A Kinetic Approach of DPPH Free Radical Assay of Ferulate-Based Protic Ionic Liquids (PILs)
Abstract
:1. Introduction
2. Results and Discussion
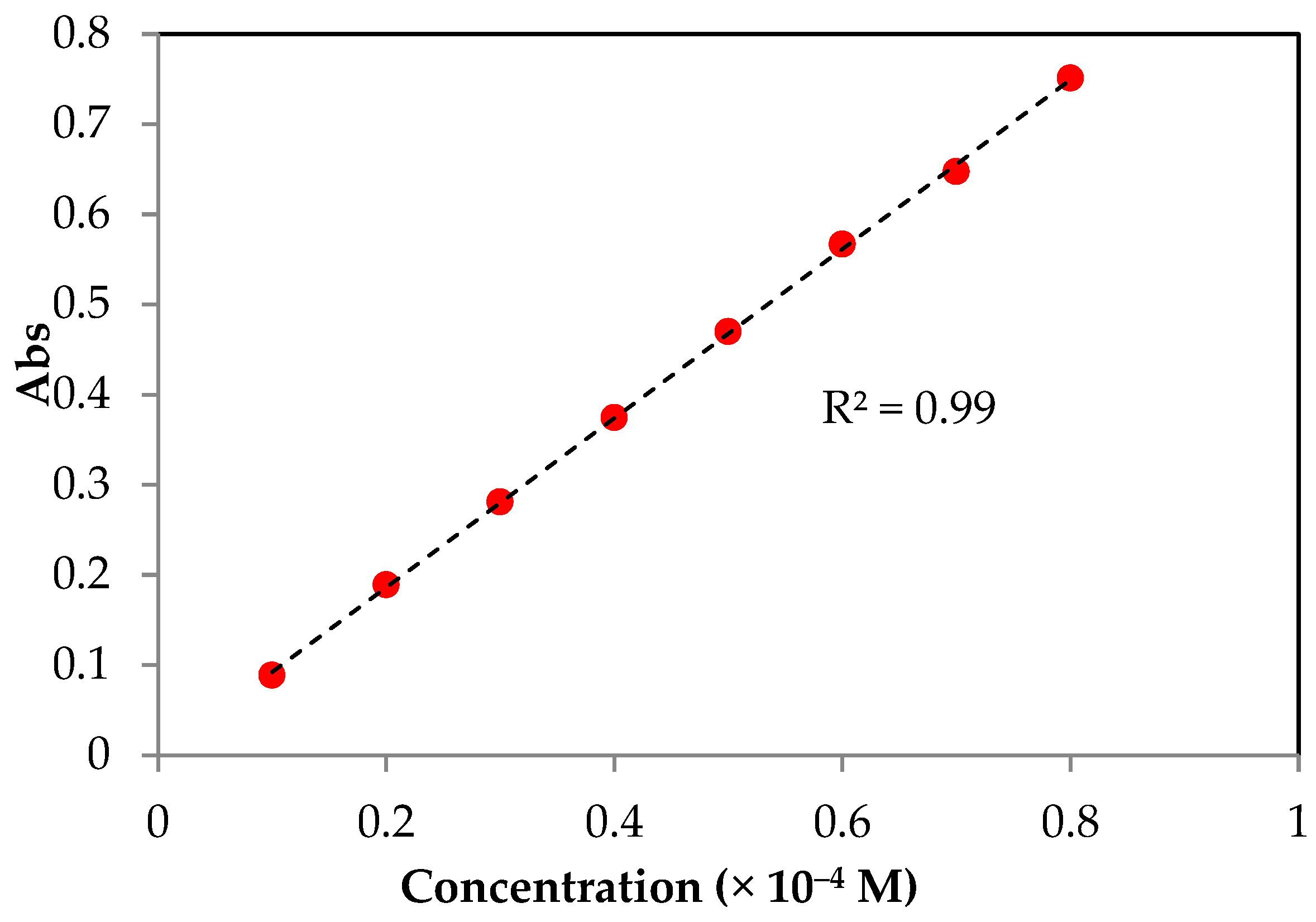
2.1. EC50
2.2. TEC50
2.3. Antiradical Efficiency (AE)
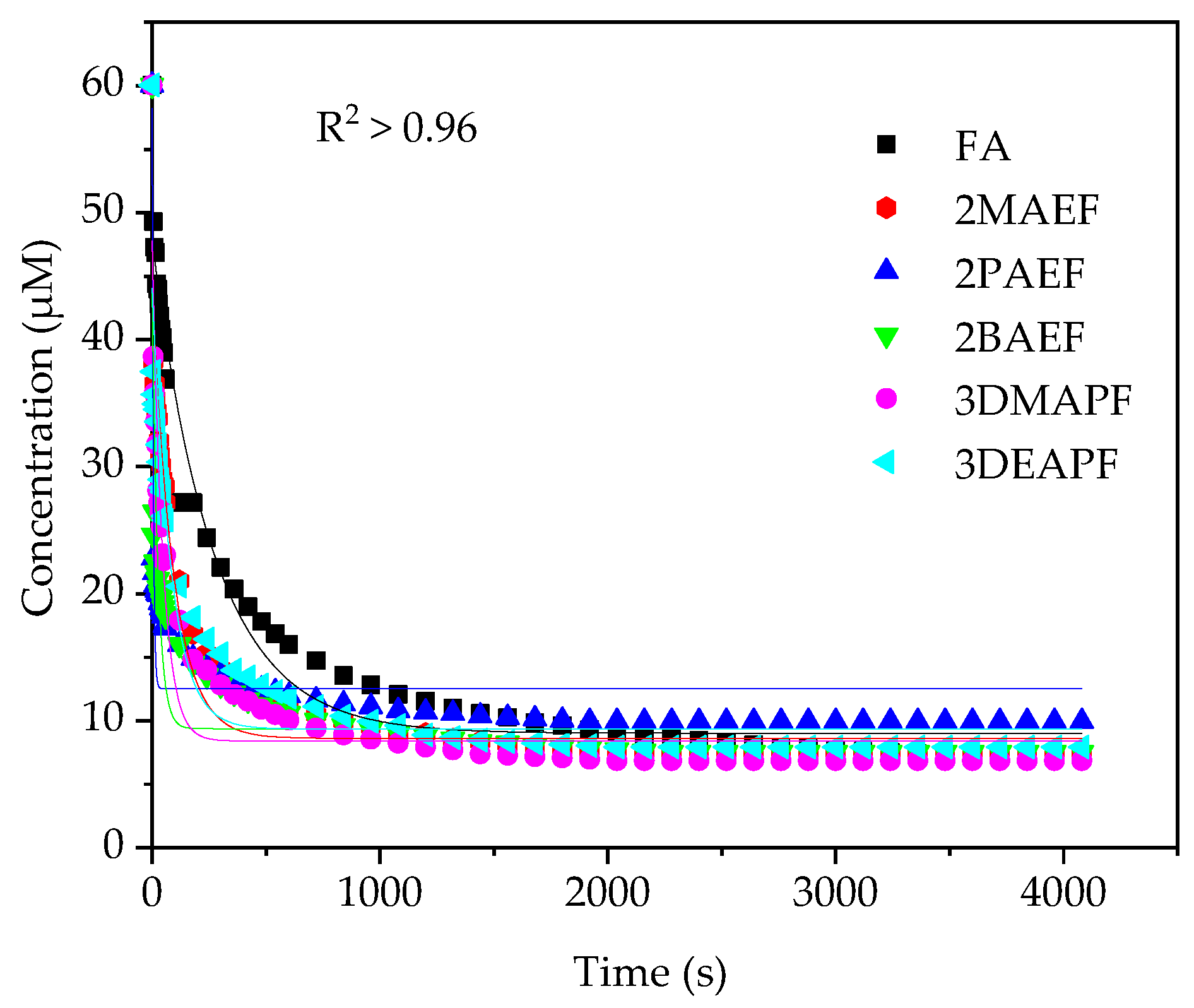
2.4. Stoichiometry of Reaction
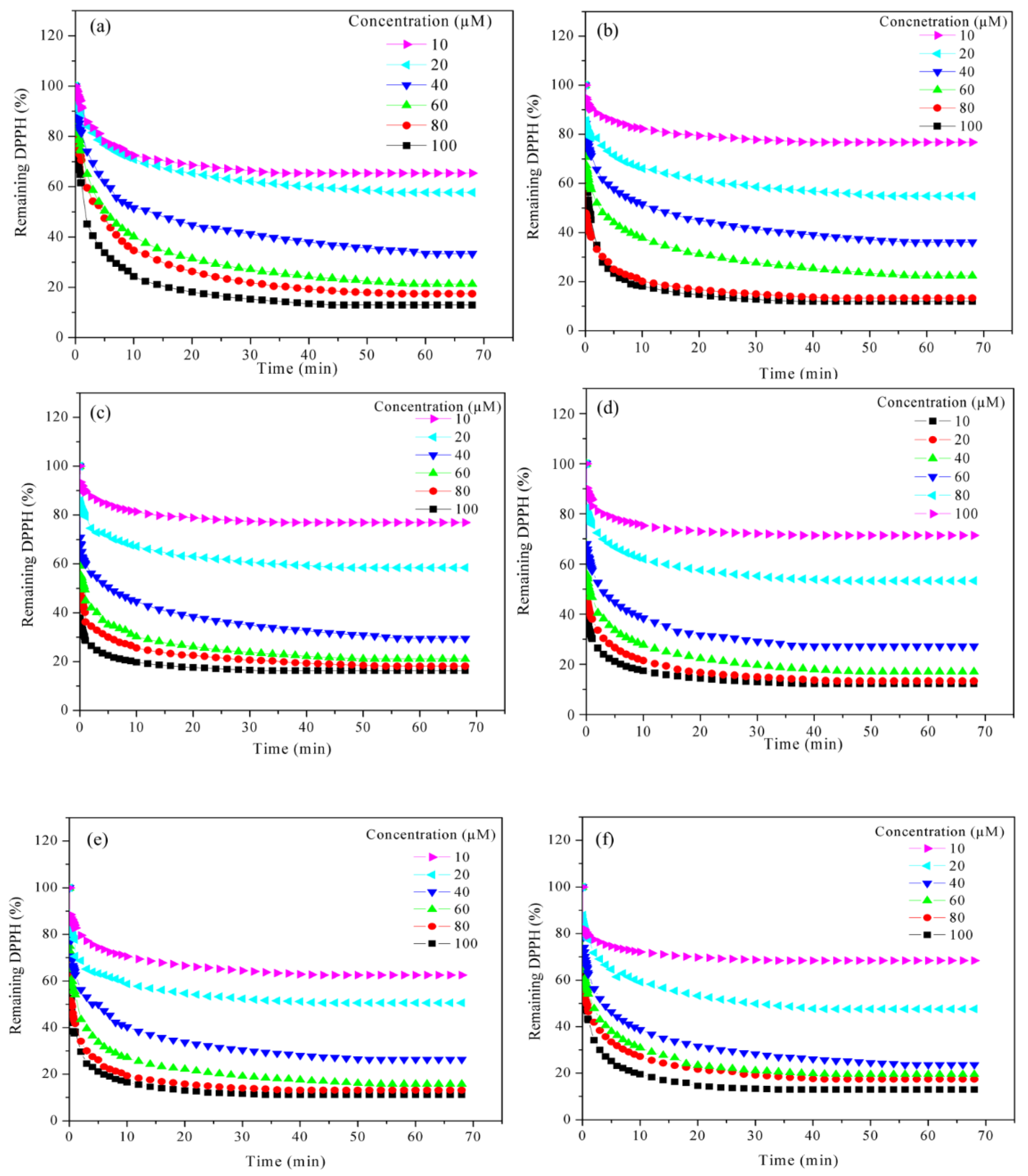
2.5. Kinetic Behaviour of PILs Towards DPPH•
3. Materials and Methods
3.1. Materials
3.2. Synthesis of PILs
3.3. Preparation of Stock Solution
3.4. DPPH free Radical Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bravo, L. Polyphenols: Chemistry, dietary sources, metabolism, and nutritional significance. Nutr. Rev. 1998, 56, 317–333. [Google Scholar] [CrossRef] [PubMed]
- Laura, A.; Alvarez-Parrilla, E.; Gonzalez-Aguilar, G.A. Fruit and Vegetable Phytochemicals: Chemistry, Nutritional Value and Stability, 1st ed.; John Wiley & Sons: Ames, IA, USA, 2009; pp. 336–340. [Google Scholar]
- Ghasemzadeh, A.; Ghasemzadeh, N. Flavonoids and phenolic acids: Role and biochemical activity in plants and human. J. Med. Plants Res. 2011, 5, 6697–6703. [Google Scholar] [CrossRef]
- Machu, L.M.; Ladislava, V.A.; Jarmila, O.; Jana, M.; Jiri, S.; Jiri, J. Phenolic content and antioxidant capacity in algal food products. Molecules 2015, 20, 1118–1133. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects-A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- De los Ángeles Fernández, M.; Espino, M.; Gomez, F.J.; Silva, M.F. Novel approaches mediated by tailor-made green solvents for the extraction of phenolic compounds from agro-food industrial by-products. Food Chem. 2018, 239, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Guo, Z.; Xu, X. Enzymatic lipophilisation of phenolic acids through esterification with fatty alcohols in organic solvents. Food Chem. 2012, 132, 1311–1315. [Google Scholar] [CrossRef]
