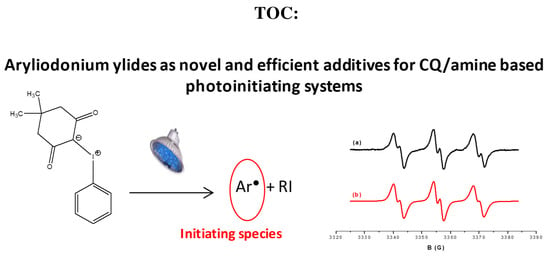
Aryliodonium Ylides as Novel and Efficient Additives for Radical Chemistry: Example in Camphorquinone (CQ)/Amine Based Photoinitiating Systems
Abstract
1. Introduction
2. Materials and Methods
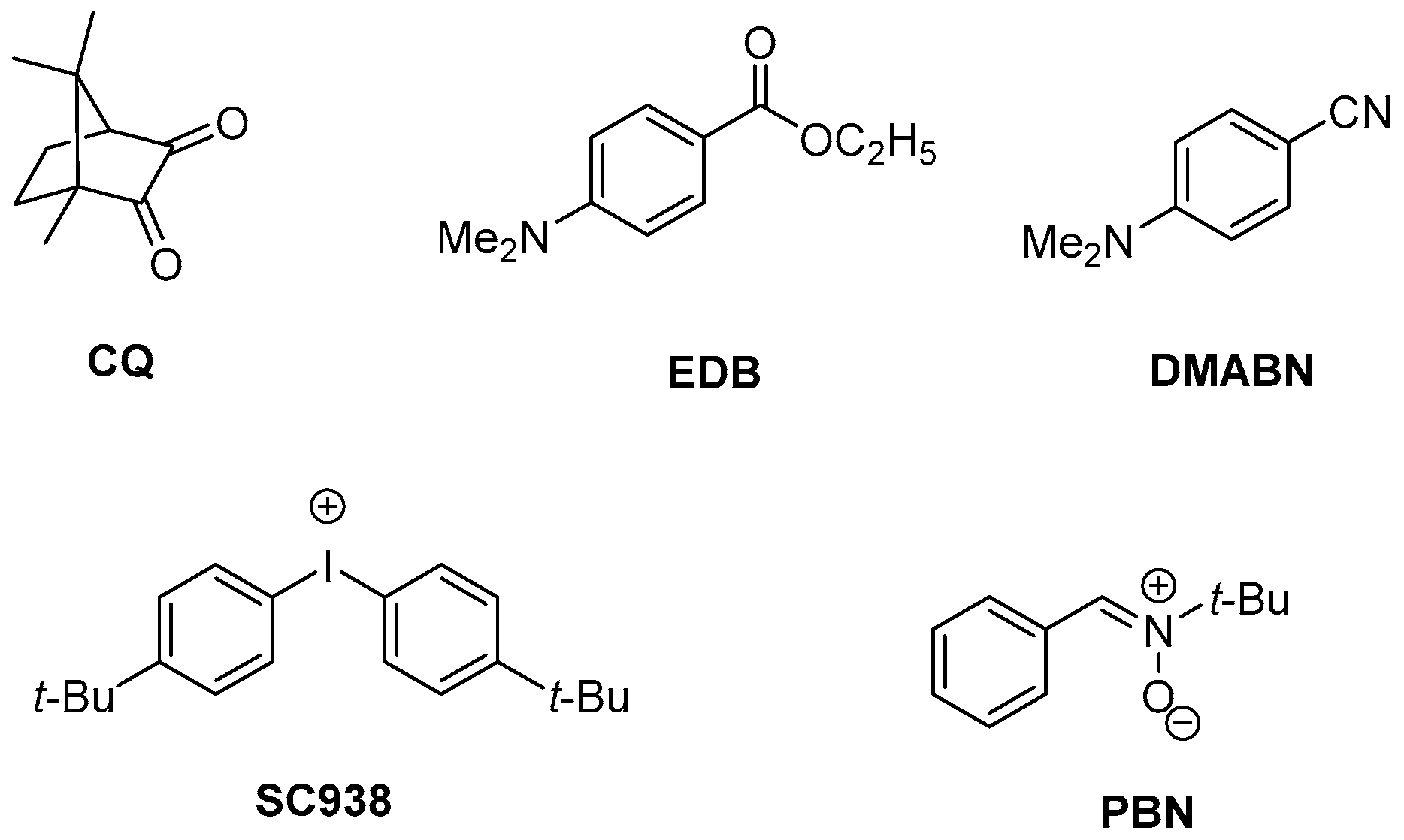
2.1. Materials
2.2. Synthesis of Aryliodonium Ylides S5 and S6
2.3. Irradiation Sources
2.4. Absorption Experiments
2.5. Photopolymerization Experiments
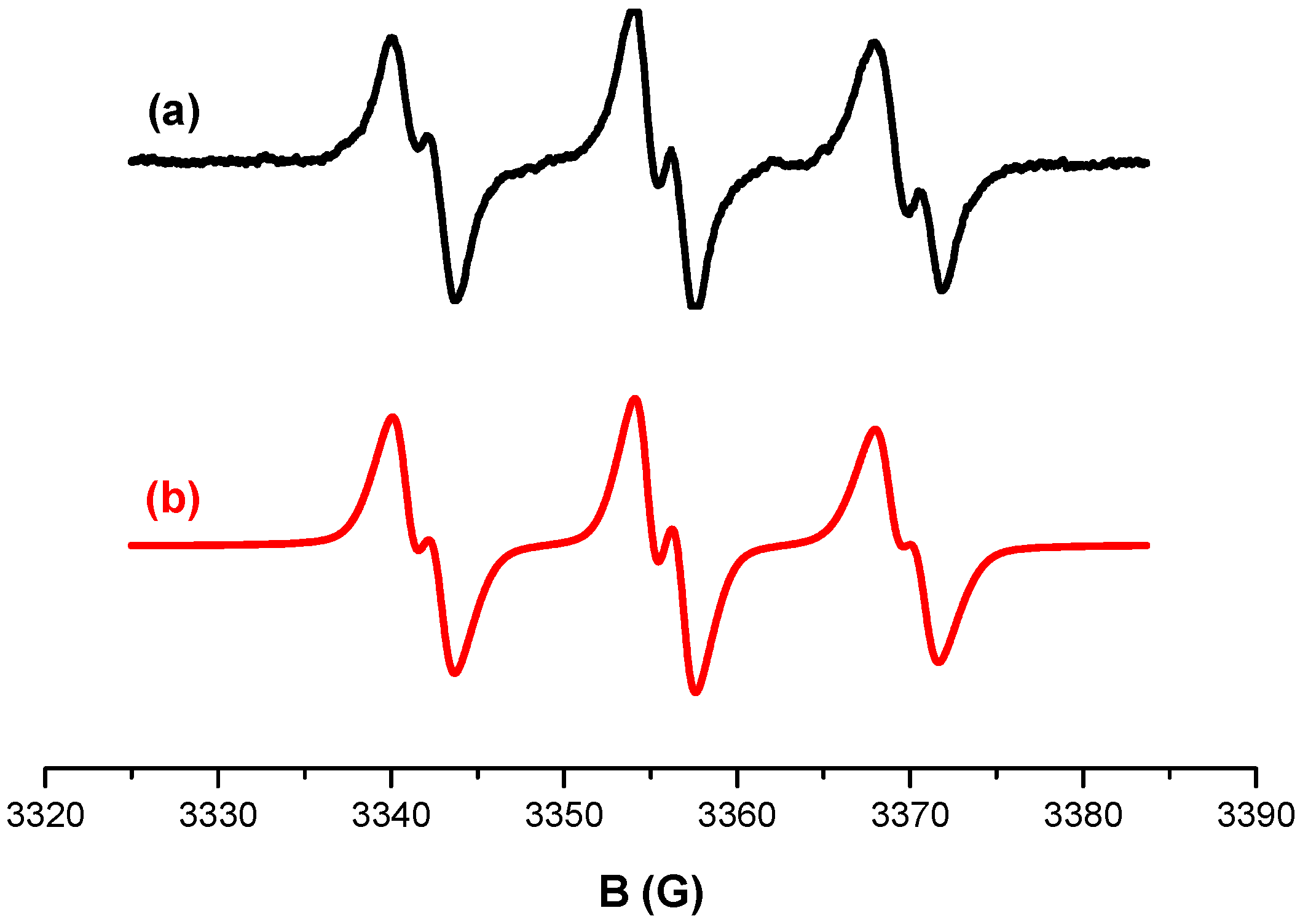
2.6. ESR Spin Trapping Experiments:
3. Results and Discussion
3.1. Photochemical Mechanism
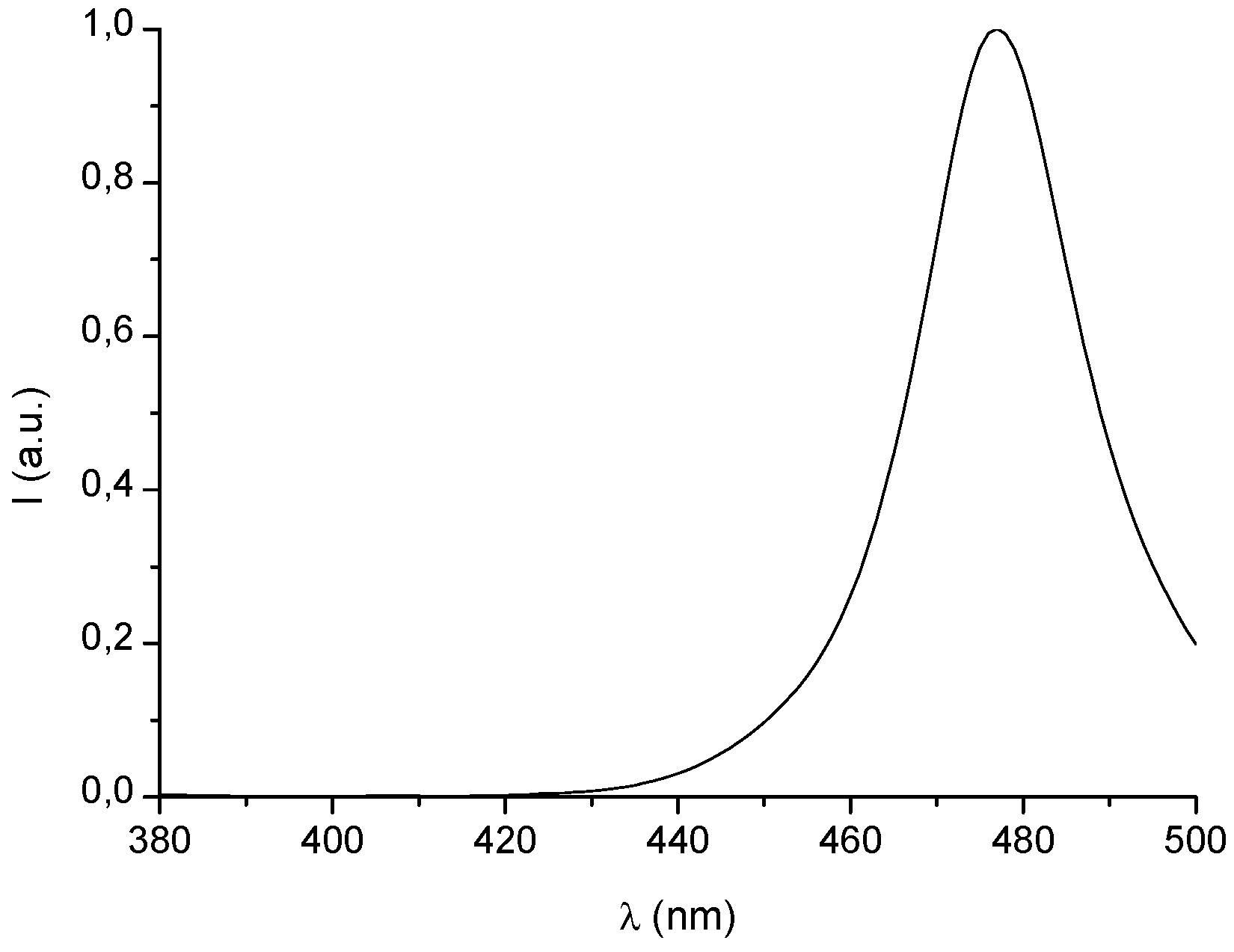
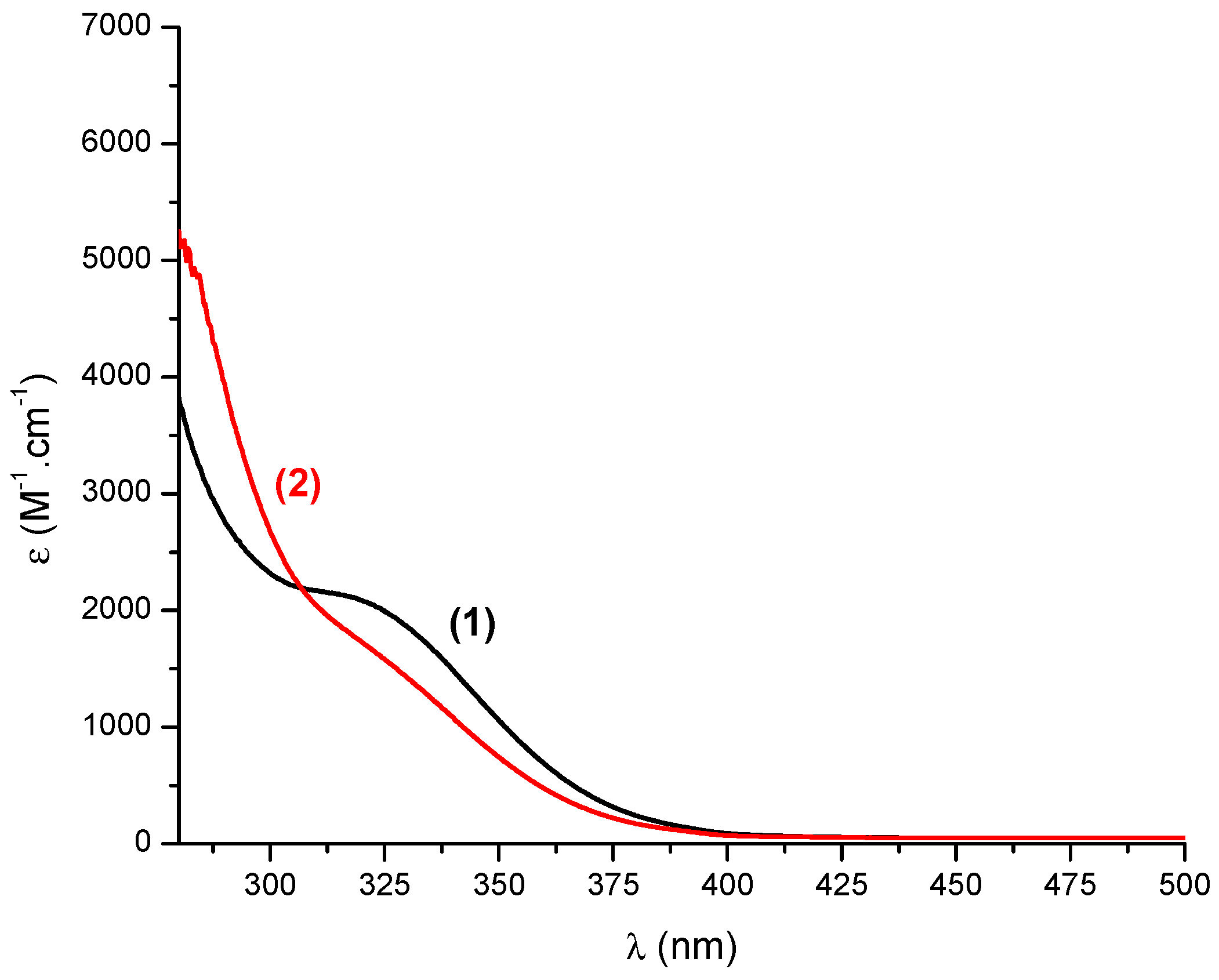
3.2. Absorption Properties
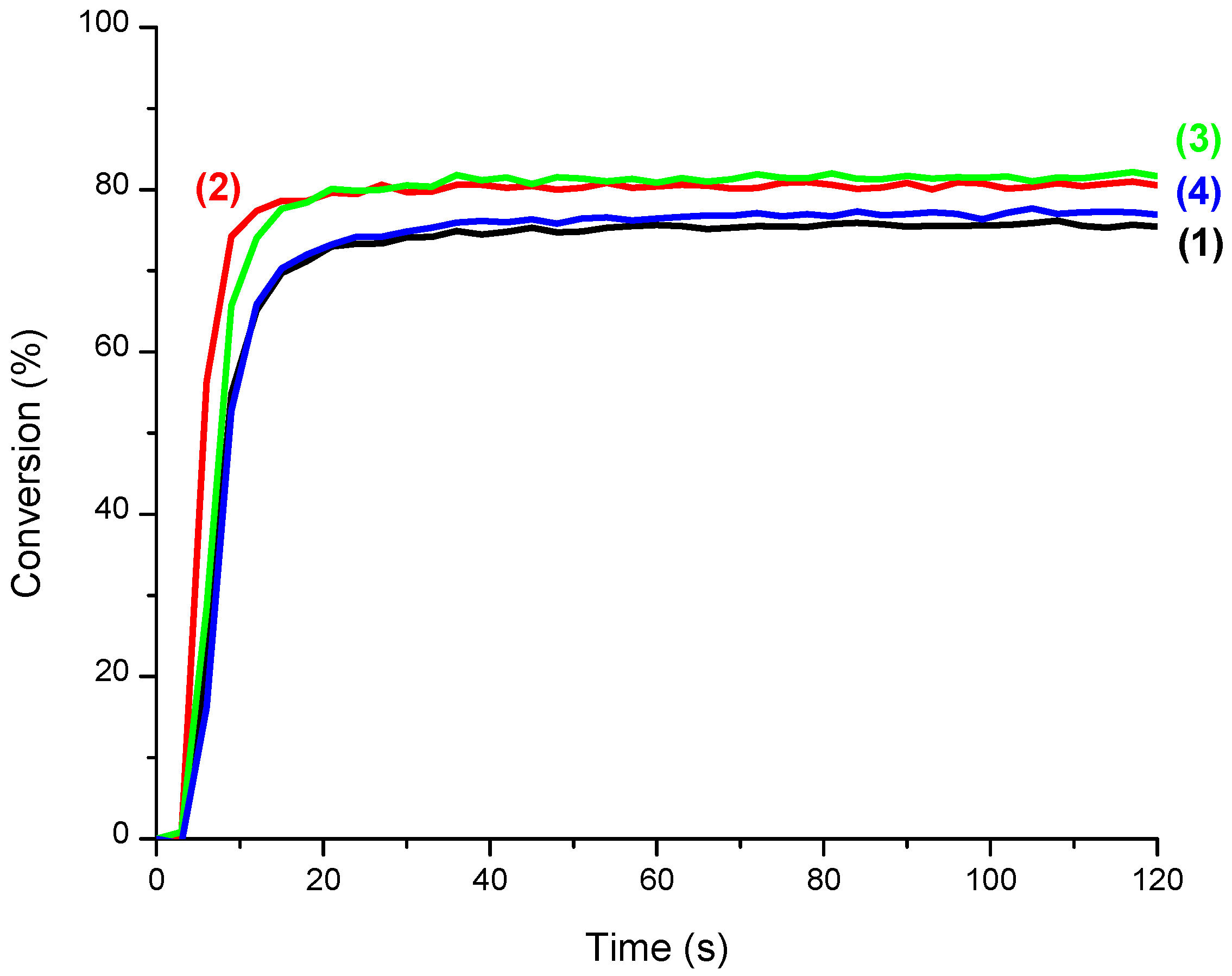
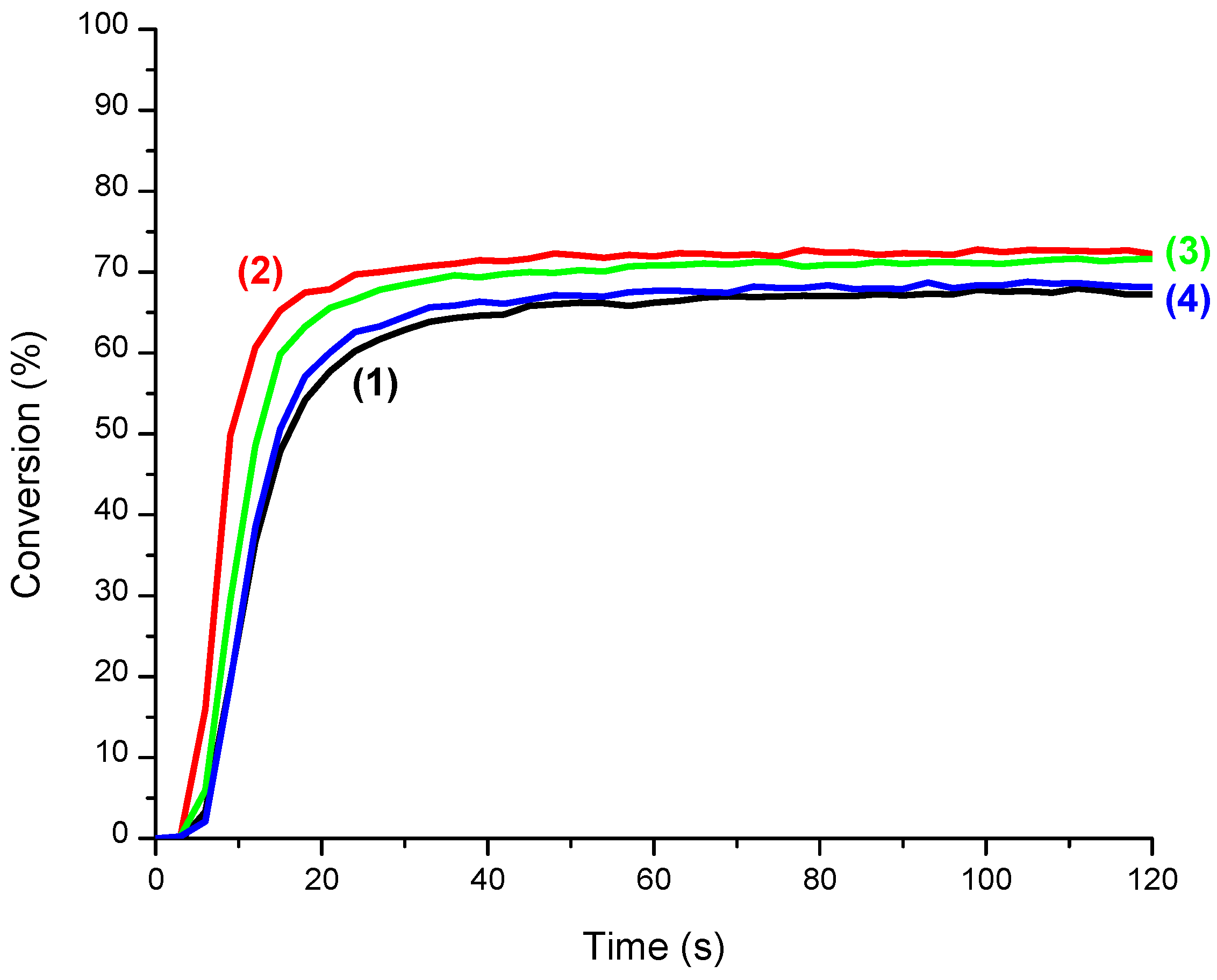
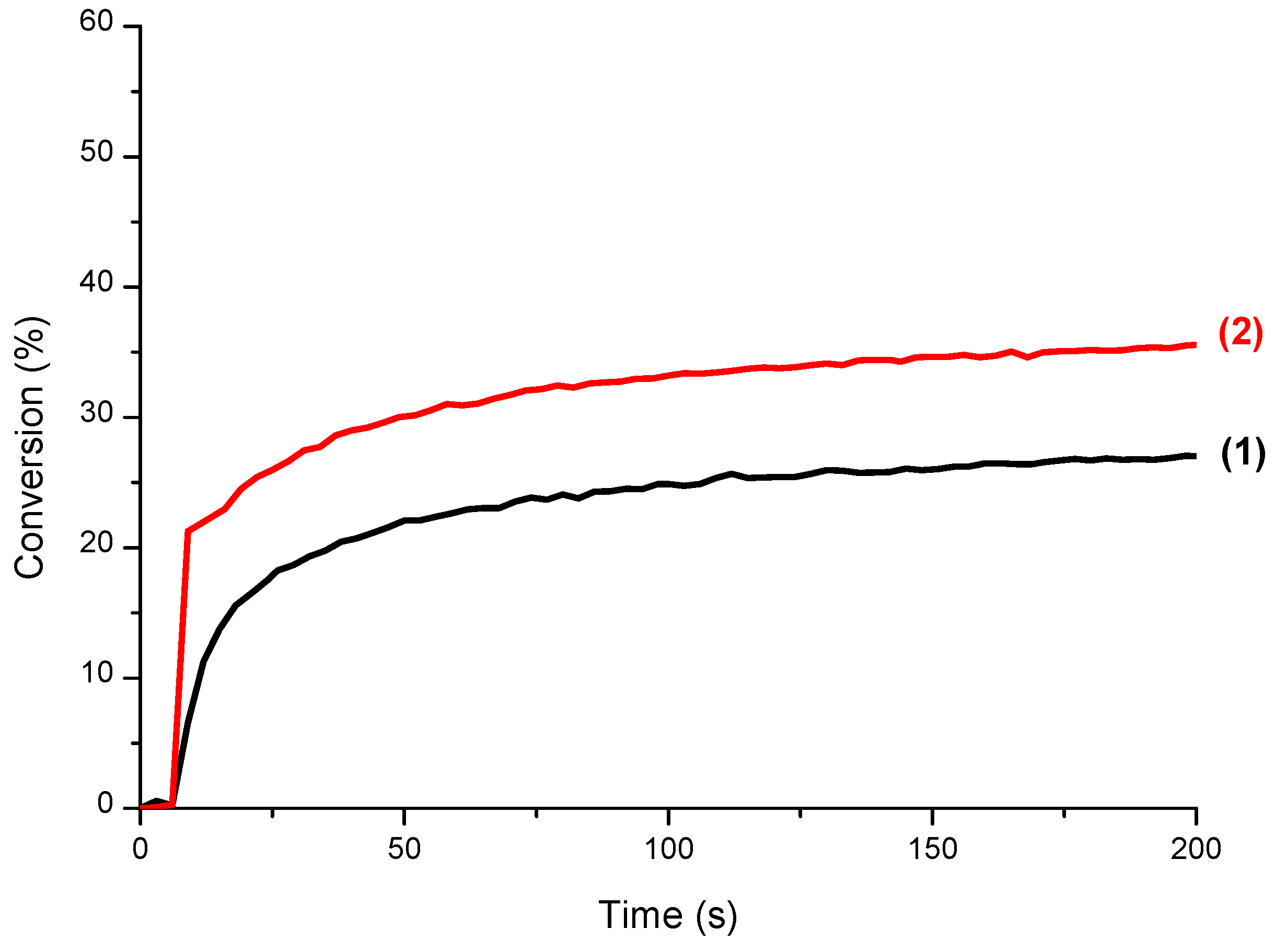
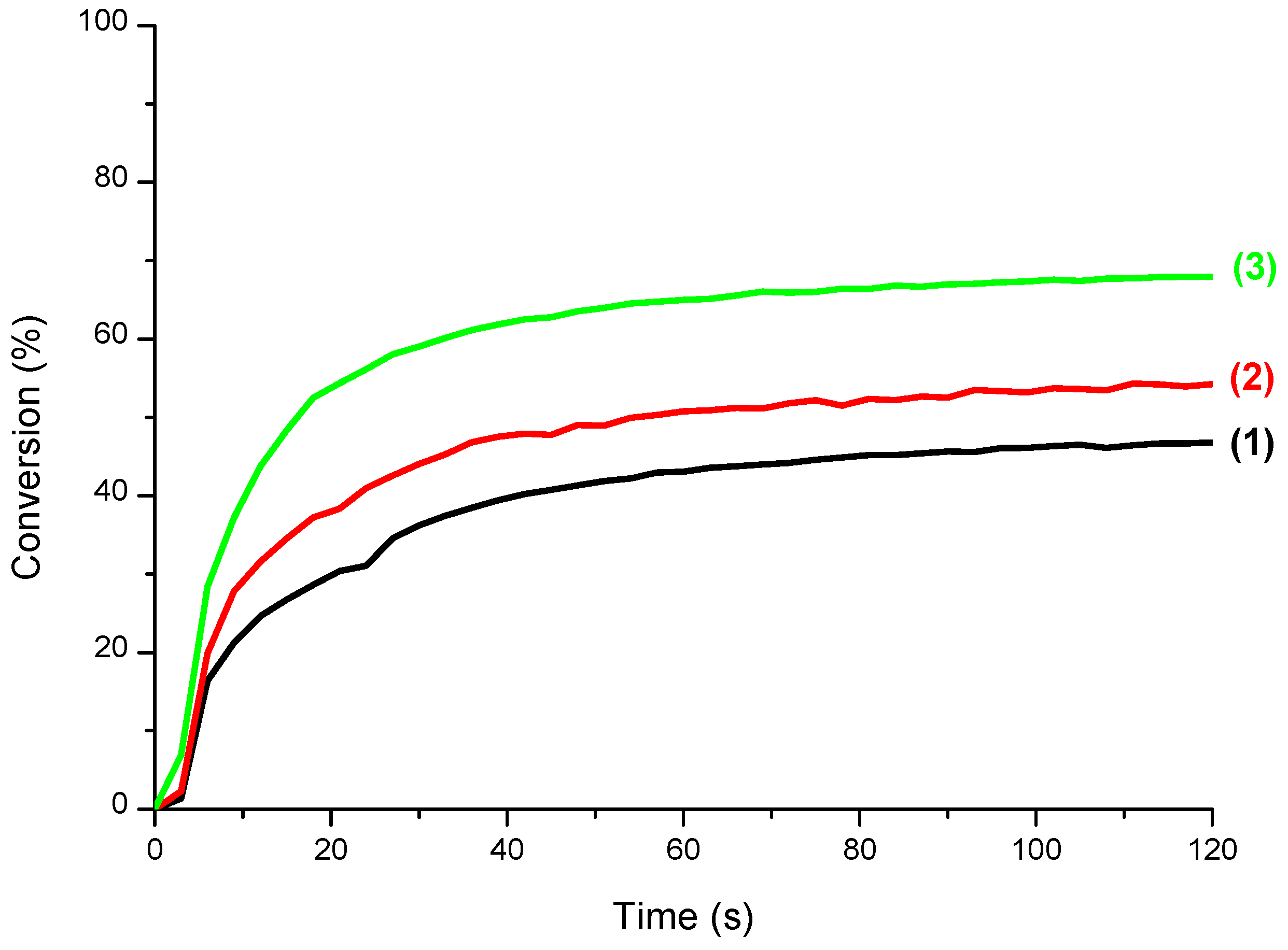
3.3. CQ/EDB/S5 and CQ/EDB/S6 Initiating Ability
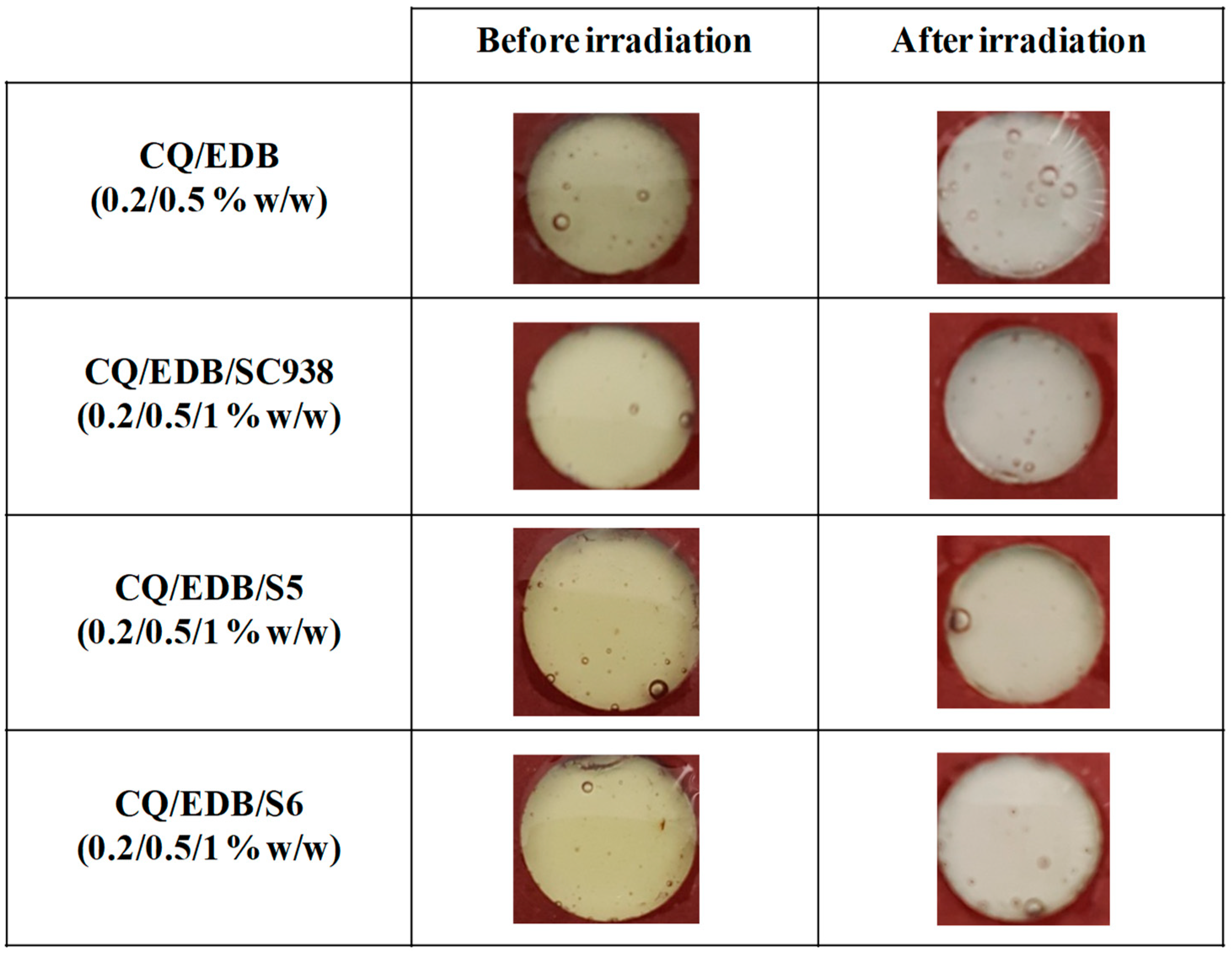
3.4. CQ/EDB/S5 and CQ/EDB/S6 for the Polymerization of Thin Samples of Methacrylates and Adhesives under Air
