Characterization of Phenolic Compounds and Antiproliferative Effects of Salvia pomifera and Salvia fruticosa Extracts
Abstract
1. Introduction
2. Results
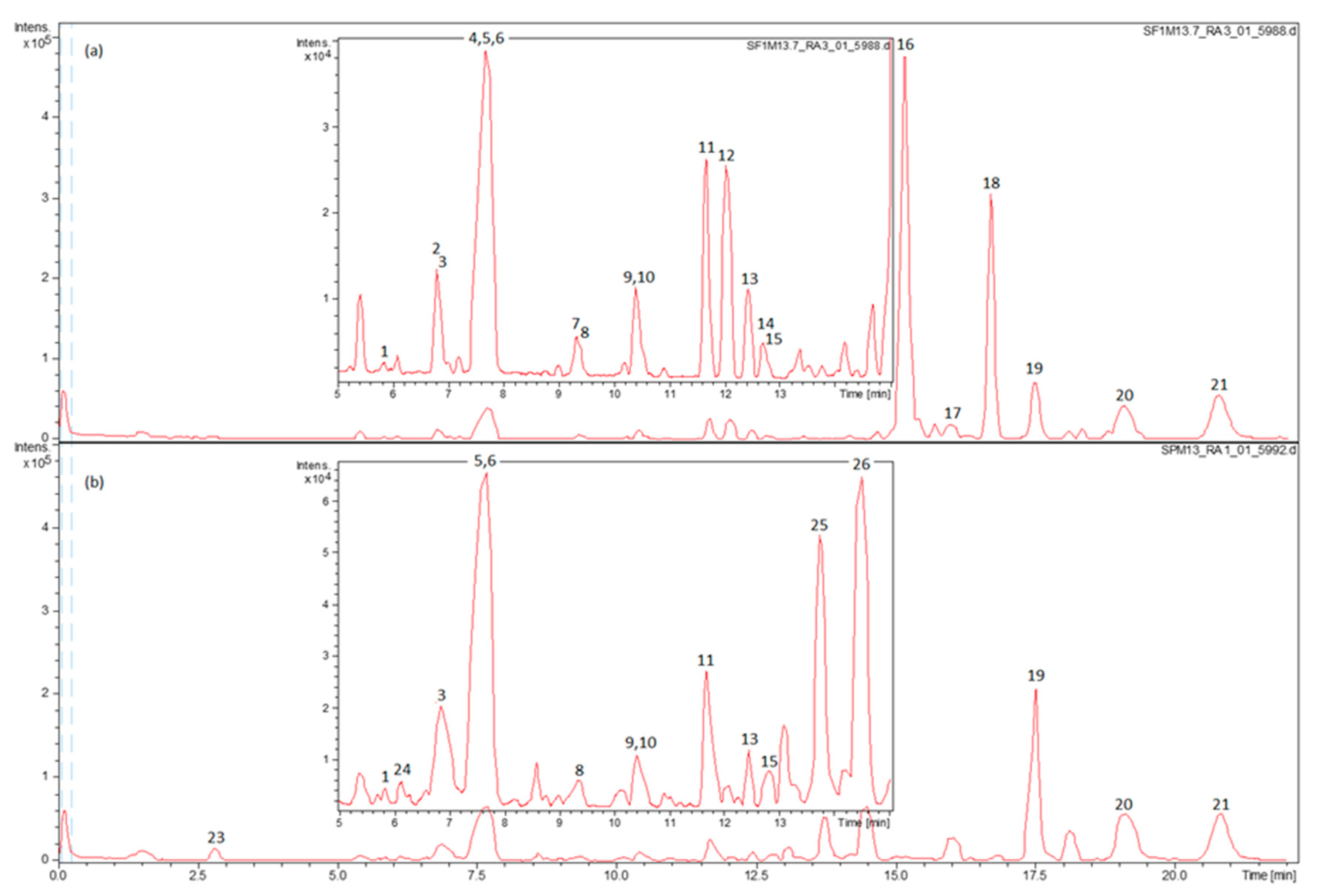
2.1. Identification of Phenolic Compounds in SF and SP by HPLC–ESI-QTOF-MS
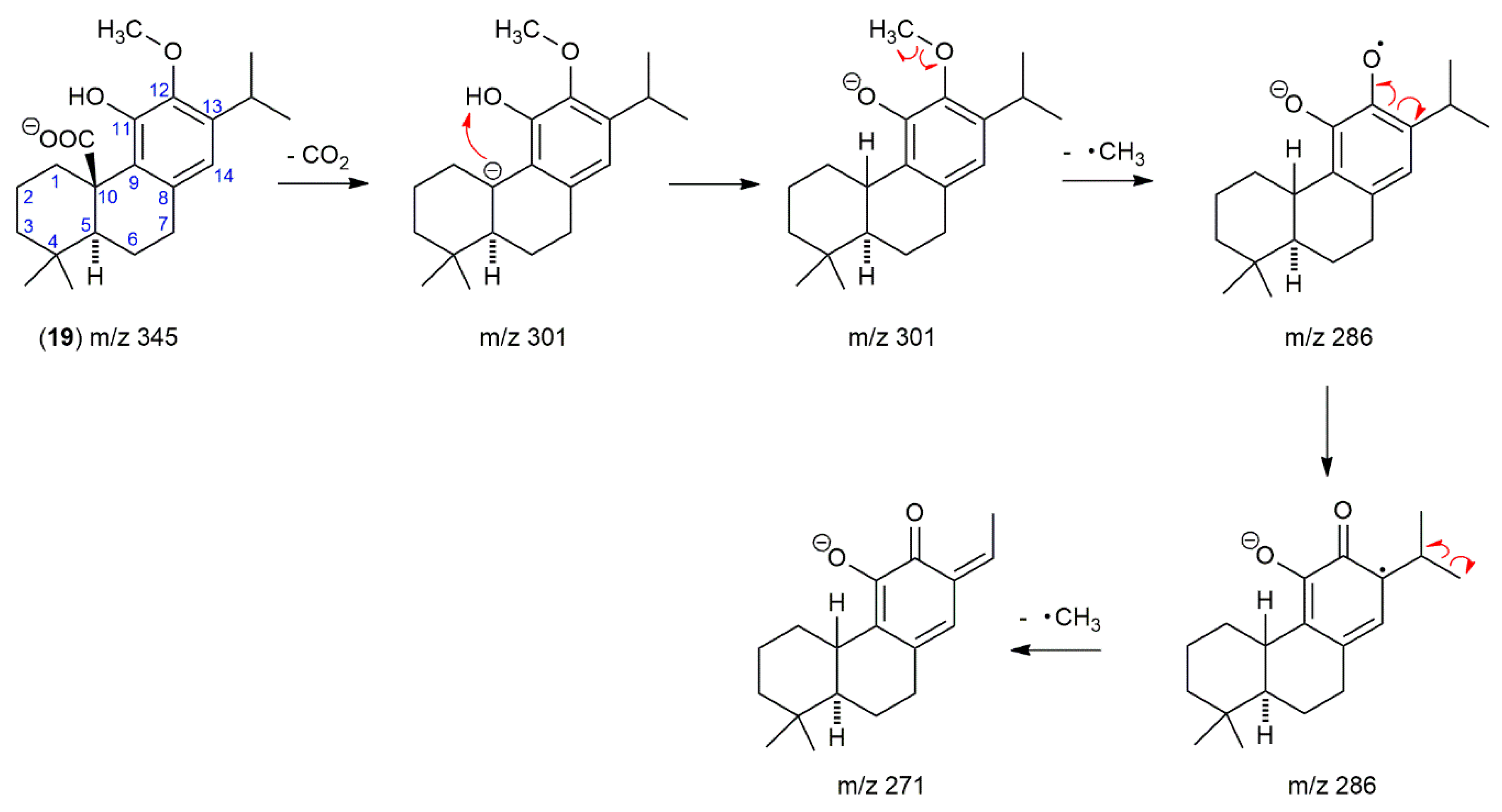
2.2. The Presence of 12-O-Methylcarnosic Acid in SF and SP Is Proved by Its Fragmentation Pathway
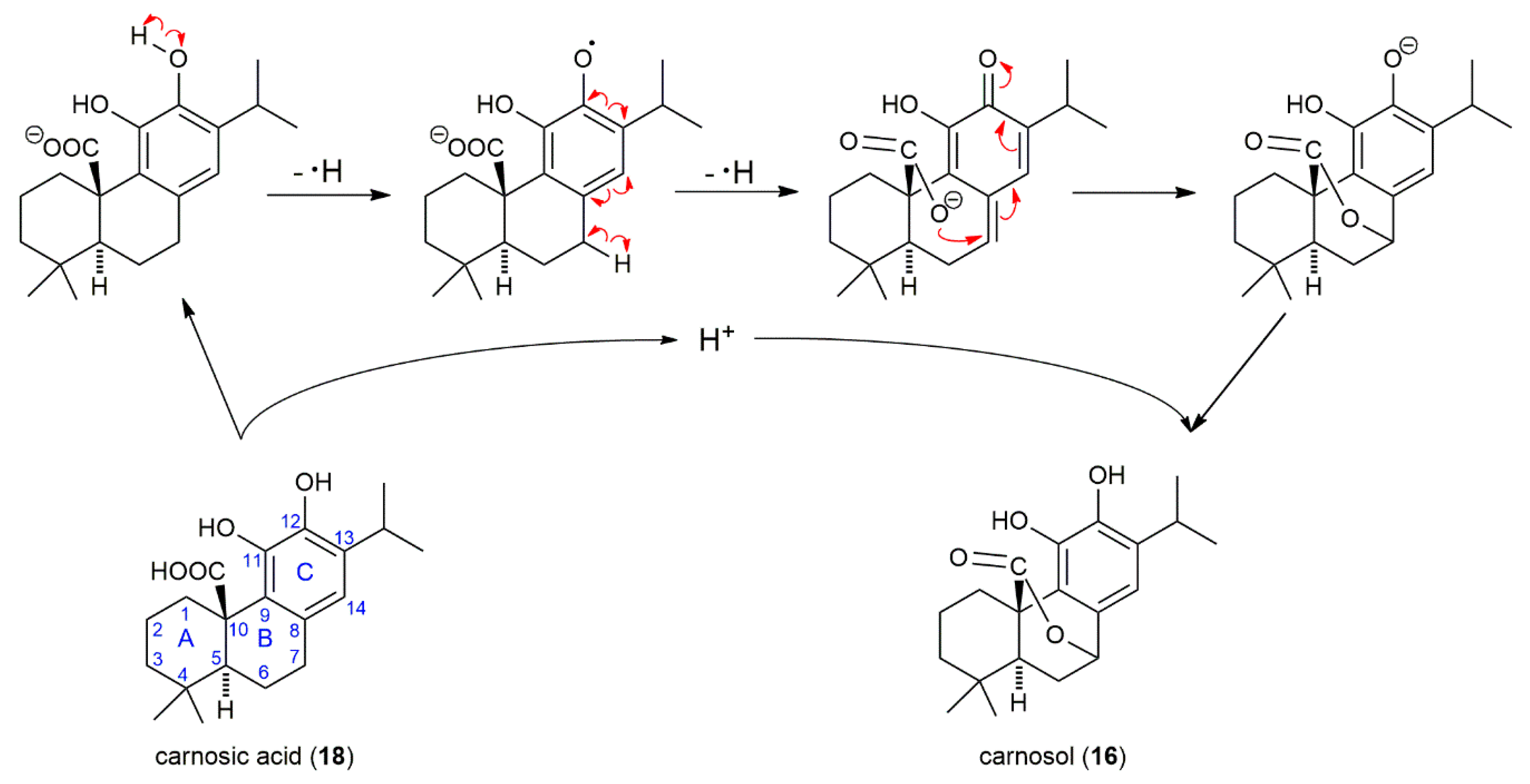
2.3. The Main Phenolic Substance of SP Is 12-O-Methylcarnosic Acid
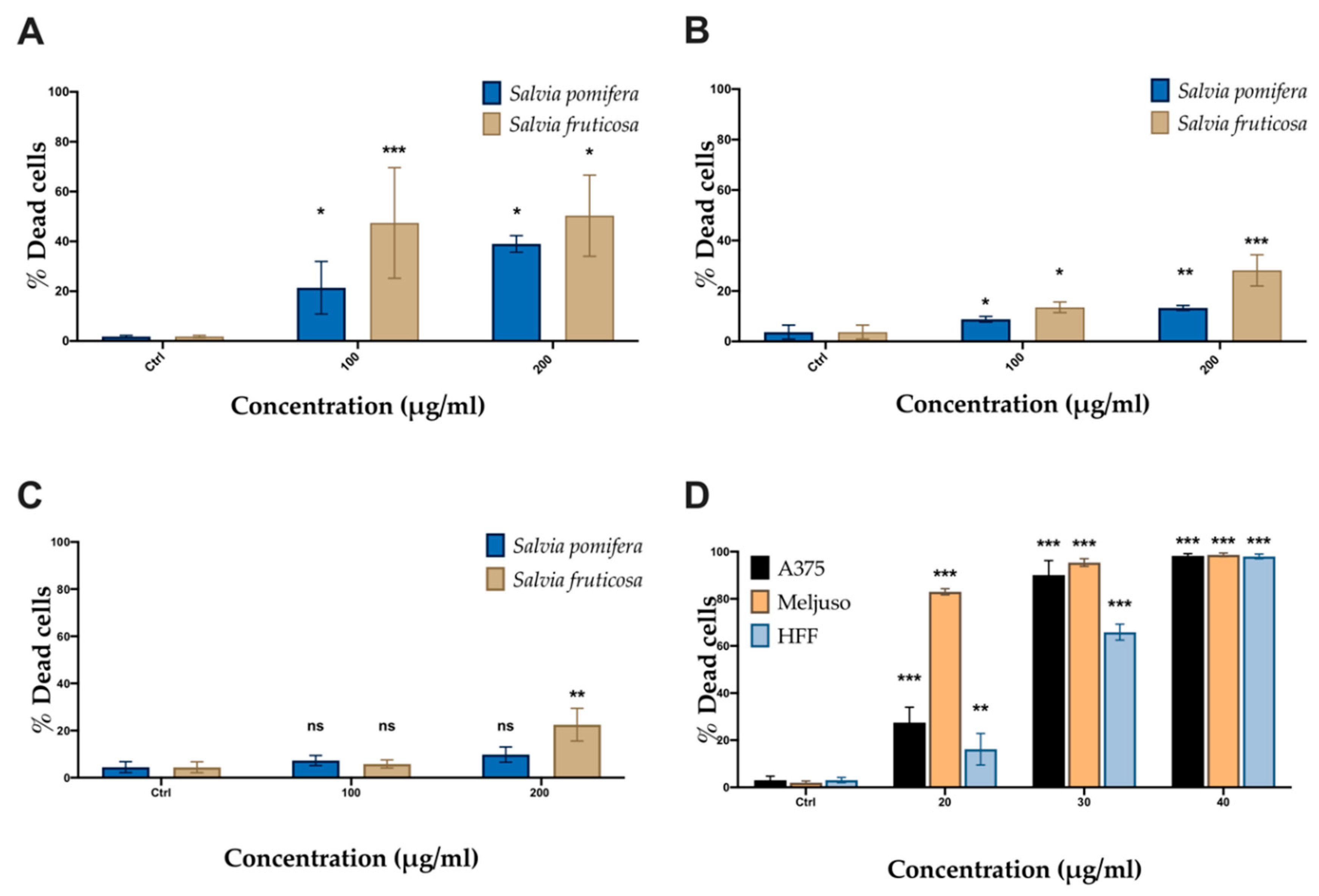
2.4. SP and SF Extracts and CA Affect Cell Viability
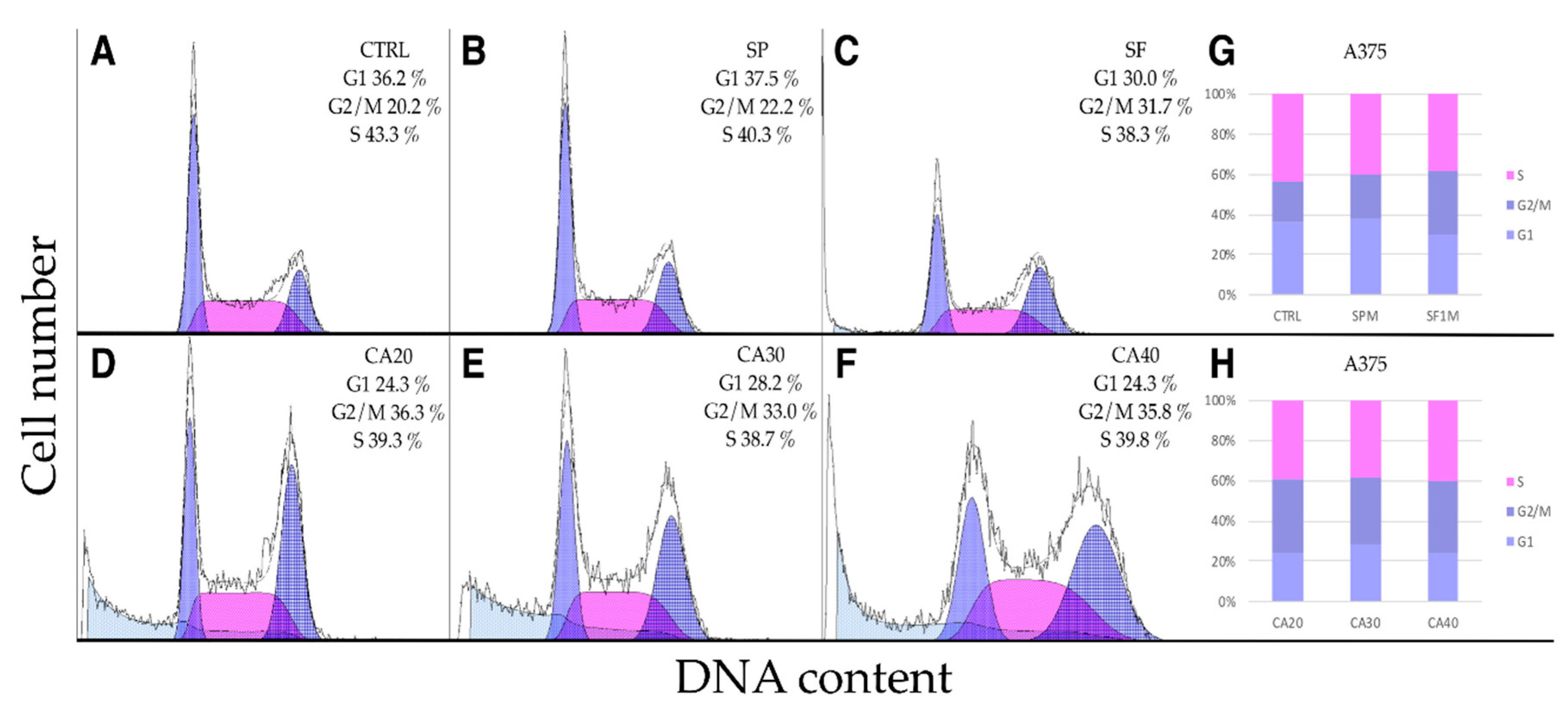
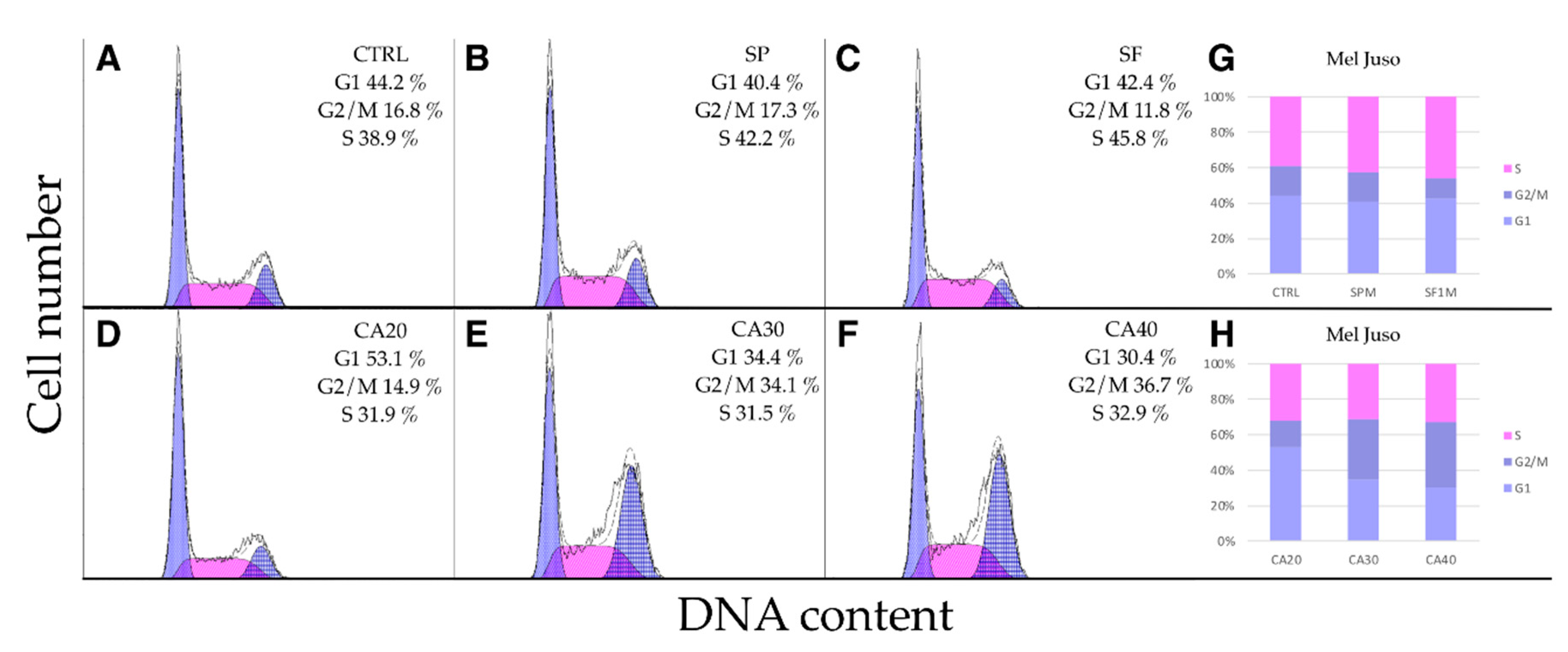
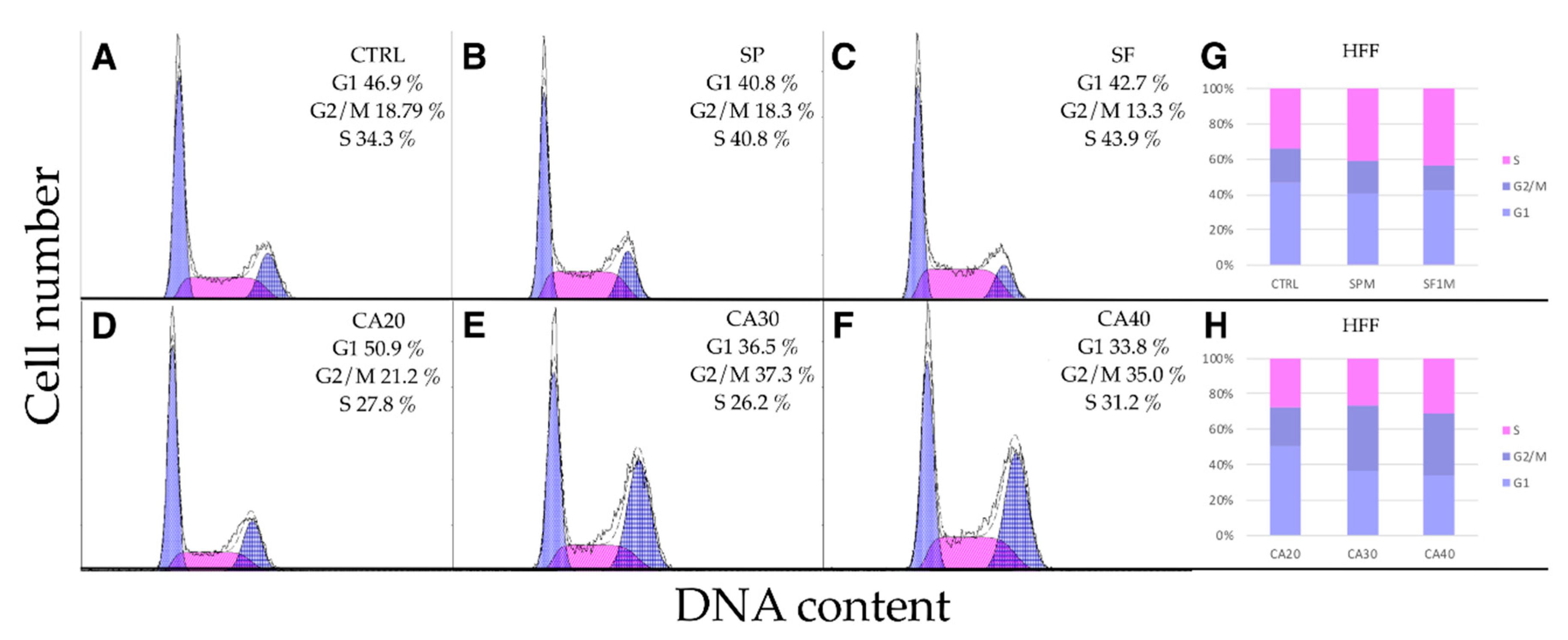
2.5. SP, SF Extracts and CA Arrest Cell Cycle
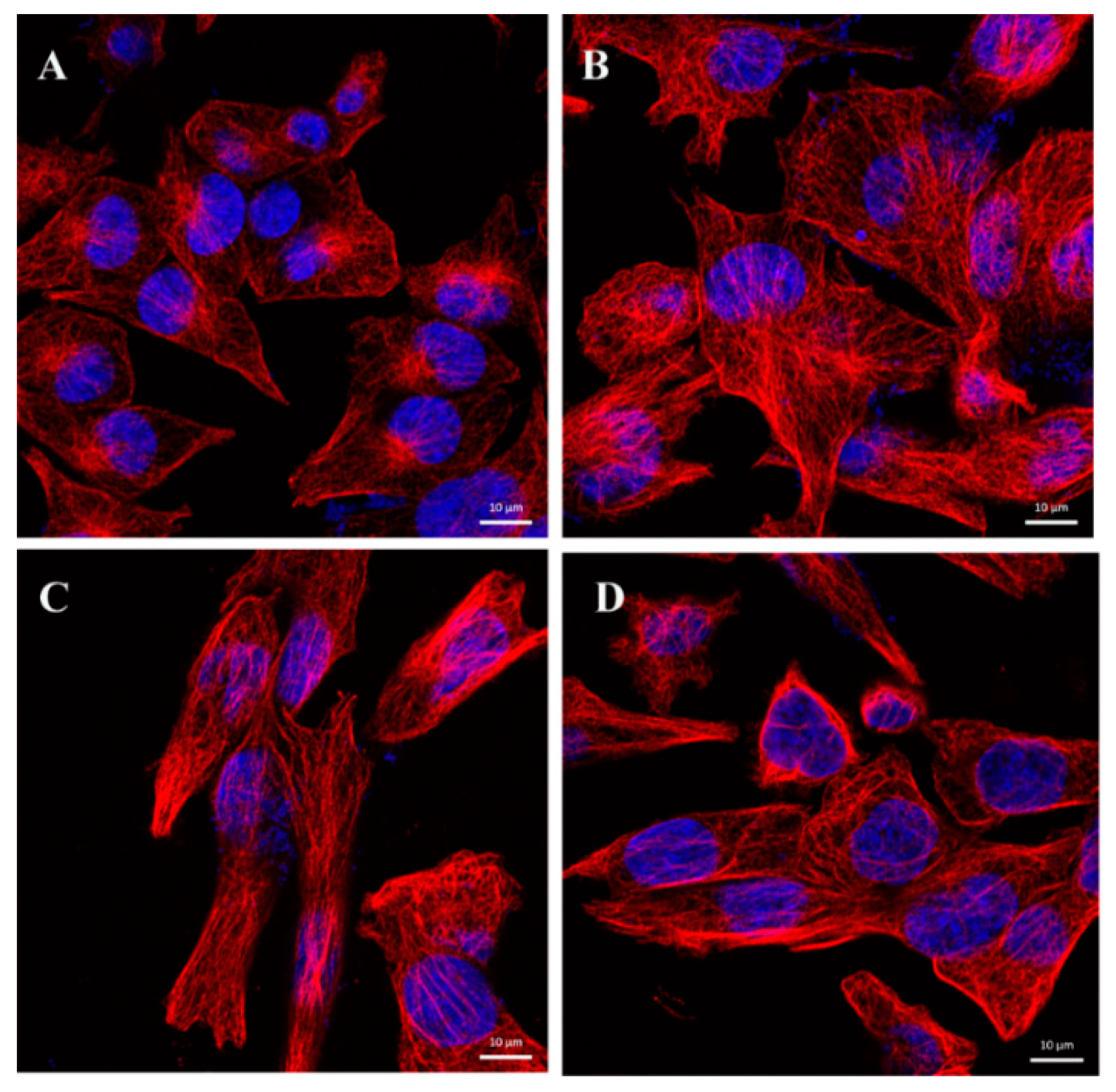
2.6. CA Changes the Dynamics of Microtubules
3. Discussion
4. Materials and Methods
4.1. Plant Material and Extract Preparation
4.2. Identification of Compounds by Liquid Chromatography-Mass Spectrometry
4.3. Cell Lines and Culture Conditions
4.4. MTT Cell Proliferation Assay
4.5. Propidium Iodide (PI) Exclusion Assay
4.6. Cell Cycle Analysis
4.7. Indirect Immunofluorescence and DAPI Staining
4.8. Statistics
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Boukhary, R.; Raafat, K.; Ghoneim, A.I.; Aboul-Ela, M.; El-Lakany, A. Anti-inflammatory and antioxidant activities of Salvia fruticosa: An HPLC determination of phenolic contents. Evid.-Based Complement. Altern. Med. 2016, 2016, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Tundis, R.; Iacopetta, D.; Sinicropi, M.S.; Bonesi, M.; Leporini, M.; Passalacqua, N.G.; Ceramella, J.; Menichini, F.; Loizzo, M.R. Assessment of antioxidant, antitumor and pro-apoptotic effects of Salvia fruticosa Mill. subsp. thomasii (Lacaita) Brullo, Guglielmo, Pavone & Terrasi (Lamiaceae). Food Chem. Toxicol. 2017, 106, 155–164. [Google Scholar] [PubMed]
- Duletić-Laušević, S.; Alimpić Aradski, A.; Šavikin, K.; Knežević, A.; Milutinović, M.; Stević, T.; Vukojević, J.; Marković, S.; Marin, P.D. Composition and biological activities of Libyan Salvia fruticosa Mill. and S. lanigera Poir. extracts. S. Afr. J. Bot. 2018, 117, 101–109. [Google Scholar] [CrossRef]
- Gali-Muhtasib, H. Anticancer and medicinal properties of essential oil and extracts of East Mediterranean sage (Salvia triloba). Adv. Phytomed. 2006, 2, 169–180. [Google Scholar]
