Cytotoxicity and Apoptosis Induction of Coumarins and Carbazole Alkaloids from Clausena harmandiana
Abstract
1. Introduction
2. Results
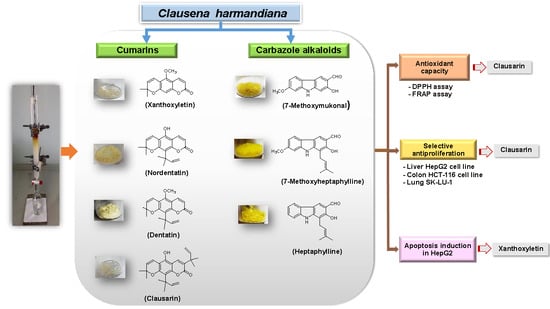
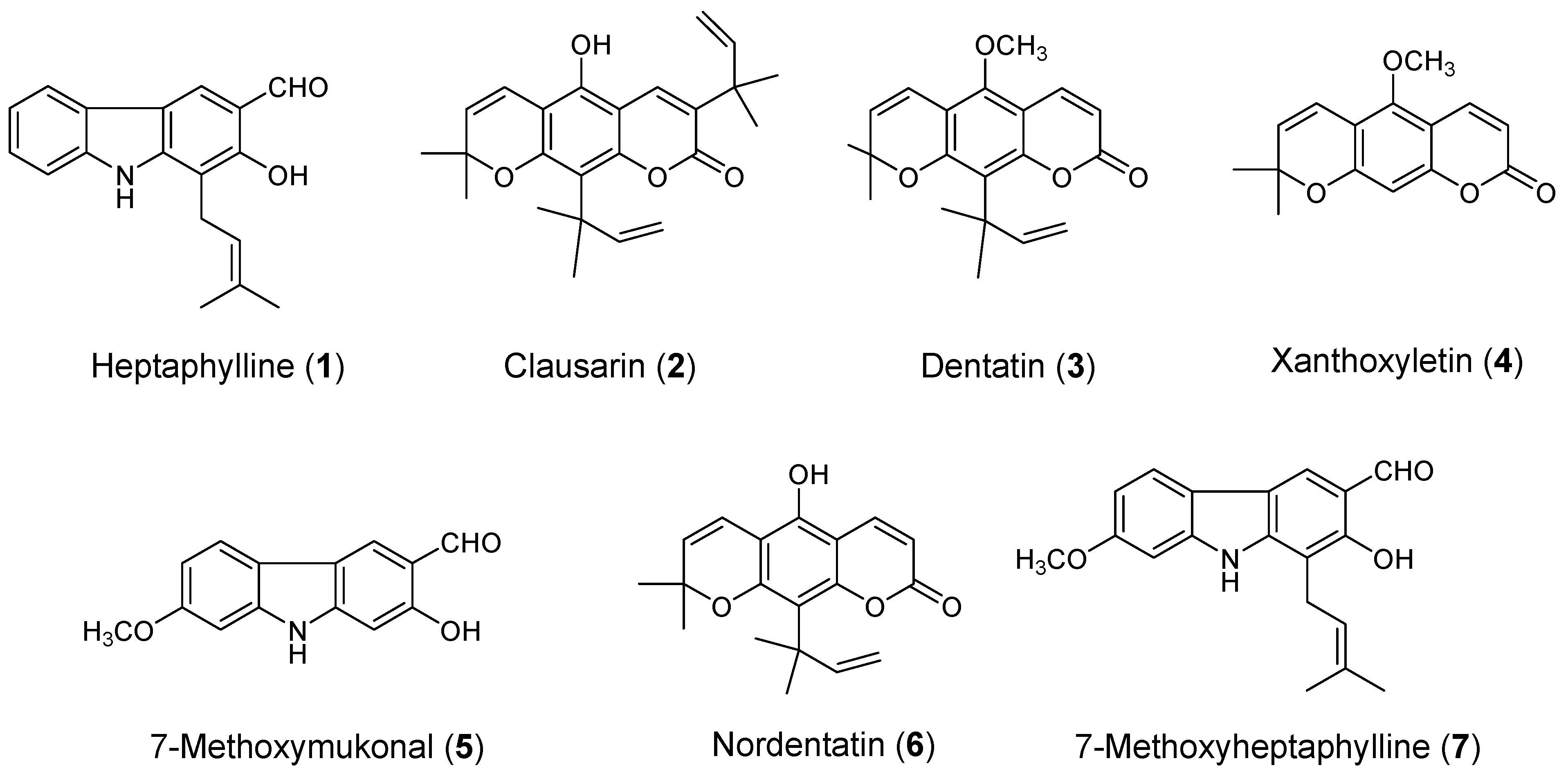
2.1. Isolation of Carbazole Alkaloids and Coumarins
2.2. Antioxidant Activity
2.3. Cytotoxicity Activity
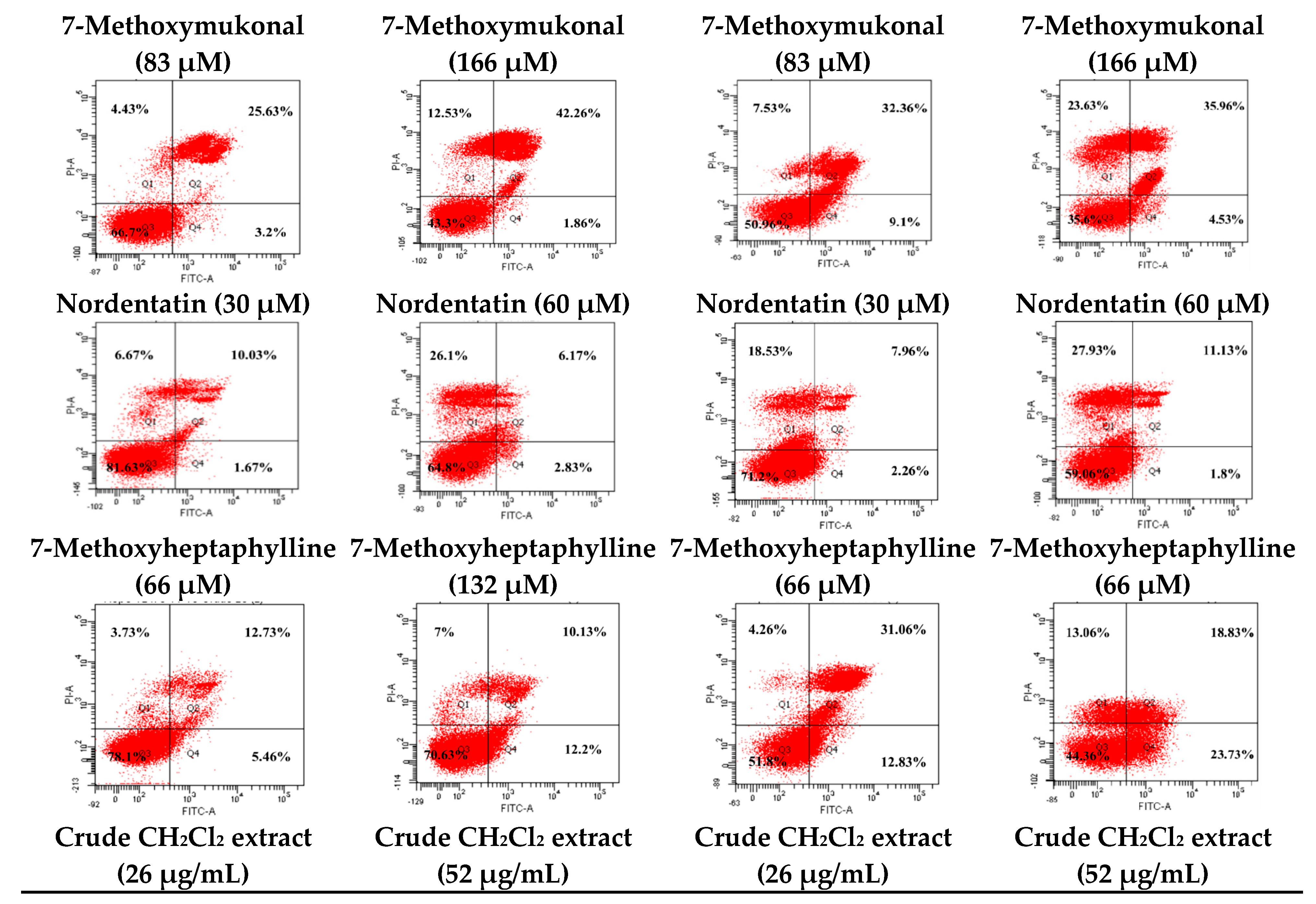
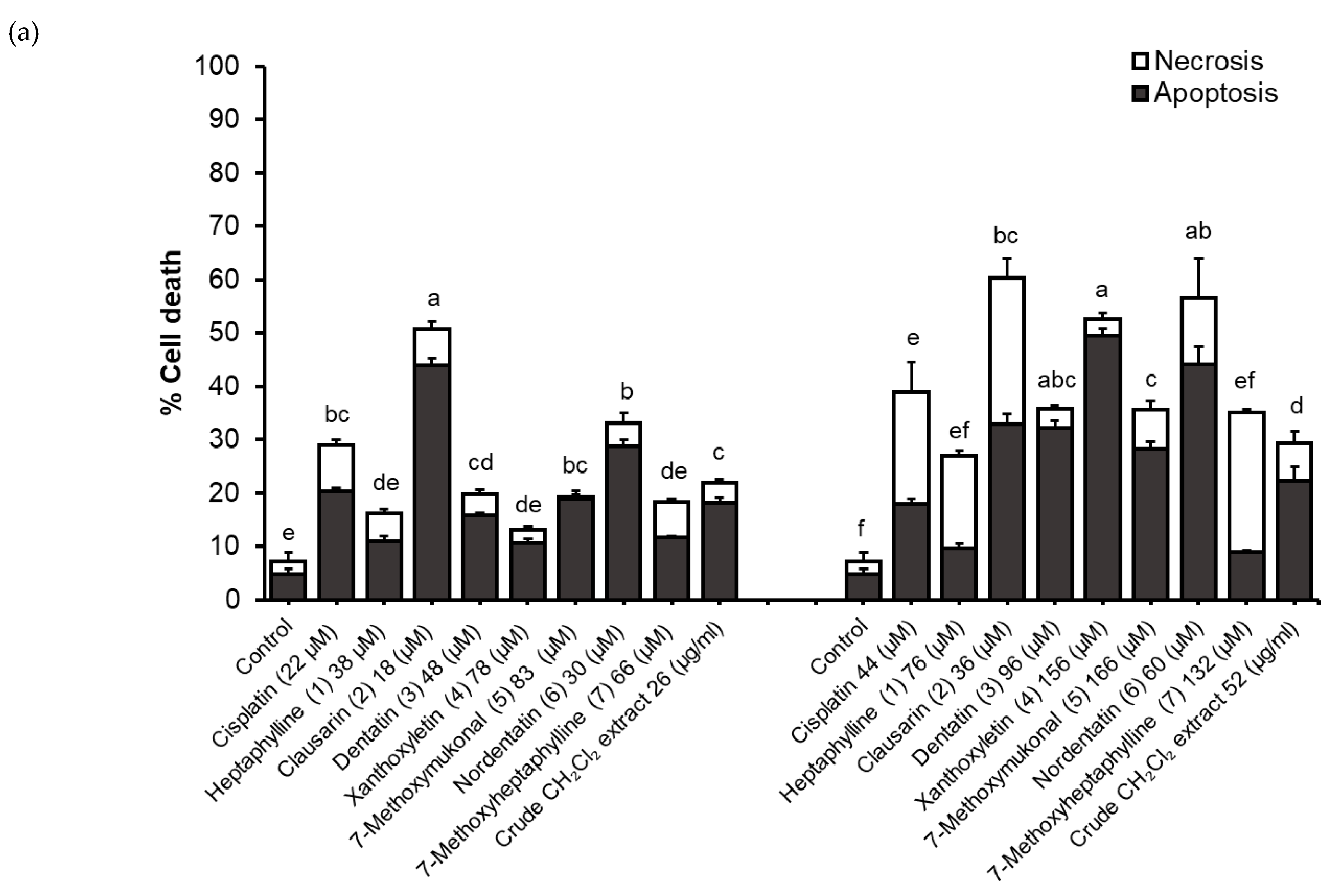
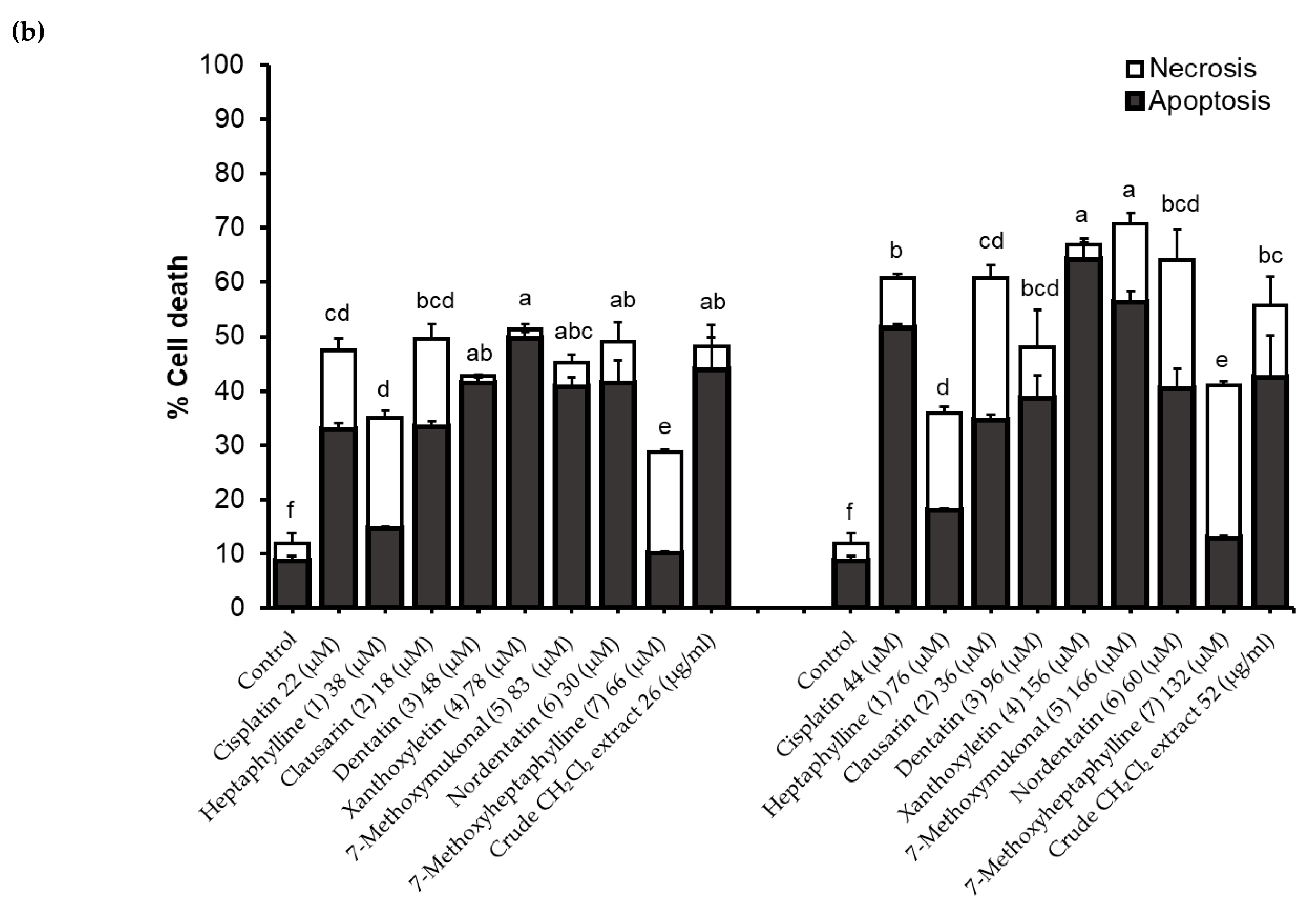
2.4. Determination of Apoptosis and Necrotic Cell Death
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Plant Material
4.3. Extraction and Isolation
4.4. Antioxidant Activity
4.4.1. DPPH Radical Scavenging Assay
4.4.2. Ferric Reducing Antioxidant Power (FRAP) Assay
4.5. Cell Lines and Cell Culture
4.5.1. Cell Viability Assay
4.5.2. Mode of Cell Death by Annexin V-FITC/PI Staining Analyzed by Flow Cytometry
4.6. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Smittinan, T. Thai Plant Names, revised edition; Royal Forestry Department: Bangkok, Thailand, 2001. (In Thai) [Google Scholar]
- Arbab, I.A.; Abdul, A.B.; Aspollah, M.; Abdullah, R.; Abdelwahab, S.I.; Ibrahim, M.Y.; Ali, L.Z. A review of traditional uses, phytochemical and pharmacological aspects of selected members of Clausena genus (Rutaceae). JMPR 2012, 6, 5107–5118. [Google Scholar] [CrossRef]