- Uysal, A.; Lazarova, I.; Zengin, G.; Gunes, Z.; Aktumsek, A.; Gevrenova, R. New Perspectives on Asphodeline lutea from Bulgaria and Turkey: Anti-mutagenic, Anti-microbial and Anti-methicillin Resistant Staphylococcus aureus (MRSA) Activity. Br. J. Pharmaceut. Res. 2016, 10, 1–10. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Tayeboon, G.S.; Miri, A.; Setzer, W.N.; Falah, F.; Kuhestani, K.; Tahanzadeh, N. Mutagenic, antimutagenic, antioxidant, anti-lipoxygenase and antimicrobial activities of Scandix pecten-veneris. Cell. Mol. Biol. 2016, 62, 8–16. [Google Scholar]
- Spilioti, E.; Jaakkola, M.; Tolonen, T.L.; Maija, V.; Vesa, C.; Ioanna, K.; Eva, K.; Sofia, M. Phenolic acid composition, antiatherogenic and anticancer potential of honeys derived from various regions in Greece. PLoS ONE 2014, 9, 94860. [Google Scholar] [CrossRef]
- Daniel, M. Medicinal Plants: Chemistry and Properties; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Robbins, R.J. Phenolic acids in foods: An overview of analytical methodology. J. Agric. Food Chem. 2003, 51, 2866–2887. [Google Scholar] [CrossRef]
- Kikugawa, M.; Tomoaki, I.; Hideshi, S. Ferulic acid and its water-soluble derivatives inhibit nitric oxide production and inducible nitric oxide synthase expression in rat primary astrocytes. Biosci. Biotechnol. Biochem. 2017, 81, 1607–1611. [Google Scholar] [CrossRef] [PubMed]
- Ramawat, K.G.; Mérillon, J.M. Phytochemistry, Botany and Metabolism of Alkaloids, Phenolics and Terpenes, Natural products; Springer: Berlin, Germany, 2013. [Google Scholar]
- Li, C.; Li, J.B. Preparation of chitosan-ferulic acid conjugate: Structure characterization and in the application of pharmaceuticals. Int. J. Biol. Macromol. 2017, 105, 1539–1543. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Pruthi, V. Potential applications of ferulic acid from natural sources. Biotechnol. Rep. 2014, 4, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Boz, H. Ferulic Acid in Cereals-A Review. Czech J. Food Sci. 2015, 33, 1–7. [Google Scholar] [CrossRef]