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fouassier, J.P.; Lalevée, J. Photoinitiators for Polymer Synthesis-Scope, Reactivity and Efficiency; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2012. [Google Scholar]
- Yagci, Y.; Jockusch, S.; Turro, N.J. Photoinitiated polymerization: Advances, challenges and opportunities. Macromolecules 2010, 43, 6245–6260. [Google Scholar] [CrossRef]
- Nakabayashi, N. Contribution of polymer chemistry to dentistry: Development of an impermeable interpenetrating polymer network to protect teeth from acid demineralization. Polym. Int. 2008, 57, 159–162. [Google Scholar] [CrossRef]
- Irving, H.; Reid, R.W. 421. The photochemical decomposition of diphenyliodonium iodide. J. Chem. Soc. 1960, 2078–2081. [Google Scholar] [CrossRef]
- Nickol, S.L.; Kampmeier, J.A. Photolysis of a charge transfer complex. Triphenylsulfonium iodide. J. Am. Chem. Soc. 1973, 95, 1908–1915. [Google Scholar] [CrossRef]
- Ortyl, J.; Popielarz, R. New photoinitiators for cationic polymerization. Polimery 2012, 57, 510–517. [Google Scholar] [CrossRef]
- Crivello, J.V. The discovery and development of onium salt cationic photoinitiators. J. Polym. Sci. Part A Polym. Chem. 1999, 37, 4241–4254. [Google Scholar] [CrossRef]
- Crivello, J.V. Cationic polymerization—Iodonium and sulfonium salt photoinitiators. In Initiators—Poly-Reactions—Optical Activity; Advances in Polymer Science; Springer: Berlin/Heidelberg, Germany, 1984; Volume 62. [Google Scholar]
- Crivello, J.V.; Lam, J.H.W. Diaryliodonium salts. A new class of photoinitiators for cationic polymerization. Macromolecules 1977, 10, 1307–1315. [Google Scholar] [CrossRef]
- Mokbel, H.; Toufaily, J.; Hamieh, T.; Dumur, F.; Campolo, D.; Gigmes, D.; Fouassier, J.P.; Ortyl, J.; Lalevée, J. Specific cationic photoinitiators for near UV and visible LEDs: Iodonium versus ferrocenium structures. J. Appl. Polym. Sci. 2015, 132, 42759. [Google Scholar] [CrossRef]