- Wangboonskul, J.D.; Pummangura, S.; Chaichantipyuth, C. Five coumarins and a carbazole alkaloids from the root bark of Clausena harmandiana. J. Nat. Prod. 1984, 47, 1058–1059. [Google Scholar] [CrossRef] [PubMed]
- Fun, H.-K.; Maneerat, W.; Laphookhieo, S.; Chantrapromma, S. Glycozolidal. Acta Cryst. Sect. E Struct. Rep. Online 2011, 67, o1811–o1812. [Google Scholar] [CrossRef] [PubMed]
- Songsiang, U.; Thongthoom, T.; Boonyarat, C.; Yenjai, C.; Claurailas, A.-D. Cytotoxic carbazole alkaloids from the roots of Clausena harmandiana. J. Nat. Prod. 2011, 74, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Lim, P.C.; Ramli, H.; Kassim, N.K.; Ali, Z.; Khan, I.A.; Shaari, K.; Ismail, A. Chemical constituents from the stem bark of Clausena excavata Burm. f. Biochem. Syst. Ecol. 2019, 82, 52–55. [Google Scholar] [CrossRef]
- Auranwiwat, C.; Laphookhieo, S.; Trisuwan, K.; Pyne, S.G.; Ritthiwigrom, T. Carbazole alkaloids and coumarins from the roots of Clausena guillauminii. Phytochem. Lett. 2014, 9, 113–116. [Google Scholar] [CrossRef][Green Version]
- Chaichantipyuth, C.; Pummangura, S.; Naowsaran, K.; Thanyavuthi, D.; Anderson, J.E.; McLaughlin, J.L. Two new bioactive carbazole alkaloids from the root bark of Clausena harmandiana. J. Nat. Prod. 1988, 51, 1285–1288. [Google Scholar] [CrossRef] [PubMed]
- Yenjai, C.; Sripontan, S.; Sriprajun, P.; Kittakoop, P.; Jintasirikul, A.; Tanticharoen, M.; Thebtaranonth, Y. Coumarins and carbazoles with antiplasmodial activity from Clausena harmandiana. Planta Med. 2000, 66, 277–279. [Google Scholar] [CrossRef]
