Effect of Ionic Composition on Physicochemical Properties of Mono-Ether Functional Ionic Liquids
Abstract
1. Introduction
2. Results and Discussion
2.1. Density and Viscosity
2.2. Electrochemical Window
2.3. Ionic Conductivity
2.4. Thermal Stability
2.5. Heat Capacity and Heat Storage Density
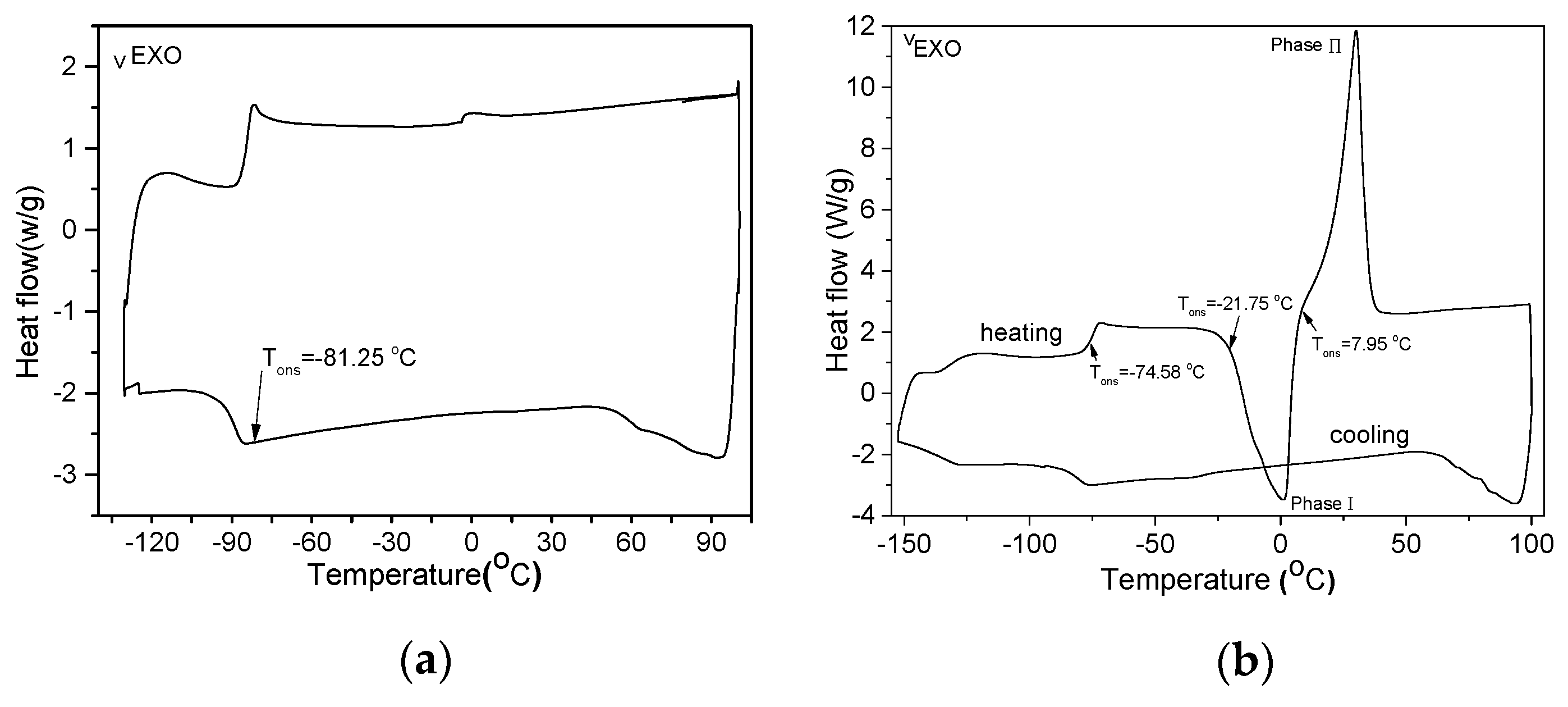
2.6. Phase Behavior
3. Materials and Methods
3.1. Materials
3.2. Methods
4. Materials and Methods
4.1. General Procedures for the Preparation of ME-FILs
4.2. Water Content Quantification
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Parvulescu, V.I.; Hardacre, C. Catalysis in ionic liquids. Chem. Rev. 2007, 107, 2615–2665. [Google Scholar] [CrossRef] [PubMed]
- Enferadi-Kerenkan, A.; Do, T.O.; Kaliaguine, S. Heterogeneous catalysis by tungsten-based heteropoly compounds. Catal. Sci. Technol. 2018, 8, 2257–2284. [Google Scholar] [CrossRef]
- Zhang, Q.H.; Zhang, S.G.; Deng, Y.Q. Recent advances in ionic liquid catalysis. Green Chem. 2011, 13, 2619–2637. [Google Scholar] [CrossRef]
- Berthod, A.M.; Ruiz-Ángel, J.; Carda-Broch, S. Ionic liquids in separation techniques. J. Chromatogr. A. 2008, 1184, 6–18. [Google Scholar] [CrossRef] [PubMed]
- Deferm, C.; Luyten, J.; Oosterhof, H.; Fransaer, J.; Binnemans, K. Purification of crude In(OH)3 using the functionalized ionic liquid betainium bis (trifluoromethylsulfonyl) imide. Green Chem. 2018, 20, 412–424. [Google Scholar] [CrossRef]
- Ma, Z.; Yu, J.; Dai, S. Preparation of inorganic materials using ionic liquids. Adv. Mater. 2010, 22, 261–285. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.X.; An, B.W.; Ji, M.; Zhang, J. Hybridization of metal–organic frameworks and task-specific ionic liquids: Fundamentals and challenges. Mater. Chem. Front. 2018, 2, 219–234. [Google Scholar] [CrossRef]
