Effect of the Algicide Thiazolidinedione 49 on Immune Responses of Bay Scallop Argopecten Irradians
Abstract
:1. Introduction
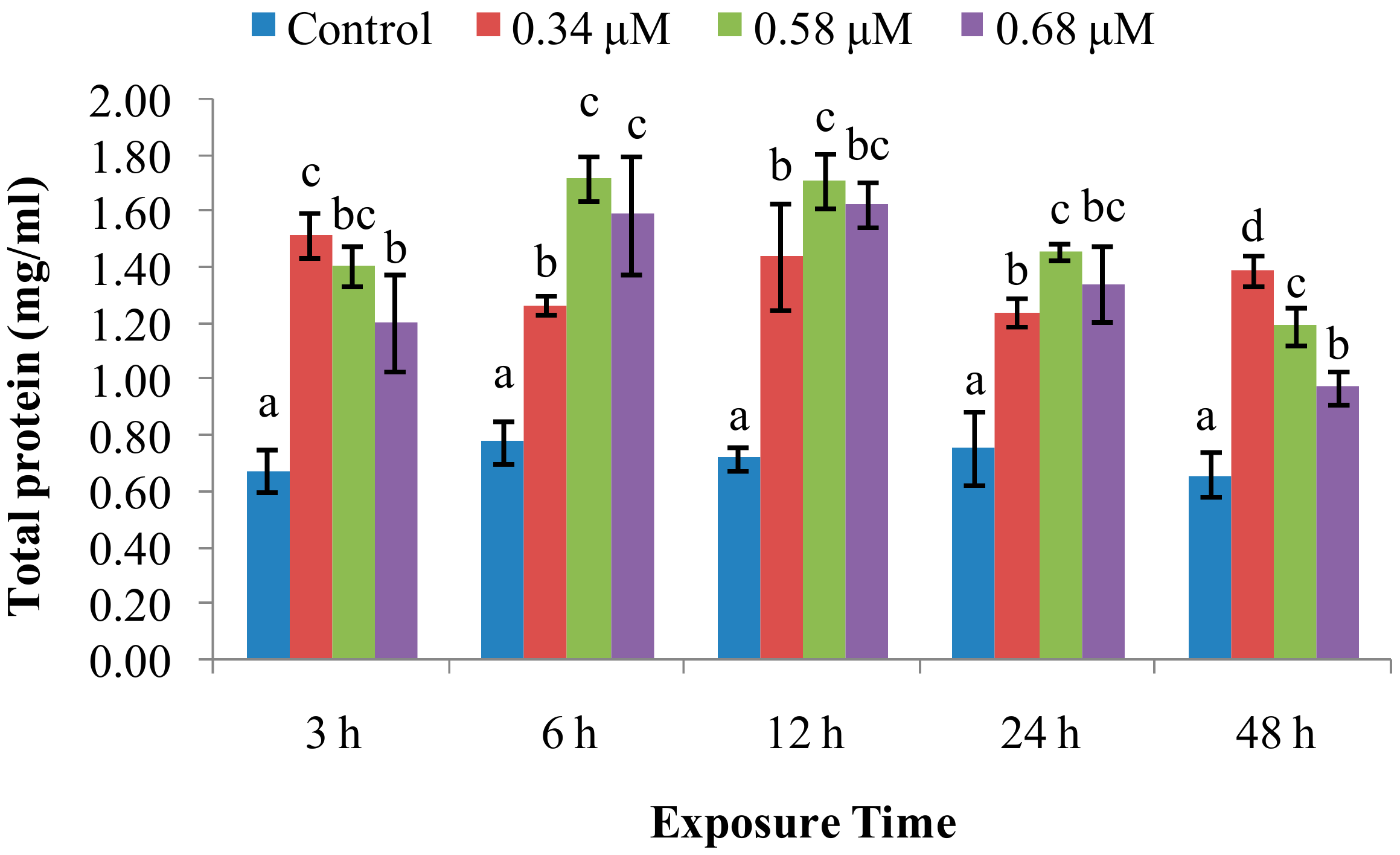
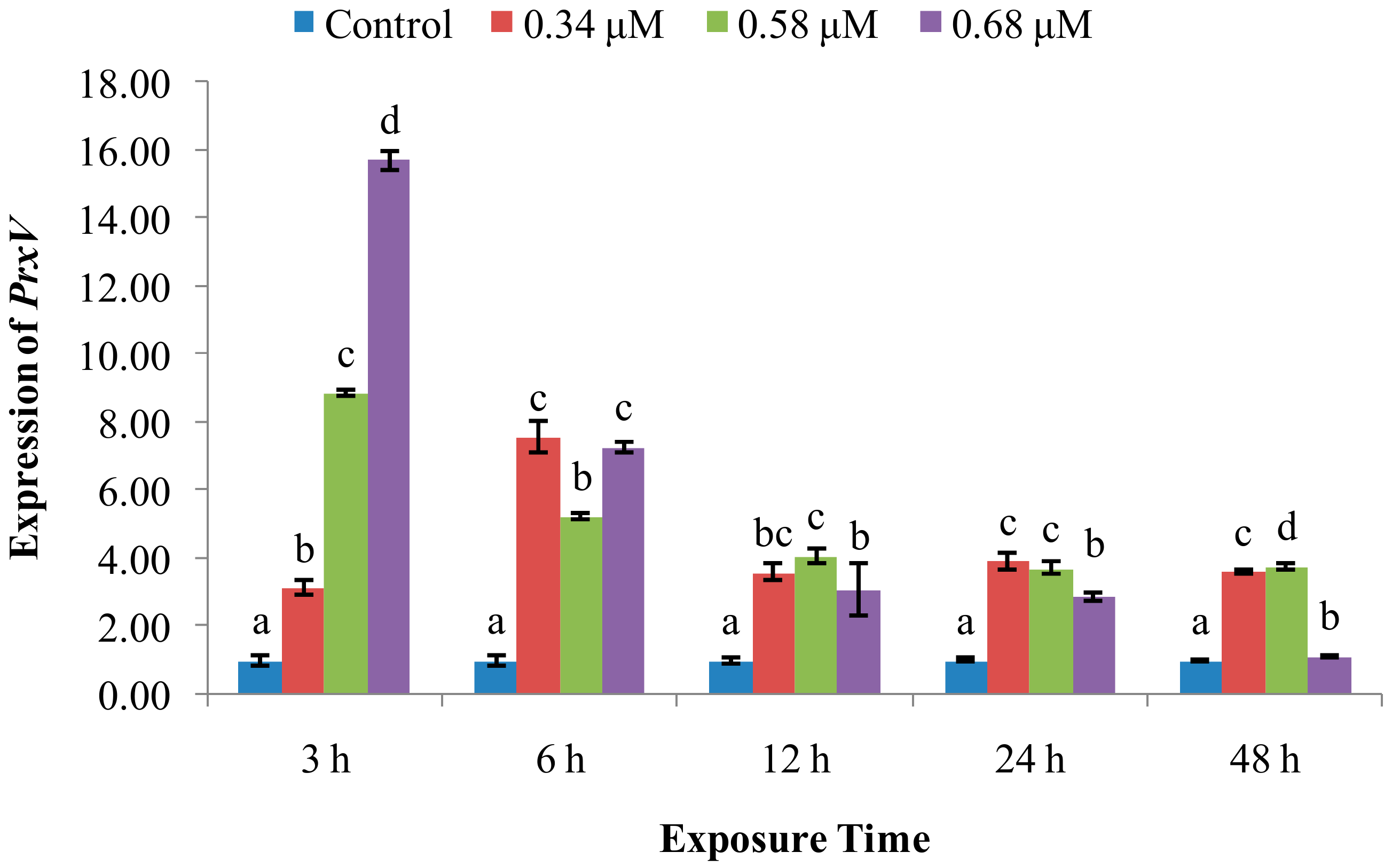
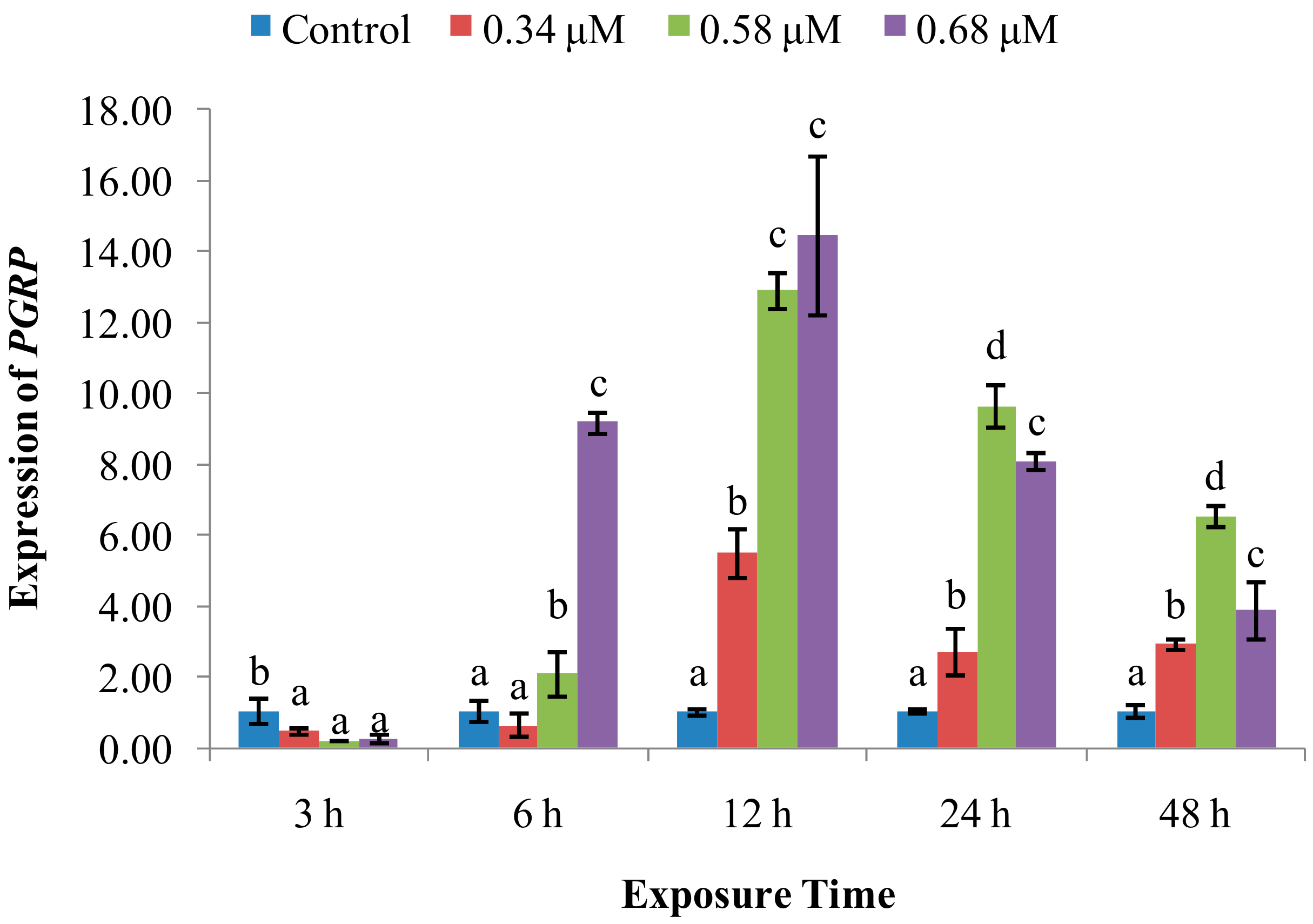
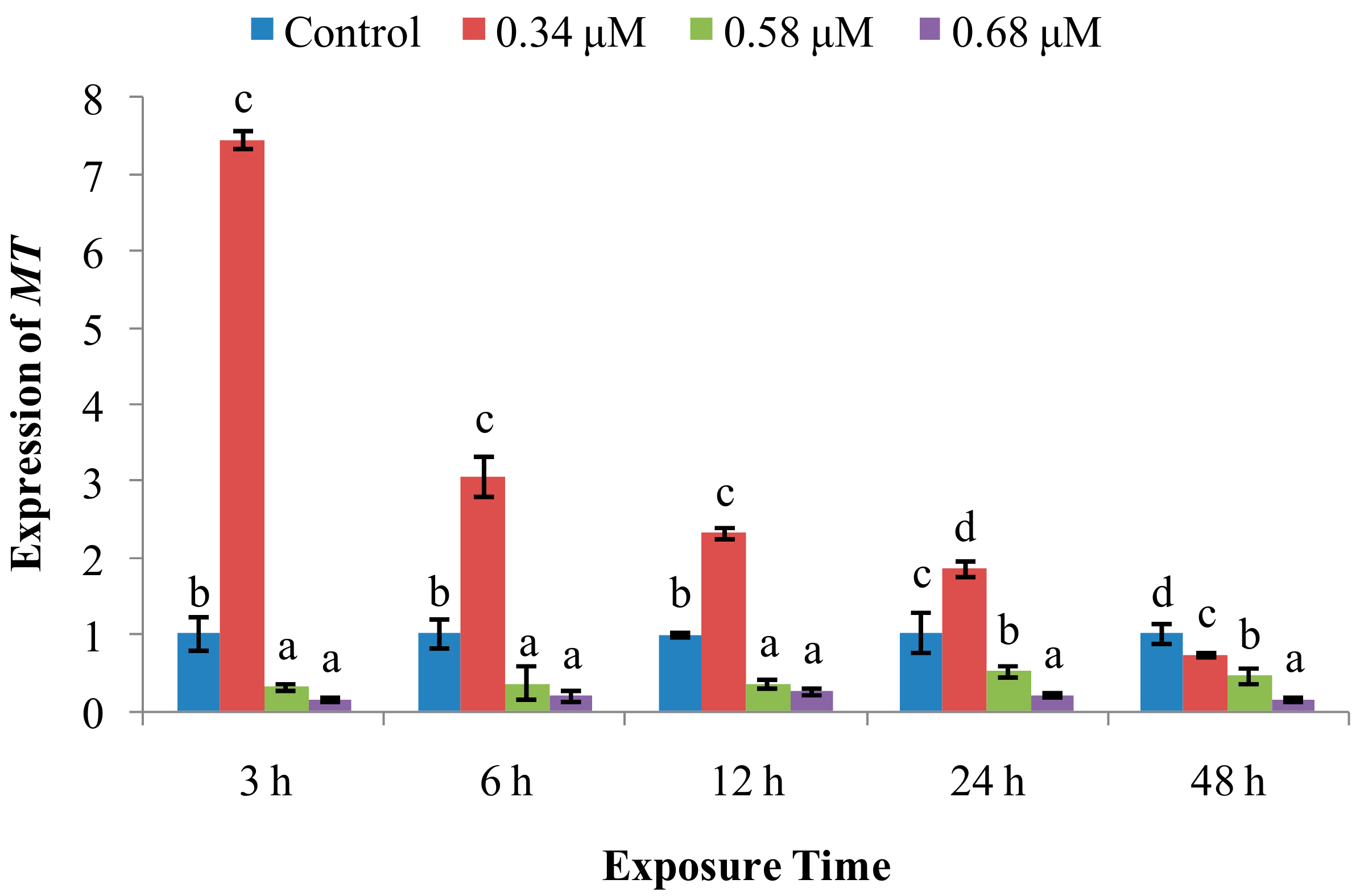
2. Results
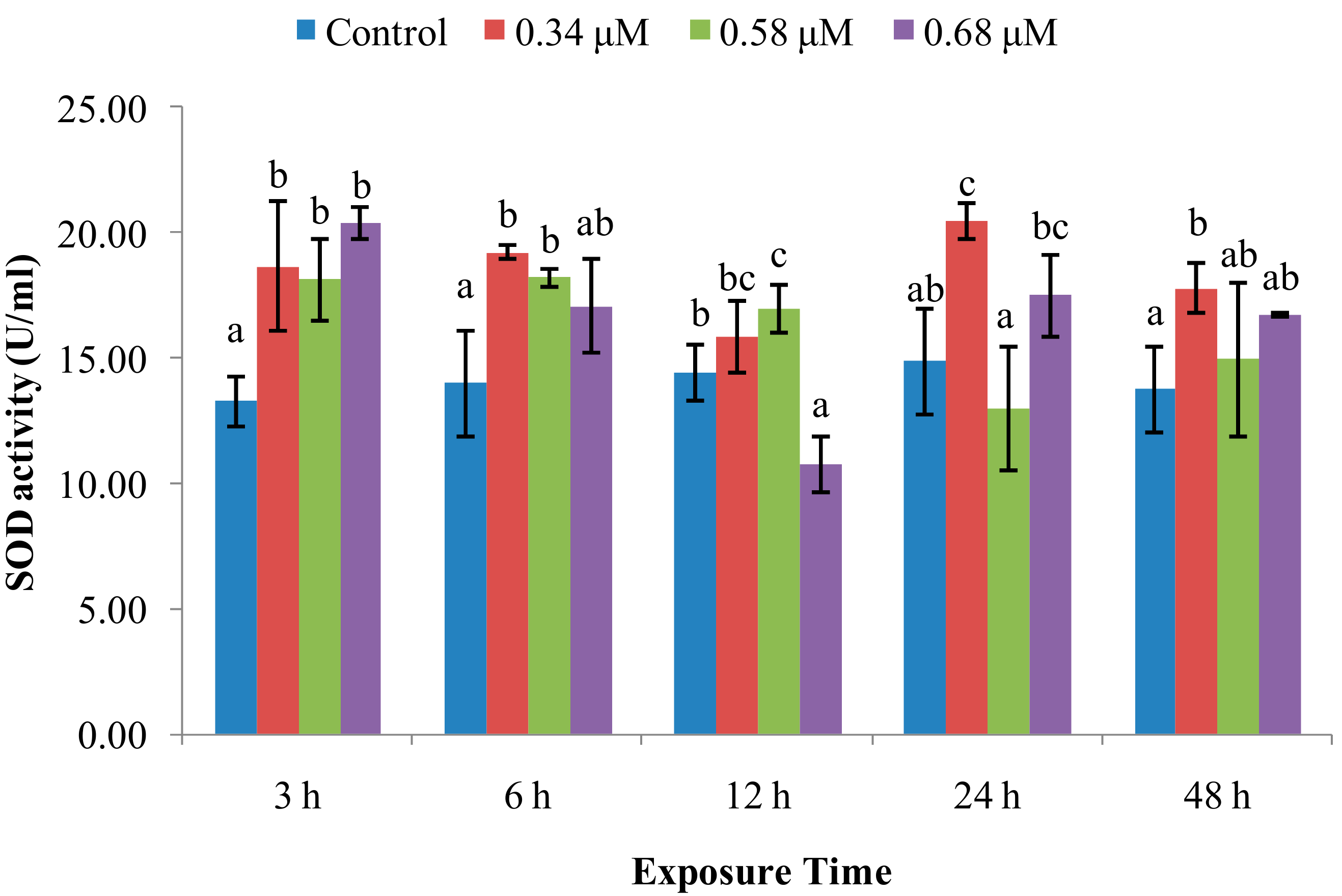
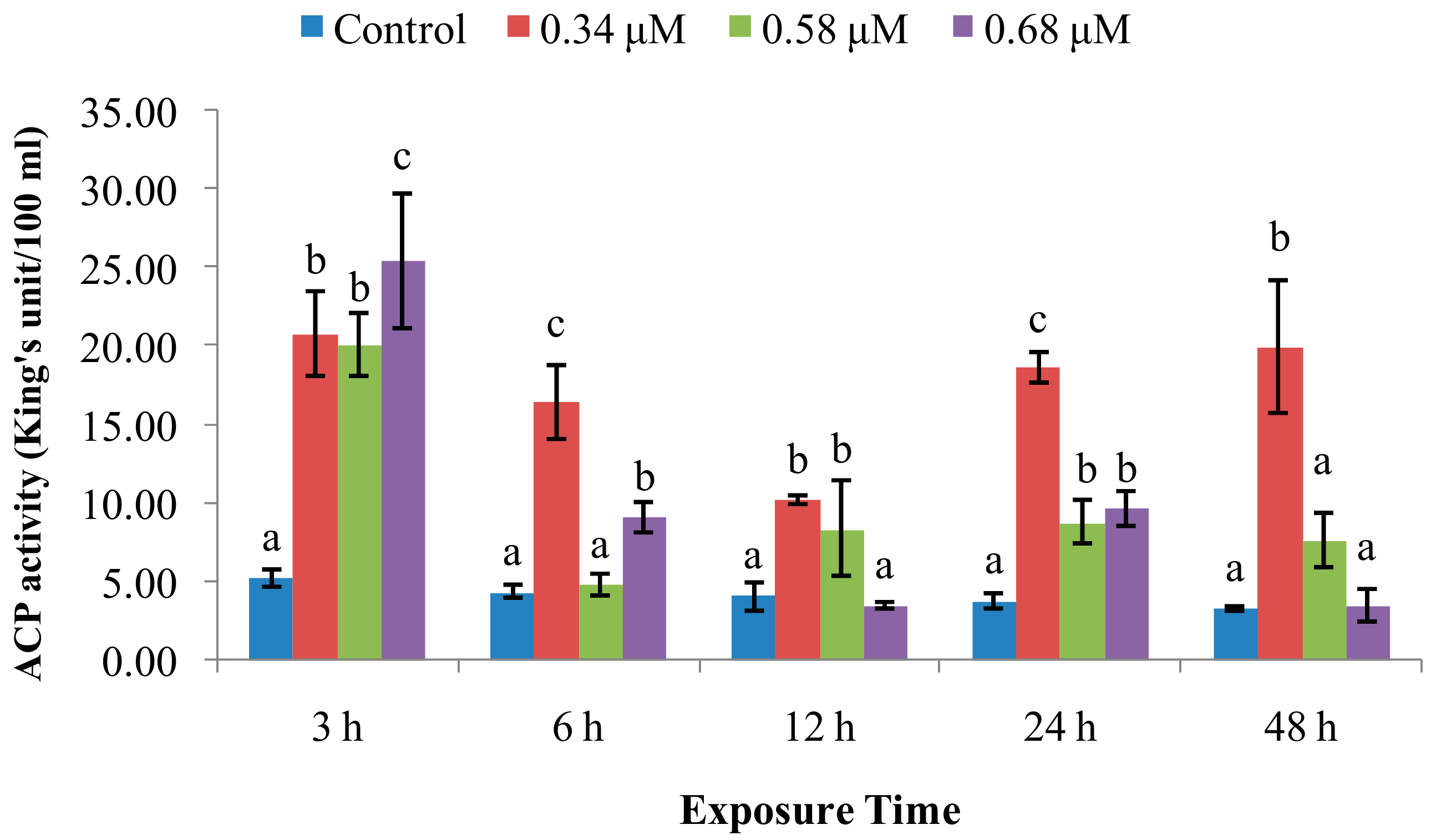
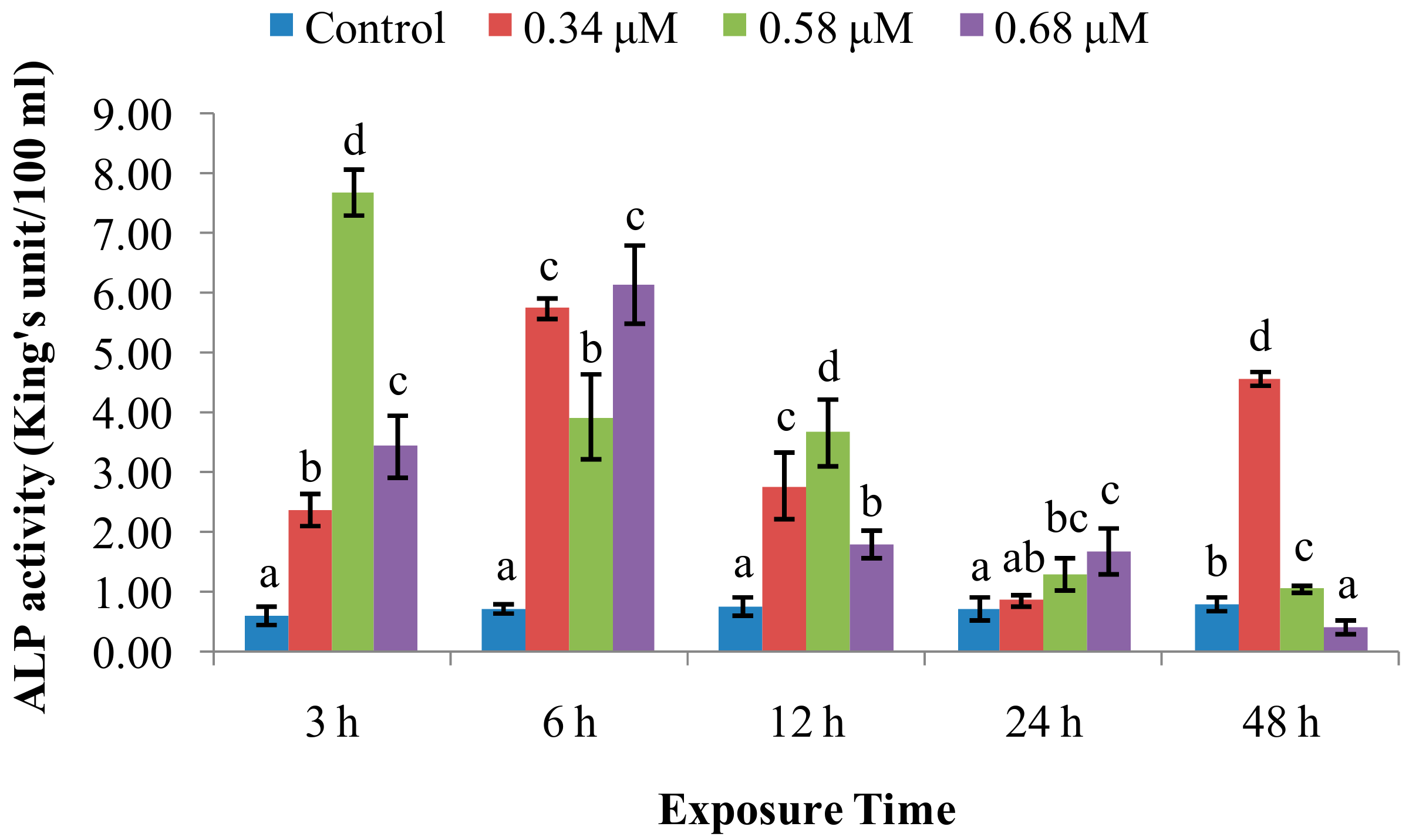
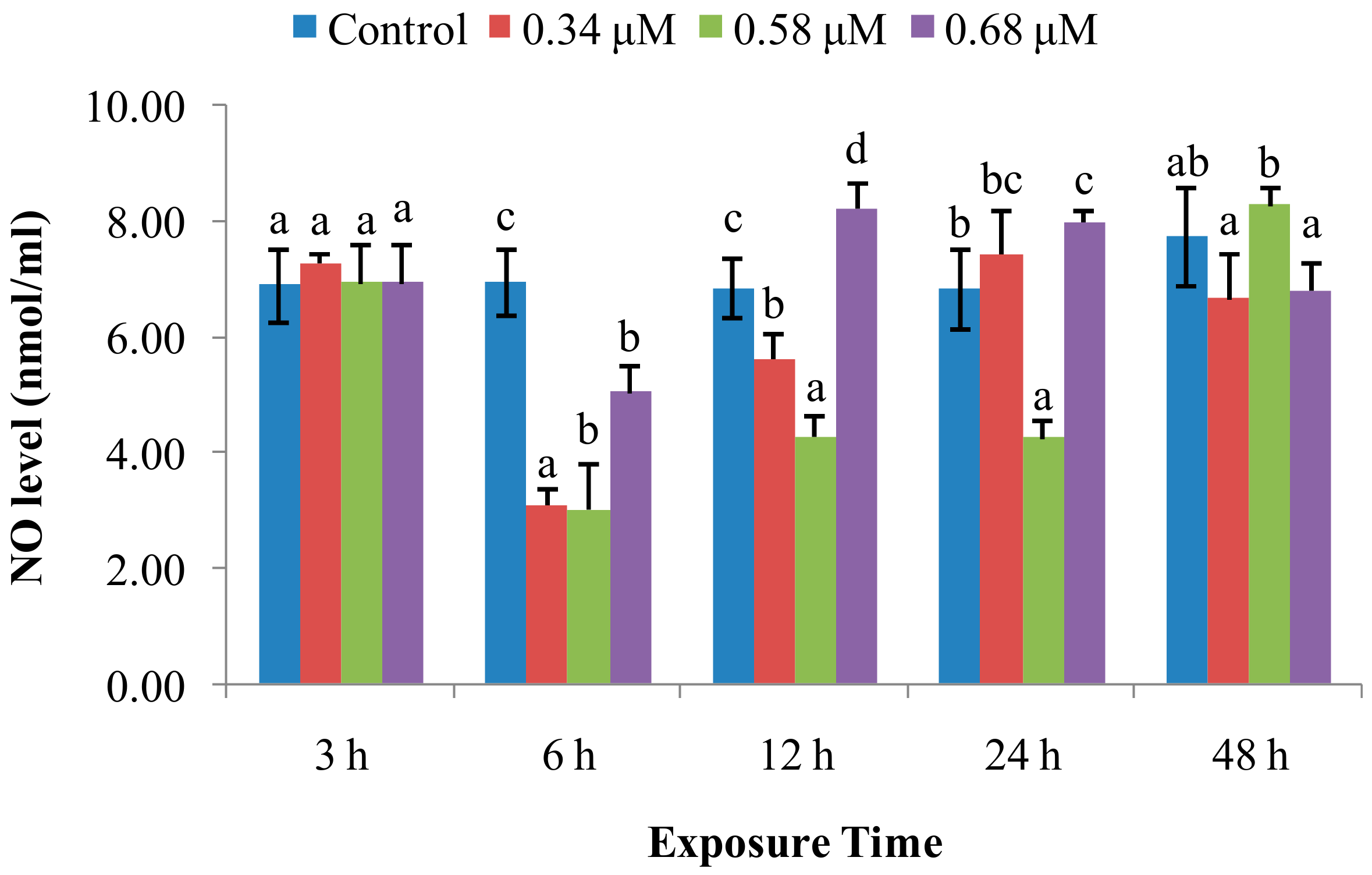
2.1. TD49 Treatment Alters Enzymatic Activities Important for Nonspecific Immune Responses
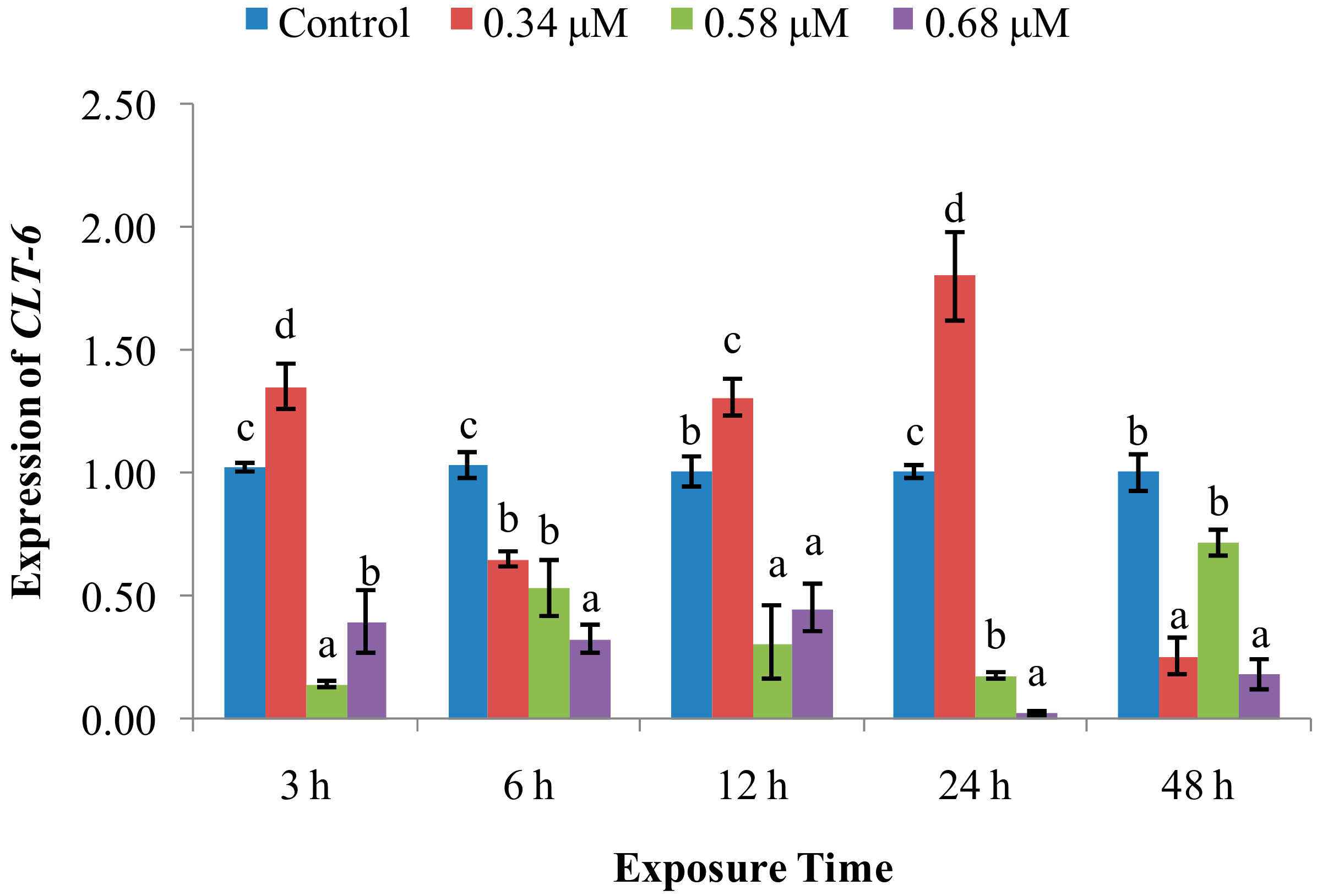
2.2. Expression of Immune System-Related Genes
3. Discussion
4. Materials and Methods
4.1. Thiazolidinedione Derivative TD49
4.2. Bay Scallop and Hemolymph Sample Collection
4.3. Measurement of Enzymatic Activity in Hemolymph
4.4. Quantitative Measurements of Gene Expression
4.5. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kim, S.J.; Yim, E.C.; Park, I.T.; Kim, S.W.; Cho, H. Comparison of the acute toxicities of novel algicides, thiazolidinedione derivatives TD49 and TD53, to various marine organisms. Environ. Toxicol. Chem. 2011, 30, 2810–2816. [Google Scholar] [CrossRef]
- Kim, Y.M.; Wu, Y.; Duong, T.U.; Ghodake, G.S.; Kim, S.W.; Jin, E.; Cho, H. Thiazolidinediones as a novel class of algicides against red tide harmful algal species. Appl. Biochem. Biotechnol. 2010, 162, 2273–2283. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhang, H.; Fu, L.; An, X.; Zhang, B.; Li, Y.; Chen, Z.; Zheng, W.; Yi, L.; Zheng, T. A novel algicide: Evidence of the effect of a fatty acid compound from the marine bacterium, Vibrio sp. BS02 on the harmful dinoflagellate, Alexandrium tamarense. PloS ONE 2014, 9, e91201. [Google Scholar] [CrossRef] [PubMed]
- Chi, C.; Giri, S.S.; Jun, J.W.; Kim, H.J.; Kim, S.G.; Yun, S.; Park, S.C. Effect of the algaecide palmitoleic acid on the immune function of the bay scallop Argopecten irradians. Molecules 2016, 21, 610. [Google Scholar] [CrossRef] [PubMed]
- Chi, C.; Giri, S.S.; Jun, J.W.; Yun, S.; Kim, H.J.; Kim, S.G.; Park, S.C. Immune response of the bay scallop, Argopecten irradians, after exposure to the algicide palmitoleic acid. Fish Shellfish Immunol. 2016, 57, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Chi, C.; Giri, S.S.; Jun, J.W.; Kim, H.J.; Kim, S.W.; Kang, J.W.; Park, S.C. Detoxification and immune transcriptomic response of the gill tissue of bay scallop (Argopecten irradians) following exposure to the algicide palmitoleic acid. Biomolecules 2018, 8, 139. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Pan, L.; Wang, J.; Yang, H.; Liu, D. Application of the biomarker responses in scallop (Chlamys farreri) to assess metals and PAHs pollution in Jiaozhou Bay, China. Mar. Environ. Res. 2012, 80, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Miao, J.; Wang, J.; Liu, J. AHH activity, tissue dose and DNA damage in different tissues of the scallop Chlamys farreri exposed to benzo [a] pyrene. Environ. Pollut. 2008, 153, 192–198. [Google Scholar] [CrossRef]