- Thongthoom, T.; Songsiang, U.; Phaosiri, C.; Yenjai, C. Biological activity of chemical constituents from Clausena harmandiana. Arch. Pharm. Res. 2010, 33, 675–680. [Google Scholar] [CrossRef]
- Sriphana, U.; Thongsri, Y.; Prariyachatigul, C.; Pakawatchai, C.; Yenjai, C. Clauraila E from the roots of Clausena harmandiana and antifungal activity against Pythium insidiosum. Arch. Pharm. Res. 2013, 36, 1078–1083. [Google Scholar] [CrossRef]
- Noipha, K.; Thongthoom, T.; Songsiang, U.; Boonyarat, C.; Yenjai, C. Carbazoles and coumarins from Clausena harmandiana stimulate glucose uptake in L6 myotubes. Diabetes Res. Clin. Pract. 2010, 90, e67–e71. [Google Scholar] [CrossRef]
- Maneerat, W.; Phakhodee, W.; Ritthiwigrom, T.; Cheenpracha, S.; Promgool, T.; Yossathera, K.; Deachathai, S.; Laphookhieo, S. Antibacterial carbazole alkaloids from Clausena harmandiana twigs. Fitoterapia 2012, 83, 1110–1114. [Google Scholar] [CrossRef]
- Maneerat, W.; Phakhodee, W.; Ritthiwigrom, T.; Cheenpracha, S.; Deachathai, S.; Laphookhieo, S. Phenylpropanoid derivatives from Clausena harmandiana fruits. Phytochem. Lett. 2013, 6, 18–20. [Google Scholar] [CrossRef]
- Songsiang, U.; Thongthoom, T.; Zeekpudsa, P.; Kukongviriyapan, V.; Boonyarat, C.; Wangboonskul, J.; Yenjai, C. Antioxidant activity and cytotoxicity against cholangiocarcinoma of carbazoles and coumarins from Clausena harmandiana. Sci. Asia 2012, 38, 75. [Google Scholar] [CrossRef]