- Ramos, A.A.; Azqueta, A.; Pereira-Wilson, C.; Collins, A.R. Polyphenolic compounds from Salvia species protect cellular DNA from oxidation and stimulate DNA repair in cultured human cells. J. Agric. Food Chem. 2010, 58, 7465–7471. [Google Scholar] [CrossRef]
- Fraihat, A.; Alatrash, L.; Abbasi, R.; Abu-Irmaileh, B.; Hamed, S.; Mohammad, M.; Abu-Rish, E.; Bustanji, Y. Inhibitory effects of methanol extracts of selected plants on proliferation of two human melanoma cell lines. Trop. J. Pharm. Res. 2018, 17, 1645–1650. [Google Scholar] [CrossRef]
- Alimpic, A.Z.; Kotur, N.; Stanković, B.; Marin, P.D.; Matevski, V.; Al Sheef, N.; Duletić-Laušević, S. The in vitro antioxidative and cytotoxic effects of selected Salvia species water extracts. J. Appl. Bot. Food Qual. 2015, 88, 115–119. [Google Scholar]
- Abu-Dahab, R.; Afifi, F.; Kasabri, V.; Majdalawi, L.; Naffa, R. Comparison of the antiproliferative activity of crude ethanol extracts of nine Salvia species grown in Jordan against breast cancer cell line models. Pharmacogn. Mag. 2012, 8, 319. [Google Scholar] [CrossRef]
- Xavier, C.P.R.; Lima, C.F.; Fernandes-Ferreira, M.; Pereira-Wilson, C. Salvia fruticosa, Salvia officinalis, and rosmarinic acid induce apoptosis and inhibit proliferation of human colorectal cell lines: The role in MAPK/ERK pathway. Nutr. Cancer 2009, 61, 564–571. [Google Scholar] [CrossRef]
- Ramos, A.A.; Pedro, D.; Collins, A.R.; Pereira-Wilson, C. Protection by Salvia extracts against oxidative and alkylation damage to DNA in human HCT15 and CO115 cells. J. Toxicol. Env. Heal Part A Curr. Issues 2012, 75, 765–775. [Google Scholar] [CrossRef]
- Stagos, D.; Portesis, N.; Spanou, C.; Mossialos, D.; Aligiannis, N.; Chaita, E.; Panagoulis, C.; Reri, E.; Skaltsounis, L.; Tsatsakis, A.M.; et al. Correlation of total polyphenolic content with antioxidant and antibacterial activity of 24 extracts from Greek domestic Lamiaceae species. Food Chem. Toxicol. 2012, 50, 4115–4124. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, N.; Okubo, A.; Yasuhira, S.; Takahashi, K.; Amano, H.; Akasaka, T.; Masuda, T.; Shibazaki, M.; Maesawa, C. Carnosic acid, an inducer of Nad(P)H quinone oxidoreductase 1, enhances the cytotoxicity of β-lapachone in melanoma cell lines. Oncol. Lett. 2018, 15, 2393–2400. [Google Scholar] [CrossRef] [PubMed]