- Silvester, D.S.; Compton, R.G. Electrochemistry in room temperature ionic liquids: A review and some possible applications. J. Phys. Chem. 2006, 220, 1247–1274. [Google Scholar] [CrossRef]
- Deferm, C.; Malaquias, J.C.; Onghena, B.; Banerjee, D.; Luyten, J.; Oosterhof, H.; Fransaer, J.; Binnemans, K. Electrodeposition of indium from the ionic liquid trihexyl (tetradecyl) phosphonium chloride. Green Chem. 2019, 21, 1517–15300. [Google Scholar] [CrossRef]
- Werner, S.; Haumann, M.; Wasserscheid, P. Ionic liquids in chemical engineering. Chem. Biomol. Eng. 2010, 1, 203–230. [Google Scholar] [CrossRef]
- Gilet, A.; Quettier, C.; Wiatz, V.; Bricout, H.; Ferreira, M.; Rousseau, C.; Monflier, E.; Tilloy, S. Unconventional media and technologies for starch etherification and esterification. Green Chem. 2018, 20, 1152–1168. [Google Scholar] [CrossRef]
- Giernoth, R. Task-specific ionic liquids. Angew. Chem. Int. Ed. 2010, 49, 2834–2839. [Google Scholar] [CrossRef] [PubMed]
- Burba, C.M. Dipolar coupling and molecular vibrations in ionic liquids. Phys. Chem. Chem. Phys. 2019, 21, 3976–3988. [Google Scholar] [CrossRef] [PubMed]
- Bates, E.D.; Mayton, R.D.; Ntai, I.; Davis, J.H., Jr. CO2 capture by a task-specific ionic liquid. J. Am. Chem. Soc. 2002, 124, 926–927. [Google Scholar] [CrossRef] [PubMed]