- Yompakdee, C.; Roytrakul, S. Molecular Target of An Anti-Cancer Compound from Leaves of Clausena harmandiana (Pierre); Chulalongkorn University: Bangkok, Thailand, 2016; Available online: http://cuir.car.chula.ac.th/handle/123456789/57101 (accessed on 24 August 2019).
- Yompakdee, C.; Yingyongnarongkul, B. Chemical Immunosuppression from Clausena harmandiana (Pierre) Research Report; Chulalongkorn University: Bangkok, Thailand, 2014. [Google Scholar] [CrossRef]
- Kitisripanya, T.; Laoburee, M.; Puengsiricharoen, L.; Pratoomtong, P.; Daodee, S.; Wangboonskul, J.; Putalun, W. Production of carbazole alkaloids through callus and suspension cultures in Clausena harmandiana. Nat. Prod. Res. 2018, 1–7. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Virani, S.; Bilheem, S.; Chansaard, W.; Chitapanarux, I.; Daoprasert, K.; Khuanchana, S.; Leklob, A.; Pongnikorn, D.; Rozek, L.S.; Siriarechakul, S.; et al. National and subnational population-based incidence of cancer in Thailand: Assessing Cancers with the highest burdens. Cancers 2017, 9, 108. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef]
- Chakraborty, S.; Rahman, T. The difficulties in cancer treatment. Ecancermedicalscience 2012. [Google Scholar] [CrossRef]