- Petiwala, S.M.; Johnson, J.J. Diterpenes from rosemary (Rosmarinus officinalis): Defining their potential for anti-cancer activity. Cancer Lett. 2015, 367, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Birtić, S.; Dussort, P.; Pierre, F.X.; Bily, A.C.; Roller, M. Carnosic acid. Phytochemistry 2015, 115, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Barni, M.V.; Carlini, M.J.; Cafferata, E.G.; Puricelli, L.; Moreno, S. Carnosic acid inhibits the proliferation and migration capacity of human colorectal cancer cells. Oncol. Rep. 2012, 27, 1041–1048. [Google Scholar] [CrossRef]
- Bahri, S.; Jameleddine, S.; Shlyonsky, V. Relevance of carnosic acid to the treatment of several health disorders: Molecular targets and mechanisms. Biomed. Pharmacother. 2016, 84, 569–582. [Google Scholar] [CrossRef] [PubMed]
- Bahri, S.; Mies, F.; Ben Ali, R.; Mlika, M.; Jameleddine, S.; Mc Entee, K.; Shlyonsky, V. Rosmarinic acid potentiates carnosic acid induced apoptosis in lung fibroblasts. PLoS ONE 2017, 12, 1–23. [Google Scholar] [CrossRef]
- Park, S.Y.; Song, H.; Sung, M.K.; Kang, Y.H.; Lee, K.W.; Park, J.H.Y. Carnosic acid inhibits the epithelial-mesenchymal transition in B16F10 melanoma cells: A possible mechanism for the inhibition of cell migration. Int. J. Mol. Sci. 2014, 15, 12698–12713. [Google Scholar] [CrossRef]
- Pesakhov, S.; Nachliely, M.; Barvish, Z.; Aqaqe, N.; Schwartzman, B.; Voronov, E.; Sharoni, Y.; Studzinski, G.P.; Fishman, D.; Danilenko, M. Cancer-selective cytotoxic Ca2+ overload in acute myeloid leukemia cells and attenuation of disease progression in mice by synergistically acting polyphenols curcumin and carnosic acid. Oncotarget 2016, 7, 31847–31861. [Google Scholar] [CrossRef]
- Kar, S.; Palit, S.; Ball, W.B.; Das, P.K. Carnosic acid modulates Akt/IKK/NF-κB signaling by PP2A and induces intrinsic and extrinsic pathway mediated apoptosis in human prostate carcinoma PC-3 cells. Apoptosis 2012, 17, 735–747. [Google Scholar] [CrossRef]
- Domingues, B.; Lopes, J.; Soares, P.; Populo, H. Melanoma treatment in review. ImmunoTargets Ther. 2018, 7, 35–49. [Google Scholar] [CrossRef]
- Sarrou, E.; Martens, S.; Chatzopoulou, P. Metabolite profiling and antioxidative activity of Sage (Salvia fruticosa Mill.) under the influence of genotype and harvesting period. Ind. Crops Prod. 2016, 94, 240–250. [Google Scholar] [CrossRef]
- Moharram, F.A.; Mahmoud, I.I.; Mahmoud, M.R.; Sabry, S. Polyphenolic profile and biological study of Salvia fruticosa. NPC Nat. Prod. Commun. 2006, 1. [Google Scholar] [CrossRef]