- Gestal, C.; Roch, P.; Renault, T.; Pallavicini, A.; Paillard, C.; Novoa, B.; Oubella, R.; Venier, P.; Figueras, A. Study of diseases and the immune system of bivalves using molecular biology and genomics. Rev. Fish. Sci. 2008, 16, 133–156. [Google Scholar] [CrossRef]
- Frantzen, M.; Regoli, F.; Ambrose, W.G.; Nahrgang, J.; Geraudie, P.; Benedetti, M.; Camus, L. Biological effects of mechanically and chemically dispersed oil on the Icelandic scallop (Chlamys islandica). Ecotoxicol. Environ. Saf. 2016, 127, 95–107. [Google Scholar] [CrossRef]
- Song, L.; Wang, L.; Zhang, H.; Wang, M. The immune system and its modulation mechanism in scallop. Fish Shellfish Immunol. 2015, 46, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Zhou, Z.; Wang, L.; Wang, L.; Yue, F.; Wang, J.; Song, L. A scallop nitric oxide synthase (NOS) with structure similar to neuronal NOS and its involvement in the immune defense. PloS ONE 2013, 8, e69158. [Google Scholar] [CrossRef] [PubMed]
- Franchini, A.; Fontanili, P.; Ottaviani, E. Invertebrate immunocytes: relationship between phagocytosis and nitric oxide production. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1995, 110, 403–407. [Google Scholar] [CrossRef]