- Nankar, R.; Prabhakar, P.; Doble, M. Hybrid drug combination: Combination of ferulic acid and metformin as anti-diabetic therapy. Phytomedicine 2017, 37, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Piotrowski, J.S.; Lu, F.; Kabbage, M.; Ralph, J.; Landick, R.C. Antimicrobial Ferulic Acid Derivatives and Uses Thereof. U.S. Patents 20150313221A1, 2 May 2014. [Google Scholar]
- Winkler-Moser, J.K.; Hwang, H.S.; Bakota, E.P.; Debra, A. Synthesis of steryl ferulates with various sterol structures and comparison of their antioxidant activity. Food Chem. 2015, 169, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.N.; Bristi, N.J.; Rafiquzzaman, M. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm. J. 2013, 21, 143–152. [Google Scholar] [CrossRef]
- Apak, M.R.; Guclu, K.; Ozyurek, M.; Bener, M.; Martinez, E.; Callewaert, D. Optical Sensor-Based Cupric Reducing Antioxidant Capacity (CUPRAC) Assay. U.S. Patents 8912004B2, 24 April 2011. [Google Scholar]
- Ioannone, F.; Di Mattia, C.D.; De Gregorio, M.; Sergi, M.; Serafini, M.; Sacchetti, G. Flavanols, proanthocyanidins and antioxidant activity changes during cocoa (Theobroma cacao L.) roasting as affected by temperature and time of processing. Food Chem. 2015, 174, 256–262. [Google Scholar] [CrossRef]
- Watanabe, M.; Watanabe, T.; Watanabe, H. Method for Producing Oyster Meat Essence Containing Large Amount of Antioxidants Having High Antioxidative Power and High ORAC Value. U.S. Patents 9943553B2, 11 April 2011. [Google Scholar]
- Niki, E. Assessment of antioxidant capacity in vitro and in vivo. Free Radical Biol. Med. 2010, 49, 503–515. [Google Scholar] [CrossRef]
- Nenadis, N.; Tsimidou, M. Observations on the estimation of scavenging activity of phenolic compounds using rapid 1, 1-diphenyl-2-picrylhydrazyl (DPPH•) tests. J. Am. Oil Chem. Soc. 2002, 79, 1191. [Google Scholar] [CrossRef]
