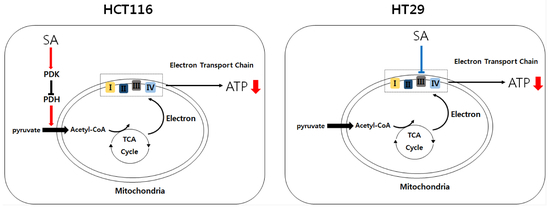
Differential Mechanism of ATP Production Occurs in Response to Succinylacetone in Colon Cancer Cells
Abstract
1. Introduction
2. Results and Discussion
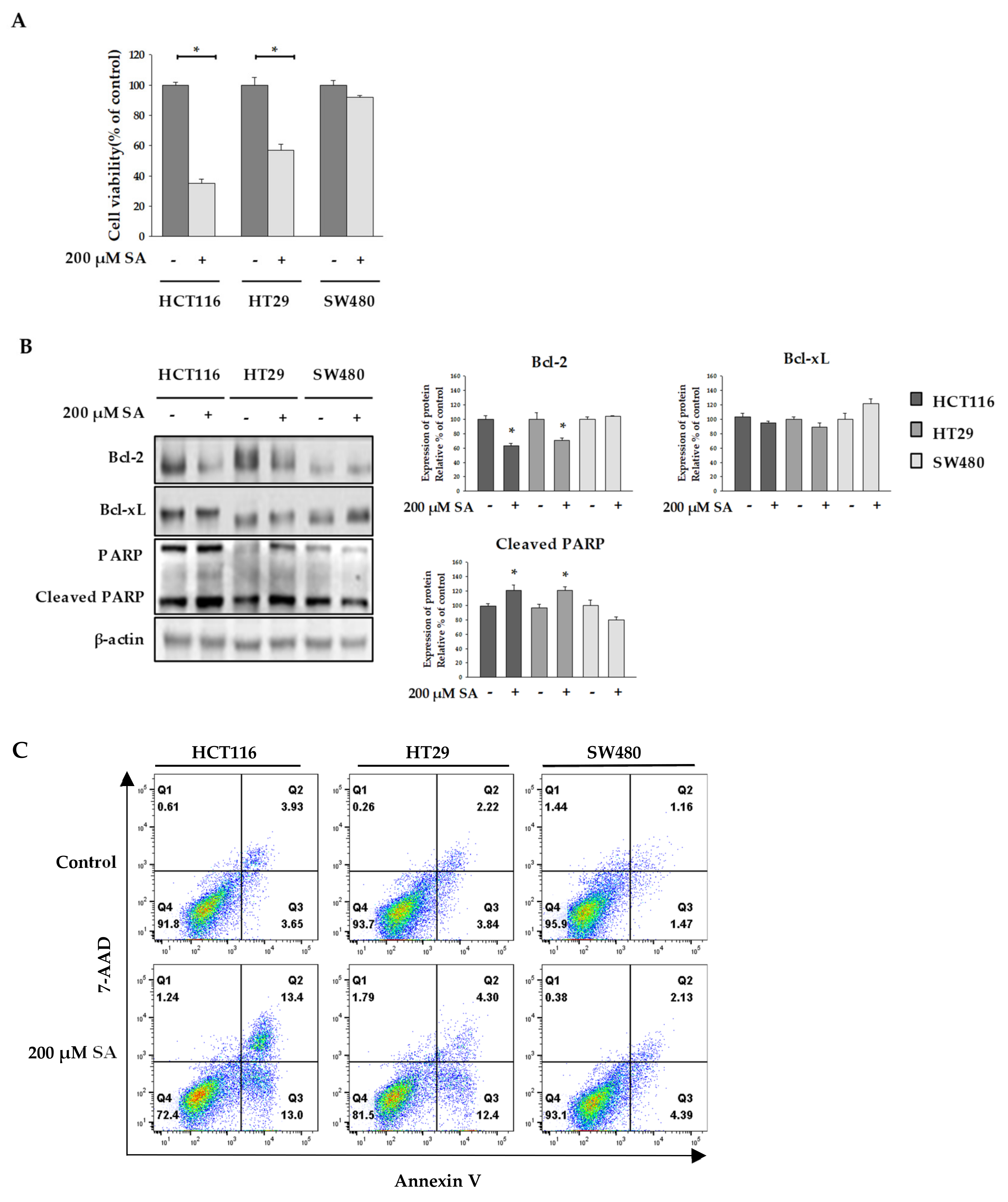
2.1. SA Effect on the Viability of Colon Cancer Cells
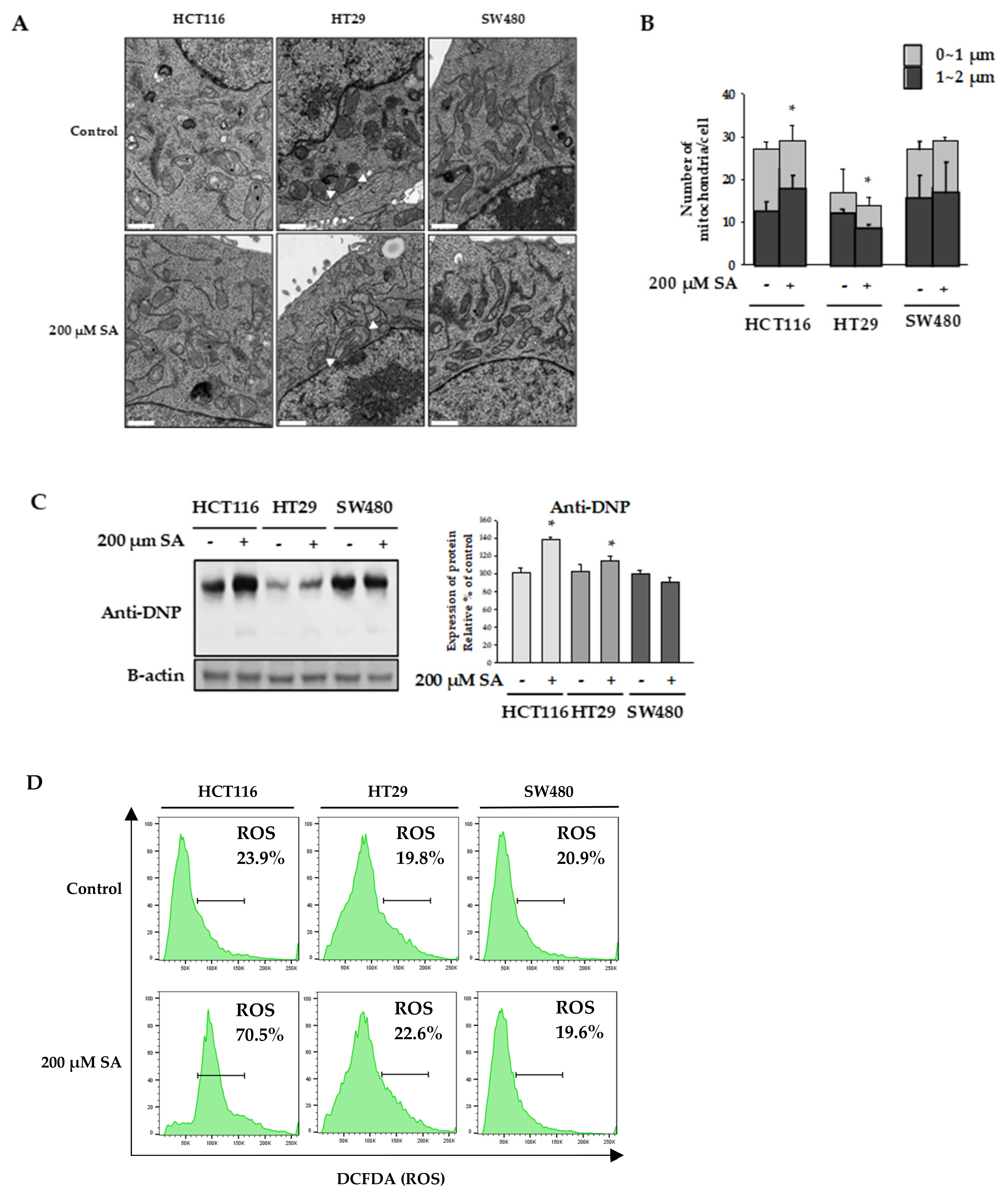
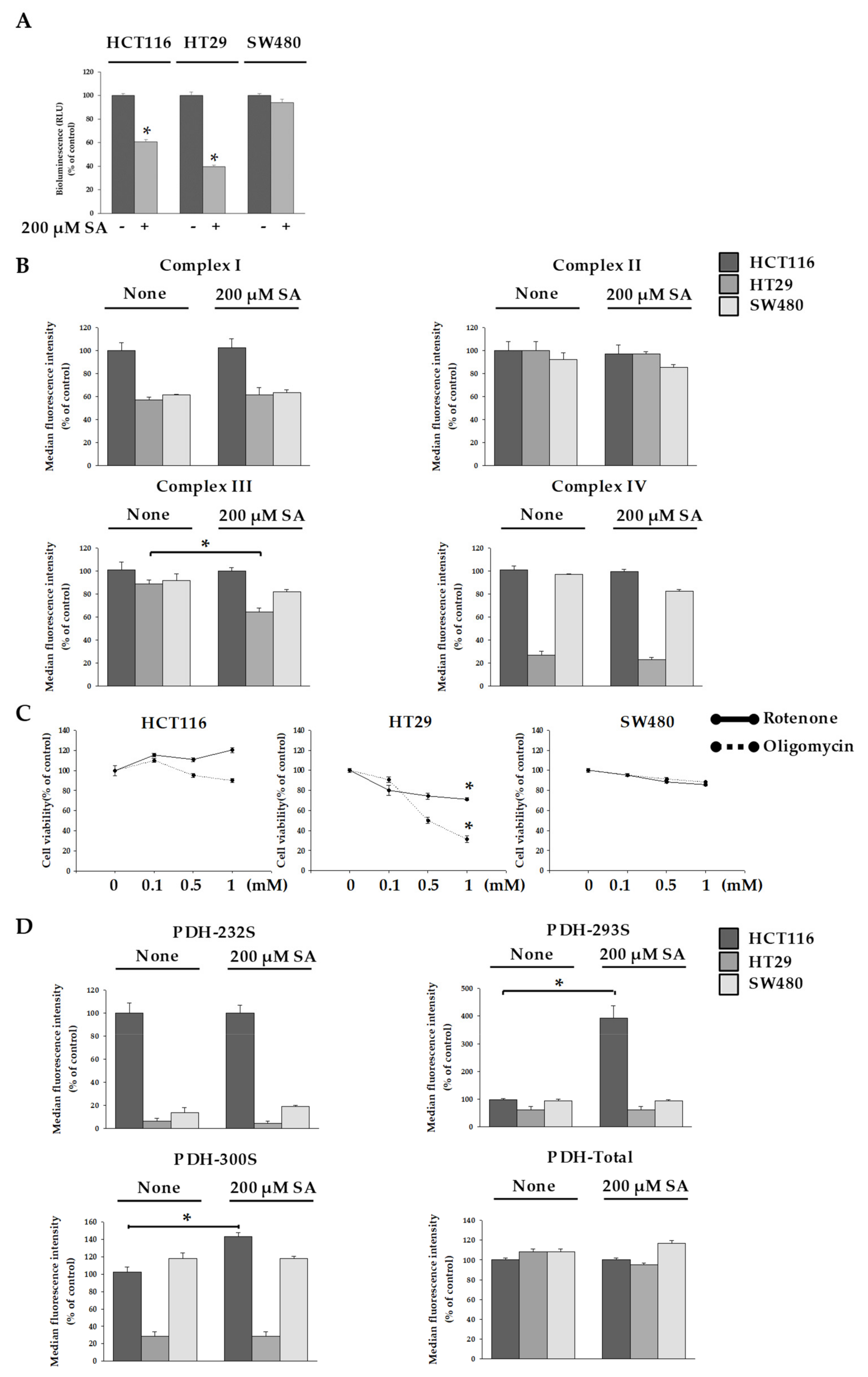
2.2. SA Decreased ATP Levels through a Differential Mechanism
2.3. Nicotinamide Nucleotide Transhydrogenase (NNT) Activity in HT29 Cells Abolished ROS Production
3. Materials and Methods
3.1. Cell Culture
3.2. Reagents
3.3. Cell Viability Measurement (MTT Assay)
3.4. Western Blotting
3.5. Transmission Electron Microscopy (TEM) Observation of Mitochondria
3.6. Measurement of PDH Activity Within Cells
3.7. Measurement of Mitochondrial Electron Transport Chain Activity
3.8. Measurement of Intracellular ATP Concentration
3.9. Determination of SA-Induced Apoptosis
3.10. Measuring Reactive Oxygen Species (ROS)
3.11. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Sassa, S.; Kappas, A. Hereditary tyrosinemia and the heme biosynthetic pathway. Profound inhibition of delta-aminolevulinic acid dehydratase activity by succinylacetone. J. Clin. Invest. 1983, 71, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.L.; Chiang, S.; Kalinowski, D.S.; Bae, D.H.; Sahni, S.; Richardson, D.R. The role of the antioxidant response in mitochondrial dysfunction in degenerative diseases: cross-talk between antioxidant defense, autophagy, and apoptosis. Oxid. Med. Cell Longev. 2019, 7. [Google Scholar] [CrossRef] [PubMed]