- Vergine, M.; Nicolì, F.; Negro, C.; Luvisi, A.; Nutricati, E.; Annunziata Accogli, R.; Sabella, E.; Miceli, A. Phytochemical profiles and antioxidant activity of Salvia species from southern Italy. Rec. Nat. Prod. 2019, 13, 205–215. [Google Scholar] [CrossRef]
- Lu, Y.; Foo, L.Y. Rosmarinic acid derivatives from Salvia officinalis. Phytochemistry 1999, 51, 91–94. [Google Scholar] [CrossRef]
- Exarchou, V.; Nenadis, N.; Tsimidou, M.; Gerothanassis, I.P.; Troganis, A.; Boskou, D. Antioxidant activities and phenolic composition of extracts from Greek oregano, Greek sage, and summer savory. J. Agric. Food Chem. 2002, 50, 5294–5299. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, H.W.; Lee, M.K.; Kim, Y.J.; Asamenew, G.; Cha, Y.S.; Kim, J.B. Phenolic profiling and quantitative determination of common sage (Salvia plebeia R. Br.) by UPLC-DAD-QTOF/MS. Eur. Food Res. Technol. 2018, 244, 1637–1646. [Google Scholar] [CrossRef]
- Exarchou, V.; Kanetis, L.; Charalambous, Z.; Apers, S.; Pieters, L.; Gekas, V.; Goulas, V. HPLC-SPE-NMR characterization of major metabolites in Salvia fruticosa Mill. extract with antifungal potential: Relevance of carnosic acid, carnosol, and hispidulin. J. Agric. Food Chem. 2015, 63, 457–463. [Google Scholar] [CrossRef]
- Kontogianni, V.G.; Tomic, G.; Nikolic, I.; Nerantzaki, A.A.; Sayyad, N.; Stosic-Grujicic, S.; Stojanovic, I.; Gerothanassis, I.P.; Tzakos, A.G. Phytochemical profile of Rosmarinus officinalis and Salvia officinalis extracts and correlation to their antioxidant and anti-proliferative activity. Food Chem. 2013, 136, 120–129. [Google Scholar] [CrossRef]
- Ayatollahi, S.A.; Shojaii, A.; Kobarfard, F.; Mohammadzadeh, M.; Choudhary, M.I. Two flavones from Salvia leriaefolia. Iran. J. Pharm. Res. 2009, 8, 179–184. [Google Scholar]
- Cuvelier, M.E.; Richard, H.; Berset, C. Antioxidative activity and phenolic composition of pilot-plant and commercial extracts of sage and rosemary. JAOCS J. Am. Oil Chem. Soc. 1996, 73, 645–652. [Google Scholar] [CrossRef]
- Topçu, G.; Öztürk, M.; Kuşman, T.; Demirkoz, A.A.B.; Kolak, U.; Ulubelen, A. Terpenoids, essential oil composition, fatty acid profile, and biological activities of Anatolian Salvia fruticosa Mill. Turk. J. Chem. 2013, 37, 619–632. [Google Scholar] [CrossRef]
- Liu, A.H.; Guo, H.; Ye, M.; Lin, Y.H.; Sun, J.H.; Xu, M.; Guo, D.A. Detection, characterization and identification of phenolic acids in danshen using high-performance liquid chromatography with diode array detection and electrospray ionization mass spectrometry. J. Chromatogr. A 2007, 1161, 170–182. [Google Scholar] [CrossRef]