- Rajan, V.K.; Muraleedharan, K. A computational investigation on the structure, global parameters and antioxidant capacity of a polyphenol, Gallic acid. Food Chem. 2017, 220, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Marxen, K.; Vanselow, K.H.; Lippemeier, S.; Hintze, R.; Ruser, A.; Hansen, U.P. Determination of DPPH radical oxidation caused by methanolic extracts of some microalgal species by linear regression analysis of spectrophotometric measurements. Sensors 2007, 7, 2080–2095. [Google Scholar] [CrossRef] [PubMed]
- Diplock, A.T.; Charuleux, J.L.; Crozier-Willi, G.; Kok, F.J.; Rice-Evans, C.; Roberfroid; Marcel Stahl, W.; Vina-Ribes, J. Functional food science and defence against reactive oxidative species. Br. J. Nutr. 1998, 80, S77–S112. [Google Scholar] [CrossRef] [PubMed]
- Terpinc, P.; Abramovič, H. A kinetic approach for evaluation of the antioxidant activity of selected phenolic acids. Food Chem. 2010, 121, 366–371. [Google Scholar] [CrossRef]
- Apak, R.; Özyürek, M.; Güçlü, K.; Çapanoğlu, E. Antioxidant activity/capacity measurement 2 Hydrogen atom transfer (HAT)-based, mixed-mode (electron transfer (ET)/HAT), and lipid peroxidation assays. J. Agric. Food Chem. 2016, 64, 1028–1045. [Google Scholar] [CrossRef] [PubMed]
- Heravi, M.M.; Haghi, B.; Morsali, A.; Ardalan, P.; Ardalan, T. Kinetic study of DPPH scavenging in the presence of mixture of Zinc and Vitamin C as an antioxidant. J. Chem. Health Risks 2012, 2, 6. [Google Scholar]
- Sekhon, B.S. Ionic liquids based active pharmaceutical ingredients. Ars Pharm. 2013, 54, 37–44. [Google Scholar]
- Czerniak, K.; Biedziak, A.; Krawczyk, K.; Pernak, J. Synthesis and properties of gallate ionic liquids. Tetrahedron 2016, 72, 7409–7416. [Google Scholar] [CrossRef]
- Czerniak, K.; Walkiewicz, F. Synthesis and antioxidant properties of dicationic ionic liquids. New J. Chem. 2017, 41, 530–539. [Google Scholar] [CrossRef]