- Weinbach, E.C.; Ebert, P.S. Effects of succinylacetone on growth and respiration of L1210 leukemia cells. Cancer Lett. 1985, 26, 253–259. [Google Scholar] [CrossRef]
- Bhandari, A.; Woodhouse, M.; Gupta, S. Colorectal cancer is a leading cause of cancer incidence and mortality among adults younger than 50 years in the USA: A SEER-based analysis with comparison to other young-onset cancers. J. Investig. Med. 2017, 65, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Gray, L.R.; Tompkins, S.C.; Taylor, E.B. Regulation of pyruvate metabolism and human disease. Cell Mol. Life Sci. 2014, 71, 2577–2604. [Google Scholar] [CrossRef] [PubMed]
- Mourier, A.; Larsson, N.G. Tracing the trail of protons through complex I of the mitochondrial respiratory chain. PLoS Biol. 2011, 9, e1001129. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y. Cancer energy metabolism: Shutting power off cancer factory. Biomol. Ther. (Seoul) 2018, 26, 39–44. [Google Scholar] [CrossRef]
- Ye, W.; Zhang, L. Heme deficiency causes apoptosis but does not increase ROS generation in HeLa cells. Biochem. Biophys. Res. Commun. 2004, 319, 1065–1071. [Google Scholar] [CrossRef]
- Kabe, Y.; Nakane, T.; Koike, I.; Yamamoto, T.; Sugiura, Y.; Harada, E.; Sugase, K.; Shimamura, T.; Ohmura, M.; Muraoka, K.; et al. Hame-dependent dimerization of PGRMC1/Sigma-2 receptor facilitates cancer proliferation and chemoresistance. Nat. Commun. 2016, 7, 11030. [Google Scholar] [CrossRef]