- Šulniūtė, V.; Pukalskas, A.; Venskutonis, P.R. Phytochemical composition of fractions isolated from ten Salvia species by supercritical carbon dioxide and pressurized liquid extraction methods. Food Chem. 2017, 224, 37–47. [Google Scholar] [CrossRef]
- Pizzale, L.; Bortolomeazzi, R.; Vichi, S.; Überegger, E.; Conte, L.S. Antioxidant activity of sage (Salvia officinalis and S fruticosa) and oregano (Origanum onites and O. indercedens) extracts related to their phenolic compound content. J. Sci. Food Agric. 2002, 82, 1645–1651. [Google Scholar] [CrossRef]
- Rangarajan, R.; Dodbiba, E.; Smuts, J.P.; Lang, J.C.; Zhang, Y.; Armstrong, D.W. Degradation study of carnosic acid, carnosol, rosmarinic acid, and rosemary extract (Rosmarinus officinalis L.) assessed using HPLC. J. Agric. Food Chem. 2012, 60, 9305–9314. [Google Scholar]
- Munné-Bosch, S.; Alegre, L.; Schwarz, K. The formation of phenolic diterpenes in Rosmarinus officinalis L. under Mediterranean climate. Eur. Food Res. Technol. 2000, 210, 263–267. [Google Scholar] [CrossRef]
- Ivanović, J.; Dilas, S.; Jadranin, M.; Vajs, V.; Babović, N.; Petrović, S.; Žižović, I. Supercritical carbon dioxide extraction of antioxidants from rosemary (Rosmarinus officinalis L.) and sage (Salvia officinalis L.). J. Serb. Chem. Soc. 2009, 74, 717–732. [Google Scholar] [CrossRef]
- Cvetkovikj, I.; Stefkov, G.; Acevska, J.; Stanoeva, J.P.; Karapandzova, M.; Stefova, M.; Dimitrovska, A.; Kulevanova, S. Polyphenolic characterization and chromatographic methods for fast assessment of culinary Salvia species from South East Europe. J. Chromatogr. A 2013, 1282, 38–45. [Google Scholar] [CrossRef]
- Abreu, M.E.; Müller, M.; Alegre, L.; Munné-Bosch, S. Phenolic diterpene and α-tocopherol contents in leaf extracts of 60 Salvia species. J. Sci. Food Agric. 2008, 88, 2648–2653. [Google Scholar] [CrossRef]
- Masuda, T.; Inaba, Y.; Takeda, Y. Antioxidant mechanism of carnosic acid: Structural identification of two oxidation products. J. Agric. Food Chem. 2001, 49, 5560–5565. [Google Scholar] [CrossRef]
- Loussouarn, M.; Krieger-Liszkay, A.; Svilar, L.; Bily, A.; Birtić, S.; Havaux, M. Carnosic acid and carnosol, two major antioxidants of rosemary, act through different mechanisms. Plant Physiol. 2017, 175, 1381–1394. [Google Scholar] [CrossRef]