- Gurkan, B.E.; de la Fuente, J.C.; Mindrup, E.M.; Ficke, L.E.; Goodrich, B.F.; Price, E.A.; Schneider, W.F.; Brenecke, J.F. Equimolar CO2 absorption by anion-functionalized ionic liquids. J. Am. Chem. Soc. 2010, 132, 2116–2117. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.M.; Luo, H.M.; Jiang, D.E.; Li, H.R.; Dai, S. Carbon dioxide capture by superbase-derived protic ionic liquids. Angew. Chem. Int. Ed. 2010, 49, 5978–5981. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Lao, D.B.; Sui, X.; Zhou, Y.F.; Nune, S.K.; Ma, X.; Troy, T.P.; Ahmed, M.; Zhu, Z.H.; Heldebrant, D.J.; et al. Two coexisting liquid phases in switchable ionic liquids. Phys. Chem. Chem. Phys. 2017, 19, 22627–22632. [Google Scholar] [CrossRef]
- Luo, S.Z.; Mi, X.L.; Zhang, L.; Liu, S.; Xu, H.; Cheng, J.P. Functionalized chiral ionic liquids as highly efficient asymmetric organocatalysts for Michael addition to nitroolefins. Angew. Chem. Int. Ed. 2006, 45, 3093–3097. [Google Scholar] [CrossRef]
- Luo, S.Z.; Zhang, L.; Cheng, J.P. Functionalized chiral ionic liquids: A new type of asymmetric organocatalysts and nonclassical chiral ligands. Chem. Asian J. 2009, 4, 1184–1195. [Google Scholar] [CrossRef]
- Itoh, H.; Naka, K.; Chujo, Y. Synthesis of gold nanoparticles modified with ionic liquid based on the imidazolium cation. J. Am. Chem. Soc. 2004, 126, 3026–3027. [Google Scholar] [CrossRef]
- Visser, A.; Swatloski, P.; Mavton, R.; Sheff, S.; Zejwierzbicki, R.; Rogers, R. Task-specific ionic liquids incorporating novel cations for the coordination and extraction of Hg 2+ and Cd 2+: Synthesis, characterization, and extraction studies. Environ. Sci. Technol. 2002, 36, 2523–2529. [Google Scholar] [CrossRef] [PubMed]