- Hua, Y.; Crivello, J.V. Development of polymeric photosensitizers for photoinitiated cationic polymerization. Macromolecules 2001, 34, 2488–2494. [Google Scholar] [CrossRef]
- Walsh, T.D.; Long, R.C. Photochemistry of aromatic ions. Photolysis of quaternary anilinium salts. J. Am. Chem. Soc. 1967, 89, 3943–3944. [Google Scholar] [CrossRef]
- Kabatc, J.; Ortyl, J.; Kostrzewska, K. New kinetic and mechanistic aspects of photosensitization of iodonium salts in photopolymerization of acrylates. RSC Adv. 2017, 7, 41619–41629. [Google Scholar] [CrossRef]
- Devoe, R.J.; Sayhun, M.R.V.; Serpone, N.; Sharma, D.K. Transient intermediates in the photolysis of iodonium cations. Can. J. Chem. 1987, 65, 2342–2349. [Google Scholar] [CrossRef]
- Dektar, J.L.; Hacker, N.P. Photochemistry of diaryliodonium salts. J. Org. Chem. 1990, 55, 639–647. [Google Scholar] [CrossRef]
- Jakubiak, J.; Allonas, X.; Fouassier, J.P.; Sionkowska, A.; Andrzejewska, E.; Linden, L.A.; Rabek, J.F. Camphorquinone-amines photoinitiating systems for the initiation of free radical polymerization. Polymer 2003, 44, 5219–5226. [Google Scholar] [CrossRef]
- Bi, Y.; Neckers, D.C. A visible light initiating system for free radical promoted cationic polymerization. Macromolecules 1994, 27, 3683–3693. [Google Scholar] [CrossRef]
- Cook, W.D. Photopolymerization kinetics of dimethacrylates using the camphorquinone/amine initiator system. Polymer 1992, 33, 600–609. [Google Scholar] [CrossRef]
- Shiraishi, A.; Kimura, H.; Oprych, D.; Schmitz, C.; Strehmel, B. Comparison between NIR and UV-sensitized radical and cationic reactivity of iodonium salts comprising anions with different coordination behavior. J. Photopolym. Sci. Technol. 2017, 30, 633–638. [Google Scholar] [CrossRef]
- Shiraishi, A.; Ueda, Y.; Schläpfer, M.; Schmitz, C.; Brömme, T.; Oprych, D.; Strehmel, B. NIR-sensitized photopolymerization with iodonium salts bearing weak coordination anions. J. Photopolym. Sci. Technol. 2016, 29, 609–615. [Google Scholar] [CrossRef]