- Lin, K.-I.; Lin, C.-C.; Kuo, S.-M.; Lai, J.-C.; Wang, Y.-Q.; You, H.-L.; Hsu, M.-L.; Chen, C.-H.; Shiu, L.-Y. Carnosic acid impedes cell growth and enhances anticancer effects of carmustine and lomustine in melanoma. Biosci. Rep. 2018, 38, BSR20180005. [Google Scholar] [CrossRef]
- Cristina, C. Rohena and Susan, L. Mooberry Recent progress with microtubule stabilizers: New compounds, binding modes and cellular activities. Nat. Prod. Rep. 2014, 31, 335–355. [Google Scholar]
- Visanji, J.M.; Thompson, D.G.; Padfield, P.J. Induction of G2/M phase cell cycle arrest by carnosol and carnosic acid is associated with alteration of cyclin A and cyclin B1 levels. Cancer Lett. 2006, 237, 130–136. [Google Scholar] [CrossRef]
- Xiang, Q.; Ma, Y.; Dong, J.; Shen, R. Carnosic acid induces apoptosis associated with mitochondrial dysfunction and Akt inactivation in HepG2 cells. Int. J. Food Sci. Nutr. 2015, 66, 76–84. [Google Scholar] [CrossRef]
- Gao, Q.; Liu, H.; Yao, Y.; Geng, L.; Zhang, X.; Jiang, L.; Shi, B.; Yang, F. Carnosic acid induces autophagic cell death through inhibition of the Akt/mTOR pathway in human hepatoma cells. J. Appl. Toxicol. 2015, 35, 485–492. [Google Scholar] [CrossRef]
- Akaberi, M.; Mehri, S.; Iranshahi, M. Multiple pro-apoptotic targets of abietane diterpenoids from Salvia species. Fitoterapia 2015, 100, 118–132. [Google Scholar] [CrossRef]
- Hammerová, J.; Uldrijan, S.; Táborská, E.; Slaninová, I. Benzo[c]phenanthridine alkaloids exhibit strong anti-proliferative activity in malignant melanoma cells regardless of their p53 status. J. Dermatol. Sci. 2011, 62, 22–35. [Google Scholar] [CrossRef]
- Slanina, J.; Pachnikova, G.; Čarnecka, M.; Porubova Koubikova, L.; Adamkova, L.; Humpa, O.; Šmejkal, K.; Slaninova, I. Identification of key structural characteristics of Schisandra chinensis lignans involved in P-glycoprotein inhibition. J. Nat. Prod. 2014, 77, 2255–2263. [Google Scholar] [CrossRef]
Sample Availability: Samples of the Salvia extracts and carnosic acid are available from the authors. |

| Compound No. | RT min | Measured Mass of [M – H]¯ | Calculated Mass of [M – H]¯ | Error in ppm | Major Fragment ions | Formula | Tentative Identification | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1 | 5.90 | 179.0346 | 179.0344 | 1.12 | 135 | C9H8O4 | Caffeic acid | [22] |
| 2 | 6.80 | 461.0722 | 461.0720 | 0.43 | 285 | C21H18O12 | Luteolin-O-glucuronide | [23] |
| 3 | 6.90 | 447.0921 | 447.0927 | 1.34 | 285 | C21H20O11 | Luteolin-O-glucoside | [22,24] |