- Hsu, E.; Barmak, K.; West, A.C.; Park, A.H.A. Advancements in the treatment and processing of electronic waste with sustainability: A review of metal extraction and recovery technologies. Green Chem. 2019, 21, 919–936. [Google Scholar] [CrossRef]
- Chaturvedi, D.; Kumar, C.; Zaidi, S.; Chaturvedi, A.K. Task specific ionic liquids: Reaction selectivity in organic synthesis. J. Org. Biomol. Chem. 2014, 2, 51–79. [Google Scholar]
- Ohno, H.; Yoshizawa-Fujita, M.; Kohno, Y. Design and properties of functional zwitterions derived from ionic liquids. Phys. Chem. Chem. Phys. 2018, 20, 10978–10991. [Google Scholar] [CrossRef] [PubMed]
- Kimizuka, N.; Nakashima, T. Spontaneous self-assembly of glycolipid bilayer membranes in sugar-philic ionic liquids and formation of ionogels. Langmuir 2001, 17, 6759–6761. [Google Scholar] [CrossRef]
- Uzagare, M.C.; Sanghvi, Y.S.; Salunkhe, M.M. Application of ionic liquid 1-methoxyethyl-3-methyl imidazolium methanesulfonate in nucleoside chemistry. Green Chem. 2003, 5, 370–372. [Google Scholar] [CrossRef]
- Itoh, T.; Matsushita, Y.; Yoshikazu Abe, S.; Han, H.; Wada, S.; Hayase, S.; Kawatsura, M.; Takai, S.; Morimoto, M.; Hirose, Y. Increased enantioselectivity and remarkable acceleration of lipase-catalyzed transesterification by using an imidazolium PEG–alkyl sulfate ionic liquid. Chem. Eur. J. 2006, 12, 9228–9237. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Jones, C.L.; Cowins, J.V. Lipase dissolution and stabilization in ether-functionalized ionic liquids. Green Chem. 2009, 11, 1128–1138. [Google Scholar] [CrossRef]
- Zhao, H.; Baker, G.A. Ionic liquids and deep eutectic solvents for biodiesel synthesis: A review. J. Chem. Technol. Biotechnol. 2013, 88, 3–12. [Google Scholar] [CrossRef]