- Hooda, J.; Cadinu, D.; Alam, M.M.; Shah, A.; Cao, T.M.; Sullivan, L.A.; Brekken, R.; Zhang, L. Enhanced heme function and mitochondrial respiration promote the progression of lung cancer cells. PloS ONE 2013, 8, e63402. [Google Scholar] [CrossRef]
- Lee, P.J.; Shin, I.; Seo, S.Y.; Kim, H.; Kim, H.P. Upregulation of both heme oxygenase-1 and ATPase inhibitory factor 1 renders tumoricidal activity by synthetic flavonoids via depleting cellular ATP. Bioorg. Med. Chem. Lett. 2014, 24, 4845–4849. [Google Scholar] [CrossRef] [PubMed]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta. 2016, 1863, 2977–2992. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Robotham, J.L.; Yoon, Y. Increased production of reactive oxygen species in hyperglycemic conditions requires dynamic change of mitochondrial morphology. Proc. Natl. Acad. Sci. USA 2006, 103, 2653–2658. [Google Scholar] [CrossRef]
- Cogliati, S.; Enriquez, J.A.; Scorrano, L. Mitochondrial cristae: Where beauty meets functionality. Trends Biochem. Sci. 2016, 41, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Atamna, H.; Newberry, J.; Erlitzki, R.; Schultz, C.S.; Ames, B.N. Biotin deficiency inhibits heme synthesis and impairs mitochondria in human lung fibroblasts. J. Nutr. 2007, 137, 25–30. [Google Scholar] [CrossRef]
- Wyatt, C.N.; Buckler, K.J. The effect of mitochondrial inhibitors on membrane currents in isolated neonatal rat carotid body type I cells. J. Physiol. 2004, 556, 175–191. [Google Scholar] [CrossRef]