| 4 | 7.60 | 445.0774 | 445.0771 | 0.67 | 269; 113 | C21H18O11 | Apigenin-O-glucuronide | [23] |
| 5 | 7.70 | 719.1537 | 719.1612 | 10.4 | 161; 323; 279; 197; 179; 135 | C36H32O16 | Sagerinic acid a | [25] |
| 6 | 7.70 | 359.0774 | 359.0767 | 1.95 | 161; 197; 179; 135 | C18H16O8 | Rosmarinic acid | [22,24,26] |
| 7 | 9.30 | 315.0509 | 315.0505 | 1.27 | 300 | C16H12O7 | Nepetin | [27] |
| 8 | 9.40 | 285.0433 | 285.0399 | 11.9 | No fragment | C15H10O6 | Luteolin | [22,24,26] |
| 9 | 10.4 | 269.0473 | 269.0450 | 8.55 | 225; 201; 159; 151; 149; 117; 107 | C15H10O5 | Apigenin | [22,24,26] |
| 10 | 10.4 | 299.0562 | 299.0556 | 2.01 | 284; 228; 137 | C16H12O6 | Hispidulin | [28] |
| 11 | 11.7 | 313.0730 | 313.0712 | 5.75 | 283; 298; 255 | C17H14O6 | Cirsimaritin | [28] |
| 12 | 12.0 | 345.1731 | 345.1702 | 8.40 | 301; 283 | C20H26O5 | Rosmanol isomer | [29] |
| 13 | 12.4 | 345.1724 | 345.1702 | 6.37 | 283 | C20H26O5 | Rosmanol isomer | [29] |
| 14 | 12.8 | 345.1737 | 345.1702 | 10.1 | 283; 301 | C20H26O5 | Rosmanol isomer | [29] |
| 15 | 12.8 | 283.0623 | 283.0607 | 5.65 | 268; 240 | C16H12O5 | Genkwanin a | [30,31] |
| 16 | 15.2 | 329.1771 | 329.1753 | 5.47 | 285 | C20H26O4 | Carnosol | [28,32] |
| 17 | 15.7 | 343.1583 | 343.1546 | 10.8 | 315; 299; 287; 244 | C20H24O5 | Rosmadial | [32] |
| 18 | 16.7 | 331.1933 | 331.1909 | 7.25 | 287; 244 | C20H28O4 | Carnosic acid | [28,32] |
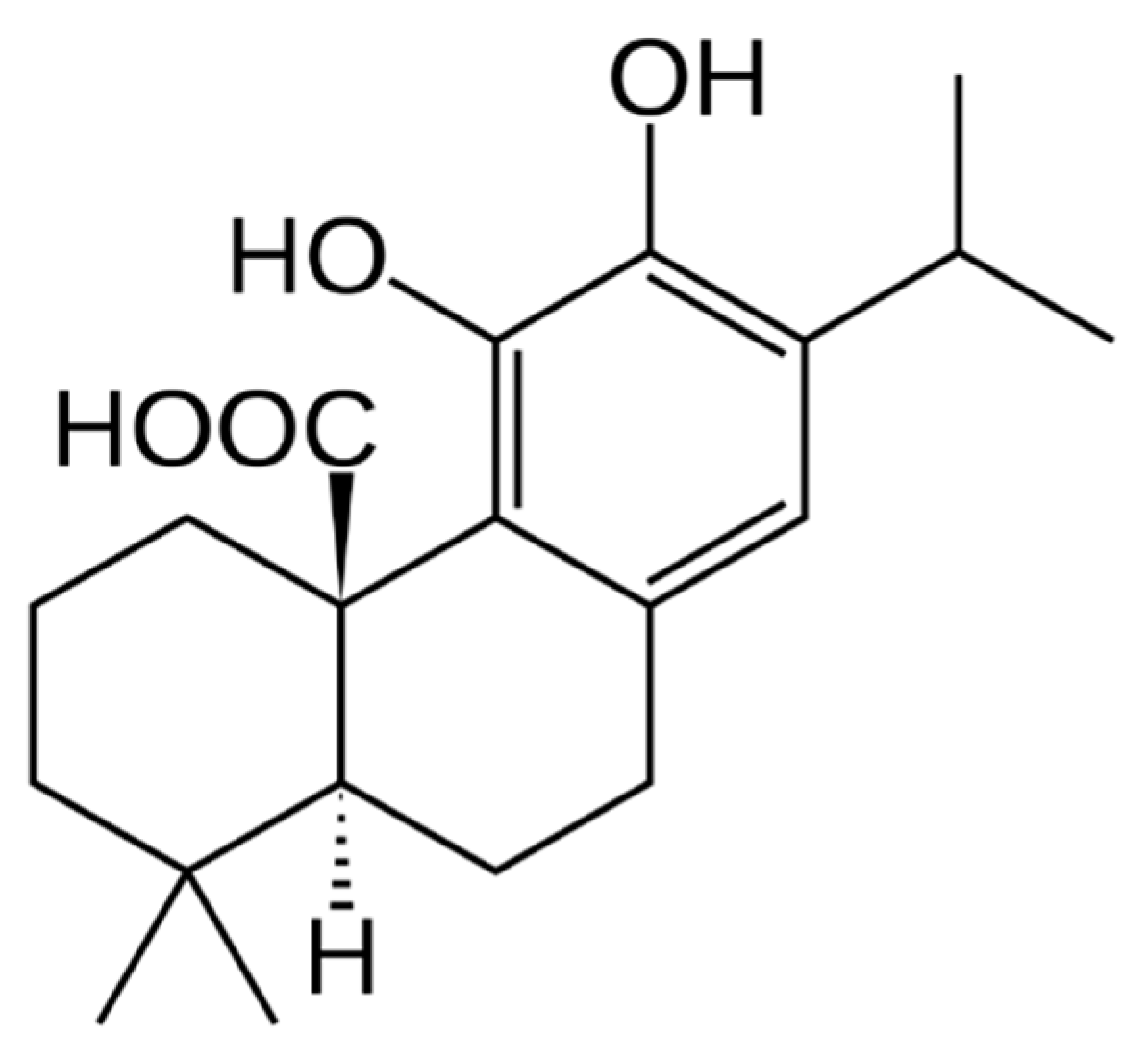
| 19 | 17.5 | 345.2088 | 345.2066 | 6.37 | 286; 301; 271 | C21H30O4 | 12-O-Methylcarnosic acid | [28,32] |
| 20 | 19.1 | 455.3530 | 455.3525 | 1.10 | No fragment | C30H48O3 | Oleanolic and ursolic acid | [32] |
| 21 | 20.8 | 283.2661 | 283.2637 | 8.47 | No fragment | C18H36O2 | Stearic acid | [32] |
| Compound no. | RT min | Measured Mass of [M – H]¯ | Calculated Mass of [M – H]¯ | Error in ppm | Major Fragment Ions | Formula | Tentative Identification | Ref. |
|---|---|---|---|---|---|---|---|---|
| 23 | 2.80 | 197.0451 | 197.0450 | 0.51 | 135; 123; 179; 151; 109 | C9H10O5 | Danshensu a | [33] |
| 1 | 5.90 | 179.0354 | 179.0344 | 5.59 | 135 | C9H8O4 | Caffeic acid a | [22] |
| 24 | 6.20 | 463.0879 | 463.0877 | 0.43 | 301; 135 | C21H20O12 | Hyperoside a | [34] |
| 3 | 6.90 | 447.0944 | 447.0927 | 3.80 | 285 | C21H20O11 | Luteolin-O-hexoside a | [22] |
| 5 | 7.70 | 719.1634 | 719.1612 | 3.06 | 161; 323 | C36H32O16 | Sagerinic acid a | [25] |
| 6 | 7.70 | 359.0773 | 359.0767 | 1.67 | 161; 197; 135 | C18H16O8 | Rosmarinic acid | [22,24,26] |