- Zhou, H.C.; Chen, J.; Ye, L.M.; Lin, H.Q.; Yuan, Y.Z. Enhanced performance of lipase-catalyzed kinetic resolution of secondary alcohols in monoether- functionalized ionic liquids. Bioresour. Technol. 2011, 102, 5562–5566. [Google Scholar] [CrossRef]
- Pernak, J.; Czepukowicz, A. New ionic liquids and their antielectrostatic properties. Ind. Eng. Chem. Res. 2001, 40, 2379–2383. [Google Scholar] [CrossRef]
- Zhao, H.; Baker, J.A.; Song, Z.Y.; Olubajo, O.; Crittle, T.; Peters, D. Designing enzyme-compatible ionic liquids that can dissolve carbohydrates. Green Chem. 2008, 10, 696–705. [Google Scholar] [CrossRef]
- Seddon, K.R.; Stark, A.; Torres, M.J. Influence of chloride, water, and organic solvents on the physical properties of ionic liquids. Pure Appl. Chem. 2000, 72, 2275–2287. [Google Scholar] [CrossRef]
- Aparicio, S.; Atilhan, M.; Karada, F. Thermophysical properties of pure ionic liquids: Review of present situation. Ind. Eng. Chem. Res. 2010, 49, 9580–9595. [Google Scholar] [CrossRef]
- Ren, S.H.; Hou, Y.C.; Wu, W.Z.; Liu, W.N. Purification of ionic liquids: Sweeping solvents by nitrogen. J. Chem. Eng. Data. 2010, 55, 5074–5077. [Google Scholar] [CrossRef]
- Pernak, J.; Syguda, A.; Pernak, A.; Nawrot, J.; Pradzyńriska, A.; Griffin, S.T.; Rogers, R.D. Choline-derivative-based ionic liquids. Chem. Eur. J. 2007, 13, 6817–6827. [Google Scholar] [CrossRef] [PubMed]
- Jacquemin, J.; Husson, P.; Padua, A.A.H.; Majer, V. Density and viscosity of several pure and water-saturated ionic liquids. Green. Chem. 2006, 8, 172–180. [Google Scholar] [CrossRef]