- Jantakoon, P.; Tadtong, S.; Puthongking, P. Neuritogenic and antioxidant activities of nordentatin from Clausena harmandiana. J. Asian Assoc. Sch. Pharm. 2012, 1, 180–186. [Google Scholar]
- Bézivin, C.; Tomasi, S.; Lohézic-Le Dévéhat, F.; Boustie, J. Cytotoxic activity of some lichen extracts on murine and human cancer cell lines. Phytomedicine 2003, 10, 499–503. [Google Scholar] [CrossRef]
- Prayong, P.; Barusrux, S.; Weerapreeyakul, N. Cytotoxic activity screening of some indigenous Thai plants. Fitoterapia 2008, 79, 598–601. [Google Scholar] [CrossRef]
- Boonyarat, C.; Yenjai, C.; Vajragupta, O.; Waiwut, P. Heptaphylline induces apoptosis in human colon adenocarcinoma cells through bid and Akt/NF-κB (p65) pathways. Asian Pac. J. Cancer Prev. 2014, 15, 10483–10487. [Google Scholar] [CrossRef]
- Pfeffer, C.M.; Singh, A.T.K. Apoptosis: A Target for anticancer therapy. Int. J. Mol. Sci. 2018, 19, 448. [Google Scholar] [CrossRef]
- Hetts, S.W. To Die or Not to Die: An overview of apoptosis and its role in disease. JAMA 1998, 279, 300–307. [Google Scholar] [CrossRef]
- Kasibhatla, S.; Tseng, B. Why target apoptosis in cancer treatment? Mol. Cancer Ther. 2003, 2, 573–580. Available online: https://mct.aacrjournals.org/content/2/6/573 (accessed on 30 August 2019).
- Martin, S.J. Cell death and inflammation: The case for IL-1 family cytokines as the canonical DAMPs of the immune system. FEBS J. 2016, 283, 2599–2615. [Google Scholar] [CrossRef]