- Klejdysz, T.; Łęgosz, B.; Czuryszkiewicz, D.; Czerniak, K.; Pernak, J. Biobased Ionic Liquids with Abietate Anion. ACS Sustainable Chem. Eng. 2016, 4, 6543–6550. [Google Scholar] [CrossRef]
- Ali, H.M.; Almagribi, W.; Al-Rashidi, M.N. Antiradical and reductant activities of anthocyanidins and anthocyanins, structure–activity relationship and synthesis. Food Chem. 2016, 194, 1275–1282. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Nie, G.; Belton, P.S.; Tang, H.; Zhao, B. Structure-activity relationship analysis of antioxidant ability and neuroprotective effect of gallic acid derivatives. Neurochem. Int. 2006, 48, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Ancerewicz, J.; Migliavacca, E.; Carrupt, P.A.; Testa, B.; Brée, F.; Zini, R.; Tillement, J.P.; Labidalle, S.; Guyot, D.; Chauvet, M.; Anne, M. Structure-property relationships of trimetazidine derivatives and model compounds as potential antioxidants. Free Radicals Biol. Med. 1998, 25, 113–120. [Google Scholar] [CrossRef]
- Villano, D.; Fernández-Pachón, M.S.; Moyá, M.L.; Troncoso, A.M.; García-Parrilla, M.C. Radical scavenging ability of polyphenolic compounds towards DPPH free radical. Talanta 2007, 71, 230–235. [Google Scholar] [CrossRef]
- Mishra, K.; Ojha, H.; Chaudhury, N.K. Estimation of antiradical properties of antioxidants using DPPH assay: A critical review and results. Food Chem. 2012, 130, 1036–1043. [Google Scholar] [CrossRef]
- Tokuda, H.; Ishii, K.; Susan, M.A.B.H.; Tsuzuki, S.; Hayamizu, K.; Watanabe, M. Physicochemical properties and structures of room-temperature ionic liquids. 3. Variation of cationic structures. J. Phys. Chem. B 2006, 110, 2833–2839. [Google Scholar] [CrossRef] [PubMed]
- Tsuzuki, S. Factors controlling the diffusion of ions in ionic liquids. ChemPhysChem 2012, 13, 1664–1670. [Google Scholar] [CrossRef] [PubMed]
- Apak, R.; Capanoglu, E.; Shahidi, F. Measurement of Antioxidant Activity and Capacity: Recent Trends and Applications, 3rd ed.; John Wiley & Sons: Chichester, UK, 2017. [Google Scholar]