- Labreuche, Y.; Lambert, C.; Soudant, P.; Boulo, V.; Huvet, A.; Nicolas, J.L. Cellular and molecular hemocyte responses of the Pacific oyster, Crassostrea gigas, following bacterial infection with Vibrio aestuarianus strain 01/32. Microbes Infect. 2006, 8, 2715–2724. [Google Scholar] [CrossRef] [PubMed]
- Chi, C.; Giri, S.S.; Jun, J.W.; Kim, H.J.; Yun, S.; Kim, S.W.; Kang, J.W.; Park, S.C. Detoxification, apoptosis, and immune transcriptomic responses of the gill tissue of bay scallop following exposure to the algicide thiazolidinedione 49. Biomolecules 2019, 9, 310. [Google Scholar] [CrossRef]
- Blaise, C.; Trottier, S.; Gagné, F.; Lallement, C.; Hansen, P.D. Immunocompetence of bivalve hemocytes as evaluated by a miniaturized phagocytosis assay. Environ. Toxicol. Chem. 2002, 17, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Hannam, M.L.; Bamber, S.D.; Moody, J.A.; Galloway, T.S.; Jones, M.B. Immune function in the Arctic Scallop, Chlamys islandica, following dispersed oil exposure. Aquat. Toxicol. 2009, 92, 187–194. [Google Scholar] [CrossRef]
- Hannam, M.L.; Bamber, S.D.; Galloway, T.S.; Moody, A.J.; Jones, M.B. Effects of the model PAH phenanthrene on immune function and oxidative stress in the haemolymph of the temperate scallop Pecten maximus. Chemosphere 2010, 78, 779–784. [Google Scholar] [CrossRef]