- Sun, J.; MacFarlane, D.R.; Forsyth, M. A new family of ionic liquids based on the 1-alkyl-2-methyl pyrrolinium cation. Electrochimica Acta 2003, 48, 1707–1711. [Google Scholar] [CrossRef]
- Herath, M.B.; Hickman, T.; Creager, S.E.; Desmarteau, D.D. A new fluorinated anion for room-temperature ionic liquids. J. Fluorine Chem. 2011, 132, 52–56. [Google Scholar] [CrossRef]
- Widegren, J.A.; Saurer, E.M.; Marsh, K.N.; Magee, J.W. Electrolytic conductivity of four imidazolium-based room-temperature ionic liquids and the effect of a water impurity. J. Chem. Thermodyn. 2005, 37, 569–575. [Google Scholar] [CrossRef]
- Takamuku, T.; Tokuda, T.; Uchida, T.; Sonoda, K.; Marekha, B.A.; Idrissi, A.; Takahashi, O.; Horikawa, Y.; Matsumura, J.; Tokushima, T.; et al. Hydrogen bonds of the imidazolium rings of ionic liquids with DMSO studied by NMR, soft X-ray spectroscopy, and SANS. Phys. Chem. Chem. Phys. 2018, 20, 12858–12869. [Google Scholar] [CrossRef]
- Passos, H.; Dinis, T.V.B.; Cláudio, A.F.M.; Freire, M.G.; Coutinho, J.A.P. Hydrogen bond basicity of ionic liquids and molar entropy of hydration of salts as major descriptors in the formation of aqueous biphasic systems. Phys. Chem. Chem. Phys. 2018, 20, 14234–14241. [Google Scholar] [CrossRef]
- Liaw, H.G.; Chen, C.C.; Chen, Y.C.; Chen, J.R.; Huang, S.K.; Liu, S.N. Relationship between flash point of ionic liquids and their thermal decomposition. Green Chem. 2012, 14, 2001–2008. [Google Scholar] [CrossRef]
- Diedrichs, A.; Gmehling, J. Measurement of heat capacities of ionic liquids by differential scanning calorimetry. Fluid Phase Equilibria 2006, 224, 68–77. [Google Scholar] [CrossRef]