- Brouckaert, G.; Kalai, M.; Krysko, D.V.; Saelens, X.; Vercammen, D.; Ndlovu, M.; Haegeman, G.; D’Herde, K.; Vandenabeele, P. Phagocytosis of necrotic cells by macrophages is phosphatidylserine dependent and does not induce inflammatory cytokine production. MBOC 2003, 15, 1089–1100. [Google Scholar] [CrossRef]
- Amaravadi, R.K.; Thompson, C.B. The roles of therapy-induced autophagy and necrosis in cancer treatment. Clin. Cancer Res. 2007, 13, 7271–7279. [Google Scholar] [CrossRef]
- Gökbulut, A.; Ozhan, O.; Satilmiş, B.; Batçioğlu, K.; Günal, S.; Sarer, E. Antioxidant and antimicrobial activities, and phenolic compounds of selected Inula species from Turkey. Nat. Prod. Commun. 2013, 8, 475–478. [Google Scholar] [CrossRef]
- Chunhakant, S.; Chaicharoenpong, C. Antityrosinase, antioxidant, and cytotoxic activities of phytochemical constituents from Manilkara zapota L. Bark. Molecules 2019, 24, 2798. [Google Scholar] [CrossRef]
- Pocasap, P.; Weerapreeyakul, N.; Thumanu, K. Alyssin and iberin in cruciferous vegetables exert anticancer activity in HepG2 by increasing intracellular reactive oxygen species and tubulin depolymerization. Biomol. Ther. 2019, 27. [Google Scholar] [CrossRef] [PubMed]
- Nonpunya, A.; Sethabouppha, B.; Rufini, S.; Weerapreeyakul, N. Cratoxylum formosum ssp. pruniflorum activates the TRAIL death receptor complex and inhibits topoisomerase I. S. Afr. J. Bot. 2018, 114, 150–162. [Google Scholar] [CrossRef]