- Huddleston, J.G.; Visser, A.E.; Reichert, W.M.; Willauer, H.D.; Broker, G.A.; Rogers, R.D. Characterization and comparison of hydrophilic and hydrophobic room temperature ionic liquids incorporating the imidazolium cation. Green Chem. 2001, 3, 156–164. [Google Scholar] [CrossRef]
- Tseng, M.C.; Liang, Y.M.; Chu, Y.H. Synthesis of fused tetrahydro-β-carbolinequinoxalinones in 1-n-butyl-2,3-dimethylimidazolium bis(trifluoromethylsulfonyl)imide ([bdmim][Tf2N]) and 1-n-butyl-2,3-dimethylimidazolium perfluorobutylsulfonate ([bdmim][PFBuSO3]) ionic liquids. Tetrahedron Lett. 2005, 46, 6131–6136. [Google Scholar] [CrossRef]
- Schank, K.; Lick, C. Ozonolytic fragmentation of phenyliodonium β-diketonates; a convenient synthesis of unsolvated vic-triketones. Synthesis 1983, 5, 392–395. [Google Scholar] [CrossRef]
- Tehfe, M.A.; Lalevée, J.; Telitel, S.; Sun, J.; Zhao, J.; Graff, B.; Morlet-Savary, F.; Fouassier, J.P. Iridium complexes incorporating coumarin moiety as catalyst photoinitiators: Towards household green LED bulb and halogen lamp irradiation. Polymer 2012, 53, 2803–2808. [Google Scholar] [CrossRef]
- Criqui, A.; Lalevée, L.; Allonas, X.; Fouassier, J.P. Electron spin resonance spin trapping technique: Application to the cleavage process of photoinitiators. Macromol. Chem. Phys. 2008, 209, 2223–2231. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds S5 and S6 are available from the authors. |

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kirschner, J.; Paillard, J.; Bouzrati-Zerelli, M.; Becht, J.-M.; Klee, J.E.; Chelli, S.; Lakhdar, S.; Lalevée, J. Aryliodonium Ylides as Novel and Efficient Additives for Radical Chemistry: Example in Camphorquinone (CQ)/Amine Based Photoinitiating Systems. Molecules 2019, 24, 2913. https://doi.org/10.3390/molecules24162913