- Koukourakis, M.I.; Giatromanolaki, A.; Sivridis, E.; Gatter, K.C.; Harris, A.L. Pyruvate dehydrogenase and pyruvate dehydrogenase kinase expression in non small cell lung cancer and tumor-associated stroma. Neoplasia 2005, 7, 1–6. [Google Scholar] [CrossRef]
- Park, S.; Jeon, J.H.; Min, B.K.; Ha, C.M.; Thoudam, T.; Park, B.Y.; Lee, I.K. Role of the pyruvate dehydrogenase complex in metabolic remodeling: Differential pyruvate dehydrogenase complex functions in metabolism. Diabetes Metab. J. 2018, 42, 270–281. [Google Scholar] [CrossRef]
- Hou, X.; Zhang, L.; Han, L.; Ge, J.; Ma, R.; Zhang, X.; Moley, K.; Schedl, T.; Wang, Q. Differing roles of pyruvate dehydrogenase kinases during mouse oocyte maturation. J. Cell Sci. 2015, 128, 2319–2329. [Google Scholar] [CrossRef]
- Pieczenik, S.R.; Neustadt, J. Mitochondrial dysfunction and molecular pathways of disease. Exp. Mol. Pathol. 2007, 83, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Hagopian, K.; Weber, K.L.; Hwee, D.T.; Van Eenennaam, A.L.; López-Lluch, G.; Villalba, J.M.; Burón, I.; Navas, P.; German, J.B.; Watkins, S.M.; et al. Complex I-associated hydrogen peroxide production is decreased and electron transport chain enzyme activities are altered in n-3 enriched fat-1 mice. PLoS ONE 2010, 5, e12696. [Google Scholar] [CrossRef] [PubMed]