Sample Availability: Samples of Clausena harmandiana extract is available from the authors. |

| Compounds | DPPH Assay | FRAP Assay | |
|---|---|---|---|
| IC50 (µM) | IC50 (µg/mL) | FRAP Value (µM FeSO4 Equivalents) | |
| Trolox | 16.8 ± 0.6b | 4.2 ± 0.1a | 37.7 ± 0.8c |
| Heptaphylline (1) | 335.1 ± 7.8g | 93.5 ± 2.2f | 1.0 ± 0.0g |
| Clausarin (2) | 6.0 ± 0.8a | 2.3 ± 0.3a | 45.2 ± 1.0b |
| Dentatin (3) | >500h | >500h | 4.7 ± 0.1f |
| Xanthoxyletin (4) | 247.1 ± 3.0e | 63.8 ± 0.8d | 5.2 ± 0.3f |
| 7-Methoxymukonal (5) | 26.2 ± 2.0c | 6.8 ± 0.5b | 47.0 ± 0.6a |
| Nordentatin (6) | 38.3 ± 2.5d | 12.0 ± 0.8c | 9.0 ± 1.1e |
| 7-Methoxyheptaphylline (7) | 313.4 ± 3.4f | 96.8 ± 1.1g | 1.4 ± 0.1g |
| Crude CH2Cl2 extract | 80.5 ± 0.7e | 14.9 ± 0.1d | |
| Compounds | IC50 (µM) and (Selectivity Index) | |||
|---|---|---|---|---|
| HepG2 | HCT116 | SK-LU-1 | Vero | |
| Cisplatin | 21.8 ± 3.0aB | 71.9 ± 2.7cC | 42.7 ± 1.9bB | inactivedB |
| (4.6) | (1.4) | (2.3) | ||
| Heptaphylline (1) | 37.8 ± 2.6aD | 74.7 ± 2.1bC | 83.1 ± 2.6cD | inactivedB |
| (2.6) | (1.3) | (1.2) | ||
| Clausarin (2) | 17.6 ± 2.1bA | 44.9 ± 1.4cA | 6.9 ± 1.6aA | 78.2±3.0dA |
| (4.4) | (1.7) | (11.3) | ||
| Dentatin (3) | 47.6 ± 2.8aE | 73.9 ± 2.5bC | 45.4 ± 2.5aB | inactivecB |
| (2.1) | (1.4) | (2.2) | ||
| Xanthoxyletin (4) | 78.2 ± 2.2aG | 79.8 ± 2.8aD | 94.4 ± 2.2bE | inactivecB |
| (1.3) | (1.3) | (1.1) | ||