| 8 | 9.40 | 285.0411 | 285.0399 | 4.21 | 133 | C15H10O6 | Luteolin a | [22,24,26] |
| 9 | 10.4 | 269.0465 | 269.0450 | 5.58 | 151; 135; 117 | C15H10O5 | Apigenin a | [22,24,26] |
| 10 | 10.4 | 299.0559 | 299.0556 | 1.00 | 284; 269; 212; 165; 136 | C16H12O6 | Hispidulin a | [28] |
| 11 | 11.7 | 313.0717 | 313.0712 | 1.60 | 283; 298; 269; 255 | C17H14O6 | Cirsimaritin a | [28] |
| 13 | 12.4 | 345.1706 | 345.1702 | 1.16 | 283; 299; 268 | C20H26O5 | Rosmanol isomer a | [29] |
| 15 | 12.8 | 283.0607 | 283.0607 | 0.00 | 268 | C16H12O5 | Genkwanin a | [30,31] |
| 25 | 13.7 | 317.2122 | 317.2117 | 1.58 | 233; 299 | C20H30O3 | Not identified diterpene | |
| 26 | 14.4 | 345.2069 | 345.2066 | 0.87 | 299; 316; 283; 263; 249 | C21H30O4 | Not identified diterpene | |
| 19 | 17.5 | 345.2066 | 345.2066 | 0.00 | 286; 301; 271 | C21H30O4 | 12-O-Methylcarnosic acid a | [28,32] |
| 20 | 19.1 | 455.3527 | 455.3525 | 0.44 | No fragment | C30H48O3 | Oleanolic and ursolic acid | [32] |
| 21 | 20.8 | 283.2639 | 283.2637 | 0.71 | No fragment | C18H36O2 | Stearic acid a | [32] |
| Extract (μg/mL) Compound (μg/mL) | A375 | Mel JuSo | HFF |
|---|---|---|---|
| Salvia pomifera (SF) | 70.29 | 76.53 | 153.5 |
| Salvia fruticosa (SP) | 57.95 | 63.57 | 72.67 |
| Carnosic acid (CA) | 7.56 | 11.75 | 21.87 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koutsoulas, A.; Čarnecká, M.; Slanina, J.; Tóth, J.; Slaninová, I. Characterization of Phenolic Compounds and Antiproliferative Effects of Salvia pomifera and Salvia fruticosa Extracts. Molecules 2019, 24, 2921. https://doi.org/10.3390/molecules24162921
Koutsoulas A, Čarnecká M, Slanina J, Tóth J, Slaninová I. Characterization of Phenolic Compounds and Antiproliferative Effects of Salvia pomifera and Salvia fruticosa Extracts. Molecules. 2019; 24(16):2921. https://doi.org/10.3390/molecules24162921
Chicago/Turabian StyleKoutsoulas, Antonios, Martina Čarnecká, Jiří Slanina, Jaroslav Tóth, and Iva Slaninová. 2019. "Characterization of Phenolic Compounds and Antiproliferative Effects of Salvia pomifera and Salvia fruticosa Extracts" Molecules 24, no. 16: 2921. https://doi.org/10.3390/molecules24162921
APA StyleKoutsoulas, A., Čarnecká, M., Slanina, J., Tóth, J., & Slaninová, I. (2019). Characterization of Phenolic Compounds and Antiproliferative Effects of Salvia pomifera and Salvia fruticosa Extracts. Molecules, 24(16), 2921. https://doi.org/10.3390/molecules24162921





