. Synlett 2011, 13, 1940–1946. [Google Scholar]
- Marvin was used for calculating pKa values of azaindoles, Marvin 16.12.12.0, ChemAxon. 2016. Available online: http://www.chemaxon.com.
Sample Availability: Samples of the compounds are not available from the authors. |
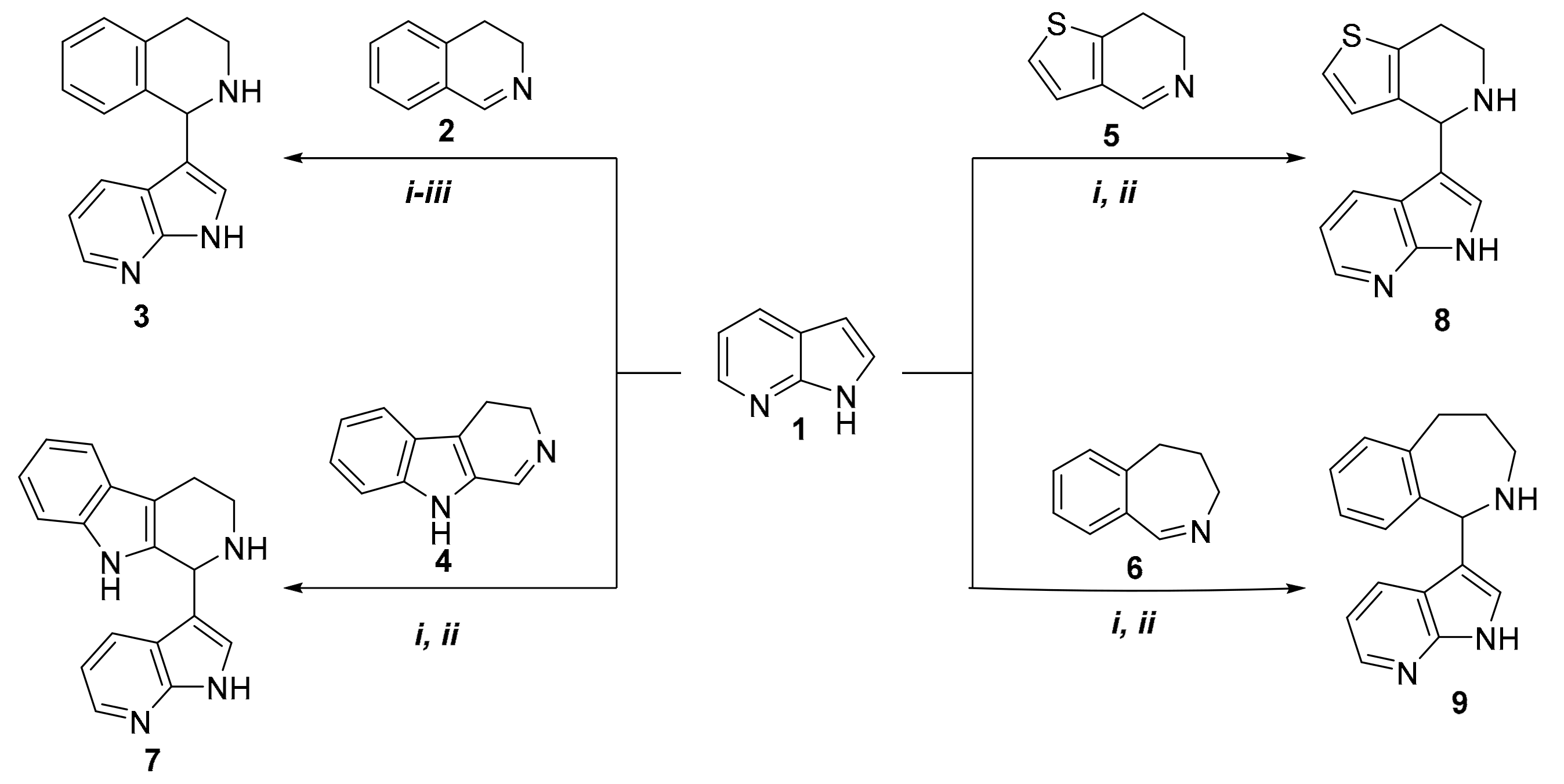
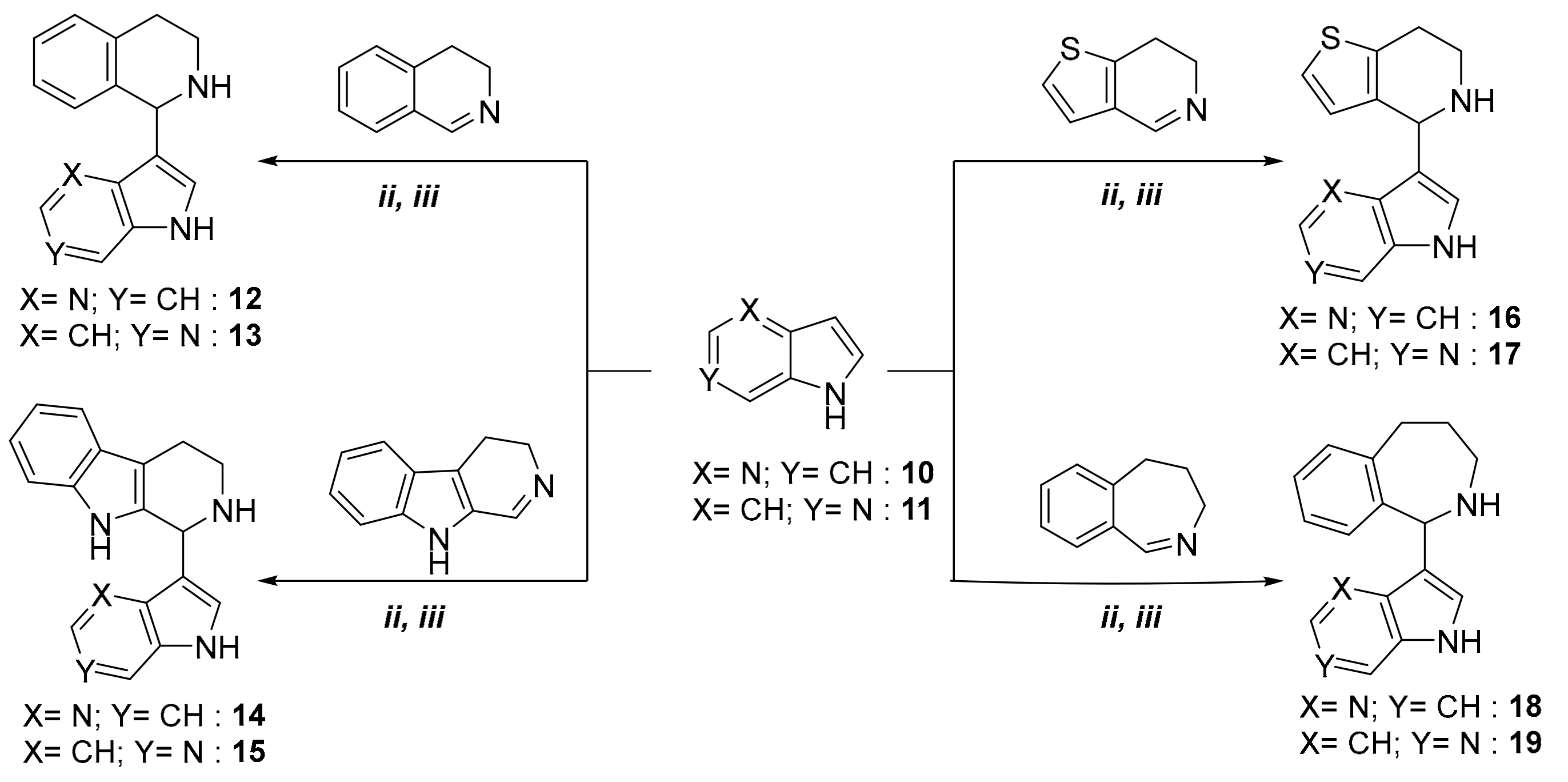
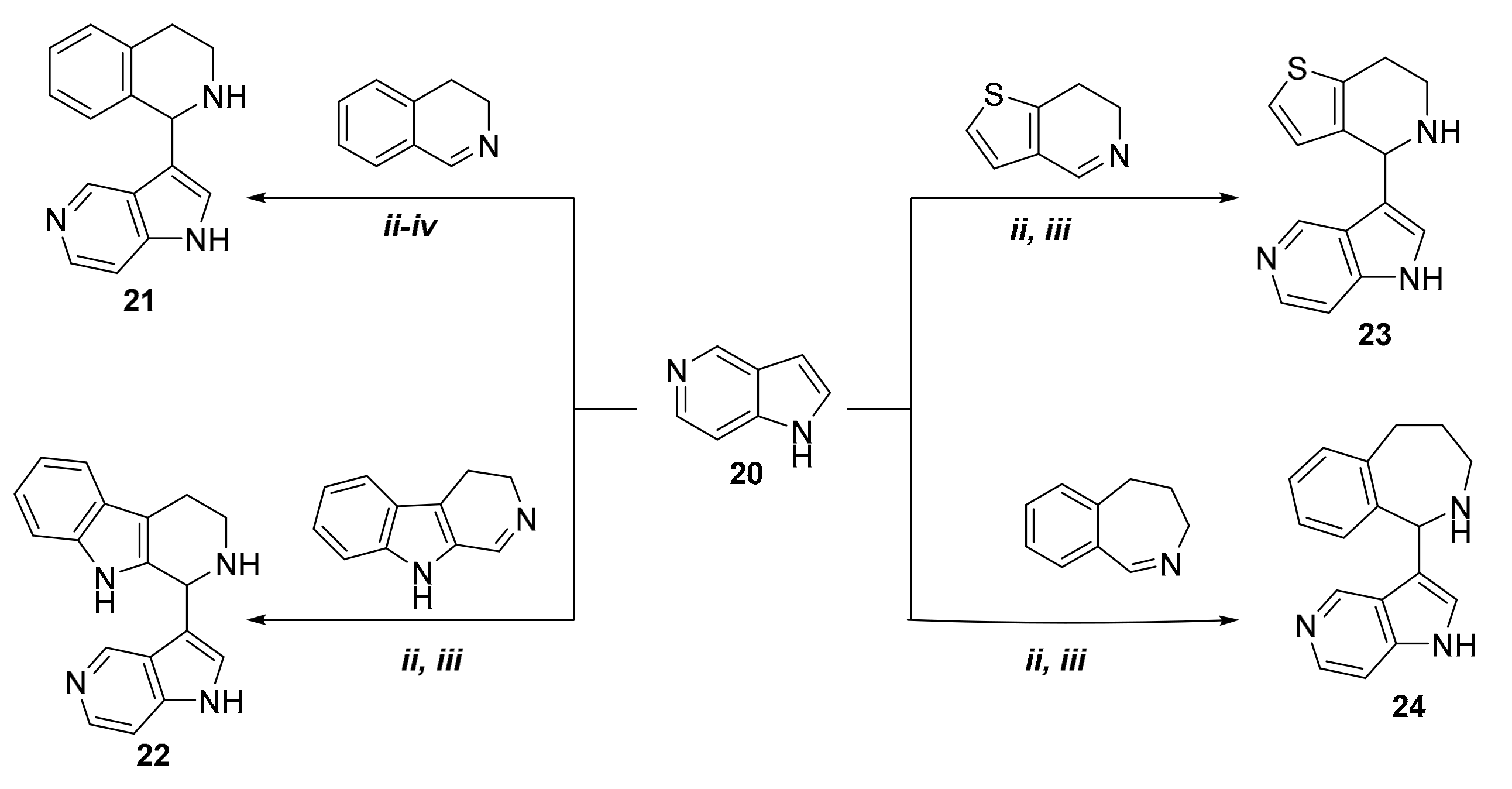
| Products | Reaction Time a | Temperature a | Yield a(%) | Reaction Time b | Temperature b | Yield b (%) |
|---|---|---|---|---|---|---|
| 3 | 18 h 10 h 6 h | 60 °C (i) 80 °C (ii) 100 °C (iii) | 31 49 28 | 2 h 4 h 2 h | 80 °C (ii) 80 °C (ii) 100 °C (iii) | 55 63 81 |