- Sanchez, M.C.; Larrauri, J.; Saura, C. A procedure to measure the antiradical efficiency of polyphenols. J. Sci. Food Agric. 1998, 76, 270–276. [Google Scholar] [CrossRef]
- Xie, J.; Schaich, K. Re-evaluation of the 2, 2-diphenyl-1-picrylhydrazyl free radical (DPPH) assay for antioxidant activity. J. Sci. Food Agric. 2014, 62, 4251–4260. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Bendary, E.; Francis, R.R.; Ali, H.M.G.; Sarwat, M.I.; El Hady, S. Antioxidant and structure–activity relationships (SARs) of some phenolic and anilines compounds. Annals Agric. Sci. 2013, 58, 173–181. [Google Scholar] [CrossRef]
- Klein, E.; Matis, M.; Lukeš, V.; Cibulková, Z. The applicability of AM1 and PM3 semi-empirical methods for the study of N–H bond dissociation enthalpies and ionisation potentials of amine type antioxidants. Polym. Degrad. Stab. 2006, 91, 262–270. [Google Scholar] [CrossRef]
- Lalevée, J.; Allonas, X.; Fouassier, J.P. N-H and α (C-H) bond dissociation enthalpies of aliphatic amines. J. Am. Chem. Soc. 2002, 124, 9613–9621. [Google Scholar] [CrossRef] [PubMed]
- Joshi, R.; Ghanty, T.K.; Mukherjee, T. Substituent effect on ionization potential, O-H bond dissociation energy and intra-molecular hydrogen bonding in salicylic acid derivatives. J. Mol. Struct. THEOCHEM 2010, 948, 47–54. [Google Scholar] [CrossRef]
- Foti, M.C.; Daquino, C.; Geraci, C. Geraci, Electron-transfer reaction of cinnamic acids and their methyl esters with the DPPH• radical in alcoholic solutions. J. Org. Chem. 2004, 69, 2309–2314. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds ferulate- based PILs are available from the authors. |

| Sample | EC50 (µM) | TEC50 | Antiradical Efficiency, AE (×10−3) | Reference |
|---|---|---|---|---|
| FA | 24.7 ± 0.40 | 53 | 0.80 ± 0.00 | [40] |
| FA | 26.0 ± 0.10 | 56 | 0.70 ± 0.05 | This work |
| 2MAEF | 25.0 ± 0.20 | 53 | 0.75 ± 0.15 | This work |
