A Strategy for Quality Control of Vespa magnifica (Smith) Venom Based on HPLC Fingerprint Analysis and Multi-Component Separation Combined with Quantitative Analysis
Abstract
:1. Introduction
2. Results and Discussion
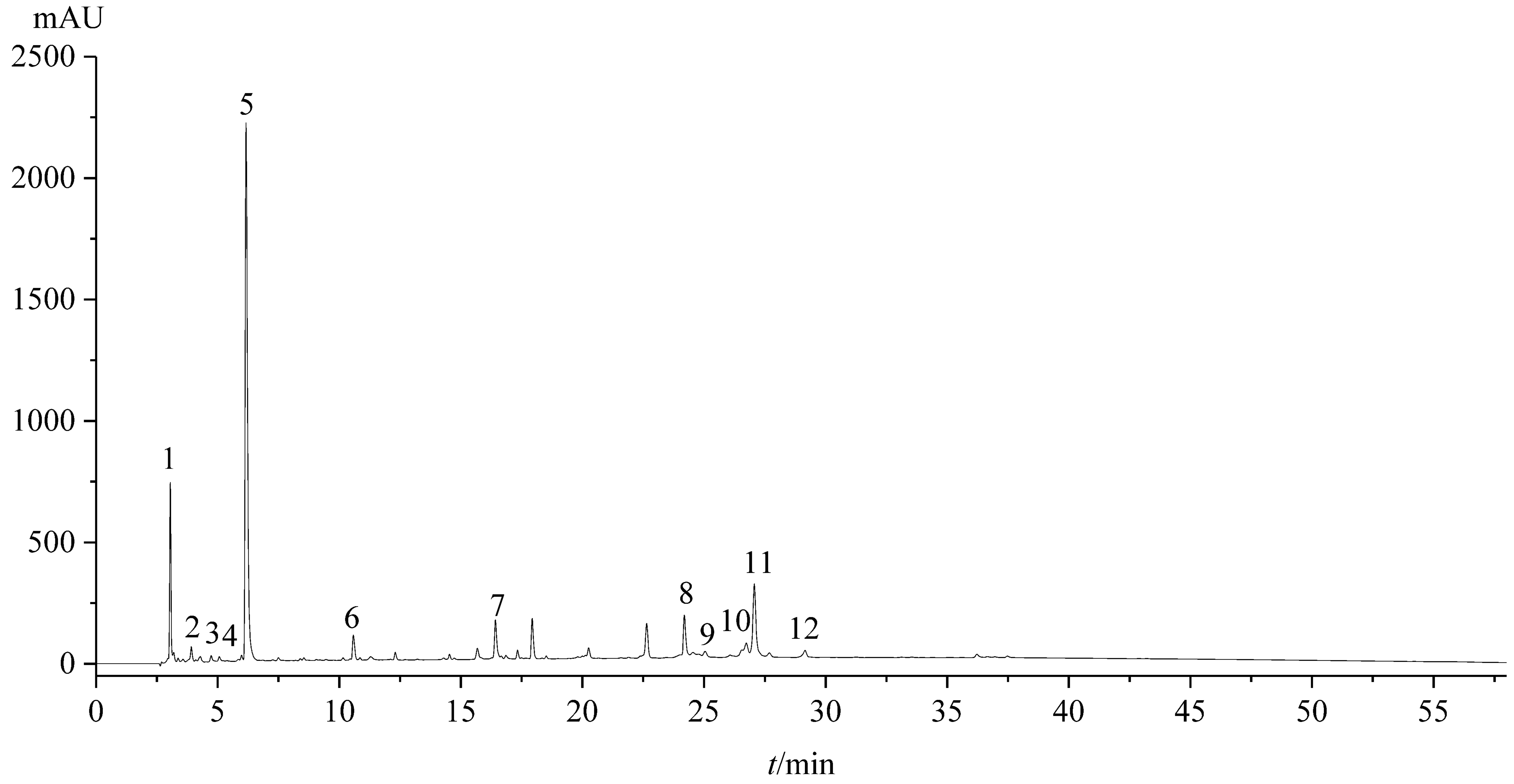
2.1. Fingerprint Analysis
2.1.1. Methodology Validation
2.1.2. Establishing the Fingerprint
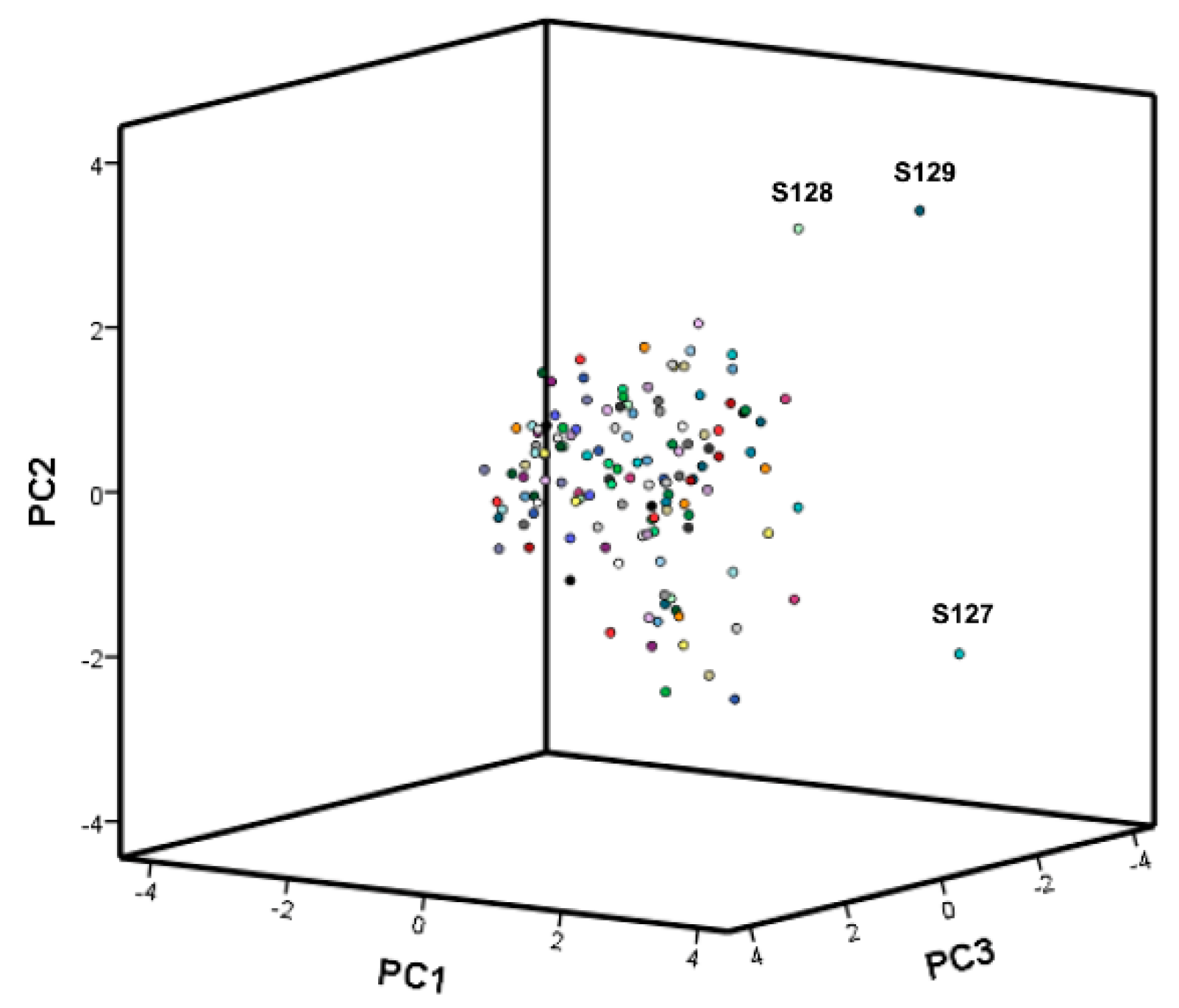
Principal Component Analysis
Hierarchical Cluster Analysis
Establishment of Standard Fingerprint
2.1.3. Similarity Analysis
2.2. Quantified Analysis
2.2.1. Identification of Major Components
2.2.2. Determination of Major Components
3. Materials and Methods
3.1. Wasps and Crude Venom
3.2. Reagents
3.3. Preparation of Sample Solutions
3.4. HPLC Diode Array Detection Analysis Instrumentation
3.5. Statistical Analysis
3.6. Purification and Identification
3.7. Determination of Major Components
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- King, G.F. Venoms as a platform for human drugs: Translating toxins into therapeutics. Expert Opin. Biol. Ther. 2011, 11, 1469–1484. [Google Scholar] [CrossRef] [PubMed]
- Habermann, E. Bee and wasp venoms. Science 1972, 177, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Jaques, R.; Schachter, M. The presence of histamine, 5-hydroxyryptamine and a potent, slow contracting substance in wasp venom. Br. J. Pharmacol. Chemother. 1954, 9, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Li, J.; Lu, Q.; Yang, H.; Zhang, Y.; Lai, R. Two families of antimicrobial peptides from wasp (Vespa magnifica) venom. Toxicon 2006, 47, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; You, D.; Xu, X.; Han, W.; Lu, Y.; Lai, R.; Meng, Q. An anticoagulant serine protease from the wasp venom of Vespa magnifica. Toxicon 2008, 51, 914–922. [Google Scholar] [CrossRef]