| 7 | 10 h | 80 °C (ii) | 56 | 2.5 h | 100 °C (iii) | 75 |
| 8 | 6 h | 80 °C (ii) | 76 | 2 h | 100 °C (iii) | 89 |
| 9 | 20 h | 80 °C (ii) | 63 | 3 h | 100 °C (iii) | 78 |
| Products | Reaction Time a | Temperature a | Yield a (%) | Reaction Time b | Temperature b | Yield b (%) |
|---|---|---|---|---|---|---|
| 12 | 20 h | 80 °C (ii) | 49 | 2.5 h | 100 °C (iii) | 70 |
| 13 | 22 h | 80 °C (ii) | 39 | 2.5 h | 100 °C (iii) | 65 |
| 14 | 16 h | 80 °C (ii) | 52 | 2.5 h | 100 °C (iii) | 69 |
| 15 | 20 h | 80 °C (ii) | 48 | 2.5 h | 100 °C (iii) | 62 |
| 16 | 7 h | 80 °C (ii) | 63 | 2.5 h | 100 °C (iii) | 85 |
| 17 | 8 h | 80 °C (ii) | 62 | 2.5 h | 100 °C (iii) | 75 |
| 18 | 21 h | 80 °C (ii) | 57 | 3 h | 100 °C (ii) | 68 |