- Frouin, H.; Pellerin, J.; Fournier, M.; Pelletier, E.; Richard, P.; Pichaud, N.; Rouleau, C.; Garnerot, F. Physiological effects of polycyclic aromatic hydrocarbons on soft-shell clam Mya arenaria. Aquat. Toxicol. 2007, 82, 120–134. [Google Scholar] [CrossRef]
- Ni, D.; Song, L.; Gao, Q.; Wu, L.; Yu, Y.; Zhao, J.; Qiu, L.; Zhang, H.; Shi, F. The cDNA cloning and mRNA expression of cytoplasmic Cu, Zn superoxide dismutase (SOD) gene in scallop Chlamys farreri. Fish Shellfish Immunol. 2007, 23, 1032–1042. [Google Scholar] [CrossRef]
- Fridovich, I. Superoxide dismutases. Annu. Rev. Biochem. 1975, 44, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Ren, J.; Liu, J. Responses of antioxidant systems and LPO level to benzo (a) pyrene and benzo (k) fluoranthene in the haemolymph of the scallop Chlamys ferrari. Environ. Pollut. 2006, 141, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, L.; Zhang, S.; Zhang, G. Cloning, genomic structure, and expression analysis of peroxiredoxin V from bay scallop Argopecten irradians. Fish Shellfish Immunol. 2011, 30, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Graham, D.B.; Jasso, G.J.; Mok, A.; Goel, G.; Ng, A.C.Y.; Kolde, R.; Varma, M.; Doench, J.G.; Root, D.E.; Clish, C.B.; et al. Nitric oxide engages an anti-inflammatory feedback loop mediated by peroxiredoxin 5 in phagocytes. Cell Rep. 2018, 24, 838–850. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.Y.; Yang, H.S.; Delaporte, M.; Zhao, S.J.; Xing, K. Immune responses of the scallop Chlamys farreri after air exposure to different temperatures. J. Exp. Mar. Biol. Ecol. 2007, 345, 52–60. [Google Scholar] [CrossRef]