Kirschner J, Paillard J, Bouzrati-Zerelli M, Becht J-M, Klee JE, Chelli S, Lakhdar S, Lalevée J. Aryliodonium Ylides as Novel and Efficient Additives for Radical Chemistry: Example in Camphorquinone (CQ)/Amine Based Photoinitiating Systems. Molecules. 2019; 24(16):2913. https://doi.org/10.3390/molecules24162913
Chicago/Turabian StyleKirschner, Julie, Julien Paillard, Mariem Bouzrati-Zerelli, Jean-Michel Becht, Joachim E. Klee, Saloua Chelli, Sami Lakhdar, and Jacques Lalevée. 2019. "Aryliodonium Ylides as Novel and Efficient Additives for Radical Chemistry: Example in Camphorquinone (CQ)/Amine Based Photoinitiating Systems" Molecules 24, no. 16: 2913. https://doi.org/10.3390/molecules24162913
APA StyleKirschner, J., Paillard, J., Bouzrati-Zerelli, M., Becht, J.-M., Klee, J. E., Chelli, S., Lakhdar, S., & Lalevée, J. (2019). Aryliodonium Ylides as Novel and Efficient Additives for Radical Chemistry: Example in Camphorquinone (CQ)/Amine Based Photoinitiating Systems. Molecules, 24(16), 2913. https://doi.org/10.3390/molecules24162913