- Yu, H.; Yang, H.; Ma, D.; Lv, Y.; Liu, T.; Zhang, K.; Lai, R.; Liu, J. Vespid chemotactic peptide precursor from the wasp, Vespa magnifica (Smith). Toxicon 2007, 50, 377–382. [Google Scholar] [CrossRef] [PubMed]
- An, S.; Chen, L.; Wei, J.F.; Yang, X.; Ma, D.; Xu, X.; Xu, X.; He, S.; Lu, J.; Lai, R. Purification and characterization of two new allergens from the venom of Vespa magnifica. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Xu, X.; Ma, D.; Zhang, K.; Lai, R. A phospholipase A1 platelet activator from the wasp venom of Vespa magnifica (Smith). Toxicon 2008, 51, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Morozzi, P.; Zappi, A.; Gottardi, F.; Locatelli, M.; Melucci, D. A quick and efficient non-targeted screening test for Saffron Authentication: Application of chemometrics to Gas-Chromatographic data. Molecules 2019, 24, 2602. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.Z.; Xie, P.S.; Chan, K. Quality control of herbal medicines. J. Chromatogr. B 2004, 812, 53–70. [Google Scholar] [CrossRef]
- Zhang, P.; Cui, Z.; Liu, Y.S.; Sheng, Y. Isolation and identification of the indolealkylamines from the traditional Chinese medicine Toad Venom. J. Shenyang Pharm. Univ. 2006, 23, 216–219. [Google Scholar]
- Welsh, J.H.; Batty, C.S. 5-Hydroxytryptamine content of some arthropod venoms and venom-containing parts. Toxicon 1963, 1, 165–170. [Google Scholar] [CrossRef]