| 2PAEF | 24.3 ± 0.14 | 50 | 0.82 ± 0.05 | This work |
| 2BAEF | 22.0 ± 0.17 | 45 | 1.01 ± 0.08 | This work |
| 3DMAPF | 20.4 ± 0.05 | 44 | 1.11 ± 0.03 | This work |
| 3DEAPF | 18.4 ± 0.12 | 42 | 1.29 ± 0.07 | This work |
| Classification | Value AE |
|---|---|
| Low | AE ≤ 1.0 × 10−3 |
| Medium | 1.0 × 10−3 < AE ≤ 5.0 × 10−3 |
| High | 5.0 × 10−3 < AE ≤ 10.0 × 10−3 |
| Very high | AE > 10.0 × 10−3 |
| Sample | EC50 (µmol of Antioxidant/ µmol of DPPH) | Stoichiometric Factor (n) 1 |
|---|---|---|
| FA | 0.43 (0.42 2) | 1.20 |
| 2MAEF | 0.41 | 1.22 |
| 2PAEF | 0.40 | 1.25 |
| 2BAEF | 0.36 | 1.39 |
| 3DMAPF | 0.33 | 1.52 |
| 3DEAPF | 0.30 | 1.67 |
| Sample | Stoichiometric Factor (n) | k2 (M−1 s−1) |
|---|---|---|
| FA | 1.16 | 109.48 |
| 2MAEF | 1.22 | 86.89 |
| 2PAEF | 1.25 | 107.20 |
| 2BAEF | 1.39 | 164.17 |
| 3DMAPF | 1.51 | 242.84 |
| 3DEAPF | 1.67 | 244.73 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, N.A.; Jumbri, K.; Ramli, A.; Abd Ghani, N.; Ahmad, H.; Lim, J.W. A Kinetic Approach of DPPH Free Radical Assay of Ferulate-Based Protic Ionic Liquids (PILs). Molecules 2018, 23, 3201. https://doi.org/10.3390/molecules23123201
Ahmad NA, Jumbri K, Ramli A, Abd Ghani N, Ahmad H, Lim JW. A Kinetic Approach of DPPH Free Radical Assay of Ferulate-Based Protic Ionic Liquids (PILs). Molecules. 2018; 23(12):3201. https://doi.org/10.3390/molecules23123201
Chicago/Turabian StyleAhmad, Nur Afiqah, Khairulazhar Jumbri, Anita Ramli, Noraini Abd Ghani, Haslina Ahmad, and Jun Wei Lim. 2018. "A Kinetic Approach of DPPH Free Radical Assay of Ferulate-Based Protic Ionic Liquids (PILs)" Molecules 23, no. 12: 3201. https://doi.org/10.3390/molecules23123201
APA StyleAhmad, N. A., Jumbri, K., Ramli, A., Abd Ghani, N., Ahmad, H., & Lim, J. W. (2018). A Kinetic Approach of DPPH Free Radical Assay of Ferulate-Based Protic Ionic Liquids (PILs). Molecules, 23(12), 3201. https://doi.org/10.3390/molecules23123201