| 19 | 25 h | 80 °C (ii) | 55 | 3 h | 100 °C (iii) | 64 |
| Products | Reaction Time a | Temperature a | Yield a (%) | Reaction Time b | Temperature b | Yield b (%) |
|---|---|---|---|---|---|---|
| 21 | 24 h 21 h 8 h 19 h c | 80 °C (ii) 100 °C (iii) 120 °C (iv) 80 °C (ii) | - - - 30 | 2.5 h 2.5 h 2 h 2.5 h c | 80 °C (ii) 100 °C (iii) 120 °C (iv) 100 °C (iii) | - - - 72 |
| 22 | 15 h c | 80 °C (ii) | 44 | 2 h c | 100 °C (iii) | 69 |
| 23 | 7 h c | 80 °C (ii) | 62 | 2 h c | 100 °C (iii) | 80 |
| 24 | 15 h c | 80 °C (ii) | 52 | 3.5 h c | 100 °C (iii) | 65 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belasri, K.; Fülöp, F.; Szatmári, I. Solvent-Free C-3 Coupling of Azaindoles with Cyclic Imines. Molecules 2019, 24, 3578. https://doi.org/10.3390/molecules24193578
Belasri K, Fülöp F, Szatmári I. Solvent-Free C-3 Coupling of Azaindoles with Cyclic Imines. Molecules. 2019; 24(19):3578. https://doi.org/10.3390/molecules24193578
Chicago/Turabian StyleBelasri, Khadija, Ferenc Fülöp, and István Szatmári. 2019. "Solvent-Free C-3 Coupling of Azaindoles with Cyclic Imines" Molecules 24, no. 19: 3578. https://doi.org/10.3390/molecules24193578
APA StyleBelasri, K., Fülöp, F., & Szatmári, I. (2019). Solvent-Free C-3 Coupling of Azaindoles with Cyclic Imines. Molecules, 24(19), 3578. https://doi.org/10.3390/molecules24193578