- Suresh, K.; Mohandas, A. Hemolymph acid phosphatase activity pattern in copper-stressed bivalves. J. Invertebr. Pathol. 1990, 55, 118–125. [Google Scholar] [CrossRef]
- Zheng, X.; Chi, C.; Xu, C.; Liu, J.; Zhang, C.; Zhang, L.; Huang, Y.; He, C.; He, C.; Jia, X. Effects of dietary supplementation with icariin on growth performance, antioxidant capacity and non-specific immunity of Chinese mitten crab (Eriocheir sinensis). Fish Shellfish Immunol. 2019, 90, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Jing, G.; Li, Y.; Xie, L.; Zhang, R. Metal accumulation and enzyme activities in gills and digestive gland of pearl oyster (Pinctada fucata) exposed to copper. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2006, 144, 184–190. [Google Scholar] [CrossRef]
- Liu, S.; Jiang, X.; Hu, X.; Gong, J.; Hwang, H.; Mai, K. Effects of temperature on nonspecific immune parameters in two scallop species: Argopecten irradians (Lamarck 1819) and Chlamys farreri (Jones & Preston 1904). Aquac. Res. 2004, 35, 678–682. [Google Scholar]
- Janeway, C.A., Jr.; Medzhitov, R. Innate immune recognition. Annu. Rev. Immunol. 2002, 20, 197–216. [Google Scholar] [CrossRef]