- Fredlake, C.P.; Crosthwaite, J.M.; Hert, D.G.; Aki, S.; Brennecke, J.F. Thermophysical properties of imidazolium-based ionic liquids. J. Chem. Eng. Data. 2005, 49, 954–964. [Google Scholar] [CrossRef]
- Fraz, C.; Diebold, G.J.; Tran, C.D.; Yu, S.F. Determination of thermal diffusivities, thermal conductivities, and sound speed of room-temperature ionic liquids by transient grafting technique. J. Chem. Eng. Data 2006, 51, 1250–1255. [Google Scholar] [CrossRef]
- Martins, M.A.R.; Neves, C.M.S.; Kurnia, S.K.; Carvalho, A.P.J.; Rocha, M.A.A.; Santos, L.M.N.B.F.; Pinho, S.P.; Freire, M.G. Densities, viscosities and derived thermophysical properties of water-saturated imidazolium-based ionic liquids. Fluid Phase Equilibria 2016, 407, 188–196. [Google Scholar] [CrossRef]
- Katayama, Y.; Dan, S.; Miura, T.; Kishi, T. Electrochemical behavior of silver in 1-Ethyl-3-methylimidazolium tetrafluoroborate molten salt. J. Electrochem. Soc. 2001, 148, C102–C105. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds ME-FIL1-7 are available from the authors. |

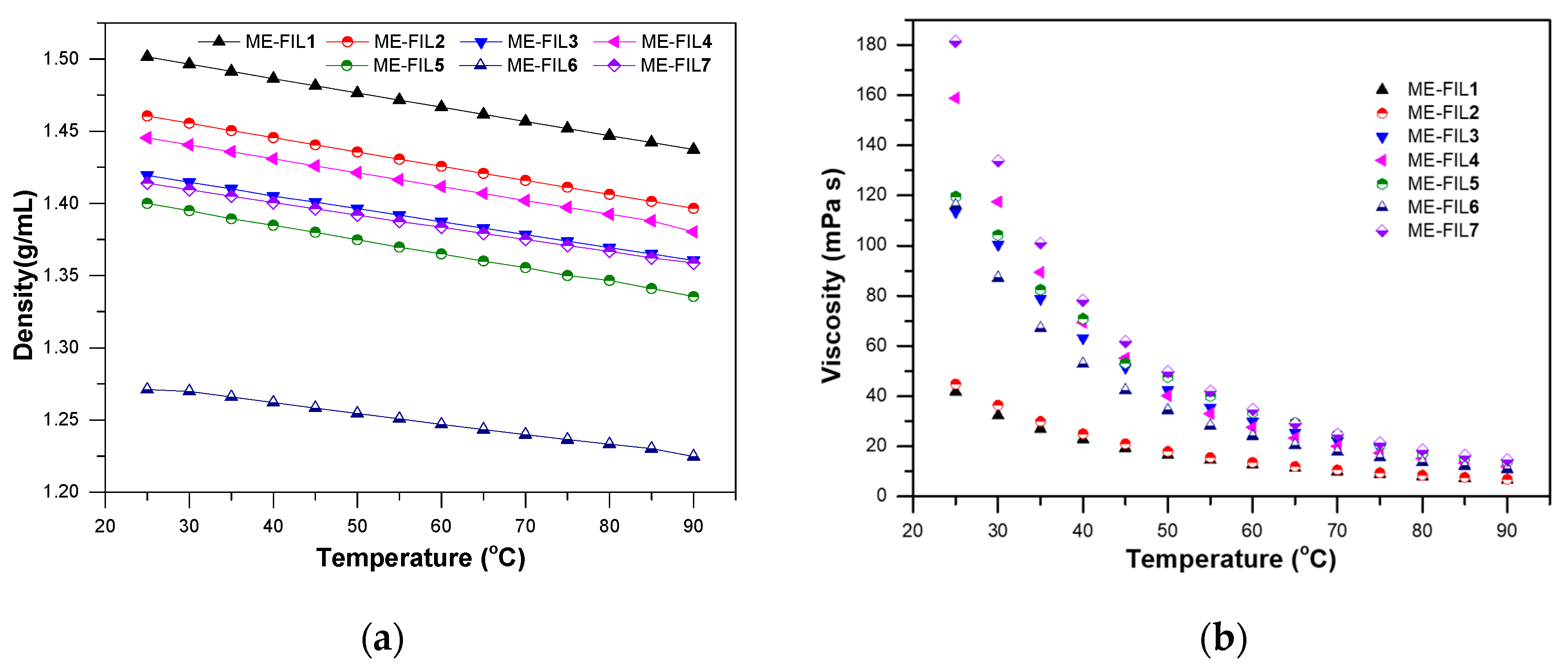
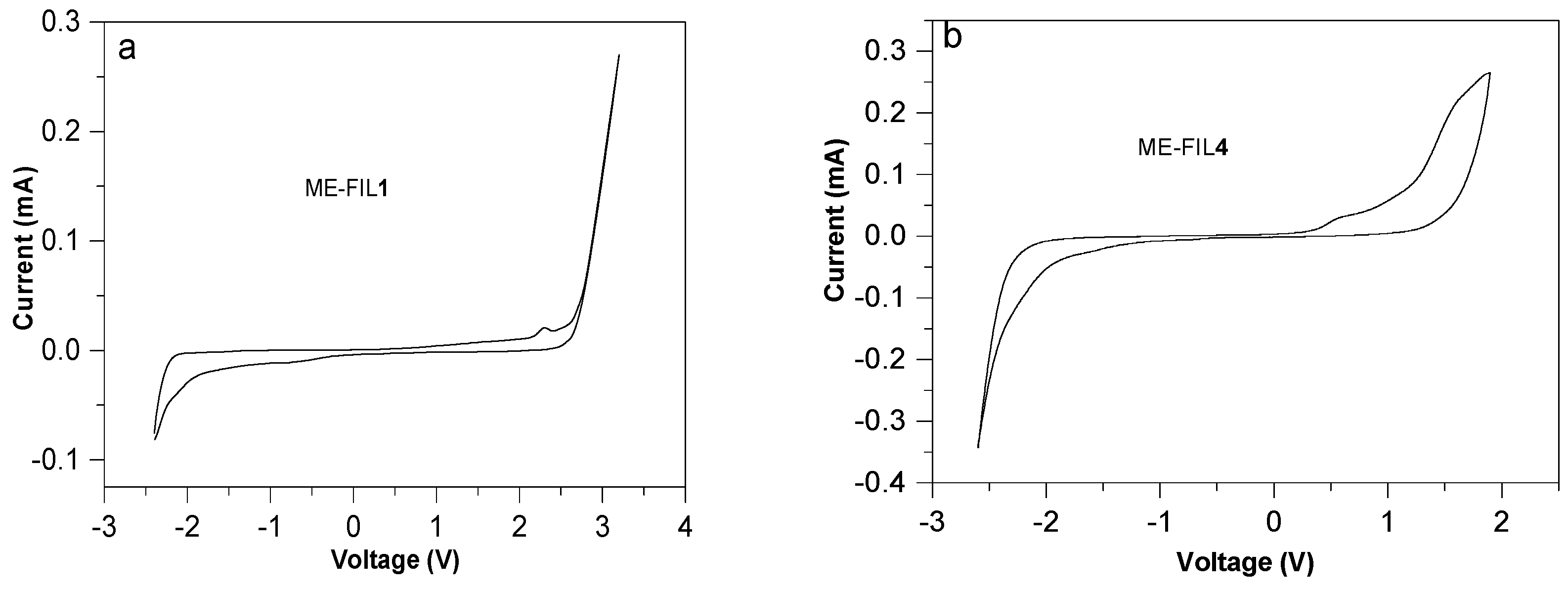
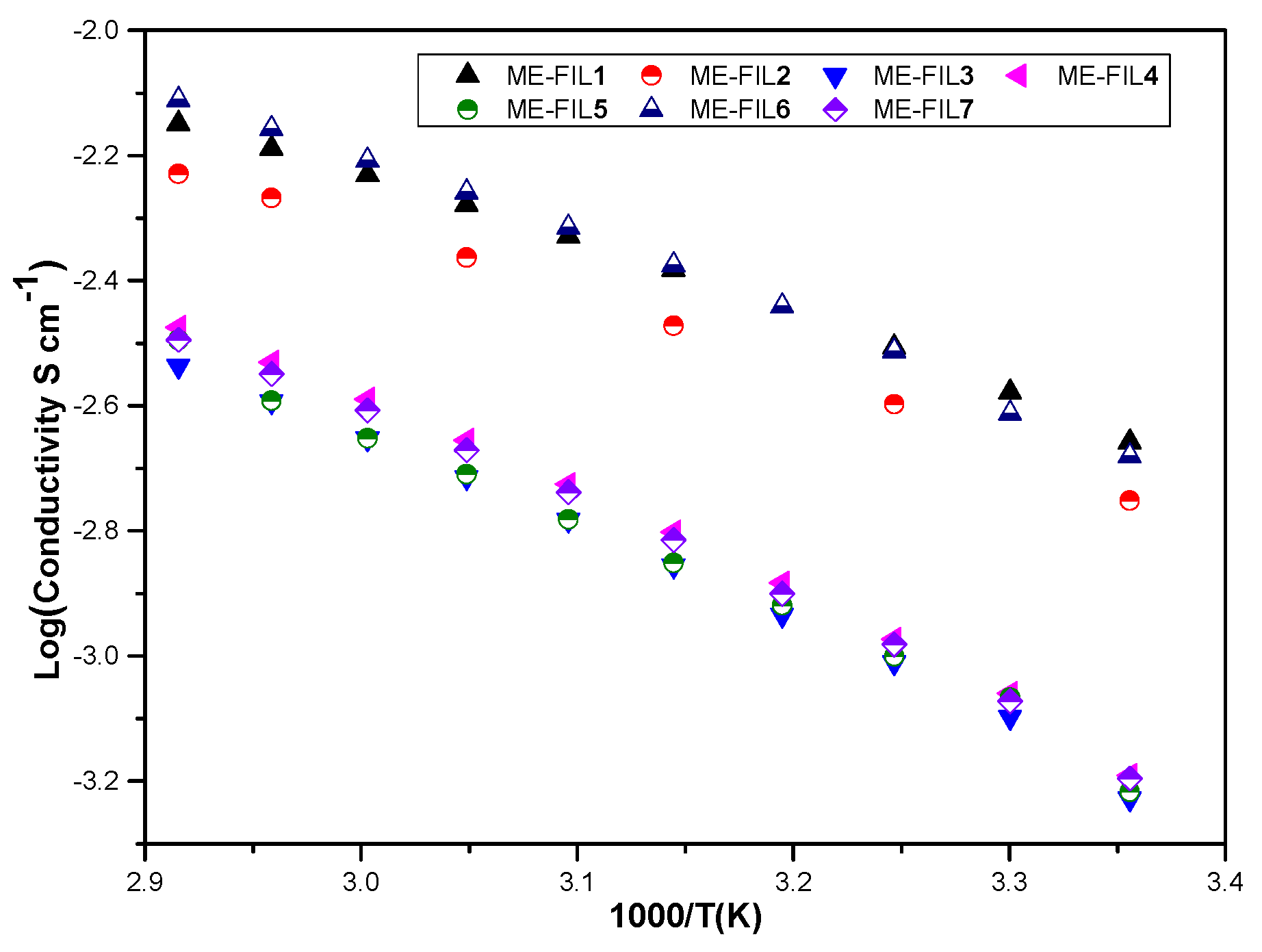
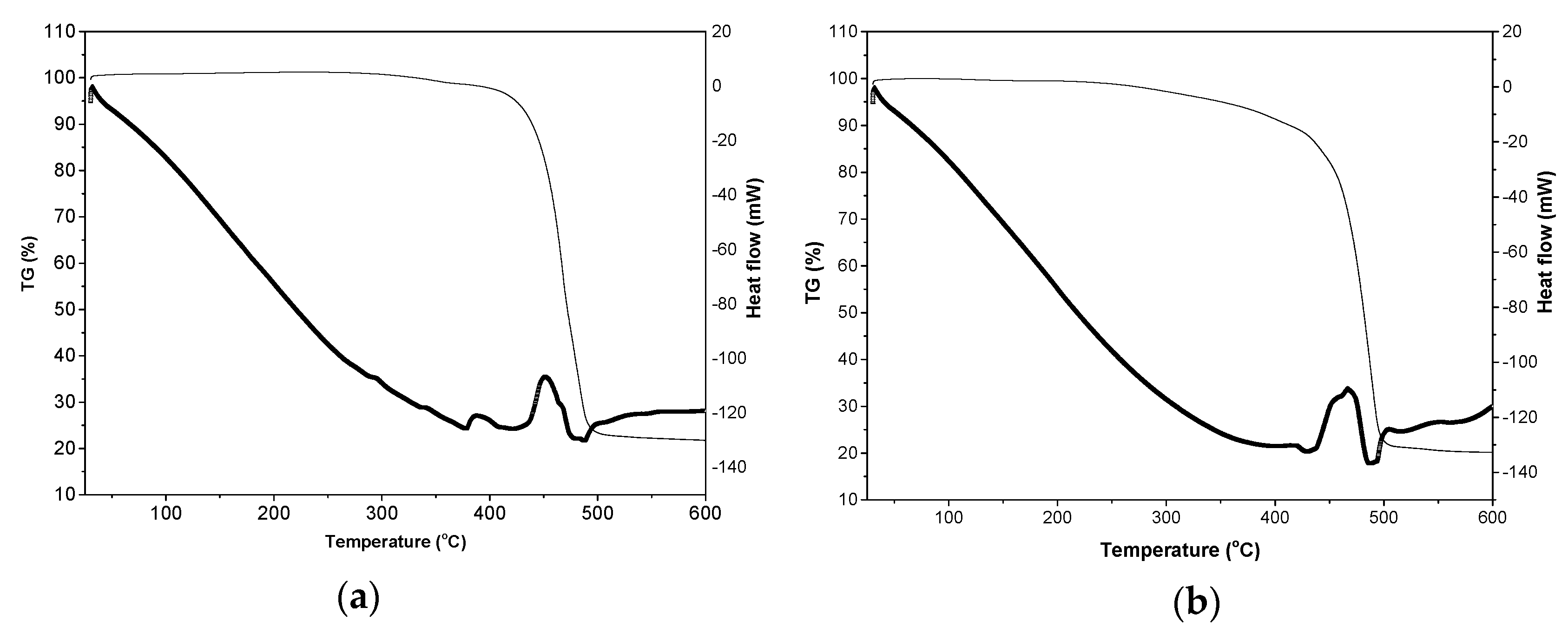
| ME-FIL | Density (g/mL) | Viscosity (cP) | Electrochemical Window (V) | Ionic Conductivity (S/m) |
|---|---|---|---|---|
| 1 | 1.501 | 41.8 | 4.7 | 2.6 × 10−1 |
| 2 | 1.459 | 44.9 | 5.0 | 2.2 × 10−1 |
| 3 | 1.420 | 113 | 3.9 | 7.3 × 10−2 |
| 4 | 1.445 | 158 | 4.8 | 5.9 × 10−2 |
| 5 | 1.400 | 109 | 4.8 | 6.4 × 10−2 |
| 6 | 1.270 | 116 | 4.5 | 2.1 × 10−1 |
| 7 | 1.413 | 181 | 4.6 | 6.4 × 10−2 |