- Ciccarese, F.; Ciminale, V. Escaping Death: Mitochondrial Redox Homeostasis in Cancer Cells. Front Oncol. 2017, 7, 117. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.J.; Woo, S.J.; Jee, J.G.; Sung, S.H.; Kim, H.P. Bisdemethoxycurcumin induces apoptosis in activated hepatic stellate cells via cannabinoid receptor 2. Molecules 2015, 20, 1277–1292. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.J.; Park, H.J.; Cho, N.; Kim, H.P. 3,5-Diethoxy-3′-hydroxyresveratrol (DEHR) ameliorates liver fibrosis via caveolin-1 activation in hepatic stellate Cells and in a mouse model of bile duct ligation injury. Molecules 2018, 23, 2833. [Google Scholar] [CrossRef]
Sample Availability: Not available. |

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, P.J.; Woo, S.J.; Yoo, H.M.; Cho, N.; Kim, H.P. Differential Mechanism of ATP Production Occurs in Response to Succinylacetone in Colon Cancer Cells. Molecules 2019, 24, 3575. https://doi.org/10.3390/molecules24193575
Lee PJ, Woo SJ, Yoo HM, Cho N, Kim HP. Differential Mechanism of ATP Production Occurs in Response to Succinylacetone in Colon Cancer Cells. Molecules. 2019; 24(19):3575. https://doi.org/10.3390/molecules24193575
Chicago/Turabian StyleLee, Phil Jun, Seung Je Woo, Hee Min Yoo, Namki Cho, and Hong Pyo Kim. 2019. "Differential Mechanism of ATP Production Occurs in Response to Succinylacetone in Colon Cancer Cells" Molecules 24, no. 19: 3575. https://doi.org/10.3390/molecules24193575
APA StyleLee, P. J., Woo, S. J., Yoo, H. M., Cho, N., & Kim, H. P. (2019). Differential Mechanism of ATP Production Occurs in Response to Succinylacetone in Colon Cancer Cells. Molecules, 24(19), 3575. https://doi.org/10.3390/molecules24193575