- Kishimura, H.; Yasuhara, T.; Yoshida, H.; Nakajima, T. Vespakinin-M, a novel bradykinin analogue containing hydroxyproline, in the venom of Vespa mandarinia Smith. Chem. Pharm. Bull. 1976, 24, 2896–2897. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Yang, H.; Xu, X.; Wang, X.; Lai, R. The first report of kininogen from invertebrates. Biochem. Biophys. Res. Commun. 2006, 347, 1099–1102. [Google Scholar] [CrossRef] [PubMed]
- Hirai, Y.; Kuwada, M.; Yasuhara, T.; Yoshida, H.; Nakajima, T. A new mast cell degranulating peptide homologous to mastoparan in the venom of Japanese hornet (Vespa xanthoptera). Chem. Pharm. Bull. 1979, 27, 1945–1946. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, Y.; Mori, C.; Morikawa, Y.; Fujimoto, H.; Mori, M.; Yasuhara, T.; Nakajima, T.; Kitada, C.; Fujino, M. Structure and activities of chemotactic peptide from the venom sac of Vespinae. Jpn. J. Pharm. 1985, 39, 212. [Google Scholar]
- Guo, Y.J.; Wang, B.; Guo, N.N.; Zhang, C.G.; Zhao, Y. Method for improving wasp venom collection by using light-emitting diodes and ultrasonic stimulation wasp. Patent CN103636567A, 19 March 2014. [Google Scholar]
- Chen, D. Qualitative and Quantitative Analysis of Main Type Materials and Purification of Abundant Components in the Southwest Vespa Venom. Master’s Thesis, Dali University, Yunnan, China, 2017. [Google Scholar]
Sample Availability: Samples of the venom available from the authors. |

 ) indicate the possible amino acid sequences.
) indicate the possible amino acid sequences.
 ) indicate the possible amino acid sequences.