| 7-Methoxymukonal (5) | 82.6 ± 2.8aH | inactivebE | inactivebF | inactivebB |
| (1.2) | (1.0) | (1.0) | ||
| Nordentatin (6) | 29.9 ± 3.2aC | 63.6 ± 2.3bB | 67.3 ± 1.2bC | inactivecB |
| (3.3) | (1.6) | (1.5) | ||
| 7-Methoxyheptaphylline (7) | 65.5 ± 2.7aF | inactivebE | inactivebF | inactivebB |
| (1.5) | (1.0) | 1.0) | ||
| Crude CH2Cl2 extract (µg/mL) | 25.7 ± 1.8a | 67.9 ± 2.6b | 67.4 ± 2.7b | inactivec |
| (3.9) | (1.5) | (1.5) | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jantamat, P.; Weerapreeyakul, N.; Puthongking, P. Cytotoxicity and Apoptosis Induction of Coumarins and Carbazole Alkaloids from Clausena harmandiana. Molecules 2019, 24, 3385. https://doi.org/10.3390/molecules24183385
Jantamat P, Weerapreeyakul N, Puthongking P. Cytotoxicity and Apoptosis Induction of Coumarins and Carbazole Alkaloids from Clausena harmandiana. Molecules. 2019; 24(18):3385. https://doi.org/10.3390/molecules24183385
Chicago/Turabian StyleJantamat, Porntip, Natthida Weerapreeyakul, and Ploenthip Puthongking. 2019. "Cytotoxicity and Apoptosis Induction of Coumarins and Carbazole Alkaloids from Clausena harmandiana" Molecules 24, no. 18: 3385. https://doi.org/10.3390/molecules24183385
APA StyleJantamat, P., Weerapreeyakul, N., & Puthongking, P. (2019). Cytotoxicity and Apoptosis Induction of Coumarins and Carbazole Alkaloids from Clausena harmandiana. Molecules, 24(18), 3385. https://doi.org/10.3390/molecules24183385