- Ni, D.; Song, L.; Wu, L.; Chang, Y.; Yu, Y.; Qiu, L.; Wang, L. Molecular cloning and mRNA expression of peptidoglycan recognition protein (PGRP) gene in bay scallop (Argopecten irradians, Lamarck 1819). Dev. Comp. Immunol. 2007, 31, 548–558. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Song, X.; Wang, L.; Kong, P.; Yang, J.; Liu, L.; Qiu, L.; Zhang, Y.; Qiu, L.; Song, L. AiCTL-6, a novel C-type lectin from bay scallop Argopecten irradians with a long C-type lectin-like domain. Fish Shellfish Immunol. 2011, 30, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Chi, C.; Giri, S.; Jun, J.; Kim, H.; Yun, S.; Kim, S.; Park, S. Marine toxin okadaic acid affects the immune function of bay scallop (Argopecten irradians). Molecules 2016, 21, 1108. [Google Scholar] [CrossRef] [PubMed]
- Vašák, M.; Meloni, G. Chemistry and biology of mammalian metallothioneins. J. Biol. Inorg. Chem. 2011, 16, 1067. [Google Scholar] [CrossRef] [PubMed]
- Giri, S.S.; Sen, S.S.; Jun, J.W.; Sukumaran, V.; Park, S.C. Immunotoxicological effects of cadmium on Labeo rohita, with emphasis on the expression of HSP genes. Fish Shellfish Immunol. 2016, 54, 164–171. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-△△CT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |

| Genes | Primer Sequence | Accession No. |
|---|---|---|
| β-actin | F: 5′CAAACAGCAGCCTCCTCGTCA 3′ | AY335441 |
| R: 5′CTGGGCACCTGAACCTTTCGTT 3′ | ||
| PrxV | F: 5′AATCAAGGAGCGGCTGGCA 3′ | HM461987 |
| R: 5′TCAACTTCTCAATCTTCCCGTCAT 3′ | ||
| CTL-6 | F: 5′CAGTTGCTACAGGGTTCG 3′ | GQ202279 |