| ME-FIL | Tda(°C) | Tccb(°C) | Tgc(°C)/ΔCpd (J/g/°C) | Tme (°C) |
|---|---|---|---|---|
| 1 | 441 | −81.53/0.15 | ||
| 2 | 430 | −81.25/0.24 | ||
| 3 | 307 | −78.64/0.77 | ||
| 4 | 410 | −68.73/1.0 | ||
| 5 | 298 | −76.18/0.89 | ||
| 6 | 269 | −75.61/0.18 | ||
| 7 | 330 | −21.75 | −74.58/0.16 | 7.95 |
| ILs | ME-FIL1 | ME-FIL2 | ME-FIL3 | ME-FIL4 | ME-FIL5 | [BMIm][BF4] b |
|---|---|---|---|---|---|---|
| Cp (J/g/°C) | 1.2 | 1.24 | 1.37 | 1.25 | 1.39 | 1.60 |
| Dha | 180.0 | 181.4 | 194.5 | 178.8 | 194.6 | 192.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, H.; Chen, L.; Wei, Z.; Lu, Y.; Peng, C.; Zhang, B.; Zhao, X.; Wu, L.; Wang, Y. Effect of Ionic Composition on Physicochemical Properties of Mono-Ether Functional Ionic Liquids. Molecules 2019, 24, 3112. https://doi.org/10.3390/molecules24173112
Zhou H, Chen L, Wei Z, Lu Y, Peng C, Zhang B, Zhao X, Wu L, Wang Y. Effect of Ionic Composition on Physicochemical Properties of Mono-Ether Functional Ionic Liquids. Molecules. 2019; 24(17):3112. https://doi.org/10.3390/molecules24173112
Chicago/Turabian StyleZhou, Hancheng, Lifei Chen, Zhuo Wei, Yongjuan Lu, Cheng Peng, Bin Zhang, Xiaojuan Zhao, Lan Wu, and Yanbin Wang. 2019. "Effect of Ionic Composition on Physicochemical Properties of Mono-Ether Functional Ionic Liquids" Molecules 24, no. 17: 3112. https://doi.org/10.3390/molecules24173112
APA StyleZhou, H., Chen, L., Wei, Z., Lu, Y., Peng, C., Zhang, B., Zhao, X., Wu, L., & Wang, Y. (2019). Effect of Ionic Composition on Physicochemical Properties of Mono-Ether Functional Ionic Liquids. Molecules, 24(17), 3112. https://doi.org/10.3390/molecules24173112