) indicate the possible amino acid sequences.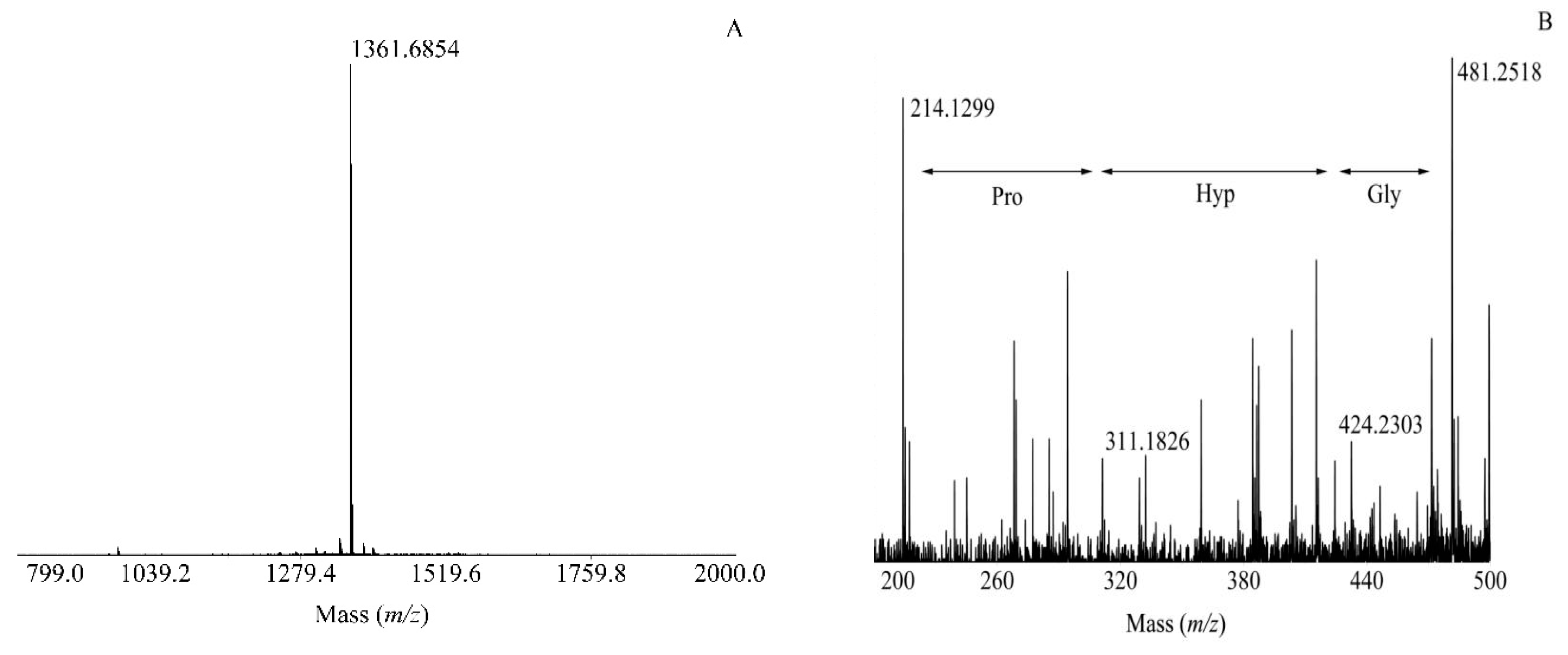
| Components | Structure | Named | Ref. |
|---|---|---|---|
| VMS1 |  | 5-Hydroxytryptamine | [11,12] |
| VMS2 | Gly–Arg–Pro–Hyp–Gly–Phe–Ser–Pro–Phe–Arg–Ile–Asp–NH2 | Vespakinin-M | [13,14] |
| VMS3 | Ile–Asn–Leu–Lys–Ala–Ile–Ala–Ala–Leu–Ala–Lys–Lys–Leu–Leu–NH2 | Mastoparan M | [15] |
| VMS4 | Phe–Leu–Pro–Ile–Ile–Gly–Lys–Leu–Leu–Ser–Gly–Leu–Leu–NH2 | Vespid chemotactic peptide M | [4,16] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, S.-T.; Luan, K.; Ni, L.-L.; Wang, Y.; Yuan, S.-M.; Che, Y.-H.; Yang, Z.-Z.; Zhang, C.-G.; Yang, Z.-B. A Strategy for Quality Control of Vespa magnifica (Smith) Venom Based on HPLC Fingerprint Analysis and Multi-Component Separation Combined with Quantitative Analysis. Molecules 2019, 24, 2920. https://doi.org/10.3390/molecules24162920
Zhou S-T, Luan K, Ni L-L, Wang Y, Yuan S-M, Che Y-H, Yang Z-Z, Zhang C-G, Yang Z-B. A Strategy for Quality Control of Vespa magnifica (Smith) Venom Based on HPLC Fingerprint Analysis and Multi-Component Separation Combined with Quantitative Analysis. Molecules. 2019; 24(16):2920. https://doi.org/10.3390/molecules24162920
Chicago/Turabian StyleZhou, Si-Tong, Kai Luan, Lian-Li Ni, Ying Wang, Shi-Meng Yuan, Yi-Hao Che, Zi-Zhong Yang, Cheng-Gui Zhang, and Zhi-Bin Yang. 2019. "A Strategy for Quality Control of Vespa magnifica (Smith) Venom Based on HPLC Fingerprint Analysis and Multi-Component Separation Combined with Quantitative Analysis" Molecules 24, no. 16: 2920. https://doi.org/10.3390/molecules24162920
APA StyleZhou, S.-T., Luan, K., Ni, L.-L., Wang, Y., Yuan, S.-M., Che, Y.-H., Yang, Z.-Z., Zhang, C.-G., & Yang, Z.-B. (2019). A Strategy for Quality Control of Vespa magnifica (Smith) Venom Based on HPLC Fingerprint Analysis and Multi-Component Separation Combined with Quantitative Analysis. Molecules, 24(16), 2920. https://doi.org/10.3390/molecules24162920