| R: 5′GGGCGTTATCTGGCTCAT 3′ | ||
| MT | F: 5′AACTTGCTGTAGTGGGAATG 3′ | EU734181 |
| R: 5′AGGCTGGAAACTGCTGTGGT 3′ | ||
| PGRP | F: 5′GGGCAAGTGTATGAGGGAAGAG 3′ | AY437875 |
| R: 5′TCCGATGAAGGAGACAGCGTAG 3′ | ||
| Cu/ZnSOD | F: 5′GTATTGAAAGGTGATTCGGAGG 3 ′ | EU563958 |
| R: 5′ATGCACATGAAAGCCATGTAGG 3 ′ |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chi, C.; Yun, S.; Giri, S.S.; Kim, H.J.; Kim, S.W.; Kang, J.W.; Park, S.C. Effect of the Algicide Thiazolidinedione 49 on Immune Responses of Bay Scallop Argopecten Irradians. Molecules 2019, 24, 3579. https://doi.org/10.3390/molecules24193579
Chi C, Yun S, Giri SS, Kim HJ, Kim SW, Kang JW, Park SC. Effect of the Algicide Thiazolidinedione 49 on Immune Responses of Bay Scallop Argopecten Irradians. Molecules. 2019; 24(19):3579. https://doi.org/10.3390/molecules24193579
Chicago/Turabian StyleChi, Cheng, Saekil Yun, Sib Sankar Giri, Hyoun Joong Kim, Sang Wha Kim, Jeong Woo Kang, and Se Chang Park. 2019. "Effect of the Algicide Thiazolidinedione 49 on Immune Responses of Bay Scallop Argopecten Irradians" Molecules 24, no. 19: 3579. https://doi.org/10.3390/molecules24193579
APA StyleChi, C., Yun, S., Giri, S. S., Kim, H. J., Kim, S. W., Kang, J. W., & Park, S. C. (2019). Effect of the Algicide Thiazolidinedione 49 on Immune Responses of Bay Scallop Argopecten Irradians. Molecules, 24(19), 3579. https://doi.org/10.3390/molecules24193579






